Abstract
In germfree mice, the administration of short-chain fatty acids (SCFA) protected the intestinal mucosa from damage produced by 1-β-d-arabinofuranosylcytosine (Ara-C). Animals receiving SCFA and Ara-C had intestinal morphologies closer to normal than the control animals, which had severe intestinal lesions. We concluded that orally administrated SCFA reduce intestinal lesions, improving the mucosa pattern of the small intestine and colon.
Our group, studying the effects of short-chain fatty acids (SCFA) in mice, demonstrated that orally administered SCFA were able to prevent the intestinal necrosis caused by 1-β-d-arabinofuranosylcytosine (Ara-C) in animals fed on commercial or elemental diets (11). However, mice have a constant production of SCFA by intestinal flora that could have contributed to the effects of oral administration.
We examined the protection seen in conventionally reared mice to see if it was maintained in germfree mice receiving SCFA orally (ad libitum) or intragastrically (single dose, 15× concentrated). Thirty germfree mice were used. They were kept germfree according to established procedures (10, 13). Four experimental groups were designed: SCFA/O+Ara-C, SCFA/IG+Ara-C, Placebo/O+Ara-C, and Placebo/IG+Ara-C, designating animals receiving oral (O) or intragastric (IG) SCFA and saline solution for 9 days and intraperitoneal Ara-C in the last 2 days.
Three control groups included SCFA/O+Sal and SCFA/IG+Sal, receiving SCFA and saline solution (Sal) instead of Ara-C, and Normal, which received water and a sterile commercial diet ad libitum for 9 days. All mice were sacrificed 4 h after the last Ara-C or saline dose. Oral SCFA solutions consisted of acetic, pronionic, and butyric acids (35, 15, and 9 mM, respectively). The intragastric solution had the same SCFA proportion in a 15× concentrated solution. The volume of intragastrically administered SCFA was adjusted to offer quantities similar to those ingested orally. Saline solutions had the same pH and osmolarity of corresponding solutions. Experimental protocols (Ara-C administration, histopathology, and morphometry) were done as described previously (11).
The oral intake of SCFA and saline was 4.47 ± 0.23 and 4.35 ± 0.15 ml/day/mouse, respectively, and the volume administered intragastrically was 0.3 ml/day/mouse.
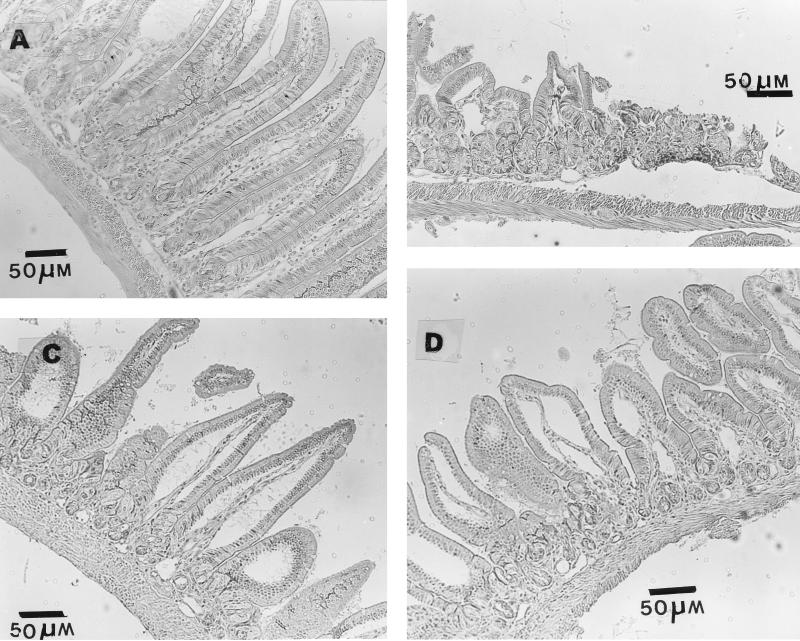
Since the histology was very similar in all control groups (Normal, SCFA/O+Sal, and SCFA/IG+Sal) the histopathology of the Sal/O+Sal group was used as the representative of the controls (Fig. 1A). Similarly, the Sal/O+Ara-C group (given saline orally and 3.6 mg of Ara-C per day per mouse intraperitoneally) was used to illustrate the lesions present in both Sal/O+Ara-C and Sal/IG+Ara-C groups, since no difference was seen between the treatments (Fig. 1B).
