Abstract
OBJECTIVES
The goal of this study was to determine the outcome of patients undergoing an elective frozen elephant trunk (FET) procedure as a redo operation following previous cardiac surgery.
METHODS
One hundred and eighteen consecutive patients underwent FET procedures between October 2010 and October 2019 at our centre. Patients were registered in a dedicated database and analysed retrospectively. Clinical and follow-up characteristics were compared between patients undergoing a FET operation as a primary (primary group) or a redo procedure (redo group) using logistic regression and Cox regression analysis. Emergency procedures (n = 33) were excluded from the analysis.
RESULTS
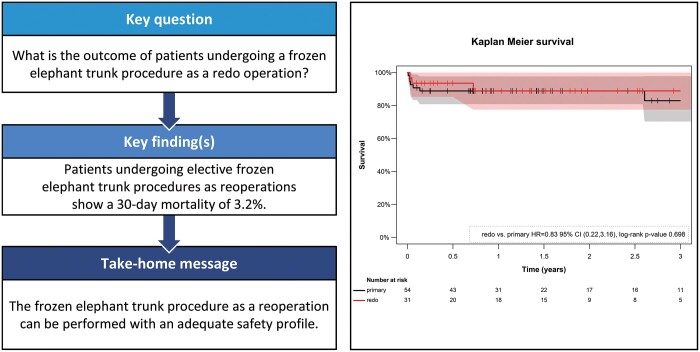
A total of 36.5% (n = 31) of the FET procedures were redo operations (redo group) and 63.5% (n = 54) of the patients underwent primary surgery (primary group). There was no significant difference in the 30-day mortality [primary group: 7.4%; redo group: 3.2%; 95% confidence interval (CI) (0.19–35.29); P = 0.63] and the 3-year mortality [primary group: 22.2%; redo group: 16.7%; 95% CI (0.23–3.23); P = 0.72] between redo and primary cases. Furthermore, the adjusted statistical analysis did not reveal significant differences between the groups in the occurrence of transient or permanent neurological deficit, paraplegia, acute renal failure and resternotomy. The redo group showed a higher rate of recurrent nerve palsy, which did not reach statistical significance [primary group: 3.7% (n = 2); redo group: 19.4% (n = 6); P = 0.091].
CONCLUSIONS
Elective FET procedures as redo operations performed by a dedicated aortic team following previous cardiac surgery demonstrate an adequate safety profile.
Keywords: Aortic aneurysm, Reoperation, Aortic arch replacement, Frozen elephant trunk, Endovascular procedures
INTRODUCTION
The frozen elephant trunk (FET) procedure is a treatment for patients with extensive thoracic aortic disease. At up to 17.1%, early mortality rates are still high following FET procedures for acute type A aortic dissection (ATAD) [1]. Therefore, in many centres, these acutely ill patients are primarily treated with hemiarch replacement rather than more extensive repair [2]. Due to chronic residual aortic dissection with persistent false-lumen perfusion, subsequent reinterventions are necessary in about 24% of these patients within 10 years after initial treatment of ATAD [3]. Because these patients frequently show complex aortic pathologies, including the aortic arch at follow-up, the FET procedure as a reoperation is necessary in many cases. Minimal data exist on the outcome of aortic arch reoperations following previous cardiac surgical procedures. Due to the improved long-term survival of patients after primary aortic arch surgery, a more consistent postoperative follow-up, and an ageing general population, aortic arch reoperations may become more frequent in the future. Therefore, we sought to study the outcome of patients undergoing a FET procedure as a reoperation.
MATERIALS AND METHODS
Ethical statement
Data acquisition was performed anonymized and retrospectively. Therefore, in accordance with German law, no ethical approval is needed and informed patient consent was waived.
Patients
From October 2010 to October 2019, a total of 118 consecutive patients underwent replacement of the aortic arch using the FET technique at our centre. Of these, 33 patients (28.0%) had redo operations (redo group) following previous cardiac surgery. In-hospital and follow-up characteristics were compared between patients undergoing FET as a primary (primary group) or a redo procedure (redo group). Anonymized patient data were collected using our dedicated institutional aortic database and analysed retrospectively.
Significantly more patients in the primary group underwent emergency treatment compared to the redo group [primary group: 36.5% (n = 31); redo group: 6.1% (n = 2); P = 0.001]. To reduce imbalances between the groups due to the high-risk profile of the emergency patients, the latter were excluded from the analysis. Among the remaining 85 patients undergoing elective surgery, 36.5% (n = 31) received the FET procedure as a reoperation (Fig. 1).
