OBJECTIVES:
Coronavirus disease 2019 has been reported to be a prothrombotic condition; however, multicenter data comparing this with other viral pneumonias in those requiring extracorporeal membrane oxygenation are lacking. We conducted a multicenter study using whole-body CT to examine the prevalence, severity, and nature of vascular complications in coronavirus disease 2019 in comparison with patients with other viral pneumonias.
DESIGN:
We analyzed whole-body CT scans for the presence of vascular thrombosis (defined as pulmonary artery thrombus, venous thrombus, systemic arterial thrombus, or end-organ infarct). The severity, distribution, and morphology of pulmonary artery thrombus were characterized. Competing risk cumulative incidence analysis was used to compare survival with discharge.
SETTING:
Three centers of the English national extracorporeal membrane oxygenation service.
PATIENTS:
Consecutive patients admitted with either coronavirus disease 2019 or noncoronavirus disease 2019 viral pneumonia admitted from January 2019.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
One-hundred thirty-six patients (45.2 ± 10.6 yr old, 39/146 [27%] female) requiring extracorporeal membrane oxygenation support underwent whole-body CT scans at admission. Of these, 86 had coronavirus disease 2019 pneumonia, and 50 had noncoronavirus disease 2019 viral pneumonia. Vascular thrombosis was seen more often in patients with coronavirus disease 2019 (odds ratio, 12.9 [95% CI 4.5–36.8]). In those with coronavirus disease 2019, 57 (73%) demonstrated pulmonary artery thrombus or pulmonary perfusion defects. Eighty-two percent of thrombus exhibited emboli-like morphology. The location of pulmonary artery thrombus and parenchymal perfusion defects was only concordant in 30% of cases. The risk of mortality was higher in those with coronavirus disease 2019 compared with noncoronavirus disease 2019 pneumonia (χ2 = 3.94; p = 0.047). Mortality was no different in coronavirus disease 2019 patients with or without vascular thrombosis (χ2 = 0.44; p = 0.51).
CONCLUSIONS:
In patients who received extracorporeal membrane oxygenation, coronavirus disease 2019 is associated with a higher prevalence of vascular thrombosis compared with noncoronavirus disease viral pneumonias. The pattern of pulmonary vascular changes suggests concurrent embolic disease and small vessel disease. Despite this, vascular thrombosis was not linked to poorer short-term prognosis in those with coronavirus disease 2019.
Keywords: coronavirus disease 2019, embolism and thrombosis, extracorporeal membrane oxygenation, tomography, x-ray computed
A high prevalence of vascular thrombosis has been reported in coronavirus disease 2019 (COVID-19), with this associated with an elevated risk of mortality (1). The prevalence of thrombus in COVID-19 varies with disease severity, reported in 5% of non-ICU cohorts and 31% of ICU cohorts in a recent meta-analysis (1).
However, other viral pneumonias have been reported to also exhibit high rates of thromboembolic events (2). Critically ill patients are generally predisposed to coagulopathy and thromboembolism due to a combination of systemic inflammation, platelet activation, endothelial dysfunction, and stasis of blood flow (3). Evidence that the prevalence or severity of vascular thrombosis is different from other viral pneumonias is limited (4). It is also not clear whether the high reported rates of pulmonary artery thrombus (PAT) is thromboembolic or an in situ thrombosis due to a combination of severe local inflammatory changes and microvascular angiopathy (5).
Identification of the true prevalence and risk of COVID-19 for vascular thrombosis as well as its nature is important as it may guide treatment, either in the use of current anti-thrombotic drugs or development of new treatments targeting a virus-specific causative pathway (6).
We conducted a multicenter study using whole-body CT to examine the prevalence, severity, and nature of vascular complications in severe COVID-19 in comparison with patients with other viral pneumonias.
METHODS
This multicenter retrospective observational study was performed at three of the five centers providing extracorporeal membrane oxygenation (ECMO) in United Kingdom. Standard procedure in these ECMO services includes the routine acquisition of a whole-body CT at the point of admission to the ECMO center to look for complications of ECMO insertion, intervenable complications of the condition requiring ECMO support, and any findings to suggest futility of care. The imaging assessments are done after ECMO has been initiated, and the patient is transferred back to the ECMO center. The study was approved by the institutional review boards of the three centers (Royal Papworth Hospital Research and Development, reference S02716) with a waiver for consent due to the observational nature of the study.
