Abstract
Eosinophilic gastrointestinal diseases, specifically eosinophilic gastritis and duodenitis, are chronic inflammatory conditions characterized by persistent gastrointestinal (GI) symptoms and elevated levels of activated eosinophils in the GI tract. Both clinical and endoscopic findings are nonspecific, no clinical or histopathologic diagnostic guidelines are published, and disease awareness is low, both among clinicians and amongst pathologists, who tend to overlook mild or moderate increases in the density of eosinophils in GI biopsy specimens. Yet, evaluating and, at times, counting eosinophils in GI biopsies may have important clinical implications: the numbers of tissue eosinophils correlate with clinical manifestations, can be used as determinants of effective management, and are used to assess the effects of treatment. A most persuasive argument for providing a count rather than a value judgment is that patients read reports, understand numbers, and use them to help to understand the course of their disease. The objective of this primer is to provide pathologists with the tools to incorporate a quantitative assessment of eosinophilia in the diagnosis of gastric and duodenal biopsy specimens and to develop a systematic approach to their evaluation, counting, and reporting. To achieve this aim, we present our general approach to the biopsy (where to count), followed by details on the characteristics of a countable eosinophil (what to count), and provide with a set of suggestions on the counting methods (how to count). We conclude with suggestions on how to report GI tissue eosinophilia in a manner that alerts clinicians and prompts pertinent management steps.
Key Words: eosinophils, eosinophilic gastrointestinal diseases, eosinophilic gastritis, eosinophilic duodenitis, quantitative methods
Eosinophilic gastrointestinal diseases (EGIDs) are chronic inflammatory conditions characterized by persistent gastrointestinal (GI) symptoms and elevated levels of activated eosinophils in the GI tract, and are clinicopathologic diagnoses.1 Whilst not perfect, the manifestations of eosinophilic esophagitis (EoE) have been characterized2 and guidelines for the clinical and histopathologic components of the diagnosis are accepted by most clinicians and pathologists.3,4 A recently reported histology scoring system for EoE evaluates features in addition to eosinophilic inflammation that correlate with symptoms, and can be used to determine remission; however, peak eosinophil count remains the gold standard for diagnosis.5–7 By contrast, eosinophilic gastritis and duodenitis (EOG/EoD) present with a constellation of generally nonspecific symptoms (early satiety, nausea and vomiting, abdominal pain and cramping, bloating, and diarrhea) that overlap with other common GI conditions, such as functional dyspepsia or irritable bowel syndrome. This broad-based clinical presentation, together with low disease awareness, perceived rarity, and the frequent absence of significant endoscopic findings8 make EGIDs an uncommon clinical suspicion. As a result, many patients face a long and frustrating delay with inappropriate care before the correct diagnosis is made.9 Similar constraints have limited both pathologists’ awareness of EGIDs and their interest for pursuing a diagnosis of EOG/EoD. Clinicians rarely list eosinophilic gastritis or duodenitis in the usually scant notes that accompany biopsy specimens, and many pathologists examine GI biopsies at low and medium power only, resorting to high-power only for targeted searches (eg, the detection of Helicobacter pylori). Therefore, mild or moderate increases in the density of eosinophils are often overlooked in GI biopsy specimens. Furthermore, few practicing pathologists see any value in spending their time enumerating GI eosinophils, as such detailed reports would rarely trigger decisive management interventions. The Food and Drug Administration (FDA) has accepted ≥30 eosinophils per high-power field (eos/hpf) in ≥5 hpfs in the stomach and in ≥3 hpfs in the duodenum as threshold values for the diagnosis of EOG and/or EoD, respectively, in certain clinical trials. In the absence of published guidelines there are few pathologists who would consider making such diagnoses without established criteria, except in those cases where eosinophils are obviously excessive.
