Abstract
OBJECTIVES
Although in younger patients indications for biological prosthesis implantation in mitral valve replacement remain controversial, recently bioprostheses use increased considerably. We present late results obtained with the Medtronic Mosaic bioprosthesis in patients aged 65 years or younger.
METHODS
Between 2007 and 2017, 67 mitral Mosaic bioprostheses were implanted in patients aged 65 years or younger (58.5 ± 6.4 years). Follow-up extended up to 13 years. Survival, freedom from structural valve degeneration, endocarditis, thromboembolic events and reoperation were considered as main clinical end points evaluated at 1, 5 and 10 years.
RESULTS
The mean follow-up was 4.7 ± 2.8 years. Overall mortality rate was 12%. At 1, 5 and 10 years, survival was 94 ± 3%, 89 ± 4% and 77 ± 9%, respectively. Freedom from structural valve degeneration was 100%, 94 ± 4% and 71 ± 21%. Freedom from endocarditis was 95 ± 3%, 90 ± 6% and 84 ± 8%. Freedom from thromboembolic events was 94 ± 3%, 90 ± 5% and 90 ± 5%. Freedom from reoperation was 94 ± 3%, 87 ± 5% and 65 ± 19%.
CONCLUSIONS
Mosaic bioprosthesis appears a valid mitral valve substitute even when employed in ≤65-year-old patients.
Keywords: Mitral valve replacement, Bioprosthesis, Survival
INTRODUCTION
Compared to mechanical cardiac valves, bioprostheses present a lower risk of thromboembolic and haemorrhagic events and do not require life-long anticoagulant therapy. However, their durability is limited as their structure tends to deteriorate, mostly in younger patients [1–3]. Because of such characteristics they are most frequently used in elderly patients. The stented porcine Mosaic bioprosthesis (MBP) was introduced in 1994 (Medtronic, Inc, Minneapolis, MN, USA) as an evolution of the Hancock II valve and approved by the American Food and Drug Administration in 2000. It presents some technical innovations: improvement of haemodynamic design introducing a supra-annular configuration, tissue antigenic reduction by glutaraldehyde fixation and antimineralization treatment with alpha-amino oleic acid to reduce calcification [4, 5]. A limited number of studies reported favourable results of the MBP in mitral position. Most studies reported good results in aortic valve replacement and in older population [4–9].
There is a trend towards using biological valves in younger patients, due to their clinical advantages and the perspective of percutaneous techniques as alternative to surgery if reoperation is indicated. The age cut-off seems to be 65 years old, when the advantages of bioprosthesis seem to offset the risk of structural valve degeneration (SVD) and of the subsequent need of redo surgery. In this study, we retrospectively evaluated the clinical results of mitral valve replacement (MVR) using an MBP in a patient population aged 65 years or younger, operated on over a 13-year period. The aim of the present study is to evaluate early and late mortality, overall survival and freedom from SVD and from major adverse cardiovascular events, to investigate if MBP remains a valid and satisfactory mitral valve substitute even in younger patients.
PATIENTS AND METHODS
The study was conducted by anonymously reviewing stored medical records. For this reason, the Institutional Review Board waived a specific Ethics Committee approval and an ad hoc patient consent.
Patient population
Between September 2007 and June 2017, 67 mitral MBP were implanted in 63 patients. Four patients underwent a second MVR with a new MBP being implanted and they were considered as new patients with a new valve.
The mean patients’ age was 58.5 ± 6.4 years. Preoperative and intraoperative patients’ characteristics are summarized in Table 1.
Table 1:
Patient demographics
| Overall, N = 67 | |
|---|---|
| Age, mean ± SD | 58.52 ± 6.43 |
| Male gender, n (%) | 35 (52) |
| Hypertension, n (%) | 41 (61) |
| Diabetes, n (%) | 11 (16) |
| Dyslipidaemia, n (%) | 20 (30) |
| Therapy with statins, n (%) | 15 (22) |
| Smoke habit, n (%) | 21 (31) |
| BMI, mean± SD | 28.04 ± 6.01 |
| BMI >30, n (%) | 23 (34) |
| CAD, n (%) | 13 (19) |
| PVD, n (%) | 13 (19) |
| Neurovascular events, n (%) | 12 (18) |
| COPD, n (%) | 20 (30) |
| Renal failure, n (%) | 8 (12) |
| Atrial fibrillation, n (%) | 24 (36) |
| NYHA ≥III, n (%) | 53 (79) |
| EF (%), mean ± SD | 61.1 ± 10 |
| Congestive heart failure, n (%) | 18 (27) |
| PASP (mmHg), mean ± SD | 41.4 ± 13.1 |
| Pulmonary hypertension (PASP > 50 mmHg), n (%) | 17 (25) |
| MV stenosis, n (%) | 13 (19) |
| MV regurgitation, n (%) | 44 (66) |
| MV stenosis and regurgitation, n (%) | 10 (15) |
| Rheumatic, n (%) | 19 (28) |
| Degenerative, n (%) | 27 (40) |
| Infective endocarditis, n (%) | 5 (7) |
| MR from myocardial ischaemia, n (%) | 3 (4) |
| MR from hypertrophic cardiomyopathy, n (%) | 2 (3) |
| Reintervention, n (%) | 21 (31) |
BMI: body mass index; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; EF: ejection fraction; MV: mitral valve; NYHA: New York Heart Association functional class; PASP: pulmonary artery systolic pression; PVD: peripheral vascular disease.