Study Population
The study included consecutive patients older than 18 years admitted for viral pneumonia between January 1, 2019, and April 30, 2020. Diagnoses were made based on a positive viral reverse transcriptase-polymerase chain reaction. Unless contraindicated, all patients were on prophylactic low molecular weight heparin prior to ECMO. Once on ECMO, all patients receive heparinization aiming activated partial thromboplastin time ratio 1.5–2 or anti-Xa 0.3–0.7—unless deep vein thrombosis/pulmonary embolus is demonstrated in which case full anticoagulation was initiated. None of the patients received routine antiplatelets unless administered for other indications.
Data Collection
Clinical data were collected from the patient’s notes at the point of referral to the ECMO center. This included past medical history, and baseline observations and biochemical markers obtained at the time of admission for ECMO.
The whole-body CT scans included a noncontrast CT head and post-IV contrast CT chest abdomen and pelvis to the upper thigh following the administration of IV contrast agent. The precise scanning protocol varied slightly between centers but ultimately provided for a minimum dataset of a systemic arterial phase chest scan and a portal venous phase abdomen and pelvis scan.
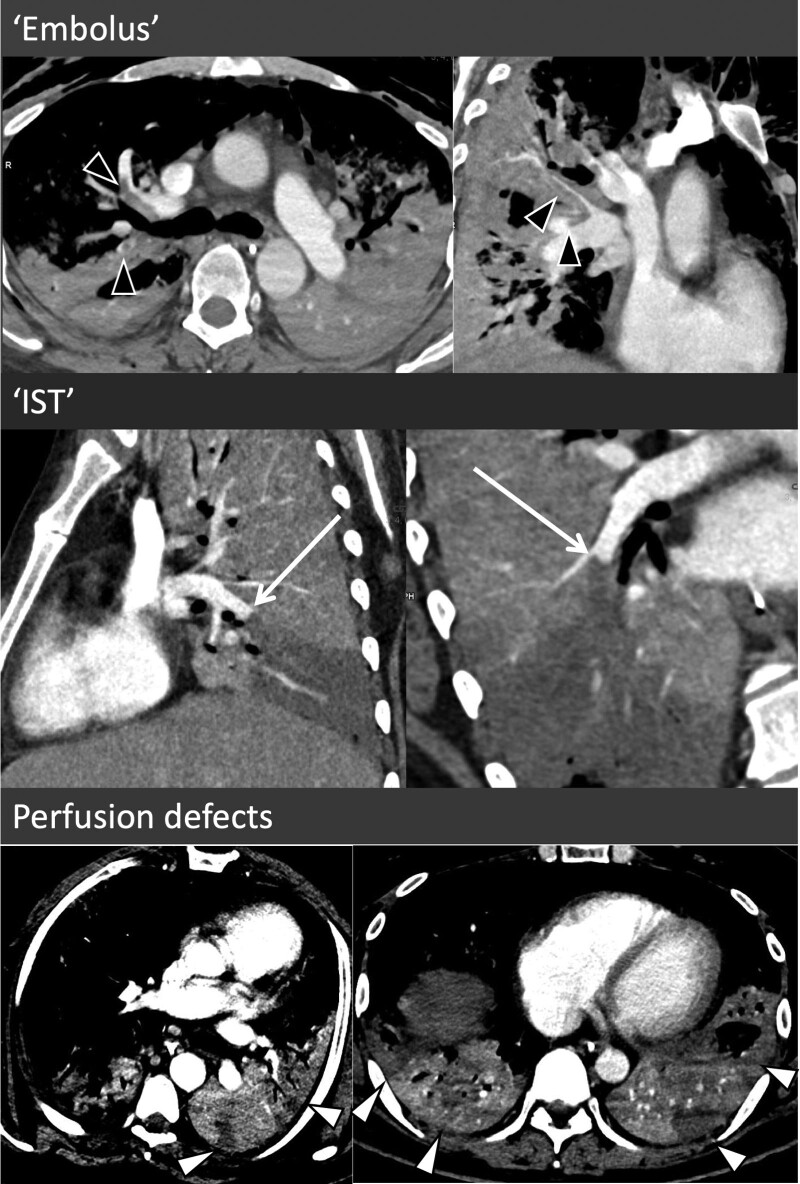
A dedicated systematic review of the CT scans was performed for the current study. Scans were reviewed for vascular thrombosis in the pulmonary and systemic circulation and venous circulation. Pulmonary arterial thrombosis (PAT) was defined as a filling defect within the pulmonary vasculature including within the central, branch, segmental, or subsegmental vessels. PAT was further characterized according to their morphology and severity. The morphology was considered consistent with embolus if it exhibited features of central filling defect within the vessel with peripheral contrast opacification, straddling bifurcations with clot extending into both branches, and visualization of contrast distal to the location of the clot (presence of this was considered strongly suggestive of embolus, but its absence was not considered exclusive of embolus). Morphology suggestive of an in situ thrombus (IST) was considered if there was complete pulmonary occlusion with a proximal concave morphology, with the clot occurring in regions of diseased lung (Fig. 1 for examples of the two morphology types). Where neither of these patterns were met nor there was overlap, the morphology was considered indeterminate. To assist in intercenter homogeneity of classification, slides with the above criteria were circulated to each center with several exemplar images of each classification contained within this. For this classification, a single reader at each center reread all the studies with PAT. Any initially scored as indeterminate was reread with the lead center before final classification was assigned. For severity, the PAT was characterized as unilateral versus bilateral, extent of distribution (central, branch, segmental, subsegmental) with this recorded based on the most proximal clot, with the number of segments involved recorded on an ordinal scale (single segment, 2–4 segments, ≥ 5 segments). The presence, location, and segmental involvement of pulmonary parenchymal hypoperfusion were also recorded (Fig. 1).