Heightened awareness and standardization of diagnostic guidelines has enhanced the detection of EoE. Increasing numbers of pathologists now evaluate the eosinophil numbers in squamous esophageal biopsy specimens and, when their density is elevated, report the highest number of eosinophils in a high-power field (hpf) (ie, peak eosinophil count). This behavior has been achieved through years of publications, consensus conferences resulting in widely accepted guidelines, and persistent nudging by gastroenterologists and patients. However, guidelines are not consistently applied, technical problems persist, and interobserver variability in the counts remain high.10
The detection and enumeration of eosinophils in gastric and duodenal specimens is more complicated than in esophageal biopsies. In contrast to the normal squamous esophageal epithelium, where there are no resident eosinophils, the gastric and intestinal mucosa normally contains yet undefined numbers of eosinophils, where they coexist with an admixture of other inflammatory cells, mostly lymphocytes and plasma cells, but also histiocytes, mast cells, and occasional neutrophils. Although the number of 30 eos/hpf as the upper limit of normal for stomach and duodenum has been proposed,11–13 others have suggested lower14–17 or higher18 thresholds. These and other issues, including population-related differences in the normal distribution of eosinophils and the clinical significance of GI eosinophilia have been thoroughly discussed in several reviews.13,15,16,19–21
The objective of this primer is to provide pathologists with the tools to incorporate the assessment of eosinophilia in the diagnosis of gastric and duodenal biopsy specimens and to develop a systematic approach to their evaluation, counting, and reporting. To achieve this aim, we will first present our general approach to the biopsy (where to count), followed by details on the characteristics of a countable eosinophil (what to count), and will conclude with a set of suggestions on the counting methods (how to count).
APPROACH TO THE BIOPSY—WHERE TO COUNT
Before placing the slide on the microscope stage, the number of pieces in a level should be counted with the naked eye to ensure that all pieces, even those that may be placed somewhat apart from the bulk of pieces, are examined. Then, each biopsy specimen should first be examined at low-power magnification (×40 and ×100) to evaluate for proper orientation and for the presence of lesions or conditions (eg, features associated with H. pylori gastritis, celiac disease, neoplasia) that would make counting eosinophils less relevant. If no other pathologic features are present, each level should then be examined at medium power magnification (×200) to detect areas with the highest eosinophil density, before switching to high power magnification (×400) and counting. Please note that throughout the text a “high-power field” refers to an area visualized on a microscope using a ×40 lens and a 22 mm Ø ocular and corresponds to an area of 0.237 mm2.
The first hpf (×400) in which eosinophils are counted is best selected from an area of high eosinophil density; the remaining hpfs should include areas, which—depending on the distribution of eosinophils in the specimen—may or may not be adjacent to the first hpf. When obtaining counts for multiple hpf, nonoverlapping areas should be counted; when obtaining a count to report for a single hpf, overlapping areas may be counted. With experience, pathologists become familiar with the selection of the best areas for evaluating the density of eosinophils and develop their own unique strategies.
The Ideal Specimen
The purpose of counting is to express the density of eosinophils in one area of the mucosa in a measurable manner that can be readily compared with measurements performed in other areas and in other specimens. Thus, our first concerns must be the integrity and how representative is the section of the mucosa as we set out to evaluate the section. Examples of ideal specimens are depicted in Figure 1. Biopsies are oriented on edge and include surface epithelium, mucosa, and muscularis mucosae. In such biopsies an observer can easily find complete hpf that contain representative parts of epithelium and lamina propria. Such specimens, however, are not always available, and a systematic approach to problematic specimens must be developed. These suggestions are given with the caveat that a pathologist may encounter areas with increased eosinophils in less-than-ideal fields. To comply with finding the fields with the highest density, one may choose to “go where the money is,” even if the money is in a suboptimal field, as long as it remains within the confines of the lamina propria.
FIGURE 1.

Ideally oriented specimens from gastric antrum (A), body (B), and duodenum (C). The blue circle represents the area of a high-power field (0.237 mm2).
