Abstract
Introduction
Heart failure (HF) is a prevalent, serious chronic illness that affects 6.5 million adults in the United States. Among patients with HF, the prevalence of attention impairment is reported to range from 15% to 27%. Although attention is fundamental to human activities including HF self‐care, cognitive interventions for patients with HF that target improvement in attention are scarce. The COgnitive intervention to Restore attention using nature Environment (CORE) study aims to test the preliminary efficacy of the newly developed Nature‐VR, a virtual reality‐based cognitive intervention that is based on the restorative effects of nature. Nature‐VR development was guided by Attention Restoration Theory. The target outcomes are attention, HF self‐care, and health‐related quality of life (HRQoL). Our exploratory aims examine the associations between attention and several putative/established HF biomarkers (eg, oxygen saturation, brain‐derived neurotrophic factor, apolipoprotein E, dopamine receptor, and dopamine transporter genes) as well as the effect of Nature‐VR on cognitive performance in other domains (ie, global cognition, memory, visuospatial, executive function, and language), cardiac and neurological events, and mortality.
Methods
This single‐blinded, two‐group randomized‐controlled pilot study will enroll 74 participants with HF. The Nature‐VR intervention group will view three‐dimensional nature pictures using a virtual reality headset for 10 minutes per day, 5 days per week for 4 weeks (a total of 200 minutes). The active comparison group, Urban‐VR, will view three‐dimensional urban pictures using a virtual reality headset to match the Nature‐VR intervention in intervention dose and delivery mode, but not in content. After baseline interviews, four follow‐up interviews will be conducted to assess sustained effects of Nature‐VR at 4, 8, 26, and 52 weeks.
Discussion
The importance and novelty of this study consists of using a first‐of‐its kind, immersive virtual reality technology to target attention and in investigating the health outcomes of the Nature‐VR cognitive intervention among patients with HF.
Keywords: apolipoprotein E, attention, augmented reality, biomarkers, brain‐derived neurotrophic factor, clinical trial protocol, cognition, dopamine receptor, dopamine transporter, genomics, heart failure, virtual reality
1. BACKGROUND
Heart failure (HF) is a prevalent, serious chronic illness affecting 6.5 million American adults. 1 The prevalence of this disease is projected to increase by 46% by 2030. 1 A major issue for patients with HF is cognitive dysfunction, which has a prevalence of 24% to 80%. 2 , 3 , 4 The most likely etiology of cognitive dysfunction in HF is inadequate cerebral blood flow. 2 , 3 , 5 In addition to memory, attention is one of the most commonly impaired cognitive domains in HF. 6 , 7 , 8 Attention is critical in supporting effective human activities, and thus poor attention function is often associated with inadequate HF self‐care (eg, lack of adherence to a low sodium diet, non‐adherence to medication, and poor symptom management). 9 , 10 , 11 As shown in past studies, inadequate HF self‐care is associated with higher mortality and hospitalizations and diminished health‐related quality of life (HRQoL). 12 , 13 Given that attention is fundamental to learning and maintaining HF self‐care, the development of interventions aiming to improve attention among patients with HF is warranted.
Approaches to developing interventions to improve attention in HF need to be guided by theory because theory provides fundamental understanding of the problem that interventions target. 14 One relevant theory is Attention Restoration Theory, which proposes that attention can be restored and improved by interacting with nature. 15 , 16 Studies guided by this theory have shown that natural restorative environment (Nature) interventions can improve attention in diverse populations. For example, engaging in nature‐related activities (eg, walking in the park, gardening) improved attention among breast cancer survivors, 17 and viewing nature pictures on computer screens improved attention among healthy adults. 18 , 19 In our pilot study consisting of a randomized controlled crossover design, a computer‐based Nature intervention using two‐dimensional pictures of nature was tested for its efficacy compared to a computer‐based active comparison intervention of two‐dimensional urban pictures among 20 participants with HF and 20 age‐matched healthy adults. The results indicated that participants with HF had poorer attention and showed small to near‐medium effects of the Nature intervention on improving attention. 20
Newer virtual reality (VR) technology can now create three‐dimensional environments to better simulate the natural environment and provide a more immersive experience than two‐dimensional computer‐based pictures. 21 , 22 Thus to extend our pilot study, our study team developed a prototype of a VR–based nature intervention (Nature‐VR) using three‐dimensional pictures delivered by a VR headset and tested its feasibility among 10 participants with HF. This feasibility study examined study completion, safety, satisfaction, and attention improvement with the Nature‐VR prototype among these 10 participants with HF. 23 The intervention was delivered by laptop computer for five participants with HF and by VR for five participants with HF. Attention was examined immediately pre‐ and post‐intervention. All 10 participants completed the study, and no adverse events were reported during or immediately after the interventions. Participants who received the intervention by the VR mode were slightly more satisfied. Participants in the computer‐based Nature intervention were provided an opportunity to try Nature‐VR at the end of the interview, with four of five participants trying and preferring Nature‐VR. 23 With regard to attention improvement, performance on tests of attention was consistently better after Nature‐VR compared with the computer‐based Nature intervention, except for the Digit Span Forward test. 23 Effect sizes were calculated using Hedge's gs. Changes in measures were modeled using a linear model with a single term for group (Nature‐VR and computer‐based Nature intervention). Ninety‐five percent confidence intervals for the effect size were estimated by generating 2000 bootstrap samples and refitting the model to each bootstrap sample. Large effect sizes were found for attention measured with Multi‐Source Interference Task (faster response time [Hedge's gs = −1.620] and higher accuracy [Hedge's gs = 1.262] on the congruent trials) and Trail Making Test A (faster response time [Hedge's gs = −1.205]) (Figure 1).
FIGURE 1.