Figure 1.
Morphologic features of pulmonary artery thrombus (PAT) and parenchymal perfusion defects. In the top two images, the PAT exhibits typical characteristic of multiple emboli with central filling defects with peripheral contrast opacification, extending into both branches at a bifurcation points with some of these exhibiting opacification of the pulmonary arteries distally (black arrowheads). In the middle two images, there is complete occlusion of the right lower lobe pulmonary artery with a large wedge perfusion defect in an area of severely disease lung. Rather than the classical crescent of an embolus, the clot is creeping along the outer edge of the vessel toward a more proximal branch (white arrow). The bottom images demonstrate two examples of parenchymal perfusion defects. IST = in situ thrombus.
The presence of end-organ infarcts and evidence of hemorrhage were also collected. End-organ infarcts were considered present in the presence of a perfusion defect in a vascular territory or whole organ hypoperfusion with a visible arterial or venous occlusion. CNS watershed infarcts or global hypoxic-ischemic injury was not classed as ischemic events as these are typically due to global hypoperfusion rather than focal vascular thrombosis. Ischemic colitis was similarly not classed as an ischemic event if it occurred in a watershed territory. Hematomas at the sites of ECMO cannulation were not classed as hemorrhagic events.
Patient status at the time of data collection was recorded using the World Health Organization COVID-19 Therapeutic Trial Synopsis seven-point ordinal scale (where 1 = deceased, 2 = ECMO, 3 = invasive ventilation, 4 = noninvasive ventilation, 5 = hospital on oxygen, 6 = hospital not requiring oxygen, and 7 = discharged). In patients transferred back to their base hospital for longer term care following discharge from the ICU, their status at the time of transfer was used, and the date of discharge was used in the analysis. In patients still in ICU, their data were censored at the point of data collection.
Outcomes
The primary outcome was the relative frequency of vascular thrombosis in COVID-19 compared with non–COVID-19 viral pneumonia. Vascular thrombosis was defined as imaging evidence of PAT, venous thrombus, systemic arterial thrombosis, or end-organ infarct.
Secondary outcomes included the relative frequency of hemorrhage and association of the presence of vascular thrombosis with clinical outcomes.
Due to a combination of patient factors and resource limitation, 22 patients with COVID-19 and one patient with non–COVID-19 pneumonia had a delay of more than 24 hours between their initiation of ECMO and their CT. The use of continuous heparin infusion while on ECMO is likely to have impacted the prevalence of both thrombosis and hemorrhage. As a result, only those patients with a CT acquired within 24 hours of ECMO initiation were included for analyses.
Statistical Analysis
Continuous variables are reported as mean ± sd, whereas categorical variables are reported as n/N (%). For continuous variables, independent t tests were used to compare the baseline variables and prevalence of vascular outcomes between those with and without COVID-19, as well as to compare characteristics between those with and without vascular thrombosis. Chi-square or Fisher exact tests were used for categorical and ordinal variables as appropriate. Logistic regression was used to compare rates of vascular thrombosis between those with and without COVID-19 and factors associated with thrombus. A competing risk analysis was performed using the “cmprsk” package within R (https://cran.r-project.org/web/packages/cmprsk/cmprsk.pdf). Cumulative incidence plots were constructed for death and survival until discharge with censoring for those still in ICU at the time of data collection. For modeling of factors associated with mortality in COVID-19, a competing risk regression was performed using a proportional hazards model as per Fine and Gray (7). Multivariable analysis was not performed due to the limited number of endpoints. Statistical significance was defined as p value of less than 0.05. All analysis was performed using SPSS v25 (IBM, Chicago, IL) and R (RStudio version 1.1.463, RStudio, Boston, MA).
