Abstract
Background
Malnutrition and sarcopenia are a growing concern in community-dwelling older adults. Hospitalization increases the risk of malnutrition and leads to a decline in functional and nutritional status at discharge. Persistent malnutrition after hospital discharge may worsen posthospital outcomes, including readmissions. The aim of this study was to determine dietary intakes and nutrient distribution patterns of community-dwelling older adults after acute hospitalization.
Method
Participants (65 years and older, n = 85) were enrolled during acute hospitalization and dietary 24-hour recalls were collected weekly for 1 month postdischarge. Analysis included change in dietary intake over recovery timeframe; daily intake of energy, protein, fruit, vegetables, and fluids; comparison of intake to recommendations; distribution of energy and protein across mealtimes; and analysis of most common food choices.
Results
Most participants did not meet current recommendations for energy, fruit, vegetables, or fluids. Average protein consumption was significantly higher than the current recommendation of 0.8 g/kg/d; however, only 55% of participants met this goal and less than 18% met the 1.2 g/kg/d proposed optimal protein intake for older adults. The protein distribution throughout the day was skewed and no one met the 0.4 g/meal protein recommendation at all meals.
Conclusions
Our findings indicate that community-dwelling older adults did not meet their nutritional needs during recovery after hospitalization. These data highlight the need for better nutritional evaluation and support of geriatric patients recovering from hospitalization.
Keywords: Dietary recall, Eating pattern, Geriatric, Malnutrition
Malnutrition, a serious problem among older adults, has negative effects on daily living and clinical outcomes (1,2). While difficult to quantify, the prevalence of malnutrition in otherwise healthy older adults living in the community has been estimated to range from 5% to 30% (3). In comparison, in patients admitted to the hospital acute care setting, malnutrition prevalence is much higher, ranging from 23% to 60%, depending on the screening tool used and severity of illness (3). The higher the intensity of care, the more likely a patient will have malnutrition (3).
Patients often experience a nutritional decline during hospitalization, which can be attributed to concomitant problems occurring during the hospital stay. Such acute care conditions include sleep deprivation and disruption, challenging and stressful situations, nihil per os (nothing by mouth) orders, inability to self-feed, physical deconditioning, and medications’ side effects. These problems also contribute to the “posthospital syndrome” (4–6), which is a period of increased vulnerability occurring after hospitalization that can lead to readmissions. One study found that nutritional status declined from admission to discharge in 31% of the patients, with others reporting this decline to be as high as 70% (7,8).
Sarcopenia, the loss of muscle mass and function with aging, can worsen the posthospital syndrome and lead to an increased risk of “catabolic crisis” in the postacute care of older adults (9). The risk of undernutrition at discharge overlaps with other risk factors for readmission; increases morbidity, mortality, and health care costs; and decreases quality of life (10,11). The Academy of Nutrition and Dietetics and the American Society for Parenteral and Enteral Nutrition have called for the development of care plans for this high-risk population. However, no models are currently in place and little research has been conducted in this population (10,12).
The primary aim of this study was to determine dietary intakes and nutrient distribution patterns of community-dwelling older adults after acute hospitalization. We also compared these findings to national standards for intake of energy, protein, fruits and vegetables, and fluid. While energy and protein intake directly impact the healing and recovery processes, fluid intake and the fibers and micronutrients contained in fruits and vegetables are also important factors that can affect recovery. The hypothesis was that older adults recovering from hospitalization have substandard dietary intakes.
Materials and Methods
Study Cohort
This nutrition analysis was carried out in a subsample of participants enrolled in 2 randomized controlled pilot trials. These studies were approved by the University of Texas Medical Branch Institutional Review Board and are registered at ClinicalTrials.gov (NCT02203656 and NCT02990533). Written informed consent was obtained from each participant prior to any study procedure. All data were collected from a single university hospital. The methods, inclusion/exclusion criteria, and intervention groups have been previously published in detail (13,14). Briefly, subjects included in this analysis were hospitalized for acute medical care. We excluded surgery, intensive care unit, hospice, or palliative care patients. Common diagnoses included congestive heart failure, gastrointestinal bleeding, respiratory infections, and metabolic disorders. Those with an active cancer diagnosis or liver or kidney failure were excluded (13,14).
Data Collection
Prior to discharge, baseline testing consisted of an enrollment interview (demographic information), the subjective global assessment (SGA) malnutrition screening tool, and a series of functional assessments (gait speed, hand grip). During weekly follow-up phone calls, a guided Automated-Self Administered 24-hour (ASA24) Dietary Assessment tool was conducted to collect the dietary intake data. The ASA24 program was used by the study team to guide the recalls and record the data. ASA24 utilizes the Food and Nutrient Database for Dietary Studies (FNDDS) and the U.S. Department of Agriculture’s (USDA) Food Pyramid Equivalents Database.
Analytical Methods
Change in intake over the recovery period
To determine if nutrient intake changed during the early stage versus late stage of posthospital recovery, recalls were compared at 2 time points. The change in nutrients intake for each participant were calculated between the first recall (earliest available recall postdischarge, typically 1 week) and the last recall (last available recall postdischarge, typically 4 weeks). Intake measured included energy (kilocalories), protein (grams), fat (grams), and carbohydrate (grams).