Special Situations
A common occurrence is the folding of mucosal fragments. Figure 2 depicts two situations in which hpf include more than one discrete mucosal area. We recommend that such fields be counted as depicted, always avoiding areas with duplication of the lamina propria. Fragmented, denuded, and poorly oriented fragments of mucosa should be rejected, as should very small fragments that do not fill at least most of the hpf. Counts performed in such specimens would very likely be deceptively low and, if reported, would fail to provide a reliable assessment of tissue eosinophilia. This assumes that a small fragment is not highly inflamed; however, there may be situations in which the greatest eosinophil density is found in a small fragment and, in such cases, the fragment should be counted.
FIGURE 2.

A, Folded duodenal mucosa. No high-power field should contain the black line, lest counts will capture densities from 2 sections of the same mucosal fragment. B, The high-power field (as depicted) does not include areas below the muscularis mucosae, in which eosinophils should not be counted.
DEFINING A COUNTABLE EOSINOPHIL—WHAT TO COUNT
Some authors have proposed a rigorous definition of eosinophils that can be counted, which includes only cells filled with eosinophilic granules and in which both lobes of the nucleus are visible.17 Applying this restrictive definition results in an underestimation of tissue eosinophilia. Histologic sections 5-µm thick often cut eosinophils perpendicularly to their bilobate nucleus, thus preventing the visualization of both lobes even though such cells are unequivocally eosinophils. Because of the size of eosinophils (12 to 17 µm in diameter), there are also instances in which the cell is sectioned is such a way that not even one fragment of nucleus is visible in a cell filled with eosinophilic granules. Therefore, we have agreed to define a countable eosinophil as a cell that has one of three different appearances: (1) intact with a bi-lobed nucleus; (2) a partial nucleus; or (3) a discrete cluster of eosinophil granules at least in part limited by a membrane, even if there is no clearly discernable nucleus. Figure 3 shows several examples of what we consider countable eosinophils. Figure 4 exemplifies aggregates of granules with an uncertain nucleus or without a nucleus; a pathologist may still elect to count such fragments based on the reasonable assumption that they can only represent an eosinophil. Granules haphazardly scattered in the lamina propria should not be counted.
FIGURE 3.
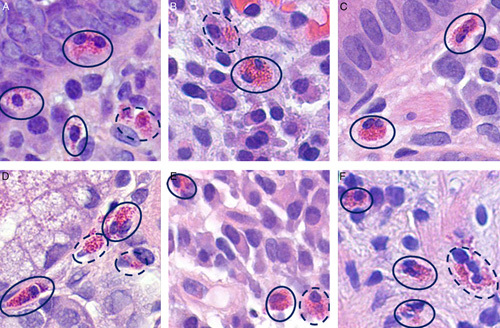
Examples of countable eosinophils. The numbers of countable eosinophils (solid line) as determined by our group is: A: 3, B: 1, C: 2, D: 1, E: 2; and F: 3. Those circled with a dotted line represent cells that would require an examination at several depths of focus to determine their precise nature.
FIGURE 4.

Examples of cells defined mostly by their granules. The numbers of unequivocal countable eosinophils (solid line) as determined by our group is: A: 1, B: 1, C: 1; D: 1. The cells and granules circled in dotted line represent cells that would either not be counted (A) or would need an examination at several fields of focus to detect the presence of nuclei (C).
A SYSTEMATIC COUNTING METHODOLOGY—HOW TO COUNT
We recommend that observers develop a counting method with which they become comfortable and adhere to it. While there is no right or wrong way to count, a consistent systematic approach will help minimize errors and provide more consistent counts over time for patients who undergo repeated endoscopies. The left panel of Figure 5 depicts a low-power view of an apparently normal oxyntic mucosa that in most circumstances one would not feel the need to examine at higher power. However, a high-power examination (right panel) shows numerous eosinophils between the intact oxyntic glands. This example illustrates how easily eosinophils can be missed if not specifically sought.