Figure 1:
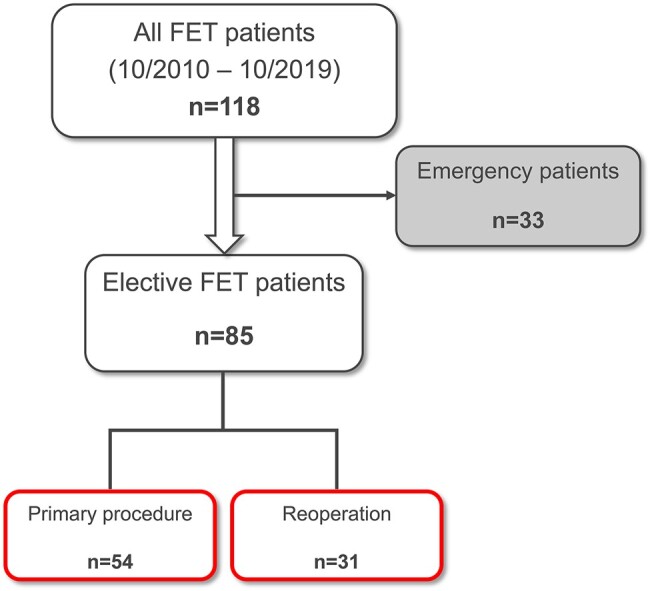
Inclusion and exclusion of patients. Emergency patients were excluded from the analysis. FET: frozen elephant trunk.
Preoperative evaluation
Prior to surgery, all redo patients were discussed in our weekly interdisciplinary aortic board meeting. The indication for the FET was weighed against performing a branched thoracic endovascular aortic repair (TEVAR). The decision for one or the other option was based on the aortic anatomy, the comorbidities and the age of the patient. Particularly younger patients with few comorbidities, patients with connective tissue diseases and patients who were not suitable for TEVAR were considered for the FET procedure as a reoperation. The indications for the FET procedure were joint interdisciplinary decisions at all times.
Surgical technique in redo cases
In redo operations, arterial cannulation of the left (zone 2, n = 20) or the right (zone 3, n = 11) subclavian artery was performed. The right femoral vein was cannulated percutaneously using Seldinger’s technique, and the cannula was advanced towards the superior vena cava under transoesophageal echocardiography guidance. Cardiopulmonary bypass (CPB) was started before resternotomy to avoid possible lacerations of the aorta or heart. The heart, the ascending aorta and the supra-aortic vessels were carefully dissected from adhesions. The left ventricle was vented via the right superior pulmonary vein. Retrograde blood cardioplegia was used for myocardial protection in all patients. Usually, no cross-clamping was performed.
Following moderate hypothermic circulatory arrest (HCA) between 24°C and 26°C, cerebral protection was performed using bilateral selective antegrade cerebral perfusion (SACP) in all patients. Therefore, in right subclavian or innominate artery cannulation, the innominate trunk was proximally clamped, and right-sided unilateral SACP was started after the intended temperature had been reached. After transection of the ascending aorta and the proximal aortic arch, a cerebral perfusion catheter was inserted into the left common carotid artery to provide bilateral SACP. In case of direct cannulation of the ascending aorta, the aortic arch or cannulation of the left subclavian artery (LSA), 2 catheters were inserted into the innominate and left carotid artery for bilateral SACP. The perfusion catheters were secured using tourniquets to prevent migration and embolization of tissue debris. The cerebral flow rate was set to 10–15 ml/kg/min with a mean pressure of 50–70 mmHg [4].
Near-infrared spectroscopy was applied to monitor cerebral oxygen saturation, and continuous carbon dioxide insufflation into the operative field was used. All redo patients were operated on by the same surgeon (C.D.) and aortic team.
Landing zone and simplified frozen elephant trunk technique
The FET procedures were performed using either a conventional FET technique with the distal anastomosis in aortic zone 3 (n = 38) or a simplified technique with an anastomosis in zone 2 (n = 47), as previously described by our group [4].
To summarize, in patients who underwent the simplified FET technique in zone 2, the LSA was exposed via a left-sided supraclavicular incision, and an 8-mm Dacron graft was sutured to the LSA (LSA T-graft). This T-graft was cannulated for full-body perfusion and blood supply for the left arm and the upper spinal perfusion collateral network. In complex residual aortic dissections with a small true lumen, a guidewire was inserted into the femoral artery and advanced towards the aortic arch to securely identify the true lumen. During HCA and SACP, the stent section of the Thoraflex hybrid prosthesis was deployed in the descending aorta, covering the origin of the LSA after ligation. The sewing collar of the prosthesis was anastomosed in zone 2. The perfusion side branch of the prosthesis was cannulated, and CPB was restarted for early antegrade lower body perfusion after clamping of the hybrid prosthesis and the side branches. Subsequently, the second and first branches of the hybrid prosthesis were anastomosed to the left common carotid and the innominate artery, respectively. Thereafter, the proximal end of the hybrid graft was anastomosed to the ascending aorta or an aortic root graft. During reperfusion on the beating heart, a retroclavicular tunnel was created by blunt dissection. Performing an extra-anatomical bypass, the LSA T-graft was pulled downwards into the upper intrathoracic aperture and anastomosed to the third branch of the Thoraflex Hybrid prosthesis (Terumo Aortic, Inchinnan, UK) in an end-to-end fashion.
Stent graft sizing
Based on the diameter of the descending aorta in the preoperative computed tomography (CT) scan, we used 10% oversized stent grafts in thoracic aneurysms to prevent type Ib endoleaks. In patients with ATAD or connective tissue disorders, no oversizing was performed. In residual dissections, the stent graft sizing was based on the maximal diameter of the true lumen measured in the CT scan and by intraoperative sizing using a hegar probe.