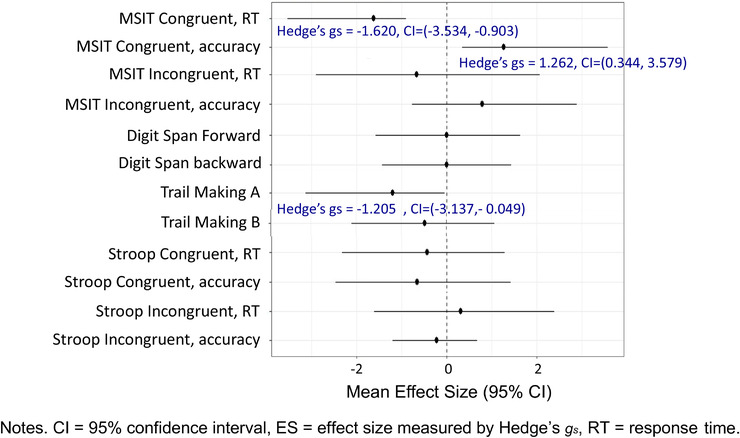
Hedge's gs effect sizes of changes on attention after Nature‐VR prototype compared to the computer‐based Nature intervention (n = 10)
Building upon the feasibility study with the prototype the Nature‐VR intervention and the active comparison condition (Urban‐VR) have now been developed. The purpose of this study is to test the preliminary efficacy of this latest version of Nature‐VR on attention, HF self‐care, and HRQoL using a two‐group single‐blinded randomized controlled design.
2. METHODS
The COgnitive intervention to Restore attention using nature Environment (CORE) study was approved by the Indiana University institutional review board. The study protocol is registered on ClinicalTrials.gov (NCT 04485507). Because the coronavirus disease 2019 (COVID‐19) pandemic has necessitated some changes to the original protocol, both the original study protocol and the modifications made in response to the pandemic are presented. In the interest of participant and researcher safety, the COVID protocol will be used until the pandemic subsides.
2.1. Study design
The proposed study is a two‐group single‐blinded randomized controlled pilot study. Participants with HF will have five data collection interviews (Figure 2). Primary and secondary outcomes will be examined at baseline, immediately post‐intervention (4 weeks), 8, 26 and 52 weeks (1 year). Follow‐up data will provide information about intervention effects over time.
FIGURE 2.
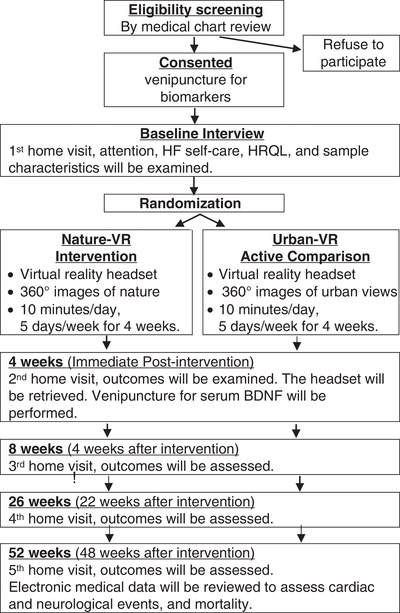
Research design and procedure
2.2. Specific aims and hypotheses
The primary aim is to evaluate the preliminary efficacy of Nature‐VR on attention among participants with HF. The secondary aims are to evaluate the preliminary efficacy of Nature‐VR on the secondary outcomes of HF self‐care and HRQoL. The study will test the following hypotheses:
Hypothesis 1: Compared with participants receiving the active comparison condition (Urban‐VR), participants receiving Nature‐VR intervention will have greater improvement in attention over time (at 4, 8, 26, and 52 weeks).
Hypothesis 2a: Compared with participants receiving the active comparison condition (Urban‐VR), participants receiving Nature‐VR intervention will have greater improvement in HF self‐care over time (at 4, 8, 26, and 52 weeks).
Hypothesis 2b: Compared with participants receiving the active comparison condition (Urban‐VR), participants receiving Nature‐VR intervention will have greater improvement in HRQoL over time (at 4, 8, 26, and 52 weeks).
Exploratory aim 1 will examine associations between attention and putative/established HF biomarkers (ie, oxygen saturation, 6 , 24 serum brain‐derived neurotrophic factor [BDNF], 20 , 25 and four genetic biomarkers—the BDNF, 26 , 27 apolipoprotein E [APOE], 28 , 29 dopamine receptor, and dopamine transporter 30 , 31 genes).
Exploratory aim 2 will examine the preliminary efficacy of Nature‐VR on other cognitive domains (ie, global cognition, memory, executive function, and language), cardiac events (ie, hospitalizations, emergency department visits), neurological events (ie, diagnosis of cognitive impairment, such as mild cognitive impairment, dementia), and mortality.
RESEARCH IN CONTEXT
Systematic review: To identify the relevant literature, the authors searched PubMed using combinations of relevant keywords (ie, chronic heart failure [HF], cognitive training/intervention, virtual and augmented reality). No studies were found of virtual reality–based cognitive interventions to improve attention or other cognitive function in HF. Studies of cognitive training/intervention in HF have been appropriately cited.
Interpretation: New, theory‐based interventions are needed to improve attention among patients with HF. This study assesses preliminary efficacy of a new virtual reality intervention using a library of 360o nature photographs for improving attention, HF self‐care, and health‐related quality of life (HRQoL) in HF.
Future directions: (1) Populations with chronic conditions, including HF, that are vulnerable to cognitive impairment need targeted interventions to improve cognitive function; (2) easy, enjoyable, cost‐effective interventions need to be developed and tested; and (3) intervention responders and non‐responders need to be characterized by examining the relationship between intervention responsiveness and patient characteristics and relevant biomarkers.
HIGHLIGHTS
Nature‐VR is a theory‐based virtual reality cognitive intervention
We describe the framework and methodology of Nature‐VR cognitive intervention study
We will test if Nature‐VR improves attention and health‐related outcomes.