Comparisons to recommendations
We compared the dietary intakes data to the general dietary guidelines usually employed in the clinical geriatric setting as detailed below.
Acceptable macronutrient distribution ranges.
The acceptable macronutrient distribution range (AMDR) for the study population was determined by calculating kilocalories from grams of protein, fat, and carbohydrate using the accepted 4, 9, and 4 kcal/g of food, respectively.
Energy.
Energy needs were based on the 2015 U.S. dietary guidelines. Calorie needs for males aged 65 years and older are estimated at 2 000 kcal/d, while females aged 65 years and older are estimated at 1 600 kcal/d (15).
Protein.
Average protein intake (g/kg) was calculated for each participant using protein intake (g) and ideal body weight (kg). Ideal body weight is often used when determining protein needs (16,17). Ideal body weight was calculated by adjusting the participant’s actual weight (kg) to the nearest weight that would give the participant a body mass index (BMI) between 22 and 27 kg/m2, which is the BMI range associated with lower mortality in older adults (16,17). The average intake was compared to the current recommended dietary allowance (RDA) of 0.8 g/kg/d for older adults and the optimal protein intake of 1.2 g/kg/d, which has been proposed in literature to improve health outcomes in older adults with acute or chronic diseases (18–20).
Fruits and vegetables.
According to the USDA MyPlate reference, it is recommended that men 51+ consume 2 cups of fruit/d and 2.5 cups of vegetables/d and women 51+ consume 1.5 cups of fruit/d and 2 cups of vegetables/d (21,22). These references were compared to the average cup equivalents of the participants.
Fluid.
Fluid needs were based on the 2005 Dietary Reference Intakes. Total daily adequate intake of fluid for men aged 65 years and older is 3.7 L and females aged 65 years and older is 2.7 L to prevent dehydration (23). This includes fluid from foods and beverages.
Meal patterns
Energy and protein intakes were assessed at each mealtime. Mealtimes were defined as follows: breakfast (6:00–10:59), lunch (11:00–15:59), dinner (16:00–20:59), and other for the remaining overnight timeframe (21:00–5:59). Differences in energy (kcal) and protein (g) between meals were compared. Meal protein consumption was also compared to the optimal 0.4 g protein/kg/meal threshold suggested for older adults that are malnourished or at risk of being malnourished (24,25).
Most common food choices
For each participant’s recall, foods reported were counted and grouped by category. Foods containing protein were grouped using ASA24 software in animal and nonanimal categories. Subcategories of animal and nonanimal proteins were also assigned. Subcategories for animal proteins included meat (beef, veal, pork, lamb, and game meat), poultry (chicken, turkey, Cornish hens, duck, goose, quail, and game birds), egg, dairy, seafood, and other animal protein. Subcategories for nonanimal proteins included grain, nut and seed, soy, other legume, fruits and vegetables, and other nonanimal protein. These subcategories were used as they are the categories utilized by the FNDDS and the USDA Food Pyramid Equivalents Database (26–28).
All reported fruits and vegetables were extracted and separated by type, processing/storage, and preparation method for consumption. Reports were tallied and reported as top fruit or vegetable choices. Types of fluid intake were determined by amounts of moisture per food. Foods providing moisture (soups, broths, stews) were counted by how many times they were reported. Small fluid amounts as determined by ASA24 software (coffee creamer, sauces, etc.) were not included in this analysis.
Statistical Analyses
Change in intake over recovery time was compared using a paired sample t test (early vs late). One-sample t tests were used to compare intake to recommendations (energy, protein, protein per meal, fruit, vegetable, and fluid). SPSS statistical software was used for all analysis. An α level of .05 determined significance.
Results
Baseline Participant Characteristics
Participant demographics are presented in Table 1. The mean age was 73 ± 8.1 years, 69% were female, and 75% were non-Hispanic White. According to BMI classification, 48% were obese, 20% were overweight, 28% were normoweight, and 4% were underweight. Using Subject Global Assessment, 56% of participants were classified as well nourished, 44% were moderately malnourished, and no participants were severely malnourished. Based on the Foundation for the National Institutes of Health (FNIH) Biomarkers Consortium Sarcopenia Project cutpoints, 28% of participants were found to be weak based on maximum hand grip and 60% were slow based on usual gait speed.
Table 1.