In the first 7 elective FET patients at our centre, the E-vita OPEN prosthesis (Jotec, Hechingen, Germany) with a stent length of 150 mm was used. Since April 2013, we implanted Thoraflex Hybrid prostheses exclusively. Initially, we used the 100-mm stent length in most patients to avoid a distal landing zone lower than T10. In zone 2 placement, however, we used the 150-mm stent length since the placement of the stent graft was at least 3–5 cm higher.
Reimplantation of supra-aortic vessels
In the initial 8 cases, the supra-aortic vessels were reimplanted using the island technique into the E-vita open prosthesis (n = 6) or the Thoraflex Hybrid prosthesis (n = 2), because most of the surgeons performing the implants were accustomed and trained to perform this technique. Due to potential benefits, like the reduced risk of cerebral emboli in severe atheromatous aneurysms and the avoidance of island aneurysms, we changed our institutional protocol and exclusively used the Thoraflex Hybrid prosthesis with the branched technique after April 2013.
Statistical analyses and follow-up
Baseline categorical variables were summarized by frequencies and percentages. These were compared between study groups using the Fisher’s exact test or the χ2 test when applicable. Continuous variables were described by the mean and standard deviation (SD). They were compared between study groups using the two-sided ‘Student’s t-test’. Because this was an exploratory study, no adjustment for multiple testing was performed [5]. Also, because this is a non-randomized study, baseline differences between groups in relevant prognostic factors can occur, which can bias the observed outcome differences between the groups. To address this, we reported treatment effects adjusted for age and the presence of a genetic aortic syndrome, according to Hickey et al. [6]. Continuous outcomes, e.g. procedural timings, were analysed in a multivariable linear model. Here, adjusted differences and confidence intervals (CIs) were reported. Binary outcomes, e.g. the 30-day mortality rate and postoperative complications, were analysed using multivariable logistic regression models applying Firth’s correction to the likelihood. These models were used to account for the small sample size and the limited number of events. Adjusted odds ratios, 95% CIs and p-values were reported from these models. CIs for early mortality were obtained using the Clopper and Pearson procedure. The level of significance was set at α = 0.05 for all analyses.
After the patients were discharged, clinical and imaging follow-up examinations were performed at 3 months, 12 months and annually thereafter and were 100% complete. The midterm survival of both groups was estimated using Kaplan–Meier curves and compared by a log-rank test. A hazard ratio of the groups and the corresponding CIs were calculated using a penalized Cox proportional hazards model. Statistical analyses were performed using IBM SPSS Version 24.0.0.0 (Armonk, NY, USA) and the logistf and coxphf-package in R 3-4.4.
RESULTS
Patient baseline characteristics are listed in Table 1. The mean age of all patients was 61.6 (SD: 13.7) years with redo patients being significantly younger [primary group: 65.6 (SD: 12.6) years; redo group: 54.7 (SD: 12.9); P < 0.001]. Due to the exclusion of emergency procedures, none of the analysed patients presented with acute type A dissection or acute aortic rupture. Significantly more patients in the redo group suffered from a genetic aortic syndrome [primary group: n = 5 (9.3%); redo group: n = 15 (48.4%); P < 0.001]. The indications for FET were thoracic aortic aneurysms with a diameter of 55 mm or rapid aortic growth of ≥5 mm/year in 45 patients (52.9%) and residual false-lumen dilatation after ATAD (Fig. 2) or retrograde dissection in 40 patients (47.1%).
Table 1:
Patient characteristics
| Primary surgery (n = 54) | Redo surgery (n = 31) | P-value | |
|---|---|---|---|
| Age (years), mean (SD) | 65.6 (12.6) | 54.7 (12.9) | <0.001 |
| Male gender, n (%) | 19 (35.2) | 23 (74.2) | 0.001 |
| Arterial hypertension, n (%) | 48 (88.9) | 23 (74.2) | 0.079 |
| Ejection fraction <45%, n (%) | 3 (5.6) | 1 (3.2) | 1.00 |
| Genetic aortic syndrome, n (%) | 5 (9.3) | 15 (48.4) | <0.001 |
| Marfan syndrome | 1 (1.9) | 12 (38.7) | <0.001 |
| Loeys-Dietz syndrome | 3 (5.6) | 0 (0.0) | 0.30 |
| Non-syndromic familial aortopathy (mutation positive) | 0 (0.0) | 2 (6.5) | 0.13 |
| Non-syndromic familial aortopathy (mutation negative) | 1 (1.9) | 1 (3.2) | 1.00 |
| EuroSCORE II, mean (SD) | 6.9 (5.2) | 9.5 (8.0) | 0.069 |
| Pathologies, n (%) | |||
| Extensive thoracic aneurysm | 39 (72.2) | 6 (19.4) | <0.001 |
| Residual dissections | 15 (27.8) | 25 (80.6) | <0.001 |
| Maximum aortic diameter (mm), mean (SD) | 59.0 (10.8) | 54.6 (11.0) | 0.082 |
| Prior TEVAR, n (%) | 8 (9.4) | 4 (12.1) | 0.74 |
| Prior cardiac surgery, n (%) | |||
| Supracoronary ascending/hemiarch replacement | 16 (51.6) | ||
| Bentall procedure | 7 (22.6) | ||
| Biological | 2 (6.3) | ||
| Mechanical | 5 (16.1) | ||
| David procedure | 6 (19.5) | ||
| Distal aortic arch replacement | 1 (3.2) | ||
| CABG | 1 (3.2) | ||
SD is reported as measure of variability. Statistically significant values are in bold (P < 0.05).