FIGURE 5.

Eosinophils are difficult to detect at low power (×40), particularly in the oxyntic mucosa. The low-power image on the left shows oxyntic mucosa in which no eosinophils can be visualized. When the area in the rectangle is enlarged (×400) several eosinophils between the oxyntic glands can be appreciated.
Once a field is selected, it is necessary to adopt a systematic approach to examination to guarantee that the entire area is examined. Using landmarks such as well-oriented crypts will increase the possibility of counting all eosinophils once, and will decrease the possibility of overcounting by counting an eosinophil more than once. Three possible approaches are provided in Figure 6. In the oxyntic mucosa, eosinophils tend to be located, sometimes literally squeezed (Fig. 7) between oxyntic glands (panel A); in the antrum, both basal and subepithelial locations are common (panel B). This distribution of eosinophils may account for the often-held impression that eosinophilic infiltrates in the stomach are more abundant in the antrum than in the corpus. In the duodenum (Fig. 8), the sub-cryptal band tends to be richer in eosinophils in cases with mild to moderate increases (panel A). When infiltrates are more marked, there are often dense aggregates within the villi, which may become wider and shorter (panel B).
FIGURE 6.

Three possible approaches to counting a high-power field. One can mentally divide a field in quadrants, as depicted in the left panel, and proceed in a conventional order (eg, from left to right, as if reading a text); alternatively, one can proceed spirally in a centrifugal or centripetal direction (center panel). A technique particularly useful for the oxyntic mucosa, where eosinophils tend to be squeezed between glands, is the “lawnmower” approach (right).
FIGURE 7.

A, In the oxyntic mucosa eosinophils tend to be squeezed between the glands (see also Fig. 4D), whereas in the antrum (B) they can be found in both subepithelial and basal locations.
FIGURE 8.

An eosinophil-rich band is often present in the duodenum between the bottom of crypts and the muscularis mucosae (A). When infiltrates are more marked, there are often dense aggregates within the villi, which may become wider and shorter (B).
Large aggregates of eosinophils in which individual cells are still identifiable can be counted, albeit with considerable investment of time and effort. In some cases of dense infiltrates, eosinophils appear to coalesce in indistinct sheets of granules and nuclear fragments (Fig. 9). In these situations, the accurate counting of individual cells is impossible; reporting should be descriptive, with an emphasis on the massive innumerable infiltrates.
FIGURE 9.

Section from the gastric antrum with sheets of partially or completely coalescent degranulated eosinophils. In these cases, an accurate count of the individual cells is impossible, and estimates need to be made.
BEYOND EOSINOPHILS
Few pathologists would embark in a thorough search for H. pylori organisms in a normal or almost normal gastric biopsy. Without the alerting features of chronic active inflammation and epithelial disarray, most observers would quickly dismiss the possibility of H. pylori gastritis, and they would be correct in the vast majority of cases.22 In contrast, relying on more readily detectable features to trigger a search for eosinophils in the stomach and duodenum would result in the systematic misdiagnosis of EGID. Again, a comparison with the esophagus may be helpful. In EoE, eosinophilic infiltrates are almost invariably accompanied by an array of histopathologic changes, which include, amongst others, basal cell hyperplasia, spongiosis, and often subepithelial fibrosis.18 In the stomach, dense eosinophilic infiltrates (>80 to 100 eos/hpf) are often accompanied by surface epithelial damage, mucin depletion, foveolar hyperplasia, and other inflammatory infiltrates, notably neutrophils (Fig. 10), but moderate (50 to 80 eos/hpf) and mild increases (30 to 50 eos/hpf) tend to be associated with subtle or insignificant mucosal changes that would not, per se, alert the attention of an observer who has not already noticed an abnormal number of eosinophils. In the duodenum, some degree of villous flattening and crypt hyperplasia may be seen in cases with marked eosinophilic infiltrates (Fig. 11), but mild to moderate increases appear to have little influence on the duodenal morphology. Therefore, while it is important to report relevant mucosal changes that may accompany eosinophilia, such changes cannot be relied upon as surrogate markers for EGID.