2.3. Sample
We will enroll 74 participants (37 in each group) to retain 30 in each group by the end of the study (projecting a 19% attrition rate). This sample size was calculated based on our previous studies. 23 , 32 For the primary outcome of attention, the effect sizes of the computer‐based nature intervention compared to those of the computer‐based urban comparison condition among 20 participants with HF ranged from 0.02 to 0.45 (Cohen's d). 32 Given that the Hedge's gs effect sizes of the three‐dimensional Nature‐VR prototype was larger compared to the computer‐based two‐dimensional nature intervention in our feasibility study (Figure 1), 23 a medium effect size was used in this sample justification. Our power analyses indicate that with a sample size of 60 (30 participants per group) we will have 84% power to detect the preliminary efficacy of the Nature‐VR intervention for improving attention compared to the Urban‐VR comparison condition among participants with HF.
Given the lack of intervention studies testing the efficacy of the Nature‐VR intervention on HF self‐care and HRQoL, our sample size calculation is based on guidelines for pilot studies. 33 , 34 A sample size of 30 for each group should allow us to estimate the effect sizes that can be used to calculate the sample sizes of future efficacy studies.
Participants will be recruited from the Indiana University Health (IUH) outpatient cardiology clinics. Inclusion criteria are (1) adults (>21 years); (2) diagnosed with chronic HF Stage C as defined by Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, and Japanese Heart Failure Society; and (3) able to communicate in English. Exclusion criteria are (1) uncorrected visual impairment; (2) major neurological disease (eg, Alzheimer's disease, Parkinson disease, etc.); and (3) major psychiatric disease (eg, schizophrenia, bipolar disease, etc.).
2.4. Procedures
Written informed consent will be obtained from all participants prior to enrollment. The interviews will be conducted at either the participant's home or our research office at Indiana University School of Nursing. Our single blinded methodology requires us to use a separate intervention and data collection research assistants (RAs). The intervention RA will collect participants’ demographic and clinical characteristics and conduct the baseline interviews. After baseline interview, the RA will randomize the participant. 1:1 randomization will be generated using SAS statistical software by the team biostatistician. The randomization results will be concealed from the principal investigator (PI) and the data collection RA. The participants will be told not to share their group assignment with the data collection RA and the PI during their follow‐up interviews. The two RAs will be instructed to not communicate with each other about randomization. The intervention RA will instruct participants on how to complete the activities for either Nature‐VR or Urban‐VR with the written intervention manual. During the 4‐week intervention phase, participants in each group will receive weekly phone calls to check if they have experienced technical issues with the VR headset and to answer questions. After the intervention phase, the data collection RA who will remain blinded to group assignment will meet participants for four follow‐up interviews. Attention and secondary outcomes will be evaluated immediately after completion of the intervention (4 weeks visit) as well as at 8, 26, and 52 weeks. During each study time point, oxygen saturation will be monitored. A pulse oximetry (eg, Nonin WristOx2) will be placed on the participants’ index finger of the non‐dominant hand. Peripheral blood for genetic biomarkers (APOE, BDNF, dopamine receptor, and dopamine transporter genes) will be collected by a phlebotomist at the outpatient cardiology clinic or by our study team member who is an RN at baseline. Peripheral blood for serum BDNF will be collected at baseline and at 4 weeks. Collected blood samples will be transferred to the Indiana Clinical and Translational Sciences Institute (CTSI) Storage Facility and analyzed at Indiana Biobank.
2.5. Intervention and active comparison condition
The intervention and active comparison condition were developed by the PI with consultation from the Advanced Visualization Lab at Indiana University to use VR technology. The development was guided by the Attention Restoration Theory, and all contents in both Nature‐VR and Urban‐VR were validated in consistency with the four qualities of the restorative environment as explained below in the Content section.
2.5.1. Nature‐VR intervention
Participants will receive a VR headset and will be asked to perform the intervention 10 minutes per day, 5 days per week over 4 weeks, for a total of 200 minutes (= 3.3 hours). This intervention dose was chosen based on four factors. First, young adults can sustain attention for 10 to 20 minutes before needing reinforcement to maintain their focus. 35 , 36 Because attention span may decrease with aging, 37 attention interventions likely need to be brief for efficacy without fatigue. Second, a meta‐analysis of interventions using nature activities suggested that 5‐minute interventions are optimal for improving self‐esteem and mood. 38 Third, in our preliminary study participants had interventions of ∼7 minutes, which led to small to medium effect sizes. 32 Finally, participants with HF in our previous feasibility study were asked about the adequacy of the intervention dose and reported it to be adequate. 23 Thus 10 minutes per day was deemed appropriate for the Nature‐VR intervention.
The 4‐week intervention duration was selected based on two previous cognitive intervention studies. First, the ACTIVE study, a large cognitive training trial in 2832 healthy older adults, supports our rationale for 4 weeks of cognitive intervention. 39 Second, Cimprich tested a Nature intervention of 36 days to improve attention among 157 participants with breast cancer and found a medium effect. 17
Content (library of nature pictures): According to Attention Restoration Theory, nature has four qualities that provide a favorable environment for restoring attention (ie, feelings of being away, soft fascination, extended feelings, and high compatibility). 15 , 16 To ensure consistency with the theory, an intervention map was developed using the four qualities and the four nature domains (vegetation, sky, water, and earth). 15 This map was used to guide our choice of nature pictures. The pictures selected for Nature‐VR were validated for consistency with the theory by three PhD‐level nurse researchers. The final 200 nature images were grouped into 20 sets (10 photographs per set) (Figure 3).
FIGURE 3.