FIG. 1.
Small intestine samples from germfree mice in the following groups: SCFA/O+Sal (A), Sal/O+Ara-C (B), SCFA/IG+Ara-C (C), and SCFA/O+Ara-C (D). The histologies of SCFA/O+Sal and Sal/O+Ara-C groups are used as representatives of control and Sal+Ara-C groups, respectively.
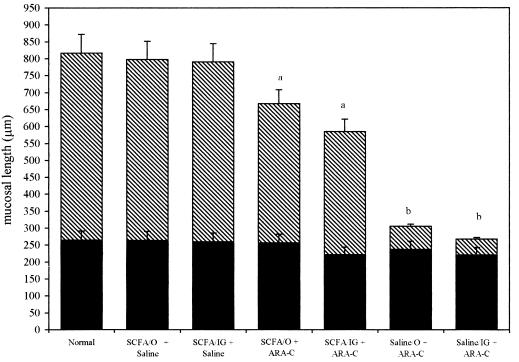
Ara-C administration induced severe intestinal lesions (shortening of villi, reduction in the number of enterocytes, inflammatory infiltration, and necrosis; Fig. 1B). SCFA administration improved the mucosa integrity, avoiding necrosis and increasing villus length, compared to the Sal+Ara-C groups (Fig. 1B to D). Morphological analysis showed that both total mucosa length and villus length were statistically lower in Sal+Ara-C groups than in SCFA and the three control groups (Fig. 2). However, the SCFA+Ara-C groups still had reductions in villus length compared to control groups.
FIG. 2.
Total mucosal length in small intestines of mice treated orally or intragastrically with SCFA or saline and Ara-C or saline intraperitoneally. Crosshatched bars represent villus length, and black bars represent crypt length. Values are means ± standard deviations. a, statistical difference (P < 0.05) on villus and total mucosal lengths between SCFA and control groups; b, statistical difference (P < 0.01) on both villus and total mucosal lengths between SCFA+Ara-C and Sal+Ara-C groups.
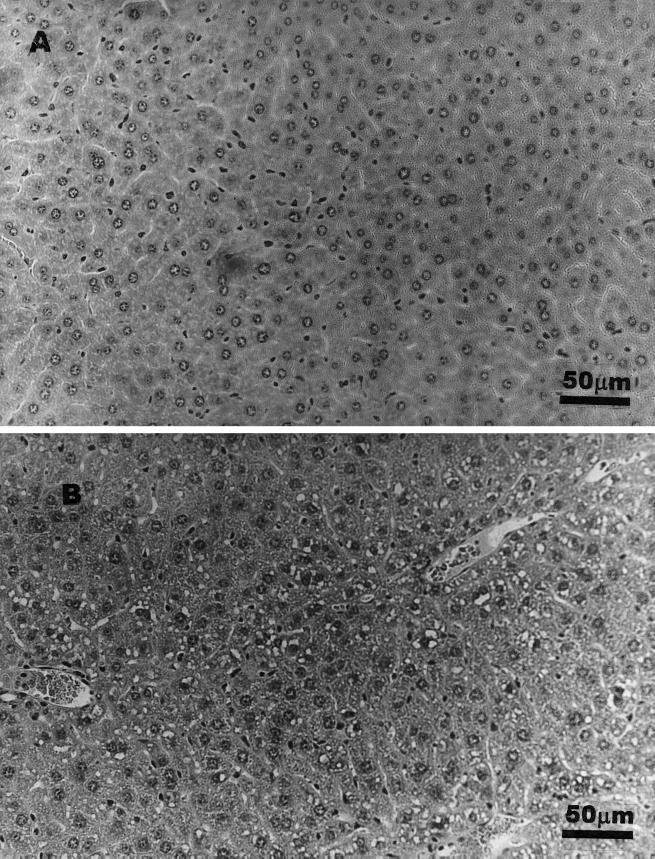
Regarding the administration route, histopathological alterations were less intensive and morphology was closer to normal in SCFA/O+Ara-C mice than in SCFA/IG+Ara-C animals (Fig. 1C and D). Shortened and fused villi were still observed in SCFA/IG+Ara-C animals, suggesting a worse villus pattern when SCFA were given as a single dose. Microvascular fat accumulation was seen only in hepatocytes from SCFA/IG animals (Fig. 3).