FIGURE 10.

In the stomach dense eosinophilic infiltrates (>80 to 100 eos/hpf) are often accompanied by surface epithelial damage, mucin depletion, foveolar hyperplasia, and other inflammatory infiltrates, notably neutrophils.
FIGURE 11.

In the duodenum, some degree of villous flattening (A) and crypt hyperplasia (B) may be seen in cases with significant eosinophilic infiltrates.
COUNTING DEVICES
Counting cells without using some type of counting device is possible and generally easier for low numbers. In these situations, experienced observers often resort to subitizing, which is the ability to make an immediate and accurate reckoning of a number of items in a group without needing to pause and actually count them.23 However, when one passes certain thresholds (10 to 20 cells per hpf) it may become confusing to memorize the numbers and keep adding to them in one’s head. Some observers find it helpful to use a simple mechanical cell counter, such as those used in hematology laboratories when differential counts were performed manually. Such devices also help minimize the number bias. It is well known that people faced with a count unconsciously gravitate toward even numbers and numbers ending in 5 and 0.24 If a machine is used and one avoids the automatic tendency to accompany the clicks with a mental count, the preference of such numbers can be avoided thus eliminating the small but real errors that subconscious rounding may cause.
DIGITAL PATHOLOGY, AUTOMATED COUNTS, AND ARTIFICIAL INTELLIGENCE
In the past several years digital pathology (the visualization of digitized slides through a computer monitor rather than a microscope) has been rapidly developing, and some programs using machine learning identify EoE biopsies with high degrees of accuracy (85%), sensitivity 82.5%), and specificity (87%).25 Algorithm-based systems to automatically detect and count certain types of cells are evolving, although for now they remain in the research domain. The evaluation of eosinophils in digitized images is rendered more difficult by the inability of using pathologists’ traditional secret weapon, focusing “up and down,” a constraint that may hinder the identification of nuclear fragments and granules. One way to compensate for these limitations is using immunochemical staining for eosinophilic peroxidase, a method that has been recently shown to facilitate the automatic detection and counting of GI eosinophils.26 Manual counting at the microscope may eventually be made obsolete by these evolving technologies. However, we suspect that for the next several years there will still be the need for pathologists to be able to diagnose—unassisted—eosinophilic conditions.
INTRAOBSERVER AND INTEROBSERVER VARIABILITY
When multiple assessments and enumerations of mucosal eosinophils are made in the same areas on the same specimen, the numbers are unlikely to be the same, irrespective of whether the assessments are made by the same observer or by different observers. The choice of the hpf may be the single most important factor affecting the counts: unless marked on a slide (or on a digitized image), it is essentially impossible that two observations include exactly the same fields for counting. Thus, to minimize variability one should make a systematic survey of each specimen, choose the hpf with the highest density of eosinophils, then proceed to other eosinophil-rich fields. Groups of pathologists who train together and agree on basic criteria tend to reach excellent κ values on almost any features, but numbers may still encompass relatively wide ranges. Thus, a reasonable goal is to reach consistency with the categorization of patients (EGID vs. no EGID), while remaining aware that borderline cases will have greater risk of being classified differently by different observers.27
REPORTING TISSUE EOSINOPHILIA
When a request to search for an eosinophilic condition accompanies a set of GI biopsies, the pathologist should attempt to follow the steps outlined above and generate a diagnosis that includes a definite statement about the presence of eosinophils in the specimens. Vague expressions, such as “increased mucosal eosinophils,” are generally ignored by clinicians. While recommendations from future guidelines cannot be predicted yet, we suggest that reporting the mean numbers of eosinophils from the observation of several hpfs could be helpful, particularly if accompanied by a description of the general distribution of the eosinophils. Some evidence is emerging that a mean count of 20 eosinophils/hpf in gastric biopsy specimens or 30 eosinophils/hpf in duodenal biopsy specimens can identify patients with EGIDs with high specificity.28 However, time constraints including quick turnaround times may preclude counting multiple hpf, but at the very least a peak eosinophil count should be provided. If no specific request to comment on eosinophilia is made, a diligent pathologist should nevertheless survey the specimens for evidence of apparently increased eosinophils. If definite increases are found, which will not happen very often, counts could be reported as detailed above, which provides a useful baseline record for monitoring therapy if needed. If not, a negative statement may be helpful to preempt clinicians’ concerns.