Examples of Nature‐VR intervention
Delivery mode: To view the photographs in the Nature‐VR intervention participants will use the Oculus Go, a head‐mounted goggle that displays 360o pictures (See Figure 3). The goggles are used to limit eye movement inside and to prevent visual distractions from outside the device. The Oculus application was developed by the Indiana University Advanced Visualization Lab to display three‐dimensional photographs for the Nature‐VR intervention. The application stores all 200 photographs, and automatically plays the 360o spherical photographs (1 minute per photograph) when the Oculus Go device is turned on and the application initiated. The application has a time‐stamping function to monitor the time spent.
2.5.2. Urban‐VR active comparison condition
The active comparison condition was developed to resemble as closely as possible the Nature‐VR intervention. Participants in the comparison condition will also receive a VR headset for research. They will be similarly instructed to perform the active comparison condition 10 minutes per day, 5 days per week over 4 weeks, for a total of 200 minutes ( = 3.3 hours). The active comparison image library will consist of 360o pictures of an urban environment. The urban views (eg, a parking structure, traffic lights, indoor images of buildings) have fewer or less of the four qualities of attention restorative stimuli, according to guiding theory. 15 , 16
2.6. Measures
Attention, our primary outcome, will be assessed using objective and subjective measures at baseline and at 4, 8, 26 and 52 weeks of follow‐up. The objective measures of attention to be used are the Multi‐Source Interference Task, Digit Span, Trail Making, and Stroop tests (Table 1). Subjective attention—a patient‐reported outcome—will also be examined using the Attentional Function Index. 40 This self‐reported questionnaire consists of 13 items that assess perceived effectiveness of behaviors requiring attention on a visual analogue scale (0 to 10). Higher scores indicate better subjective attention. 40
TABLE 1.
Measures of attention
| Measures | Description | Reliability | Validity |
|---|---|---|---|
| Multi‐Source Interference Task | This computerized test examines the cingulo‐fronto‐parietal cognitive/attention network. 52 , 53 In this task participants are asked to identify a target number that is different from two other numbers displayed on a computer screen. There are two types of trials: congruent and incongruent. For the congruent trials a target number is always matched by its position on a button (eg, 100, 020, or 223), whereas for incongruent trials the target number is never matched with its position on a button (eg, 010, 233, or 232). A faster response time and lower error rate indicates better attention. In this study, congruential trial scores will be used to assess sustained attention, and differences between congruent and incongruent trials will be used to assess directed attention. | Reliable activation of the dorsal anterior cingulate cortex and prefrontal cortex was found during the test in a meta‐analysis of nine studies with healthy adults. 54 | Construct validity was confirmed among 22 patients with HF. 55 |
| Digit Span Test | This test measures attention, specifically, attention span. 56 Participant are asked to repeat the sequence of numbers that they were told. The test has two parts, Forward, repeating the numbers in the same sequence, and Backward, repeating the numbers backwards from the last to the first number. The numbers of digits participants repeated correctly are scored. Higher scores indicate better attention. | Test‐retest reliability coefficients ranged between .66 and .89. 56 | Construct validity was supported by comparing healthy and closed head injury patients. 57 |
| Trail Making Test | This test measures attention requiring visual tracking and task switching. 58 , 59 There are two parts: A and B. In Part A, participants are asked to connect a series of randomly arrayed circles numbered from 1 to 25 in order as quickly as possible. In Part B, participants are asked to connect a series of 25 circles numbered from 1 to 13 randomly intermixed with letters from A to L, alternating between numbers and letters in ascending order. The time to complete including the time for correction of any errors is recorded, called response time. A longer response time indicates poorer attention. In this study, Part A scores will be used to assess sustained attention and differences between Part A and B will be used to assess directed attention. | Test‐retest reliability ranged from.86 to 96 in 20 patients with HF. 32 | Construct validity was supported among healthy adults and patients with closed head injury. 57 |
| Stroop Test | This test measures attention involving in selective processing of different visual features while ignoring distractions on the test (letters and ink colors of color words). 60 A computerized Stroop test was programmed using E‐Prime software. Participants are asked to read the letters or ink colors of four color words (ie, red, blue, yellow, and green) and press the designated keyboard on the laptop computer. There are two types of trials. In congruent trials, the color names have same letters and print colors (eg, red in red ink). In incongruent trials, the color names do not match to the print colors (eg, red in blue ink). There are two conditions: switching and non‐switching. In switching conditions, the commands are switched from word to color, or color to word. In non‐switching conditions, the commands are the same (color to color, or word to word). Differences in performance between switching and non‐switching conditions will be used to assess attention switching. Congruential trial scores will be used to assess sustained attention and differences between congruent and incongruent trials will be used to assess directed attention. | Reliability was satisfactory. 61 , 62 | Construct validity was supported in patients with traumatic brain injury. 63 Impaired performance on Stroop test was most common in patients with frontal lobe lesions in a meta‐analysis. 64 |
HF self‐care will be examined by the Self‐Care of Heart Failure Index v7.2. 41 This self‐report questionnaire consists of 39 items. Higher scores indicate better self‐care and scores ≥70 indicate adequate self‐care.
HRQoL will be measured by the Minnesota Living with Heart Failure Questionnaire. 42 , 43 This self‐report questionnaire consists of 21 items that ask participants to rate how their HF condition has impacted their physical and emotional health. Lower scores indicate better HRQoL. This measure is predictive of mortality, and a 4.84‐point change in the total score is clinically meaningful. 44
In addition to the attention measures described above we will use the Montreal Cognitive Assessment (MoCA) to measure global cognition, the Hopkins Verbal Learning Test (HVLT) and Craft Story to assess verbal memory, the Benson Figure Copy to assess visuospatial memory, Category Fluency to assess language, and Digit Symbol to assess executive function. Health events and mortality will be collected by reviewing electronic medical records (EMRs) and doing online searches for obituaries.