Participants’ Baseline Characteristics Including Nutrition and Physical Function Measures (n = 85) (29)
| Female | 69% |
| Age | 73.4 ± 8.1 |
| Race/ethnicity | |
| White, non-Hispanic | 75% |
| White, Hispanic | 13% |
| Black, non-Hispanic | 12% |
| Household income | |
| <$30 000 | 47% |
| $30 000–$50 000 | 13% |
| >$50 000 | 29% |
| Unknown | 11% |
| Discharge diagnosis | |
| Cardiovascular disease | 39% |
| Pulmonary disease | 21% |
| Gastrointestinal disease | 15% |
| Metabolic disease | 8% |
| Other | 17% |
| Circumstances that limit quality/quantity of food | |
| Allergies/disease | 15% |
| Trouble eating | 13% |
| Not enough money | 11% |
| Nutrition and physical function measures | |
| BMI | |
| Underweight (<20) | 4% |
| Normoweight (20–24.9) | 28% |
| Overweight (25–29.9) | 20% |
| Obese (>30) | 48% |
| Subject Global Assessment Score (30) | |
| Well nourished | 55% |
| Moderately malnourished | 45% |
| Usual gait speeda | |
| Slow | 60% |
| Not slow | 40% |
| Maximum hand gripa | |
| Weak | 28% |
| Not weak | 72% |
| Do you feel like you are malnourished or at risk? | |
| Yes | 15% |
| Are you worried about the amount of muscle that you have lost with aging? | |
| Yes | 62% |
Notes: BMI = body mass index, FNIH = Foundation for the National Institutes of Health Biomarkers Consortium Sarcopenia Project. Values are reported as a percentage ormean ± SD.
aBased on FNIH cutpoints.
Change in Intake Over Recovery Timeframe, Early vs Late
A total of 205 ASA24 recalls were collected after discharge, with an average of 2.4 recalls collected for each participant (range 1–5). Of the 85 participants, 77 had more than one dietary recall collected, which were used in this analysis. There were no significant differences in average 24-hour recalls from early recovery (Week 1 or earliest available recall) to late recovery (Week 4 or last available recall) for energy, grams of protein, grams of total fat, and grams of carbohydrate as shown in Table 2. Thus, for each of the 77 participants with more than one recall on file, all available recalls were averaged, and the recall average was used throughout the rest of the analysis. For the remaining analyses, the recall data from all 85 participants were included.
Table 2.
Energy Intake Over Time of Older Adults After Discharge From the Hospital (n = 77)
| Energy (kcal) | Protein (g) | Fat (g) | Carbohydrates (g) | |
|---|---|---|---|---|
| Early recovery (first recall) |
1476.5 ± 636.1 | 62.2 ± 30.1 | 60.6 ± 30.2 | 173.3 ± 85.5 |
| Late recovery (last recall) |
1522.5 ± 680.0 | 60.8 ± 27.9 | 64.8 ± 33.8 | 176.8 ± 98.1 |
| Difference (late-early) | 46.0 | 1.4 | 4.3 | 3.4 |
| p value | .593 | .746 | .330 | .787 |
Notes: Values reported as a mean ± SD. There were no significant differences in average 24-h recalls from early recovery (Week 1 or earliest available recall) to late recovery (Week 4 or last available recall) for energy, grams of protein, grams of total fat, and grams of carbohydrate. Thus, for each participant, all their available recalls were averaged, and the recall average was used throughout the rest of the analysis. For the remaining analyses, all 85 participants were included.
Group Nutrient Intakes vs Recommendations
Average macronutrient distribution range
On average, the macronutrient distribution range was 17.4% protein, 37.4% fat, and 45.8% carbohydrates. This distribution falls within the recommended ranges of the AMDR except fat, which fell just above the recommendation of 20%–35%.
Comparison to recommendations
Table 3 reports the current recommendation for energy, protein, fruit, vegetable, and fluid and the average intake of the participants. Most recommendations are gender specific except protein, which was compared to the current RDA (0.8 g/kg/d) and the proposed optimal standard (1.2 g/kg/d) for older adults.
Table 3.
Average Daily Consumption of Energy (kilocalories), Protein (grams protein per kilogram ideal body weight), Fruit (cup equivalent), Vegetables (cup equivalent), and Fluids (milliliters) of Older Adults Measured Over the First 30 d Following Discharge From the Hospital
| Daily Recommendations | Recommendation | Average Consumption | % (n) Met the Recommendation | p Value |
|---|---|---|---|---|
| Energy (kcal) | ||||
| Male | 2 000a | 1 626 ± 539.4 | 27% (7/26) | ↓.002 |
| Female | 1 600a | 1 401 ± 538.5 | 34% (20/59) | ↓.006 |
| Protein (g/kg) | ||||
| Current RDA | 0.8a | 0.90 ± 0.34 | 55% (47/85) | ↑ .006 |
| Proposed standard | 1.2b | 0.90 ± 0.34 | 18% (15/85) | ↓ <.001 |
| Fruit (cup equivalent) | ||||
| Male | 2a | 0.83 ± 0.93 | 8% (2/26) | ↓ <.001 |
| Female | 1.5a | 0.83 ± 0.87 | 20% (12/59) | ↓ <.001 |
| Vegetables (cup equivalent) | ||||
| Male | 2.5a | 1.69 ± 1.26 | 8% (2/26) | ↓.003 |
| Female | 2a | 1.30 ± 0.94 | 20% (12/59) | ↓ <.001 |
| Fluid (mL) | ||||
| Male | 3 700a | 1 937 ± 703.3 | 4% (1/26) | ↓ <.001 |
| Female | 2 700a | 1 903 ± 688.7 | 14% (8/59) | ↓ <.001 |
Notes: RDA = recommended dietary allowance. Recommendations, p value, and percent (%) of participants that met the recommendation. ↓: actual intake lower than recommendation; ↑: actual intake higher than recommendation. Most participants did not meet current recommendations for energy, fruit, vegetables, or fluids. While protein consumption was on average significantly higher than the current RDA of 0.8 g/kg/d, only half of the participants met the recommendation.