CABG: coronary artery bypass grafting; SD: standard deviation; TEVAR: thoracic endovascular aortic repair.
Figure 2:
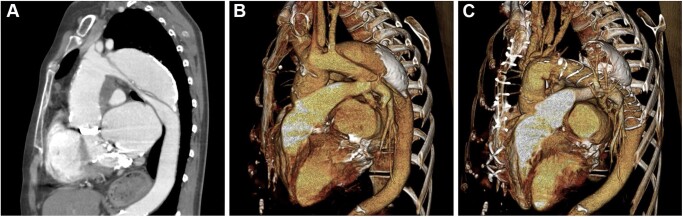
Computed tomography (CT) scans of a 57-year-old female patient with residual dissection after type A dissection and thoracic false lumen aneurysm measuring 6.0 cm in diameter. The patient had a biological Bentall procedure in combination with a biological mitral valve replacement 28 years earlier and mechanical mitral and aortic valve replacements 16 years earlier. The persistent false lumen aneurysm was treated by performing a frozen elephant trunk (FET) procedure. (A) CT scan prior to the FET procedure. (B) 3-Dimensional reconstruction of the preoperative CT scan. (C) CT scan after the FET procedure.
Table 2 depicts data on surgical techniques and additional procedures. There were no significant differences between the primary and the redo group in the frequency of a distal anastomosis in landing zone 2, the ascending or additional procedures. In redo cases, more frequently a guidewire was used for the proper identification of the true lumen [primary group: n = 3 (3.6%); redo group: n = 7 (21.2%); P = 0.005].
Table 2:
Surgical technique and additional procedures
| Primary surgery (n = 54), n (%) | Redo surgery (n = 31), n (%) | P-value | |
|---|---|---|---|
| FET hybrid prosthesis | 0.25 | ||
| Jotec E-vita Open Plus Hybrid | 3 (5.6) | 4 (12.9) | |
| Thoraflex™ Hybrid Plexus | 51 (94.4) | 27 (87.1) | |
| Supra-aortic vessels | 1.00 | ||
| Island technique | 5 (9.3) | 3 (9.7) | |
| Branched technique | 49 (90.7) | 28 (90.3) | |
| True lumen guidewire | 3 (3.6) | 7 (21.2) | 0.005 |
| Zone of distal anastomosis | 0.20 | ||
| Arch zone 2 | 27 (50.0) | 20 (64.5) | |
| Arch zone 3 | 27 (50.0) | 11 (35.5) | |
| Ascending procedures | |||
| Supracoronary replacement | 38 (70.4) | 27 (87.1) | 0.080 |
| Biological Bentall procedure | 8 (14.8) | 3 (9.7) | 0.74 |
| David procedure | 8 (14.8) | 1 (3.2) | 0.15 |
| Additional AVR | 4 (7.4) | 4 (12.9) | 0.46 |
| Additional CABG | 6 (11.1) | 2 (6.5) | 0.71 |
| Additional TVR | 1 (1.9) | 0 (0.0) | 1.00 |
AVR: aortic valve replacement or repair; CABG: coronary artery bypass graft; FET: frozen elephant trunk; TVR: tricuspid valve repair. Statistically significant values are in bold (P < 0.05).
Table 3 shows the intraoperative data. In redo cases, the lowest body temperature was significantly lower compared to that of the primary cases [adjusted difference = −1.0°C; 95% CI (−1.6, −0.5); P ≤ 0.001]. Furthermore, redo patients showed an increased extracorporeal circulation (ECC) time [primary group: 232.2 (SD: 61.5) min; redo group: 269.5 (SD: 65.4) min; adjusted difference = 36.4 min; 95% CI (4.9–68.0); P = 0.024] and a higher HCA time [primary group: 50.3 (SD: 20.7) min; redo group: 57.6 (SD: 25.9) min; adjusted difference = 12.1 min; 95% CI (0.8–23.3); P = 0.036].