Demographic (ie, age, education, gender, socioeconomic status, handedness, body mass index, smoking history), and clinical (ie, ejection fraction, HF type and severity, B‐type natriuretic peptide, medications, cardiac devices, depressive symptoms, comorbidity) characteristics will be collected at baseline and used as possible covariates.
Pulse oximetry will be used to monitor oxygen saturation at each study time point. Venipuncture for serum BDNF will be performed at baseline and 4 weeks. Venipuncture for genetic biomarkers (ie, APOE, BDNF, dopamine receptor, and dopamine transporter genes) will be performed at baseline.
2.7. Statistical analysis plan
Distributions based on density plots will be examined for normality of the data and to detect outliers. If not normally distributed, non‐parametric methods will be used. Baseline equivalencies between the groups will be evaluated using independent t‐tests and chi‐square tests. Observed scores of outcome variables at pre‐ and post‐intervention will be presented for both groups. An intent‐to‐treat approach will be used to ensure that observed differences between groups are not attributable to differential dropout. 45 Analyses for each study aim will be performed using the statistical package SAS at an alpha of .05 or less.
To test the effect of Nature‐VR on attention (hypothesis 1), HF self‐care, and HRQoL (hypotheses 2a and 2b, respectively), we will use linear mixed models to model attention performance over time with group, time, and group by time interaction variables. Adherence to the intervention (ie, minutes spent using Nature‐VR, which is objective evidence collected by using time‐stamping function of the Oculus application in the device), HF severity, comorbidity, and gender will be tested as potential covariates. The mixed models will produce valid estimates for missing data at random.
We will employ three analytic strategies to demonstrate the relationships between attention and possible biomarkers. The relationships of baseline attention with oxygen saturation, BDNF serum levels over time, BDNF Met, APOE ε4, 7‐repeat of dopamine receptor and 10‐repeat of dopamine transporter alleles will be examined using analysis of covariance (ANCOVA) models, while adjusting for participants’ ages. The changes in oxygen saturation and serum BDNF levels in relation to attention changes at 4‐week follow‐up will be examined using ANCOVA models adjusting for age. Those biomarkers that show significant associations with attention will be added to models for hypotheses 1, 2a, and 2b to examine different levels of responsiveness to the intervention based on the biomarkers.
We will assess the effect of Nature‐VR on the other cognitive domains (exploratory aim 2) using linear mixed models similar to the analysis plan for hypothesis 1, 2a, and 2b. Binomial logistic regressions will be used to test the preliminary efficacy of Nature‐VR on cardiac and neurological events and mortality adjusting for covariates.
Effect sizes of Nature‐VR will be examined by omega‐squared for the interaction terms. 46 Ninety‐five percent confidence intervals will be estimated using the non‐parametric bootstrap method.
2.8. Potential difficulties and alternative approaches
Although HF prevalence is similar for men and women, 1 women are underrepresented in HF research studies. 47 To address this issue we will recruit equal numbers of women and men by monitoring monthly recruitment. If the enrollment of women is <50% for three consecutive months, we will modify the recruitment brochure to be more attractive to women (eg, more pictures of women) and identify more outpatient clinics for recruitment of women. Second, the African American population in the United States is 12.6%. To recruit a diverse sample, study invitations for African American will be prioritized during recruitment. Finally, using a VR headset may be challenging to some participants. To help participants with the use of this technology, written instructions and a phone helpline will be provided.
2.9. Research conduct during the COVID‐19 pandemic
The CORE study is being conducted during the worldwide pandemic of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) (coronavirus disease 2019 [COVID‐19]). The COVID‐19 pandemic has led to study protocol modifications to meet the COVID‐19 restrictions for human research. Given that our study population is adults with HF who are identified as high risk for more serious symptoms of COVID‐19, we are conducting the study with a remote option without direct physical contact with participants. Major changes are recruitment over the phone with verbal consent, data collection by phone interviews, saliva sample collection, and drop‐off–based intervention delivery. Because of the remote option, necessary changes were made to measures. Detailed information is presented in Table 2. The modified study protocol using a remote data collection and intervention delivery has been approved by the university institutional review board.
TABLE 2.
Protocol changes responding to the COVID‐19 pandemic
| Study procedure | Changes in methods | Descriptions |
|---|---|---|
| Recruitment | Phone recruitment and verbal consent | Possibly eligible adults with HF will be contacted by phone. A copy of the informed consent and HIPAA form will be sent to possibly eligible participants via email or mail prior to the verbal consent and authorization. We will obtain verbal consent without a written signature on the consent form and HIPAA form after explaining our study procedures with our informed consent and HIPAA forms. Participants will be asked if they have any questions before they provide verbal consent to participate in the study. |
| Data collection | Phone interviews |
To avoid physical contact, data will be collected by phone interviews instead of face‐to‐face interviews at baseline, 4, 8, 26, and 52 weeks. Our original protocol included two computerized (ie, Multi‐Source Interference Task, Stroop Test) and two paper‐pencil based cognitive tests (ie, Benson Figure Copy, Digit Symbol tests) that cannot be administered via phone. Thus, the Oral Trail Making (primary outcome measure) and Digit Span tests will be used to assess attention. A blind version of the MoCA will be used to assess global cognition. Verbal Fluency will be administered to examine executive function. In addition to Category Fluency, the Verbal Naming Test will be administered to examine language. The Hopkins Verbal Learning Test and Craft Story will be administered as planned to assess verbal memory. Oxygen saturation will not be monitored during the phone interviews. |
| Saliva samples |
Venipuncture was planned to measure serum BDNF levels and collect DNA for genetic biomarkers (ie, BDNF Met, APOE ε4, 7‐repeat of dopamine receptor, and 10‐repeat of dopamine transporter alleles). Due to the need to collect data remotely, saliva samples will instead be collected for genetic biomarkers using the OGR‐600 saliva kit (DNA Genotek). The saliva sample collection kit will be delivered to the participants by the intervention RA with the intervention kit. The intervention RA will instruct how to collect saliva sample and ask to leave their samples outside their front doors for collection by the RA. The intervention RA will deliver the saliva sample to the storage facility on campus. Oxygen saturation and serum BDNF will not be collected in this modified study protocol. |
|
| Intervention delivery | Drop‐off based remote option of delivery |
A study team member will call participants to set up a time to drop off the intervention kit (virtual reality headset, intervention manual binder). The intervention kit will be dropped off outside the participant's front door after which the participant will be called to let them know the kit has been delivered. Instructions for how to use the intervention kit will be given in the follow up phone call. If a participant wants to receive the intervention in person, the intervention RA may give the intervention instructions to the participant outdoors in person. Throughout the entire process, adequate social distancing of 6 feet or more will be maintained. After the intervention phase, participants will be asked to leave the intervention kit outside their front door for a pick up at a pre‐arranged date and time. |
Abbreviations: APOE, apolipoprotein E; BDNF, brain‐derived neurotrophic factor; DNA, deoxyribonucleic acid; HIPAA, Health Insurance Portability and Accountability Act; MoCA, Montreal Cognitive Assessment; RA, research assistant.