Bioprosthesis choice was based on clinical contraindication or psychological and professional incompatibility with life-long oral anticoagulation therapy, desire of pregnancy (1 patient) or unspecified personal preference, in accordance with the surgeon’s opinion. Most frequent mitral valve diseases observed were myxomatous degenerative (40%) and rheumatic (28%). Three patients (4%) presented severe mitral regurgitation with posterior chordal tethering from myocardial ischaemia. In 2 patients (3%) with myocardial septal hypertrophy, mitral regurgitation was caused by bulging of the interventricular septum and systolic anterior motion of the anterior mitral leaflet. Thirty-one patients (46%) presented significant calcification of the mitral annulus. Five patients had endocarditis (7%), one of the native mitral valve, 4 of a prosthetic valve. For 21 patients (31%), our operation was the second mitral valve intervention. Eleven patients were reoperated for valve prosthesis failure (8 biological and 3 mechanical valves), 6 for valve repair failure and 2 had undergone postinfarction ventricular septal defect repair. One patient had undergone previous coronary artery bypass grafting and another one for aortic MVR after previous mitral commissurotomy. In 2 cases, previous percutaneous mitral valvuloplasty had been performed. In 28 patients (41%), associated procedures were performed.
Sixty patients (89%) were operated through full sternotomy and bicaval cannulation for cardiopulmonary bypass. After aortic cross-clamping, warm antegrade blood cardioplegia was administered for temporary cardiac arrest, usually left atrium was entered through left atriotomy, in 13 (19%) cases through biatrial transseptal approach according to Guiraudon technique [10]. The mitral valve was removed and the subvalvular apparatus was preserved when possible. The left atrial appendage was closed in 16 (24%) patients. MBP was rinsed in saline solution and implanted in supra-annular position with interrupted mattress pledgeted sutures. Seven patients (10%) underwent MVR through a video-assisted 5–6-cm right thoracotomy, femoral arterial and venous cannulation and Custodiol cardioplegia. A 33-mm bioprosthesis was implanted in 11 patients (16%), 31 mm in 17 (25%), 29 mm in 16 (24%), 27 mm in 8 (12%) and 25 mm in 12 (18%).
After surgery, patients in sinus rhythm were treated with life-long acetylsalicylic acid and 3-week subcutaneous enoxaparin. Patients in atrial fibrillation received life-long acetylsalicylic acid, initial percutaneous calcium heparin, at 1 week followed by long-term Warfarin anticoagulation with an international normalized ratio 2 level target. The mean follow-up was 4.7 ± 2.8 years, extended up to 13 years. An outpatient clinical evaluation with a transthoracic echocardiogram was performed every year. The follow-up was completed in 97% patients (65 patients). Two patients were not available at medical follow-up during the current year; therefore, data of their last postoperative control were included.
Five main clinical end points were considered: survival, freedom from SVD, freedom from endocarditis, freedom from thromboembolic events and freedom from reoperation. Morbidity and mortality were classified according to the Guidelines of the Society of Thoracic Surgeons, the American Association of Thoracic Surgery and the European Association of Cardiothoracic Surgeons [11, 12]. A follow-up transthoracic echocardiogram was yearly performed to evaluate the MBP status and the degree of degeneration. To evaluate the bioprosthesis condition, besides the clinical status of the patient, haemodynamic and morphological parameters were evaluated at echocardiography. We therefore considered the progression of valve dysfunction (stenosis and/or regurgitation) and the evaluation of the systo-diastolic leaflets excursion, along with possible signs of bioprosthesis structural anomalies (exclusive of infection or thrombosis), such as leaflet tears or flail, increased presence of leaflet thickening, fibrosis and calcifications. SVD was considered severe, and surgery indicated, in the presence of severe mitral regurgitation with effective regurgitant orifice area ≥40 mm2 or of severe mitral stenosis with transprosthetic mean pressure gradient >8 mmHg [6, 11–19].
Statistical analysis
Statistical analysis was performed using Statistical Package for Social Sciences (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0: IBM Corp, Armonk, NY, USA). Five main outcome end points (survival, freedom from SVD, freedom from endocarditis, freedom from thromboembolic events and freedom from reoperation) were analysed with Kaplan–Meier survival curve.