FIG. 3.
Hepatic normal histology (A) and liver fat accumulation (B) seen in animals receiving a highly concentrated solution of SCFA intragastrically.
Colon lesions were less intensive than those in the small intestines of orally or intragastrically treated groups. This finding could be due to the lower turnover of colonocytes than that of enterocytes. The administration route (oral or intragastric) did not influence colonic histology (data not shown).
SCFA+Ara-C animals had a greater colon length than mice in the Sal+Ara-C group (data not shown). No differences were seen between SCFA+Sal and SCFA+Ara-C groups. Colonic morphometry was similar between oral and intragastric treatments.
The results show that the same quantity of SCFA used in conventionally reared mice offered similar protection against Ara-C damage in germfree animals when both intragastric or oral solutions were administered, suggesting that the protective effect of SCFA was independent of endogenous production.
In the present study, hepatic fat accumulation was related to the intragastric administration of SCFA. In our previous studies using conventionally reared animals, intestinal and hepatic fat accumulation was seen only when high doses were administered (11). It is known that SCFA are rapidly metabolized by the gastrointestinal tract. The SCFA not metabolized by the mucosal epithelium are transported to the liver and can be incorporated into longer-chain fatty acid and/or may originate ketone bodies (4, 12). Propionate can be used as a substrate for gluconeogenesis (1). Probably, the high concentrations of SCFA (as those given intragastrically) induced a higher captation of SCFA by the intestinal tract and liver and their metabolization to fatty acids or other lipids with the consequent lipid accumulation.
Independently of the presence of fat accumulation, SCFA administered both orally and intragastrically were able to improve intestinal mucosa integrity when compared to the results for the Sal+Ara-C groups. Although animals in the SCFA/O+Sal group had a moderately better morphology of intestinal mucosa than those receiving SCFA intragastrically, the villus length of animals receiving SCFA intragastrically and Ara-C was about five times greater than that of mice in the Sal+Ara-C group and similar to that of mice in the SCFA/O+Ara-C groups, confirming the advantages of the administration of SCFA during chemotherapy, even as a single dose.
It is now clear that the trophic effect of SCFA is due not only to the simple provision of energy to the host but also to the combination of local action and systemic metabolism of SCFA (2, 3, 6, 7). The result of all these SCFA actions is a faster recovery of intestinal and colonic mucosa damaged by Ara-C treatment. We have demonstrated that this protection can be obtained by oral doses of SCFA.
These results indicate a new potential clinical use for SCFA. Chemotherapy for leukemia and other neoplasias leads to mucosal atrophy, diarrhea and malnutrition caused by alterations in absorption, and sometimes death. Besides trophic effects, SCFA administration has been described as useful in the prevention or treatment in vivo and in vitro of several diseases (5) and cancers (14), especially colon cancer (8, 9), inducing cell cycle arrest and apoptosis or interfering with the expression of oncogenes. However, a clinical trial in humans is needed to determine if a similar reduction in drug toxicity would be obtained.
Acknowledgments
This work was supported by Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG) (grant CBS/1242) and Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (grant 400544/94.6).
Footnotes
This work was performed at the Laboratório de Gnotobiologia e Nutrição, Departamento de Bioquímica e Imunologia, ICB-UFMG, Belo Horizonte, MG, Brazil.