Table 3:
Intraoperative data
| Primary surgery (n = 54), mean (SD) | Redo surgery (n = 31), mean (SD) | Adjusted difference (95% CI) | P-value | |
|---|---|---|---|---|
| Extracorporeal circulation, time (min) | 232.2 (61.5) | 269.5 (65.4) | 36.4 (4.9, 68.0) | 0.024 |
| Aortic clamp time (min) | 120.9 (44.0) | 121.5 (41.7) | −2.5 (−23.9, 18.9) | 0.82 |
| Circulatory arrest time (min) | 50.3 (20.7) | 57.6 (25.9) | 12.1 (0.8, 23.3) | 0.036 |
| Cerebral perfusion time (min) | 70.6 (22.6) | 78.4 (28.0) | 10.9 (−1.6, 23.3) | 0.086 |
| Lowest body temperature (°C) | 24.4 (1.0) | 22.6 (6.0) | −1.0 (−1.6, −0.5) | <0.001 |
SD is reported as measure of variability. Statistically significant values are in bold (P < 0.05).
CI: confidence interval; SD: standard deviation.
Table 4 shows the early deaths and the postoperative complications. The 30-day mortality was 7.4% [95% CI (2.1–17.9)] and 3.2% [95% CI (0.1–16.7)] in the primary and redo groups, respectively [adjusted odds ratio = 3.12; 95% CI (0.19–35.29); P = 0.37]. The 1 death after redo surgery occurred on postoperative day 18 of acute pancreatitis after an uneventful initial course. No unexpected aortic injury or severe uncontrolled bleeding occurred while re-entering the sternum in redo procedures. There was a trend towards a higher rate of recurrent nerve palsy in the redo group. However, this was not statistically significant [primary group: n = 2 (3.7%); redo group: n = 6 (19.4%); P = 0.091]. Redo patients with stent deployment in zone 2 showed a significantly lower rate of recurrent nerve injuries compared to zone 3 patients [zone 2: n = 1 (5.0%); zone 3: n = 5 (45.5%); P = 0.013; Fisher’s exact test]. There were no significant differences in the rate of transient neurological deficits, permanent neurological deficits, paraplegia, acute renal failure and resternotomy between the groups.
Table 4:
Early mortality and postoperative complications
| Primary surgery (n = 54), n (%) | Redo surgery (n = 31), n (%) | Adjusted OR (95% CI) | P-value | |
|---|---|---|---|---|
| Overall 30-day mortality | 4 (7.4) | 1 (3.2) | 3.12 (0.19–35.29) | 0.37 |
| Transient neurological deficit | 3 (5.6) | 0 (0.0) | 0.17 (0.00–1.93) | 0.17 |
| Permanent neurological deficit | 5 (9.3) | 2 (6.5) | 5.02 (0.59–46.27) | 0.13 |
| Paraplegia | 3 (5.6) | 0 (0.0) | 0.32 (0.00–4.28) | 0.43 |
| Recurrent nerve palsy | 2 (3.7) | 6 (19.4) | 3.96 (0.81–25.24) | 0.091 |
| Acute renal failure | 10 (18.5) | 5 (16.1) | 1.64 (0.39–6.64) | 0.49 |
| Resternotomy | 5 (9.3) | 4 (12.9) | 2.27 (0.50–10.35) | 0.28 |
CI: confidence interval; OR: odds ratio.
Three patients in the primary group suffered from postoperative paraplegia. All of these received a 100-mm stent graft prosthesis. The first had undergone complete endovascular repair of the entire descending and abdominal aorta; the second sustained an iatrogenic aortic dissection after coronary artery bypass grafting; and the third suffered from a massive thoracic aneurysm with thrombus formation in the descending aorta. Thus, we speculate that the paraplegia was unrelated to the FET procedure itself.
No significant differences occurred in the ventilation time [primary group: 40.2 (SD: 158.6) h; redo group: 30.1 (SD: 62.4) h; P = 0.74], the rate of tracheotomy [primary group: n = 2 (3.7%); redo group: n = 1 (3.2%); P = 1.00], the rate of transfusion of packed red blood cell units [primary group: n = 47 (87.0%); redo group: n = 30 (96.8%); P = 0.25] or the blood loss during the first 24 h after surgery [primary group: 781.3 (SD: 719.0) ml; redo group: 820.7 (SD: 498.9) ml; P = 0.79].
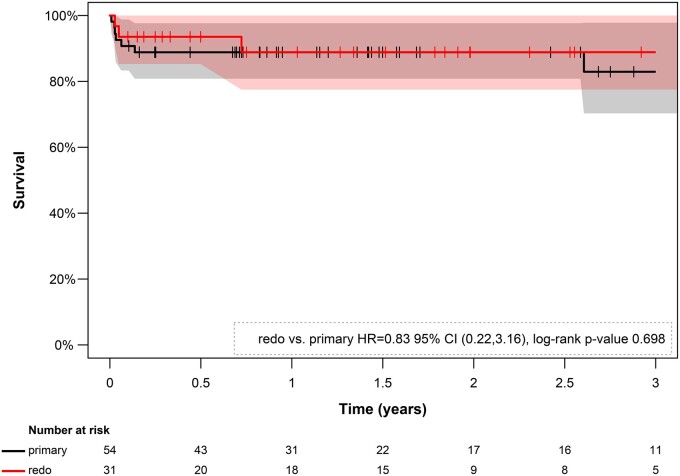
Figure 3 shows the estimated Kaplan–Meier survival curves of the primary and redo FET groups. There was no significant difference in midterm survival between groups with an estimated 3-year survival rate of 77.8% in the primary group and 83.3% in the redo group [hazard ratio = 0.85; 95% CI (0.23–3.23) penalized Cox regression analysis; P = 0.72; log-rank test]. During a mean follow-up time of 20.1 months (142.1 patient-years), subsequent TEVAR after FET was performed in 38 patients (44.7%) with no significant difference between the groups [primary group: n = 26 (48.1%); redo group: n = 12 (38.7%); P = 0.40].