RESULTS
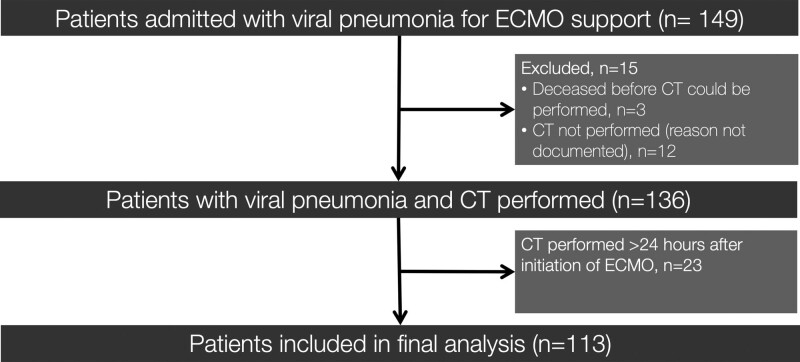
A total of 149 patients supported with venovenous ECMO were admitted. We excluded 12 who did not have a CT, three who died after ECMO initiation and before CT, and 23 who had a CT greater than 24 hours after admission (Fig. 2). The remaining 113 patients (44.7 ± 10.5 yr old; 35/113 [31%] female) included 64 with COVID-19 pneumonia and 49 with non–COVID-19 viral pneumonia.
Figure 2.
Study flow chart of participants included in the study. ECMO = extracorporeal membrane oxygenation.
No difference in age was present between those with and without COVID-19. Those with COVID-19 were more likely to be male (COVID: 49/64 [77%], non-COVID: 30/49 [59%]; p = 0.048) and had a higher prevalence of background hypertension (COVID: 14/64 [23%], non-COVID: 0/50 [0%]; p < 0.001) and diabetes (COVID: 16/64 [25%], non-COVID: 3/49 [6%]; p = 0.01) (Table 1).
TABLE 1.
Comparison of Baseline Variables Between Those With Coronavirus Disease 2019 and Noncoronavirus Disease 2019 Viral Pneumonias
| Clinical Variables | COVID 2019 | Non-COVID | p |
|---|---|---|---|
| N | 64 | 49 | |
| Age, yr, mean ± sd | 45.0 ± 9.4 | 44.2 ± 11.9 | 0.71 |
| Sex, female, n (%) | 15 (23) | 20 (41) | 0.048 |
| Body mass index, mean ± sd | 28.9 ± 7.0 | 29.1 ± 7.7 | 0.93 |
| Hypertension, n (%) | 14 (23) | 0 (0) | < 0.001 |
| Cardiovascular disease, n (%) | 1 (2) | 0 (0) | 1 |
| Chronic kidney disease, n (%) | 1 (2) | 0 (0) | 1 |
| Diabetes, n (%) | 16 (25) | 3 (6) | 0.01 |
| Preexisting lung disease, n (%) | 9 (14) | 11 (22) | 0.25 |
| Asthma | 8 (13) | 7 (14) | |
| Other | 1 (1) | 4 (8) | |
| Viral etiology, n (%) | |||
| Severe acute respiratory syndrome coronavirus 2 | 64 (100) | 0 | |
| Influenza | 0 | 32 (65) | |
| Respiratory syncytial virus | 0 | 2 (4) | |
| Rhinovirus | 0 | 4 (8) | |
| Coronavirus (non-COVID) | 0 | 2 (4) | |
| Adenovirus | 0 | 3 (6) | |
| Other | 0 | 6 (13) | |
| Vitals at admission to extracorporeal membrane oxygenation, mean ± sd | |||
| Heart rate, beats/min | 88.8 ± 20.5 | 91.9 ± 19.3 | 0.51 |
| Systolic BP, mm Hg | 116 ± 20 | 108 ± 13 | 0.03 |
| Diastolic BP, mm Hg | 68 ± 15 | 56 ± 7 | 0.001 |
| Pao2, kPa | 12.8 ± 8.9 | 14.7 ± 7.1 | 0.34 |
| Baseline biochemical markers | |||
| WBC, 109/L | 12.9 ± 7.2 | 11.0 ± 9.1 | 0.24 |
| Lymphocytes, 109/L | 0.85 ± 0.46 | 0.67 ± 0.56 | 0.07 |
| Platelet, 109/L | 300 ± 120 | 178 ± 87 | < 0.001 |
| C-reactive protein, mg/L | 258 ± 130 | 192 ± 101 | 0.004 |
| d-dimer, mg/L | 13.6 ± 20.0 | a | |
| Prothrombin time, s | 13.5 ± 2.2 | 15.4 ± 4.0 | 0.002 |
| Activated partial thromboplastin time, s | 40.2 ± 21.2 | 50.0 ± 27.9 | 0.046 |
| Fibrinogen, g/L | 7.72 ± 9.94 | 4.09 ± 2.59 | 0.01 |
| Urea, mmol/L | 14.7 ± 9.9 | 11.3 ± 7.0 | 0.04 |
| Creatinine, μmol/L | 177 ± 190 | 154 ± 104 | 0.42 |
| Acute Physiology and Chronic Health Evaluation score | 13.9 ± 6.1 | 14.4 ± 4.1 | 0.70 |
BP = blood pressure, COVID = coronavirus disease.