3. DISCUSSION
Participant enrollment was initiated in July 2020 to evaluate the preliminary efficacy of the Nature‐VR intervention for improving attention, HF self‐care, and HRQoL compared to the Urban‐VR active comparison condition. In this new study, 74 participants with HF will be enrolled and followed up 52 weeks (1 year) after baseline.
The Nature‐VR is theoretically sound and innovative. The incorporation of VR technology into this restorative cognitive intervention will provide immersive experiences by visually simulating a real natural environment. The use of this immersive technology is ideal for participants who have limited access to natural environments (eg, due to limited physical function, inclement weather, or urban living with limited access to green space) and less opportunity to engage with nature directly. VR technology is safe and may increase the strength of the intervention effect, as demonstrated by past studies, including our feasibility study. 48 , 49 , 50 , 51
Investigating the effects of Nature‐VR on HF self‐care and HRQoL will contribute to understanding the relationships between cognitive dysfunction and the health outcomes as well as provide another approach to improve HF management if efficacious. Previous studies have been more focused on educating patients to change self‐care behaviors. Cognitive interventions may support the patients’ learning during the education, improve perception of the HF symptoms, and help make decisions on managing the symptoms.
The Nature‐VR has great potential to improve attention and prevent attention impairment, and may lead to better self‐care and HRQoL among patients with HF. The Nature‐VR intervention can be delivered in patients’ homes at low cost (eg, the Oculus Go, head‐mounted goggles costs $300). If findings are as hypothesized, the Nature‐VR and Urban‐VR interventions will be used to test full‐scale efficacy of the Nature‐VR intervention with a sample size powered from the effect sizes calculated from this study. If no effect is seen, this pilot study will contribute to the development of knowledge about attention changes over time in HF, future design of biobehavioral cognitive interventions, and development of possible biomarkers associated with attention.
CONFLICT OF INTEREST
Miyeon Jung received the American Heart Association Career Development Award [19CDA34520006] to support this CORE study. Liana G. Apostolova, Debra K. Moser, Sujuan Gao, and Susan J. Pressler have served as mentors on the grant. Jeff L. Rogers is a consultant on the grant. All authors have no other relationships/activities/interests to disclose related to the content of this submission.
ACKNOWLEDGMENT
The authors acknowledge Tassie Gniady, PhD and Matthew Mercer at Indiana University Research Technologies for their assistance in developing the Nature‐VR and Urban‐VR; Laura Hays, PhD, RN at Indiana University School of Nursing for her support for content validity of Nature‐VR and Urban‐VR; and Christine Feak at University of Michigan for her support in editing this manuscript. This work was financially supported by the American Heart Association Career Development Award [19CDA34520006].
Jung M, Apostolova LG, Moser DK, et al. Virtual reality cognitive intervention for heart failure: CORE study protocol. Alzheimer's Dement. 2022;8:e12230. 10.1002/trc2.12230
REFERENCES
- 1. Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67‐e492. [DOI] [PubMed] [Google Scholar]
- 2. Pressler SJ. Cognitive functioning and chronic heart failure: a review of the literature (2002‐July 2007). J Cardiovasc Nurs. 2008;23(3):239‐249. [DOI] [PubMed] [Google Scholar]
- 3. Pressler SJ, Subramanian U, Kareken D, et al. Cognitive deficits in chronic heart failure. Nurs Res. 2010;59(2):127‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leto L, Feola M. Cognitive impairment in heart failure patients. J Geriatr Cardiol. 2014;11(4):316‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vogels RL, Scheltens P, Schroeder‐Tanka JM, et al. Cognitive impairment in heart failure: a systematic review of the literature. Eur J Heart Fail. 2007;9(5):440‐449. [DOI] [PubMed] [Google Scholar]
- 6. Jung M, Jonides J, Northouse L, et al. Poorer attention in heart failure is related to increased attentional demands and oxygen saturation. Circulation. 2015;132(Suppl 3):A18863‐A18863. https://www.ahajournals.org/doi/abs/10.1161/circ.132.suppl_3.18863 [Google Scholar]
- 7. Serber SL, Kumar R, Woo MA, et al. Cognitive test performance and brain pathology. Nurs Res. 2008;57(2):75‐83. [DOI] [PubMed] [Google Scholar]
- 8. Woo MA, Kumar R, Macey PM, et al. Brain injury in autonomic, emotional, and cognitive regulatory areas in patients with heart failure. J Card Fail. 2009;15(3):214‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dickson VV, Knafl GJ, Riegel B. Predictors of medication nonadherence differ among black and white patients with heart failure. Res Nurs Health. 2015;38(4):289‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dickson VV, Lee CS, Riegel B. How do cognitive function and knowledge affect heart failure self‐care? J Mix Methods Res. 2011;5(2):167‐189. 10.1177/1558689811402355 [DOI] [Google Scholar]
- 11. Alosco ML, Spitznagel MB, Cohen R, et al. Cognitive impairment is independently associated with reduced instrumental ADLs in persons with heart failure. J Cardiovasc Nurs. 2012;27(1):44‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dickson VV, Tkacs N, Riegel B. Cognitive influences on self‐care decision making in persons with heart failure. Am Heart J. 2007;154(3):424‐431. [DOI] [PubMed] [Google Scholar]
- 13. Riegel B, Moser DK, Buck HG, et al. Self‐care for the prevention and management of cardiovascular disease and stroke: a scientific statement for healthcare professionals from the American Heart Association. J Am Heart Assoc. 2017;6(9):e006997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fleury J, Sidani S. Using theory to guide intervention research, in Intervention research and evidence‐based quality improvement: designing, conducting, analyzing, and funding. Springer Publishing Company; 2018:55.