CONCLUSIONS
In contrast to our medieval scholastic predecessors, who relied on philosophical arguments to resolve the question of the number of angels who could dance on the head of a pin, we now have the tools to determine with a reasonable degree of accuracy how many eosinophils are present in an area of the GI mucosa that we define as a hpf. These numbers show good correlation with clinical manifestations, can serve as the determinant of effective management, and are used to assess the effects of treatment. A further, and possibly the most persuasive argument for providing a count rather than a value judgement is that patients read reports, understand numbers, and use them to help to understand the course of their disease. Will we deprive them of that right?
Footnotes
Conflicts of Interest and Source of Funding: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
Contributor Information
Kevin O. Turner, Email: kevin.turner@informdx.com.
Margaret H. Collins, Email: Margaret.Collins@cchmc.org.
Marjorie M. Walker, Email: marjorie.walker@newcastle.edu.au.
Robert M. Genta, Email: rmgenta@gastropath.com.
REFERENCES
- 1. Gonsalves N. Eosinophilic gastrointestinal disorders. Clin Rev Allergy Immunol. 2019;57:272–285. [DOI] [PubMed] [Google Scholar]
- 2. Reed CC, Dellon ES. Eosinophilic esophagitis. Med Clin North Am. 2019;103:29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dellon ES, Gonsalves N, Hirano I, et al. ACG clinical guideline: evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol. 2013;108:679–692. [DOI] [PubMed] [Google Scholar]
- 4. Dellon ES, Liacouras CA, Molina-Infante J, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: proceedings of the AGREE conference. Gastroenterology. 2018;155:1022.e10–1033.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aceves S, Collins MH, Rothenberg ME, et al. Consortium of Eosinophilic Gastrointestinal Disease Researchers. Advancing patient care through the Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR). J Allergy Clin Immunol. 2020;145:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Collins MH, Martin LJ, Alexander ES, et al. Newly developed and validated eosinophilic esophagitis histology scoring system and evidence that it outperforms peak eosinophil count for disease diagnosis and monitoring. Dis Esophagus. 2017;30:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Collins MH, Martin LJ, Wen T, et al. Eosinophilic esophagitis histology remission score: significant relations to measures of disease activity and symptoms. J Pediatr Gastroenterol Nutr. 2020;70:598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pesek RD, Reed CC, Collins MH, et al. Association between endoscopic and histologic findings in a multicenter retrospective cohort of patients with non-esophageal eosinophilic gastrointestinal disorders. Dig Dis Sci. 2020;65:2024–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chehade M. Eosinophilic gastrointestinal disorders: the journey to diagnosis remains arduous. Ann Allergy Asthma Immunol. 2020;124:229–230. [DOI] [PubMed] [Google Scholar]
- 10. Dellon ES, Aderoju A, Woosley JT, et al. Variability in diagnostic criteria for eosinophilic esophagitis: a systematic review. Am J Gastroenterol. 2007;102:2300–2313. [DOI] [PubMed] [Google Scholar]
- 11. Genta RM, Sonnenberg A, Turner K. Quantification of the duodenal eosinophil content in adults: a necessary step for an evidence-based diagnosis of duodenal eosinophilia. Aliment Pharmacol Ther. 2018;47:1143–1150. [DOI] [PubMed] [Google Scholar]