Figure 3:
Kaplan–Meier estimates of survival by group (primary vs redo frozen elephant trunk procedure). The CIs are presented in the background in black and rose, respectively. The estimation does not reveal a significant difference in the survival between the groups during a 3-year follow-up period (P = 0.75). CI: confidence interval; HR: hazard ratio.
DISCUSSION
Reoperations in thoracic aortic disease after previous aortic surgery have been reported to show an increased mortality of up to 22.9% compared to primary procedures [7]. However, redo surgery has become more frequent [7] as the outcome in aortic arch surgery has improved, and consistent follow-up with better imaging techniques is available. Data on early and late outcomes of this growing number of redo procedures are of utmost importance. We report a case series of 31 patients who had the FET procedure as a reoperation.
Preoperative evaluation
Reoperations are generally known to be more challenging and time-consuming due to the surgical complexity of severe tissue adhesions, especially when fabric tissue like aortic grafts is present. In progressive aneurysmal disease, aortic tissue or grafts may have direct contact with the sternum, thereby increasing the risk of injury and uncontrolled bleeding by severe lacerations of aortic tissue and surrounding structures. Thus, preoperative imaging techniques like CT angiography with 3-dimensional reconstruction are of utmost importance for proper planning of the surgical strategy to decrease surgical risk and to perform aortic arch reoperations successfully.
Intraoperative data
For aortic reoperations, we started ECC before resternotomy via arterial cannulation of the left or right subclavian artery and percutaneous cannulation of the right femoral vein for safety reasons. This approach explains the higher ECC times in patients having redo procedures. By using ECC early, pulsatile flow and blood pressure could significantly be lowered to avoid possible lacerations of retrosternal structures, e.g. aorta, graft or the right ventricle. Using this technique, no unexpected aortic injury or severe uncontrolled bleeding occurred when we re-entered the sternum. Our surgical approach in redo aortic surgery follows our standard aortic arch protocol using moderate HCA, bilateral SACP for cerebral protection and retrograde blood cardioplegia for myocardial protection in all patients. In redo aortic surgery, however, the body temperature was significantly lower to increase the safety of the anticipated more complex procedure. The increased complexity of redo procedures led to higher ECC and circulatory arrest times. Although CPB time is known as a risk factor for early mortality and acute kidney injury after aortic arch surgery [8, 9], the patients of our cohort who had redo procedures did not show statistically significant higher complication rates.
Early deaths
Little comparable data on the outcome of aortic procedures as reoperations have been published to date. The prior reported early mortality of aortic reoperations ranges from 5.5% to 22.9% [7, 10, 11]. In our study cohort, patients undergoing elective FET procedures as reoperations showed a low 30-day mortality of 3.2%. This low mortality is in line with a recent analysis by Berger et al. of 63 patients undergoing FET as a reoperation. Berger et al. [12] reported an in-hospital mortality of about 3% as well. However, in contrast to most prior studies, emergency cases and therefore patients with ATAD were excluded from our analysis. With a mean age of 55 years, the redo group was significantly younger and showed a high rate of connective tissue disorders. Thus, conclusions for treatment advice for older patients with extensive aortic disease and previous cardiac operation(s) should be drawn with caution.
Because age is reported to be a risk factor for impaired outcome after aortic surgery [10, 11], endovascular approaches might be a suitable or even better alternative in older patients with previous cardiac procedures. In a retrospective analysis of 141 patients, Rylski et al. [7] showed that endovascular interventions for descending aortic pathologies after surgical repair for DeBakey type I or II dissections are associated with lower in-hospital and 5-year deaths compared to open surgery. Tsilimparis et al. [13] demonstrated that the treatment of residual aortic arch dissection with a branched endovascular repair is feasible and safe, showing low 30-day mortality and incidence of stroke of 5.0% each. Although surgical aortic replacement remains the gold standard and long-term data on endovascular procedures are pending, early results of endovascular approaches using fenestrated and branched stent grafts in patients with extensive aortic arch pathologies are promising [13–15]. The growing range of treatment options for these complex pathologies should be discussed by an interdisciplinary aortic team to ensure optimal patient selection and improvement of procedural outcomes.