- 15. Kaplan S. The restorative benefits of nature: toward an integrative framework. J Environ Psychol. 1995;15(3):169‐182. [Google Scholar]
- 16. Kaplan S. Meditation, restoration, and the management of mental fatigue. Environ Behav. 2001;33(4):480‐506. [Google Scholar]
- 17. Cimprich B, Ronis DL. An environmental intervention to restore attention in women with newly diagnosed breast cancer. Cancer Nurs. 2003;26(4):284‐292. [DOI] [PubMed] [Google Scholar]
- 18. Berman MG, Jonides J, Kaplan S. The cognitive benefits of interacting with nature. Psychol Sci. 2008;19(12):1207‐1212. [DOI] [PubMed] [Google Scholar]
- 19. Gamble KR, Howard JH Jr, Howard DV. Not just scenery: viewing nature pictures improves executive attention in older adults. Exp Aging Res. 2014;40(5):513‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pressler SJ, Titler M, Koelling TM, et al. Nurse‐enhanced computerized cognitive training increases serum brain‐derived neurotrophic factor levels and improves working memory in heart failure. J Card Fail. 2015;21(8):630‐641. [DOI] [PubMed] [Google Scholar]
- 21. Freina L, Ott M, A literature review on immersive virtual reality in education: state of the art and perspectives. in The International Scientific Conference eLearning and Software for Education. 2015. “ Carol I” National Defence University.
- 22. Desai PR, Desai PN, Ajmera KD, Mehta K. A review paper on oculus rift‐A virtual reality headset. Int J Eng Trends Technol. 2014;13(4):175‐179. [Google Scholar]
- 23. Jung M, Gradus‐Pizlo I, Berman MG, et al. Feasibility of a novel virtual reality‐based cognitive intervention to improve attention in people with heart failure. J Card Fail. 2018;24(8):S74‐S75. [Google Scholar]
- 24. Bennett SJ, Sauvé MJ, Shaw RM. A conceptual model of cognitive deficits in chronic heart failure. J Nurs Scholarsh. 2005;37(3):222‐228. [DOI] [PubMed] [Google Scholar]
- 25. Vinogradov S, Fisher M, Holland C, et al. Is serum brain‐derived neurotrophic factor a biomarker for cognitive enhancement in schizophrenia?. Biol Psychiatry. 2009;66(6):549‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lim YY, Villemagne VL, Laws SM, et al. Effect of BDNF Val66Met on memory decline and hippocampal atrophy in prodromal Alzheimer's disease: a preliminary study. PLoS One. 2014;9(1):e86498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu C, Wang Z, Fan M, et al. Effects of BDNF Val66Met polymorphism on brain metabolism in Alzheimer's disease. Neuroreport. 2010;21(12):802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pressler SJ, Harrison JM, Titler M, et al. APOE ε4 and memory among patients with heart failure. West J Nurs Res. 2017;39(4):455‐472. [DOI] [PubMed] [Google Scholar]
- 29. Wolk DA, Dickerson BC, Initiative TASDN. Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional—executive network function in Alzheimer's disease. Proc Natl Acad Sci. 2010;107(22):10256‐10261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hasler R, Salzmann A, Bolzan T, et al. DAT1 and DRD4 genes involved in key dimensions of adult ADHD. Neurol Sci. 2015;36(6):861‐869. [DOI] [PubMed] [Google Scholar]
- 31. Li D, Sham PC, Owen MJ, He L. Meta‐analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder (ADHD). Hum Mol Genet. 2006;15(14):2276‐2284. [DOI] [PubMed] [Google Scholar]
- 32. Jung M, Jonides J, Northouse L, Berman MG, Koelling TM, Pressler SJ. Randomized crossover study of the natural restorative environment intervention to improve attention and mood in heart failure. J Cardiovasc Nurs. 2017;32(5):464‐479. [DOI] [PubMed] [Google Scholar]
- 33. Moore CG, Carter RE, Nietert PJ, Stewart PW. Recommendations for planning pilot studies in clinical and translational research. Clin Transl Sci. 2011;4(5):332‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health. 2008;31(2):180‐191. [DOI] [PubMed] [Google Scholar]
- 35. David Cornish M, Dukette D. The Essential 20: Twenty Components of an Excellent Health Care Team. Dorrance Publishing; 2009. [Google Scholar]
- 36. Johnstone AH, Percival F. Attention breaks in lectures. Educ Chem. 1976;13(2):49‐50. [Google Scholar]
- 37. Commodari E, Guarnera M. Attention and aging. Aging Clin Exp Res. 2008;20(6):578‐584. [DOI] [PubMed] [Google Scholar]
- 38. Bratman GN, Hamilton JP, Daily GC. The impacts of nature experience on human cognitive function and mental health. Ann N Y Acad Sci. 2012;1249(1):118‐136. [DOI] [PubMed] [Google Scholar]
- 39. Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. J Am Med Ass. 2002;288(18):2271‐2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cimprich B, Visovatti M, Ronis DL. The Attentional Function Index—a self‐report cognitive measure. Psychooncology. 2011;20(2):194‐202. [DOI] [PubMed] [Google Scholar]