- 12. Lwin T, Melton SD, Genta RM. Eosinophilic gastritis: histopathological characterization and quantification of the normal gastric eosinophil content. Mod Pathol. 2011;24:556–563. [DOI] [PubMed] [Google Scholar]
- 13. Hurrell JM, Genta RM, Melton SD. Histopathologic diagnosis of eosinophilic conditions in the gastrointestinal tract. Adv Anat Pathol. 2011;18:335–348. [DOI] [PubMed] [Google Scholar]
- 14. Ronkainen J, Aro P, Walker MM, et al. Duodenal eosinophilia is associated with functional dyspepsia and new onset gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2019;50:24–32. [DOI] [PubMed] [Google Scholar]
- 15. Walker MM, Potter M, Talley NJ. Eosinophilic gastroenteritis and other eosinophilic gut diseases distal to the oesophagus. Lancet Gastroenterol Hepatol. 2018;3:271–280. [DOI] [PubMed] [Google Scholar]
- 16. Powell N, Walker MM, Talley NJ. Gastrointestinal eosinophils in health, disease and functional disorders. Nat Rev Gastroenterol Hepatol. 2010;7:146–156. [DOI] [PubMed] [Google Scholar]
- 17. Koutri E, Patereli A, Noni M, et al. Distribution of eosinophils in the gastrointestinal tract of children with no organic disease. Ann Gastroenterol. 2020;33:508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Collins MH. Histopathologic features of eosinophilic esophagitis and eosinophilic gastrointestinal diseases. Gastroenterol Clin North Am. 2014;43:257–268. [DOI] [PubMed] [Google Scholar]
- 19. Sunkara T, Rawla P, Yarlagadda KS, Gaduputi V. Eosinophilic gastroenteritis: diagnosis and clinical perspectives. Clin Exp Gastroenterol. 2019;12:239–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walker MM, Potter MD, Talley NJ. Tangible pathologies in functional dyspepsia. Best Pract Res Clin Gastroenterol. 2019;40–41:101650. [DOI] [PubMed] [Google Scholar]
- 21. Turner KO, Sinkre RA, Neumann WL, et al. Primary colonic eosinophilia and eosinophilic colitis in adults. Am J Surg Pathol. 2017;41:225–233. [DOI] [PubMed] [Google Scholar]
- 22. Glickman JN, Noffsinger A, Nevin DT, et al. Helicobacter infections with rare bacteria or minimal gastritis: expecting the unexpected. Dig Liver Dis. 2015;47:549–555. [DOI] [PubMed] [Google Scholar]
- 23. Liu W, Zheng P, Huang S, et al. Subitizing, unlike estimation, does not process sets in parallel. Sci Rep. 2020;24:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rozwat A, Roberts DK. Even-number measurement bias with Goldmann applanation tonometry. J Glaucoma. 2020;29:124–126. [DOI] [PubMed] [Google Scholar]
- 25. Czyzewski T, Daniel N, Rochman M, et al. Machine learning approach for biopsy-based identification of eosinophilic esophagitis reveals importance of global features. Eng Med Biol. 2021;2:218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hasan SH, Taylor S, Garg S, et al. Diagnosis of pediatric non-esophageal eosinophilic gastrointestinal disorders by eosinophil peroxidase immunohistochemistry. Pediatr Dev Pathol. 2021. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stucke EM, Clarridge KE, Collins MH, et al. Value of an additional review for eosinophil quantification in esophageal biopsies. J Pediatr Gastroenterol Nutr. 2015;61:65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reed CC, Genta RM, Youngblood BA, et al. Mast cell and eosinophil counts in gastric and duodenal biopsy specimens from patients with and without eosinophilic gastroenteritis. Clin Gastroenterol Hepatol. 2021;19:2102–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]