ad-dimer was not routinely collected in extracorporeal membrane oxygenation admission bloods pre COVID.
The prevalence of vascular thrombosis was 38 of 64 (59%) for patients with COVID-19 and five of 49 (10%) for patients with non–COVID-19 pneumonia (Table 2). PAT made up the majority of these, with 33 of 64 COVID-19 patients (52%) having PAT, five of 64 (8%) having venous thrombosis, and four of 64 (6%) demonstrating end-organ ischemia. Of those with end-organ ischemia, three patients had strokes, two had splenic infarcts, and one had ischemic colitis secondary to portal vein thrombosis. No systemic arterial thrombosis was evident.
TABLE 2.
Comparison of Imaging Findings Between Those With Coronavirus Disease 2019 and Noncoronavirus Disease 2019 Viral Pneumonias in Those With CT Performed With 24 Hours of Initiation of Extracorporeal Membrane Oxygenation
| CT Findings | COVID 2019 | Non-COVID | OR (95% CI) | p |
|---|---|---|---|---|
| N | 64 | 49 | ||
| Vascular thrombosis, n (%) | 38 (59) | 5 (10) | 12.9 (4.5–36.8) | < 0.001 |
| Pulmonary artery thrombus | 33 (52) | 5 (10) | 9.4 (3.3–26.7) | < 0.001 |
| Venous thrombus | 5 (8) | 1 (2) | 4.1 (0.5–36.0) | 0.21 |
| Arterial | 0 | 0 | 1 | |
| End-organ ischemiaa,b, n (%) | 4 (6) | 0 (0) | 1 | |
| Brain | 3 (5) | 0 | ||
| Spleen | 2 (3) | 0 | ||
| Renal | 0 | 0 | ||
| Bowel | 1 (2) | 0 | ||
| Hemorrhagea, n (%) | 10 (16) | 4 (8) | 2.1 (0.6–7.1) | 0.24 |
| Brain | 9 (14) | 3 (6) | ||
| Abdomen | 1 (2) | 1 (2) | ||
| Other | 0 (0) | 0 (0) |
COVID = coronavirus disease, OR = odds ratio.
aSubtotals may add up to more than total as ischemia/hemorrhage could occur in more than one territory.
bOR not calculable as zero events in the non-COVID 2019 group.
There was no difference in the location or extent of PAT between COVID-19 and non-COVID pneumonia (Supplementary Table S1, http://links.lww.com/CCM/G849). Pulmonary parenchymal perfusion defects were significantly more common in COVID (42/64; 66%) than non-COVID pneumonia (7/49; 14%) (Supplementary Table S1, http://links.lww.com/CCM/G849). In those with COVID-19, the majority of thrombi exhibited embolus-like features (82%), with only 12% exhibiting IST-like features and 6% being indeterminate. Fifty-seven (73%) demonstrated evidence of PAT or parenchymal perfusion defects. Of these, PAT occurred in isolation in five patients (8%) with no perfusion defect, 14 patients (22%) had perfusion defects with no pulmonary emboli, and 28 (44%) had both PAT and perfusion defect. Thirty-six percent of PAT were in regions of the lung most severely affected by consolidation and ground glass changes, and the location of PAT and perfusion defects were only concordant in 30% of cases.