REFERENCES
- 1.Cross T A, Paul C, Oberhansli R. Ketogenesis in the living rats followed by 13C-NMR spectroscopy. Biochemistry. 1984;23:6398–6402. doi: 10.1021/bi00321a018. [DOI] [PubMed] [Google Scholar]
- 2.Frankel W L, Zhang W, Singh A, Klurfeld D M, Don S, Sakata T, Rombeau J L. Mediation of the trophic effects of short chain fatty acids on the rat jejunum and colon. Gastroenterology. 1994;106:385–390. doi: 10.1016/0016-5085(94)90595-9. [DOI] [PubMed] [Google Scholar]
- 3.Harada E, Kato S. Effect of short chain fatty acids on the secretory response of the ovine exocrine pancreas. Am J Physiol. 1983;244:G284–G290. doi: 10.1152/ajpgi.1983.244.3.G284. [DOI] [PubMed] [Google Scholar]
- 4.Hoverstad T, Bohmer T, Fausa O. Absorption of short chain fatty acids from human colon measured by the 14CO2 breath test. Scand J Gastroenterol. 1982;17:373–378. doi: 10.3109/00365528209182070. [DOI] [PubMed] [Google Scholar]
- 5.Kim Y. Short chain fatty acid in ulcerative colitis. Nutr Rev. 1998;56:17–24. doi: 10.1111/j.1753-4887.1998.tb01654.x. [DOI] [PubMed] [Google Scholar]
- 6.Kvietys P R, Granger D N. Effects of volatile fatty acids on blood flow and oxygen uptake by dog colon. Am J Physiol. 1981;80:962–969. [PubMed] [Google Scholar]
- 7.Linseisen J, Wolfran G. Efficacy of different triglycerides in total parenteral nutrition for preventing atrophy of the gut in traumatized rats. J Parenter Enteral Nutr. 1997;21:21–26. doi: 10.1177/014860719702100121. [DOI] [PubMed] [Google Scholar]
- 8.Lupton L R. Short chain fatty acids and colon tumorigenesis. In: Cummings J H, Rombeau J L, Sakata T, editors. Physiological and clinical aspects of short chain fatty acids. New York, N.Y: Cambridge University Press; 1995. pp. 307–318. [Google Scholar]
- 9.Medina V, Afonso J J, Alvarez-Arguelles H, Hernandez C, Gonzalez F. Sodium butyrate inhibits carcinoma development in a 1,2-dimethylhydrazine-induced rat colon cancer. J Parenter Enteral Nutr. 1998;22:14–17. doi: 10.1177/014860719802200114. [DOI] [PubMed] [Google Scholar]
- 10.Pleasents J R. Gnotobiotics. In: Melby E C Jr, Altman N H, editors. CRC handbook of laboratory animal science. Vol. 1. Cleveland, Ohio: CRC Press; 1974. pp. 119–174. [Google Scholar]
- 11.Ramos M G, Bambirra E A, Cara D C, Vieira E C, Alvarez-Leite J I. Oral administration of short chain fatty acids reduces the intestinal mucositis caused by treatment with Ara-c in mice fed commercial or elemental diets. Nutr Cancer. 1997;26:211–219. doi: 10.1080/01635589709514577. [DOI] [PubMed] [Google Scholar]
- 12.Remesy C, Demigné C. Partition and absorption of volatile fatty acids in the alimentary canal of the rat. Ann Rech Vet. 1976;7:39–55. [PubMed] [Google Scholar]
- 13.Wagner M. Determination of germfree status. Ann N Y Acad Sci. 1959;78:89–101. [Google Scholar]
- 14.Young G P, Gibson P R. Butyrate and human cancer cell. In: Cummings J H, Rombeau J L, Sakata T, editors. Physiological and clinical aspects of short chain fatty acids. New York, N.Y: Cambridge University Press; 1995. pp. 319–336. [Google Scholar]