Perioperative complications
Adjusted statistical analyses do not reveal a significant difference between elective primary and redo procedures in the examined outcome parameters transient neurological deficit, permanent neurological deficit, paraplegia, acute renal failure and resternotomy. Explanations might be the younger patient age and the high rate of patients with a genetic aortic syndrome, mainly presenting with residual dissections in the redo cohort. These patients usually have less severe vessel calcification and organ deterioration. Furthermore, dissection of adhesions in redo aortic surgery was set to a minimum to avoid aortic manipulation and laceration of surrounding tissue leading to unintentional injury, bleeding and perioperative stroke [16]. Our simplified FET technique with the distal anastomosis in arch zone 2 follows this concept. It avoids complex dissection of adhesions in the region of the distal aortic arch or proximal descending aorta, especially in redo operations [4]. Besides, by moving the distal anastomosis into aortic arch zone 2, the risk of recurrent nerve injury may be reduced due to anatomical considerations. Indeed, a subset of patients having redo procedures with a distal anastomosis in arch zone 2 showed a significantly lower rate of recurrent nerve injuries compared to patients who had a zone 3 distal anastomosis. This fact and the reduction of other complications by using landing zone 2 have been shown previously by our and other groups [4, 17–19].
Follow-up of patients with aortic diseases
Although the FET technique is considered a one-stage treatment for aortic arch and proximal descending pathologies, patients with concomitant distal thoracic or thoraco-abdominal aortic disease are likely to require further surgical or endovascular treatment at a later stage. In these cases, the FET is indicated as a first step of the treatment concept [20]. Kreibich et al. [21] reported that 33% of patients who had an FET procedure needed reinterventions, which agrees with our data. We show that 44.7% of patients of this cohort require TEVAR to treat distal pathologies after the FET procedure. These high reintervention rates underline the importance of consistent follow-up CT or magnetic resonance tomography scans after FET procedures to prevent life-threatening complications.
Limitations
The main limitations of our study are its retrospective nature and the small number of patients. Due to the limited number of patients and events, the reported CIs for the adjusted effects are large. Furthermore, the baseline characteristics of the groups significantly differed with regard to age, sex and underlying disease. This issue was at least partly addressed by multiple adjustments for confounding imbalances. Nevertheless, due to the limited number of events, we could not adjust for all parameters differing between the groups. Therefore, selection bias may still be an issue. Furthermore, the redo cases were all performed by 1 experienced aortic surgeon who was well trained using the FET technique. In contrast, primary procedures were performed by other surgeons as well. In addition, redo cases were mostly performed during a period when the aortic team was already trained and experienced with the FET technique. For further evaluation, higher patient numbers and multicentre analyses are needed.
CONCLUSION
Our data demonstrate that young patients with residual dissections or progressive thoracic aneurysmal formations after previous aortic surgery can undergo an FET procedure as a reoperation with low risk when treated electively and by a specialized aortic team in a high-volume centre.
Conflict of interest: Christian Detter is a proctor for Terumo Aortic. Despite that, we declare no conflicts of interests.
ABBREVIATIONS
- ATAD
Acute type A aortic dissection
- CI
Confidence interval
- CPB
Cardiopulmonary bypass
- CT
Computed tomography
- ECC
Extracorporeal circulation
- FET
Frozen elephant trunk
- HCA
Hypothermic circulatory arrest
- LSA
Left subclavian artery
- SACP
Selective antegrade cerebral perfusion
- SD
Standard deviation
- TEVAR
Thoracic endovascular aortic repair
Author contributions
Till Joscha Demal: Conceptualization; Data curation; Formal analysis; Visualization; Writing—original draft; Writing—review & editing. Lennart Bax: Data curation; Visualization; Writing—original draft; Writing—review & editing. Jens Brickwedel: Writing—original draft; Writing—review & editing. Tilo Kölbel: Writing—original draft; Writing—review & editing. Eik Vettorazzi: Formal analysis; Writing—original draft; Writing—review & editing. Franziska Sitzmann: Data curation. Hermann Reichenspurner: Writing—original draft; Writing—review & editing. Christian Detter: Conceptualization; Formal analysis; Writing—original draft; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks the anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
- 1. Leontyev S, Tsagakis K, Pacini D, Di Bartolomeo R, Mohr FW, Weiss G. et al. Impact of clinical factors and surgical techniques on early outcome of patients treated with frozen elephant trunk technique by using EVITA open stent-graft: results of a multicentre study. Eur J Cardiothorac Surg 2016;49:660–6. [DOI] [PubMed] [Google Scholar]