On binary logistic regression, the diagnoses of COVID-19 were associated with an odds ratio (OR) of 12.9 (95% CI, 4.5–36.8; p < 0.001) for vascular thrombosis. On multiple variable regression in a model including age, sex, hypertension, and diabetes, the risk remained elevated with an OR for vascular thrombosis of 7.4 (95% CI, 2.4–22.5; p < 0.001). COVID-19 was associated with an OR of 11.5 (95% CI, 4.4–29.7; p < 0.001) for pulmonary parenchymal perfusion defect. If these were considered evidence of thrombotic event, then 51 of 64 patients (79.7%) with COVID had evidence of thrombosis of their admission study, with an OR of 20.1 (95% CI, 7.6–53.1; p < 0.001) for thrombosis.
The prevalence of hemorrhage was 10 of 64 (16%) in the COVID-19 patients and four of 49 (8%) in non–COVID-19 patients (OR, 2.1 [95% CI, 0.6–7.1]) (Table 2). Intracranial hemorrhage accounted for the majority of these events, with nine of 64 patients (14%) with COVID-19 having evidence of an intracranial bleed and one of 64 (2%) having an intramuscular rectus sheath hematoma.
There was no difference in baseline clinical variables, observations, nor biochemical markers in COVID-19 patients, with and without vascular thrombosis (Supplementary Table S2, http://links.lww.com/CCM/G849).
At the time of data collection, follow-up of a median of 13 days (range, 0–36 d) was available for the COVID-19 patients and 15 days (range, 1–67 d) for the non–COVID-19 patients.
On May 30, 2020, of the 64 COVID patients, 18 of 64 (28 %) had died, 13 of 64 (20%) remained on ECMO, nine of 64 (14 %) were off ECMO support but remained on ventilatory support, eight of 64 (13 %) remained in ICU on noninvasive ventilation, seven of 64 (11 %) remained in hospital on oxygen therapy, three of 64 (5 %) were in hospital without supplemental oxygen therapy, and six of 64 (9 %) had been discharged. In those with non–COVID-19 viral pneumonia, 15 of 50 (30%) had died, and 35 of 50 (70%) survived to discharge.
In those patients with COVID-19, there was no difference in the cumulative prevalence of death (χ2 = 0.43; p = 0.51) or discharge (χ2 = 0.57; p = 0.45) between those with or without vascular thrombosis on CT (Supplemental Fig. S1, http://links.lww.com/CCM/G849). Nor was there any difference in the cumulative prevalence of death (χ2 = 3.62; p = 0.057) or discharge (χ2 = 0.53; p = 0.47) between those with or without hemorrhage. In univariate competing risk regression, age, history of CVD, CKD, d-dimer levels, fibrinogen, and troponin were positively associated with mortality in those with COVID-19 (Supplementary Table S3, http://links.lww.com/CCM/G849).
DISCUSSION
In this multicenter study of patients with severe viral pneumonia requiring ECMO support, we found that those with COVID-19 had a 12-fold greater risk of vascular thrombosis compared with other viral pneumonias. Despite this, there was no difference in outcomes between COVID-19 patients with and without vascular thrombosis.
Vascular thrombosis rates in COVID-19 range from 3–23% of patients admitted to hospital requiring ward level care (8, 9) to 10–69% in patients admitted to ICU (10–12). Concerns have been raised that this is an overestimate as most studies report the cumulative event rates, with a further risk of confirmation bias (11). In one study comparing COVID-19 patients with a historical control, 66% of COVID-19 patients compared with only 28% of the historic cohort received CT pulmonary angiogram due to a hyperawareness of a potential COVID-19 thrombosis interaction leading to greater efforts to investigate for it (12). Our findings of a 59% vascular thrombosis rate confirm a high prevalence of vascular thrombosis when a systematic approach free from referral bias is undertaken. This is similar to the 51% prevalence reported in a smaller single-center study of COVID-19 patients requiring ECMO (13).