Energy
Energy consumption ranged broadly, from 317 to 3 095 kcal/d. The energy intake of the male participants, 1626.1 ± 539.4 kcal/d, was significantly lower than the recommended 2 000 kcal/d (p = .002) with only 27% meeting the recommendation. The energy intake of the female participants, 1401.6 ± 538.5 kcal/d, was also significantly lower than the recommended 1 600 kcal/d (p = .006) with only 34% meeting the recommendation.
Protein
Protein consumption ranged from 12 to 141 g/d. Average protein consumption, 0.90 ± 0.34 g/kg ideal body weight/d, was significantly higher than the RDA (p = .006). However, only 55% of participants met the RDA (0.8 g/kg/d), and even fewer (18%) met the 1.2 g/kg/d recommendation for optimal intake in older adults.
Fruits and vegetables
The range for fruit consumption was 0–3.9 cups/d. Fruit consumption was significantly lower (p < .001) than recommendations for both males and females, with only 8% and 20% meeting the recommendations, respectively. The range for vegetable consumption was 0–6.6 cups/d. Vegetable consumption was also significantly lower than the recommendation for both males (p = .003) and females (p < .001) with 20% and 16% meeting the recommendations, respectively.
Fluid
The range for fluid intake was 586–3 710 mL/d. Total fluid intake was significantly below the recommendations for males and females (p < .001) with 4% and 14% meeting the recommendations, respectively.
Meal Patterns
Daily energy distribution
Participants consumed an average of 1471.0 ± 549.6 kcal/d with a range from 316.6 to 3095.3 daily kcal. This was distributed over meals as follows: breakfast 298.7 ± 175.3 kcal, lunch 498.4 ± 305.7 kcal, dinner 602.9 ± 330.6 kcal, and other 71.8 ± 147.5 kcal.
Daily protein distribution
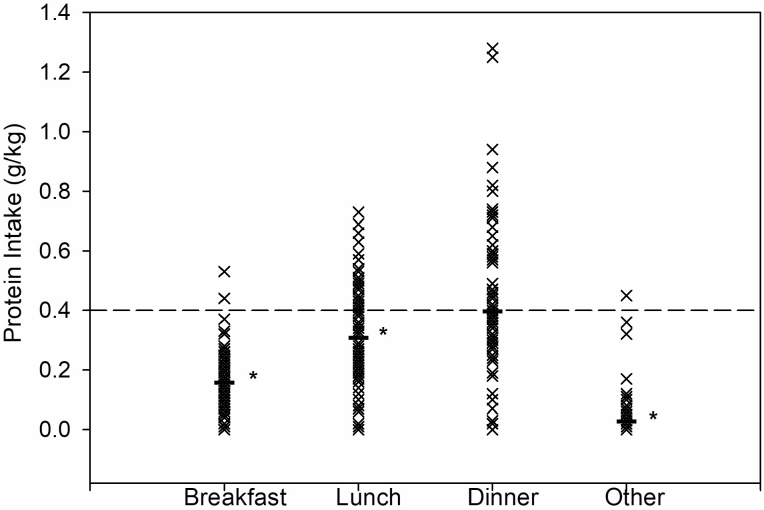
Participants consumed an average of 62.1 ± 23.7 g protein/d with a range from 11.5 to 140.5 g protein daily. Protein was distributed over meals as follows: breakfast 11.0 ± 7.4 g, lunch 21.4 ± 13.2 g, dinner 27.6 ± 15.9 g, and other 2.2 ± 5.3 g. Per meal consumption of protein per kilogram of ideal body weight was: breakfast 0.2 ± 0.1, lunch 0.3 ± 0.2, dinner 0.4 ± 0.2, and other 0.0 ± 0.1 (Figure 1). Protein consumption at breakfast, lunch, and other were significantly below the 0.4 g/kg threshold (p < .001), while dinner was not significantly different (p = .9). Only 2% of participants met the threshold at breakfast, 33% at lunch, and 41% at dinner. No participants met the 0.4 g/kg threshold for all 3 meals, 21% met it for 2 meals, 34% met it for one meal, and 45% of the participants did not meet the threshold at any meal.
Figure 1.
Mean protein intake for each participant by meal. Protein intake at each mealtime of older adults following discharge from a hospital admission for an acute medical illness. Mealtime defined as follows: breakfast (6:00–10:59), lunch (11–15:59), dinner (16:00–20:59), and other (21:00–5:59). Bars represent mean for each meal. The dashed line represents 0.4 g/kg/meal recommendation for older adults. Protein intake calculated individually by grams protein per ideal body weight in kilograms. *Significantly lower than the proposed 0.4 g/kg/meal threshold. Distribution of protein throughout the meals was skewed. No one met the per meal protein recommendation of 0.4 g of protein/kg/meal at all 3 main meals.