- 2. Parikh N, Trimarchi S, Gleason TG, Kamman AV, di Eusanio M, Myrmel T. et al. Changes in operative strategy for patients enrolled in the International Registry of Acute Aortic Dissection interventional cohort program. J Thorac Cardiovasc Surg 2017;153:S74–79. [DOI] [PubMed] [Google Scholar]
- 3. Geirsson A, Bavaria JE, Swarr D, Keane MG, Woo YJ, Szeto WY. et al. Fate of the residual distal and proximal aorta after acute type A dissection repair using a contemporary surgical reconstruction algorithm. Ann Thorac Surg 2007;84:1955–64. [DOI] [PubMed] [Google Scholar]
- 4. Detter C, Demal TJ, Bax L, Tsilimparis N, Kölbel T, von Kodolitsch Y. et al. Simplified frozen elephant trunk technique for combined open and endovascular treatment of extensive aortic diseases. Eur J Cardiothorac Surg 2019;56:738–45. [DOI] [PubMed] [Google Scholar]
- 5. Bender R, Lange S.. Adjusting for multiple testing—when and how? J Clin Epidemiol 2001;54:343–9. [DOI] [PubMed] [Google Scholar]
- 6. Hickey GL, Dunning J, Seifert B, Sodeck G, Carr MJ, Burger HU. et al. Statistical and data reporting guidelines for the European Journal of Cardio-Thoracic Surgery and the Interactive CardioVascular and Thoracic Surgery. Eur J Cardiothorac Surg 2015;48:180–93. [DOI] [PubMed] [Google Scholar]
- 7. Rylski B, Beyersdorf F, Desai ND, Euringer W, Siepe M, Kari FA. et al. Distal aortic reintervention after surgery for acute DeBakey type I or II aortic dissection: open versus endovascular repair. Eur J Cardiothorac Surg 2015;48:258–63. [DOI] [PubMed] [Google Scholar]
- 8. Xu S, Liu J, Li L, Wu Z, Li J, Liu Y. et al. Cardiopulmonary bypass time is an independent risk factor for acute kidney injury in emergent thoracic aortic surgery: a retrospective cohort study. J Cardiothorac Surg 2019;14: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Preventza O, Price MD, Simpson KH, Cooley DA, Pocock E, de la Cruz KI. et al. Hemiarch and total arch surgery in patients with previous repair of acute type I aortic dissection. Ann Thorac Surg 2015;100:833–8. [DOI] [PubMed] [Google Scholar]
- 10. Bajona P, Quintana E, Schaff HV, Daly RC, Dearani JA, Greason KL. et al. Aortic arch surgery after previous type A dissection repair: results up to 5 years. Interact CardioVasc Thorac Surg 2015;21:81–6. [DOI] [PubMed] [Google Scholar]
- 11. Quintana E, Bajona P, Schaff HV, Dearani JA, Daly RC, Greason KL. et al. Open aortic arch reconstruction after previous cardiac surgery: outcomes of 168 consecutive operations. J Thorac Cardiovasc Surg 2014;148:2944–50. [DOI] [PubMed] [Google Scholar]
- 12. Berger T, Kreibich M, Mueller F, Rylski B, Kondov S, Schröfel H. et al. The frozen elephant trunk technique for aortic dissection is safe after previous aortic repair. Eur J Cardiothorac Surg 2020;59:130–6. [DOI] [PubMed] [Google Scholar]
- 13. Tsilimparis N, Detter C, Heidemann F, Spanos K, Rohlffs F, Von Kodolitsch Y. et al. Branched endografts in the aortic arch following open repair for DeBakey Type i aortic dissection. Eur J Cardiothorac Surg 2018;54:517–23. [DOI] [PubMed] [Google Scholar]
- 14. Makaloski V, Tsilimparis N, Rohlffs F, Heidemann F, Debus ES, Kölbel T.. Endovascular total arch replacement techniques and early results. Ann Cardiothorac Surg 2018;7:380–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Verscheure D, Haulon S, Tsilimparis N, Resch T, Wanhainen A, Mani K. et al. Endovascular treatment of post type A chronic aortic arch dissection with a branched endograft. Ann Surg 2019;published ahead-of-print. [DOI] [PubMed] [Google Scholar]
- 16. Moz M, Misfeld M, Leontyev S, Borger MA, Davierwala P, Mohr FW.. Aortic arch reoperation in a single centre: early and late results in 57 consecutive patients. Eur J Cardiothorac Surg 2013;44:e82–6. [DOI] [PubMed] [Google Scholar]
- 17. Leone A, Di Marco L, Coppola G, Amodio C, Berardi M, Mariani C. et al. Open distal anastomosis in the frozen elephant trunk technique: initial experiences and preliminary results of arch zone 2 versus arch zone 3. Eur J Cardiothorac Surg 2019;56:564–71. [DOI] [PubMed] [Google Scholar]
- 18. Tsagakis K, Wendt D, Dimitriou AM, Thielmann M, Shehada S-E, El GM. et al. The frozen elephant trunk treatment is the operation of choice for all kinds of arch disease. J Cardiovasc Surg (Torino) 2018;59:540–6. [DOI] [PubMed] [Google Scholar]
- 19. Di Bartolomeo R, Murana G, Di Marco L, Alfonsi J, Gliozzi G, Amodio C. et al. Is the frozen elephant trunk frozen? Gen Thorac Cardiovasc Surg 2019;67:111–7. [DOI] [PubMed] [Google Scholar]
- 20. Czerny M, Schmidli J, Adler S, van den Berg JC, Bertoglio L, Carrel T. et al. Editor’s Choice—Current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: an expert consensus document of the European Association for Cardio-Thoracic Surgery (EACTS) & the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2019;57:165–98. [DOI] [PubMed] [Google Scholar]
- 21. Kreibich M, Berger T, Rylski B, Chen Z, Beyersdorf F, Siepe M. et al. Aortic reinterventions after the frozen elephant trunk procedure. J Thorac Cardiovasc Surg 2020;159:392–9.e1. [DOI] [PubMed] [Google Scholar]