Postmortem examinations show increased thrombosis, microangiopathy, and endothelialitis in COVID-19 compared with other viral pneumonias (14). Consistent with these autopsy findings, we observed both a high prevalence of PAT and peripheral wedge perfusion defects. The predominate morphologic appearance of the PAT was that of thromboembolism, further supported by its distribution in regions of lung which were least affected by consolidation. As consolidation induces regional hypoxia which in turn induces vasoconstriction, higher blood flow in the regions of normal lung aeration is relatively increased, increasing the likelihood of emboli ending up in the segments. Although we only observed peripheral venous thrombus in 8% of our cohort, which might suggest that the PAT was not embolic, this may be due to the CT scan not covering the whole length of the legs and could only assess for proximal venous thrombosis. Previous studies using ultrasound to scan the whole leg report rates of 15–46% of screened hospital patients with COVID-19 with 58% reported in postmortem studies (15–18). That 37% of wedge parenchymal perfusion defects occurred without visible PAT, and when the two were present in the same patient, their distribution was only concordant in 30% of cases supports the notion of separate parallel pathophysiologic process driving micro- and macrovascular disease. A recent study has shown that the Spike protein of the COVID virus directly damages vascular mitochondria (19). Thus, the perfusion defects may represent microangiopathy secondary to this, whereas the macrovascular thrombosis is secondary to a severe inflammatory and hypercoaguable state, although further work is necessary to better explore these hypotheses.
The need for an understanding of the processes that lead to thrombosis is heightened by the finding of a 12-fold higher rate of vascular thrombosis in COVID-19 compared with other viral pneumonias. A 26% PAT prevalence was reported in 107 ICU patients at a single center with COVID-19 compared with a 7% prevalence admitted with influenza in 2019 (20). Helms et al (12) reported a three-fold higher rate of PAT in 150 COVID-19 patients, although the study compared this with a historical cohort of acute respiratory distress syndrome patients admitted to ICU rather than a viral pneumonia cohort. Our multicenter study in consecutive patients confirms these findings of an elevated thrombotic risk particular to COVID-19. The collective trial group (Anti-thrombotics for Adults Hospitalized With COVID-19, Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community- Acquired Pneumonia, and Antithrombotic Therapy to Ameliorate Complications of COVID-19) found no benefit of routine therapeutic dose anticoagulation in intensive care patients, whereas the Intermediate vs Standard-Dose Prophylactic Anticoagulation in Critically-ill Patients With COVID-19: An Open Label Randomized Controlled Trial (INSPIRATION) trial found no benefit of intermediate-dose anticoagulation (21, 22). Thus, despite our findings of a high prevalence of vascular thrombosis, current evidence does not support more aggressive preemptive treatment. It does however highlight the high prevalence of such findings, which if identified in a routine and systematic manner as performed in the current study may identify those with most to gain from more aggressive therapy. The ongoing antiplatelet arms of the REMAP-CAP and RECOVERY trial will provide further evidence on alternate approaches to tackling the thrombotic complications of COVID-19.
There are several limitations to the current study. Included patients were all suffering from a severe form of pulmonary disease justifying the use of ECMO. Due to the wide geographical catchment areas of the ECMO centers, data availability on treatment prior to transfer is incomplete limiting comment on, or comparison of differences between, pretransfer treatment approaches between the two groups. The first wave of the COVID-19 pandemic was a national emergency and resulted in an acute increase in intensive care workload. As a result, differences in care may have resulted between the groups as highlighted by the delay in acquisition of the CT in 27% of COVID patients, but only 2% of non-COVID pneumonias. We have attempted to address this issue comparing those with immediate CT scans between the groups but cannot exclude further downstream differences in care which could influence results. Our assessment of perfusion defects was limited to visual assessment of contrast-enhanced CT, rather than dedicated dual-energy CT which could more directly quantify perfusion. Thus, subtle hypoperfusion will have been overlooked. Indeed, a dual-energy study in COVID-19 patients on ECMO showed hypoperfusion in all patients despite PAT only being evident in 39%, further supporting a parallel microvascular and macrovascular process (23).
In conclusion, in patients who received ECMO, COVID-19 is associated with a significantly higher prevalence of vascular thrombosis compared with non-COVID viral pneumonias of similar severity. The pattern of pulmonary vascular changes suggests concurrent embolic disease and local small vessel disease with further work required to explore this issue. Despite this, vascular thrombosis was not linked to poorer short-term prognosis in those with COVID-19.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Drs. Weir-McCall, Galea, Screaton, and Vuylsteke were involved in the design of the study, delivery of the study, interpretation of the results, and writing of the report. Drs. Mun Mak, Joshi, Agrawal, Toshner, Benedetti, Brozik, Machin, Das, Marusa, Sun, and Camporota were involved in the delivery of the study and writing of the report. Drs. Jacob and Rodrigues were involved in the design of the study and writing of the report. All authors have reviewed the final version of the article.
Supported, in part, by the National Institute for Health Research Cambridge Biomedical Research Centre.