Food preferences
Animal sources (62.1% ± 14.4%) provided more protein than the nonanimal sources (36.0% ± 13.9%). The top 5 sources of protein based on the percent of total grams of protein provided were grains (19.4% ± 10.3%), meat (18.0% ± 19.0%), dairy (15.0% ± 12.9%), fruits and vegetables (11.1% ± 7.3%), and poultry (9.6% ± 11.6%).
The top 4 fruit categories consumed were tropical, berries, core, and citrus. The top 5 fruits consumed included bananas, apples, dried cranberries/raisins, grapes, and oranges. Most fruit was consumed raw (77%), with the remaining split between canned and dried. The top 5 vegetables included leafy greens (lettuce 63%, cabbage 22%, spinach 16%), potatoes (white potato 86%, sweet potato 14%), tomatoes, beans, and onions.
The primary source of fluid in this study cohort was water, which every single participant reported consuming at least once. This was followed by coffee/tea, milk, juice, soft drinks, and soup/stew.
Discussion
The current study is the first to investigate trends in dietary intake among community-dwelling older adults over the 30 days following discharge after hospitalization for an acute medical illness. The main findings include: (a) no significant difference in nutrient intake across time from the early to the late phase of recovery; (b) the majority of participants did not meet current recommendations for energy, fruit, vegetables, or fluids; (c) while protein consumption was on average significantly higher than the current RDA of 0.8 g/kg/d, only half of the participants met the recommendation; (d) distribution of protein throughout the meals was skewed and no one met the per meal protein recommendation of 0.4 g of protein/kg/meal at all 3 main meals.
The prevalence of malnutrition in our population was quite high, 45%, which is not surprising and consistent with previous research that found 23%–60% of older adults admitted into acute care to be malnourished (3). Yet, 48% of participants were classified obese by BMI. Prevalence of obesity has been increasing in older adults, which is of particular concern because the combination of obesity and sarcopenia (sarcopenic obesity) increases the risk of mobility limitations, metabolic diseases, reduced quality of life, and increased mortality (31–34). It is estimated that around 12% of older adults have sarcopenic obesity (35). In our participants, we found 60% were classified slow, 28% weak, and 45% had low muscle mass using FNIH cutoff points (29). In our total sample, 17% were classified as having sarcopenia, and of those who had sarcopenia 31% were obese. Thus, it is alarming that few of our participants met the protein recommendations considering that they not only had or were at risk of sarcopenia but also required a higher protein consumption for optimal recovery from illness.
We also found that dietary intakes did not change over the 30 days after hospital discharge, indicating that malnutrition persists well beyond the index hospitalization. All participants had conditions that would increase nutritional needs during posthospital recovery. However, most participants continued to not meet the current nutritional recommendations during recovery from hospitalization and consumed approximately 400 kcal less energy than healthy, community-dwelling older adults (36). An additional concern is that only about 1 in 3 participants met the energy recommendation, highlighting the large variability in caloric consumption among seniors. It is also not surprising that participants in this study did not meet the recommended daily amount of fruits and vegetables. Our data are consistent with previous reports documenting that nearly half of older adults do not consume the recommended 5 daily servings of fruits and vegetables (37). Higher intake of fruits and vegetables is beneficial in many chronic diseases due to their micronutrient and fiber content, and is associated with better nutrition status, quality of life, and survival in older adults (38,39). Finally, participants did not meet the recommended fluid intake. There is little information in the literature on fluid intake in community-dwelling older adults. Dehydration is associated with poor outcomes and serious medical problems in the elderly, including renal injury, confusion, and delirium (40,41). The current fluid recommendations aim at preventing dehydration.
The current RDA for protein is 0.8 g/kg of protein a day. New evidence shows that this amount may not be optimal. The International PROT-AGE Study Group recommended 1–1.2 g/kg/d for healthy older adults to help maintain and regain lean body mass and function, and up to 1.2–1.5 g/kg/d for older adults with acute or chronic diseases (20). Our participants recovering from acute illness averaged 0.90 g/kg/d. While this amount was significantly higher than the RDA of 0.8 g/kg/d, only 55% of participants achieved the RDA indicating broad variation within this patient population. When compared to the higher 1.2 g/kg/d recommendation for acutely ill older adults, only 18% met the recommendation. These findings are consistent with other studies reporting protein intakes of 0.89–1.06 g/kg/d in older adults (17,42). In our study, of all protein consumed, 62.1% was animal-based. This is consistent to other reports of animal protein consumption in older adults of 61%–65% (17,43). Due to the anabolic resistance of aging, which reduces the capacity of skeletal muscle to store amino acids at low intakes, it has been found that a balanced protein feeding pattern across the 3 main meals is more favorable to elicit muscle protein anabolism. Moore et al. found 0.4 g protein/kg/meal to be an adequate recommendation for older adults to reach optimal postprandial muscle protein synthesis (24). This amount corresponds to ~30 g of protein/meal for a 70 kg individual (44). Consistent with previous reports (17,25), we found that our participants distributed their daily protein unevenly across the mealtimes, with the least amount of protein at breakfast and the most at dinner. Moreover, 45% of participants did not meet the 0.4 g protein/kg/meal threshold at any meal, and no participant consumed enough protein to meet that threshold at every meal.