- 41. Riegel B, Barbaranelli C, Carlson B, et al. Psychometric testing of the revised self‐care of heart failure index. J Cardiovasc Nurs. 2019;34(2):183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rector TS, Cohn JN. Assessment of patient outcome with the Minnesota Living with Heart Failure questionnaire: reliability and validity during a randomized, double‐blind, placebo‐controlled trial of pimobendan. Am Heart J. 1992;124(4):1017‐1025. https://www.sciencedirect.com/science/article/abs/pii/0002870392909866 [DOI] [PubMed] [Google Scholar]
- 43. Rector TS, Kubo SH, Cohn JN. Validity of the Minnesota Living with Heart Failure questionnaire as a measure of therapeutic response to enalapril or placebo. Am J Cardiol. 1993;71(12):1106‐1107. [DOI] [PubMed] [Google Scholar]
- 44. Bennett SJ, Oldridge NB, Eckert GJ, et al. Comparison of quality of life measures in heart failure. Nurs Res. 2003;52(4):207‐216. [DOI] [PubMed] [Google Scholar]
- 45. Little R, Yau L. Intent‐to‐treat analysis for longitudinal studies with drop‐outs. Biometrics. 1996;52:1324‐1333. [PubMed] [Google Scholar]
- 46. Olejnik S, Algina J. Generalized eta and omega squared statistics: measures of effect size for some common research designs. Psychol Methods. 2003;8(4):434. [DOI] [PubMed] [Google Scholar]
- 47. Pressler SJ. Women with heart failure are disproportionately studied as compared with prevalence: a review of published studies from 2013. J Cardiovasc Nurs. 2016;31(1):84‐88. [DOI] [PubMed] [Google Scholar]
- 48. Parsons TD, Rizzo AA. Affective outcomes of virtual reality exposure therapy for anxiety and specific phobias: a meta‐analysis. J Behav Ther Exp Psychiatry. 2008;39(3):250‐261. [DOI] [PubMed] [Google Scholar]
- 49. Bohil CJ, Alicea B, Biocca FA. Virtual reality in neuroscience research and therapy. Nat Rev Neurosci. 2011;12(12):752‐762. [DOI] [PubMed] [Google Scholar]
- 50. Wilson CJ, Soranzo A. The use of virtual reality in psychology: a case study in visual perception. Comput Math Methods Med. 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Adams R, Finn P, Moes E, Flannery K, Rizzo AS. Distractibility in attention/deficit/hyperactivity disorder (ADHD): the virtual reality classroom. Child Neuropsychol. 2009;15(2):120‐135. https://www.tandfonline.com/doi/full/10.1080/09297040802169077?casa_token=HANBGDj9zjsAAAAA%3A--WU9Sayk_CSNw1ollIx7FaMNbmZYx3eLMr3Ft4eKOdMIsffrkZ4if_wEF8MFEF1O7jjC4TFDPZN [DOI] [PubMed] [Google Scholar]
- 52. Bush G, Shin LM, Holmes J, Rosen BR, Vogt BA. The multi‐source interference task: validation study with fMRI in individual subjects. Mol Psychiatry. 2003;8(1):60‐70. [DOI] [PubMed] [Google Scholar]
- 53. Bush G, Shin LM. The multi‐source interference task: an fMRI task that reliably activates the cingulo‐frontal‐parietal cognitive/attention network. Nat Protoc. 2006;1(1):308‐313. [DOI] [PubMed] [Google Scholar]
- 54. Deng Y, Wang X, Wang Y, Zhou C. Neural correlates of interference resolution in the multi‐source interference task: a meta‐analysis of functional neuroimaging studies. Behav Brain Funct. 2018;14(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jung M, Jonides J, Berman MG, Northouse L, Koelling TM, Pressler SJ. Construct validity of the multi‐source interference task to examine attention in heart failure. Nurs Res. 2018;67(6):465‐472. [DOI] [PubMed] [Google Scholar]
- 56. Lezak M, et al. Neuropsychological Assessment. Oxford University Press; 2012. [Google Scholar]
- 57. Shum DH, McFarland KA, Bain JD. Construct validity of eight tests of attention: comparison of normal and closed head injured samples. Clin Neuropsychol. 1990;4(2):151‐162. [Google Scholar]
- 58. Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8(3):271‐276. 10.2466/pms.1958.8.3.271 [DOI] [Google Scholar]
- 59. Bowie CR, Harvey PD. Administration and interpretation of the Trail Making Test. Nat Protoc. 2006;1(5):2277‐2281. [DOI] [PubMed] [Google Scholar]
- 60. Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18(6):643. [Google Scholar]
- 61. Jensen AR, Rohwer WD Jr. The Stroop color‐word test: a review. Acta Psychol (Amst). 1966;25:36‐93. [DOI] [PubMed] [Google Scholar]
- 62. Franzen MD, Tishelman AC, Sharp BH, Friedman AG. An investigation of the test‐retest reliability of the stroop colorword test across two intervals. Arch Clin Neuropsychol. 1987;2(3):265‐272. [PubMed] [Google Scholar]
- 63. Ponsford J, Kinsella G. Attentional deficits following closed‐head injury. J Clin Exp Neuropsychol. 1992;14(5):822‐838. [DOI] [PubMed] [Google Scholar]
- 64. Demakis GJ. Frontal lobe damage and tests of executive processing: a meta‐analysis of the category test, stroop test, and trail‐making test. J Clin Exp Neuropsychol. 2004;26(3):441‐450. [DOI] [PubMed] [Google Scholar]