Dr. Weir-McCall received support for article research from Research Councils UK. Dr. Toshner received funding from Bayer and Actelion/Jansen; he disclosed he is a member of MorphogenIX scientific advisory board. Drs. Toshner and Jacob received funding from GlaxoSmithKline. Dr. Rodrigues received funding from Sanofi. Drs. Rodrigues and Jacob received funding from National Health Service Digital. Dr. Jacob received funding from Boehringer Ingelheim and Roche; he received support for article research from Wellcome Trust/Charity Open Access Fund. Dr. Jacob is supported by a grant 209553/Z/17/Z from the Wellcome Trust and the National Institute for Health Research University of College London Biomedical Research Center. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Malas MB, Naazie IN, Elsayed N, et al. : Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: A systematic review and meta-analysis [Internet]. EClinicalMedicine 2020; 29–30:100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal PP, Cinti S, Kazerooni EA: Chest radiographic and CT findings in novel swine-origin influenza A (H1N1) virus (S-OIV) infection. AJR Am J Roentgenol 2009; 193:1488–1493 [DOI] [PubMed] [Google Scholar]
- 3.Walsh TS, Stanworth SJ, Prescott RJ, et al. ; Writing Committee of the Intensive Care Study of Coagulopathy Investigators: Prevalence, management, and outcomes of critically ill patients with prothrombin time prolongation in United Kingdom intensive care units. Crit Care Med 2010; 38:1939–1946 [DOI] [PubMed] [Google Scholar]
- 4.Levi M, Thachil J, Iba T, et al. : Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol 2020; 7:e438–e440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Price LC, McCabe C, Garfield B, et al. : Thrombosis and COVID-19 pneumonia: The clot thickens! Eur Respir J 2020; 56:2001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paranjpe I, Fuster V, Lala A, et al. : Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol 2020; 76:122–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94:496–509 [Google Scholar]
- 8.Stoneham SM, Milne KM, Nuttal E, et al. : Thrombotic risk in COVID-19: A case series and case–Control study. Clin Med (Northfield Il) 2020; 20:e76–e81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bompard F, Monnier H, Saab I, et al. : Pulmonary embolism in patients with COVID-19 pneumonia. Eur Respir J 2020; 56:2001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui S, Chen S, Li X, et al. : Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost 2020; 18:1421–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klok FA, Kruip MJHA, van der Meer NJM, et al. : Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020; 191:145–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helms J, Tacquard C, Severac F, et al. : High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med 2020; 46:1089–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirsadraee S, Gorog DA, Mahon CF, et al. : Prevalence of thrombotic complications in ICU-treated patients with coronavirus disease 2019 detected with systematic CT scanning. Crit Care Med 2021; 49:804–815 [DOI] [PubMed] [Google Scholar]
- 14.Ackermann M, Verleden SE, Kuehnel M, et al. : Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med 2020; 49:804–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wichmann D, Sperhake J, Lütgehetmann M, et al. : Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med 2020; 25:M20–2003 [DOI] [PubMed] [Google Scholar]
- 16.Demelo-Rodríguez P, Cervilla-Muñoz E, Ordieres-Ortega L, et al. : Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb Res 2020; 192:23–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Feng X, Zhang D, et al. : Deep vein thrombosis in hospitalized patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: Prevalence, risk factors, and outcome. Circulation 2020; 142:114–128 [DOI] [PubMed] [Google Scholar]
- 18.Voicu S, Bonnin P, Stépanian A, et al. : High prevalence of deep vein thrombosis in mechanically ventilated COVID-19 patients. J Am Coll Cardiol 2020; 76:480–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lei Y, Zhang J, Schiavon CR, et al. : SARS-CoV-2 Spike protein impairs endothelial function via downregulation of ACE 2. Circ Res 2021; 128:1323–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poissy J, Goutay J, Caplan M, et al. ; Lille ICU Haemostasis COVID-19 Group: Pulmonary embolism in patients with COVID-19: Awareness of an increased prevalence. Circulation 2020; 142:184–186 [DOI] [PubMed] [Google Scholar]
- 21.The REMAP-CAP, ACTIV-4a, ATTACC Investigators, Zarychanski R: Therapeutic anticoagulation in critically ill patients with COVID-19 – Preliminary report. medRxiv 2021.03.10.21252749 [Google Scholar]
- 22.Sadeghipour P, Talasaz AH, Rashidi F, et al. : Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: The INSPIRATION randomized clinic. JAMA 2021; 325:1620–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel BV, Arachchillage DJ, Ridge CA, et al. : Pulmonary angiopathy in severe COVID-19: Physiologic, imaging, and hematologic observations. Am J Respir Crit Care Med 2020; 202:690–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.