This study has also some limitations. Food recalls can underreport dietary intakes and underestimate dietary intakes. However, the dietary guidelines we compared our data to are based on large cohort studies and clinical trials, many of which conducted using dietary recalls. Another limitation is that the relatively small sample did not allow us to stratify the dietary intakes relative to the specific disease needs, primary discharge diagnosis, comorbidities, and activity level. Thus, we elected to draw comparisons between measured postdischarge dietary intakes and dietary guidelines usually employed by geriatricians in the clinical setting. Our results highlight the risk of malnutrition geriatric patients experience after hospital discharge to home, and the need for future larger and more detailed nutritional studies in this patient population. A second weakness was the collection of the dietary recalls over the phone. Occasionally, we were unable to reach study participants within the expected time window or could not complete the call due to participants’ hearing impairment, but in most cases we completed the recall on another day with help from family members. For all participants, we collected an in-person final recall during the 1-month follow-up visit. We also standardized data collection and analysis by using the ASA24 software. Another limitation regards generalizability of the findings. On average, our participants tended to be healthier and more independent than the average hospitalized geriatric patient as we included only individuals who were independent and community-dwelling before hospitalization and were discharged back to home. Thus, we expect that malnutrition would be worse in sicker, functionally dependent patients. A final weakness is that we compared habitual intake to AMDR/USDA recommendations rather than individual dietary recommendations. We elected to provide comparisons with the general dietary guidelines as those are usually employed in the clinical setting, including transition of care from hospital to home. While we agree that considering individual energy recommendations based on the Harris Benedict equation, comorbidity, and actigraphy could have provided more detailed information (and perhaps uncovered higher levels of malnutrition), it is noteworthy that these patients did not meet the general USDA dietary guidelines for healthy individuals for energy, fruit, vegetables, or fluids. This highlights the importance of getting dietary counseling during hospitalization and immediately after discharge to correct deficiencies and improve recovery.
To our knowledge, this is the first study to analyze nutrient intake patterns of community-dwelling older adults after hospitalization. The finding that the participants did not meet the current nutrient intake recommendations is concerning, considering that 55% of the participants were considered “well nourished” based on the initial SGA screening questionnaire. Nutrition tends to be overlooked during and after hospitalization, likely due to the more pressing health problems relative to the hospital admission. However, the importance of adequate nutrition cannot be overstated. An improved nutritional status in times of increased needs, such as during recovery after hospitalization, could potentially reduce readmission rates, decrease costs, and improve quality of life. Future research should focus on eating habits and food preferences to guide the development of pragmatic and effective interventions. For instance, we found that older adults need to increase protein consumption and this intervention would be most beneficial at breakfast and lunch mealtimes. Also, our population did not meet the recommended amount of daily fruit intake, and their most reported fruit have a soft texture, suggesting a role for poor dentition in fruit selection. Thus, any recommendation should consider the patient’s chewing/swallowing abilities. Small pilot programs have demonstrated the potential efficacy of nutritional interventions using meal delivery services or supplements after hospital discharge (14,45) and could guide the development of future interventions. In conclusion, this study showed that dietary intakes and intake patterns of geriatric patients recovering from a hospitalization are inadequate and persistent in most individuals. This study highlights the importance of collecting dietary recall information—in addition to using the basic malnutrition screening questionnaires—in geriatric patients during hospitalization and immediately after discharge to correct deficiencies that may hamper recovery.
Acknowledgment
We thank the study participants for their willingness to be involved in our study.
Funding
This work was supported by the National Dairy Council (1229), University of Texas Medical Branch (UTMB) Claude D. Pepper Older Americans Independence Center (P30 AG024832) from the National Institute on Aging, and the UTMB Clinical and Translational Science Award (UL1 TR001439, TL1 TR001440, and KL2TR001441) from the National Center for Advancing Translational Sciences. The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Conflict of Interest
None declared.
Author Contributions
R.R.D.: study design, data acquisition, data analysis, and writing of the manuscript; E.H., A.M., and K.H.: data analysis; S.G. and N.R.: data acquisition; and E.V.: study design and writing of the manuscript.
References
- 1. Souza TT, Sturion CJ, Faintuch J. Is the skeleton still in the hospital closet? A review of hospital malnutrition emphasizing health economic aspects. Clin Nutr. 2015;34(6):1088–1092. doi: 10.1016/j.clnu.2015.02.008 [DOI] [PubMed] [Google Scholar]
- 2. Colby S, Ortman J. Projections of the size and composition of the U.S. population: 2014 to 2060. Current Population Reports P25–1143. U.S. Census Bureau; 2014. [Google Scholar]
- 3. Agarwal E, Miller M, Yaxley A, Isenring E. Malnutrition in the elderly: a narrative review. Maturitas. 2013;76(4):296–302. doi: 10.1016/j.maturitas.2013.07.013 [DOI] [PubMed] [Google Scholar]
- 4. Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–102. doi: 10.1056/NEJMp1212324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coker RH, Hays NP, Williams RH, Wolfe RR, Evans WJ. Bed rest promotes reductions in walking speed, functional parameters, and aerobic fitness in older, healthy adults. J Gerontol A Biol Sci Med Sci. 2015;70(1):91–96. doi: 10.1093/gerona/glu123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Agarwal E, Ferguson M, Banks M, Bauer J, Capra S, Isenring E. Nutritional status and dietary intake of acute care patients: results from the Nutrition Care Day Survey 2010. Clin Nutr. 2012;31(1):41–47. doi: 10.1016/j.clnu.2011.08.002 [DOI] [PubMed] [Google Scholar]
- 7. Braunschweig C, Gomez S, Sheean PM. Impact of declines in nutritional status on outcomes in adult patients hospitalized for more than 7 days. J Am Diet Assoc. 2000;100(11):1316–1322; quiz 1323. doi: 10.1016/S0002-8223(00)00373-4 [DOI] [PubMed] [Google Scholar]
- 8. Kirkland LL, Shaughnessy E. Recognition and prevention of nosocomial malnutrition: a review and a call to action! Am J Med. 2017;130(12):1345–1350. doi: 10.1016/j.amjmed.2017.07.034 [DOI] [PubMed] [Google Scholar]
- 9. English KL, Paddon-Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care. 2010;13(1):34–39. doi: 10.1097/MCO.0b013e328333aa66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. White JV, Stotts N, Jones SW, Granieri E. Managing postacute malnutrition (undernutrition) risk. JPEN J Parenter Enteral Nutr. 2013;37(6):816–823. doi: 10.1177/0148607113492339 [DOI] [PubMed] [Google Scholar]
- 11. National Alliance for Infusion Therapy and the American Society for Parenteral and Enteral Nutrition Public Policy Committee and Board of Directors. Disease-related malnutrition and enteral nutrition therapy: a significant problem with a cost-effective solution. Nutr Clin Pract. 2010;25(5):548–554. doi: 10.1177/0884533610378524 [DOI] [PubMed] [Google Scholar]
- 12. White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy of Nutrition and Dietetics Malnutrition Work Group; A.S.P.E.N. Malnutrition Task Force; A.S.P.E.N. Board of Directors. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112(5):730–738. doi: 10.1016/j.jand.2012.03.012 [DOI] [PubMed] [Google Scholar]
- 13. Deer RR, Dickinson JM, Fisher SR, Ju H, Volpi E. Identifying effective and feasible interventions to accelerate functional recovery from hospitalization in older adults: a randomized controlled pilot trial. Contemp Clin Trials. 2016;49:6–14. doi: 10.1016/j.cct.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deer RR, Goodlett SM, Fisher SR, et al. A randomized controlled pilot trial of interventions to improve functional recovery after hospitalization in older adults: feasibility and adherence. J Gerontol A Biol Sci Med Sci. 2018;73(2):187–193. doi: 10.1093/gerona/glx111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. U.S. Department of Agriculture; U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2015–2020. 8th ed. http://health.gov/dietaryguidelines/2015/guidelines/. Accessed August 30, 2021.
- 16. Winter JE, MacInnis RJ, Wattanapenpaiboon N, Nowson CA. BMI and all-cause mortality in older adults: a meta-analysis. Am J Clin Nutr. 2014;99(4):875–890. doi: 10.3945/ajcn.113.068122 [DOI] [PubMed] [Google Scholar]
- 17. Berner LA, Becker G, Wise M, Doi J. Characterization of dietary protein among older adults in the United States: amount, animal sources, and meal patterns. J Acad Nutr Diet. 2013;113(6):809–815. doi: 10.1016/j.jand.2013.01.014 [DOI] [PubMed] [Google Scholar]
- 18. Courtney-Martin G, Ball RO, Pencharz PB, Elango R. Protein requirements during aging. Nutrients. 2016;8(8). doi: 10.3390/nu8080492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nowson C, O’Connell S. Protein requirements and recommendations for older people: a review. Nutrients. 2015;7(8):6874–6899. doi: 10.3390/nu7085311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bauer J, Biolo G, Cederholm T, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013;14(8):542–559. doi: 10.1016/j.jamda.2013.05.021 [DOI] [PubMed] [Google Scholar]
- 21. U.S. Department of Agriculture (USDA). All About the Fruit Group. Washington, DC: USDA. https://www.choosemyplate.gov/fruit. Accessed August 30, 2021. [Google Scholar]
- 22. U.S. Department of Agriculture (USDA). All About the Vegetable Group. Washington, DC: USDA. https://www.choosemyplate.gov/vegetables. Accessed August 30, 2021. [Google Scholar]
- 23. Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. Washington, DC: National Academies Press; 2004. [Google Scholar]
- 24. Moore DR, Churchward-Venne TA, Witard O, et al. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci. 2015;70(1):57–62. doi: 10.1093/gerona/glu103 [DOI] [PubMed] [Google Scholar]
- 25. Cardon-Thomas DK, Riviere T, Tieges Z, Greig CA. Dietary protein in older adults: adequate daily intake but potential for improved distribution. Nutrients 2017;9(3):184. doi: 10.3390/nu9030184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kirkpatrick SI, Subar AF, Douglass D, et al. Performance of the automated self-administered 24-hour recall relative to a measure of true intakes and to an interviewer-administered 24-h recall. Am J Clin Nutr. 2014;100(1):233–240. doi: 10.3945/ajcn.114.083238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bowman SA, Clemens JC, Friday JE, et al. USDA Food Patterns Equivalents Database 2007–08: Methodology and User Guide. http://www.ars.usda.gov/SP2UserFiles/Place/12355000/pdf/fped/FPED_0708.pdf. Accessed December 14, 2017.
- 28. U.S. Department of Agriculture–Agricultural Research Service. The USDA Food and Nutrient Database for Dietary Studies: 2011–2012: Documentation and User Guide. http://www.ars.usda.gov/SP2UserFiles/Place/12355000/pdf/fndds/fndds_2011_2012_doc.pdf. Accessed December 15, 2017.
- 29. Alley DE, Shardell MD, Peters KW, et al. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci. 2014;69(5):559–566. doi: 10.1093/gerona/glu011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. da Silva Fink J, Daniel de Mello P, Daniel de Mello E. Subjective global assessment of nutritional status—a systematic review of the literature. Clin Nutr. 2015;34(5):785–792. doi: 10.1016/j.clnu.2014.12.014 [DOI] [PubMed] [Google Scholar]
- 31. Deutz NE, Bauer JM, Barazzoni R, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. 2014;33(6):929–936. doi: 10.1016/j.clnu.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Benton MJ, Whyte MD, Dyal BW. Sarcopenic obesity: strategies for management. Am J Nurs. 2011;111(12):38–44; quiz 45. doi: 10.1097/01.NAJ.0000408184.21770.98 [DOI] [PubMed] [Google Scholar]
- 33. Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60(3):324–333. doi: 10.1093/gerona/60.3.324 [DOI] [PubMed] [Google Scholar]
- 34. Rantanen T, Harris T, Leveille SG, et al. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J Gerontol A Biol Sci Med Sci. 2000;55(3):M168–M173. doi: 10.1093/gerona/55.3.m168 [DOI] [PubMed] [Google Scholar]
- 35. Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11(6):693–700. doi: 10.1097/MCO.0b013e328312c37d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Johnston R, Poti JM, Popkin BM. Eating and aging: trends in dietary intake among older Americans from 1977–2010. J Nutr Health Aging. 2014;18(3):234–242. doi: 10.1007/s12603-013-0387-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nicklett EJ, Kadell AR. Fruit and vegetable intake among older adults: a scoping review. Maturitas. 2013;75(4):305–312. doi: 10.1016/j.maturitas.2013.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Anderson AL, Harris TB, Tylavsky FA, et al. ; Health ABC Study. Dietary patterns and survival of older adults. J Am Diet Assoc. 2011;111(1):84–91. doi: 10.1016/j.jada.2010.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bernstein M, Munoz N; Academy of Nutrition and Dietetics. Position of the Academy of Nutrition and Dietetics: food and nutrition for older adults: promoting health and wellness. J Acad Nutr Diet. 2012;112(8):1255–1277. doi: 10.1016/j.jand.2012.06.015 [DOI] [PubMed] [Google Scholar]
- 40. El-Sharkawy AM, Watson P, Neal KR, et al. Hydration and outcome in older patients admitted to hospital (The HOOP prospective cohort study). Age Ageing. 2015;44(6):943–947. doi: 10.1093/ageing/afv119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Warren JL, Bacon WE, Harris T, McBean AM, Foley DJ, Phillips C. The burden and outcomes associated with dehydration among US elderly, 1991. Am J Public Health. 1994;84(8):1265–1269. doi: 10.2105/ajph.84.8.1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fulgoni VL, 3rd. Current protein intake in America: analysis of the National Health and Nutrition Examination Survey, 2003–2004. Am J Clin Nutr. 2008;87(5):1554s–1557s. doi: 10.1093/ajcn/87.5.1554S [DOI] [PubMed] [Google Scholar]
- 43. Smit E, Nieto FJ, Crespo CJ, Mitchell P. Estimates of animal and plant protein intake in US adults: results from the Third National Health and Nutrition Examination Survey, 1988–1991. J Am Diet Assoc. 1999;99(7):813–820. doi: 10.1016/S0002-8223(99)00193-5 [DOI] [PubMed] [Google Scholar]
- 44. Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care. 2009;12(1):86–90. doi: 10.1097/MCO.0b013e32831cef8b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Buys DR, Campbell AD, Godfryd A, et al. Meals enhancing nutrition after discharge: findings from a pilot randomized controlled trial. J Acad Nutr Diet. 2017;117(4):599–608. doi: 10.1016/j.jand.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]