RESULTS
In-hospital results
Hospital mortality was 3%. Two patients died, both were redo surgery in critically ill patients with preoperative cardiogenic shock. Seven patients (10%) required surgical revision for bleeding, 6 patients (9%) presented complete atrioventricular block, 5 of them (7%) required permanent pacemaker implantation. Postoperative stroke occurred in a patient with atrial fibrillation who had previously undergone mitral and aortic valve replacement for rheumatic disease (1%). Twenty patients (30%) required blood transfusions. Neither early endocarditis nor early paravalvular leaks were observed. In all patients, the discharge echocardiogram showed normally functioning MBP (mean gradient 5.7 ± 2.4 mmHg). Postoperative left ventricular ejection fraction was 54.1 ± 11.9% (Table 2).
Table 2:
Operative and postoperative data
| Overall, N = 67 | |
|---|---|
| Reintervention, n (%) | 21 (31) |
| Combined procedures, n (%) | 28 (42) |
| Maze procedure, n (%) | 4 (6) |
| TVPL, n (%) | 6 (9) |
| AVR/AVPL, n (%) | 7 (10) |
| TVPL and AVPL, n (%) | 1 (1) |
| CABG, n (%) | 5 (7) |
| Miomectomy, n (%) | 2 (3) |
| Pericardiectomy, n (%) | 1 (1) |
| IVD defect repair, n (%) | 1 (1) |
| CABG and VAn repair, n (%) | 1 (1) |
| CPB time (min), mean ± SD | 119.5 ± 53,9 |
| Aortic cross-clamping time (min), mean ± SD | 82.3 ± 34.1 |
| Early postoperative outcome | |
| In-hospital mortality, n (%) | 2 (3) |
| Surgical revision for bleeding, n (%) | 7 (10) |
| Complete AV block, n (%) | 6 (9) |
| Permanent PMK implantation, n (%) | 5 (7) |
| Neurovascular events, n (%) | 1 (1) |
| Transfusions, n (%) | 20 (30) |
| MT MPG (mmHg), mean ± SD | 5.7 ± 2.4 |
| EF (%), mean ± SD | 54.1 ± 11.9 |
AV: atrioventricular; AVPL: aortic valve plasty; AVR: aortic valve replacement; CABG: coronary artery bypass grafting; CPB: cardiopulmonary bypass; EF: ejection fraction; IVD: interventricular septum; MT MPG: mitral transprosthetic mean pressure gradient; PMK: pacemaker; TVPL: tricuspid valve plasty; Van: ventricular aneurysm.
Survival
Overall 10-year survival was 88%.Overall mortality was 12% (8 patients) for a linearized mortality rate of 2.73% valve-years.
Late mortality rate was 9% (6 patients). At 10 and 83 months from surgery, a valve-related death occurred in 2 55- and 60-year-old patients, from infective endocarditis with septic shock. Other causes of death were type A aortic dissection (1%), pulmonary embolism (1%), progressive heart failure and not valve-related cardiac arrest (2 cases, 3%). Overall survival at 1, 5 and 10 years was 94 ± 3%, 89 ± 4% and 77 ± 9%, respectively (Fig. 1).
Figure 1:
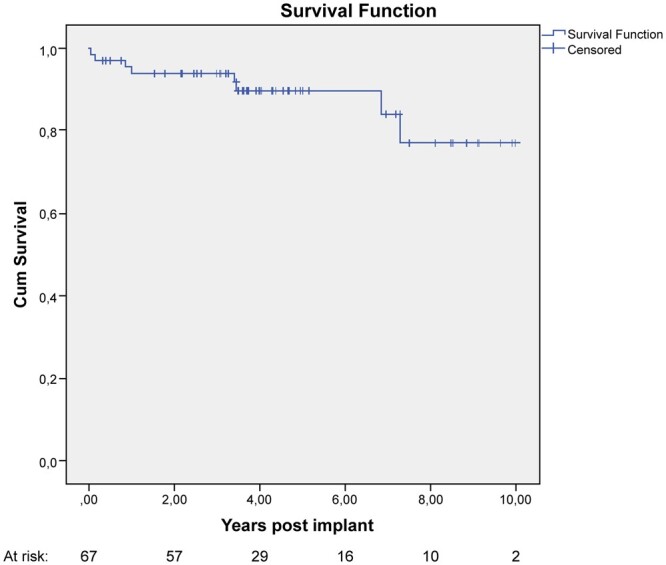
Kaplan–Meier curve showing survival after Mosaic bioprosthesis.
SVD
SVD was observed in 3 patients (4%): 2 patients underwent successful new bioprosthetic valve implantation and 1 patient had transcatheter mitral valve-in-valve procedure. In a 64-year-old patient, reoperation occurred 9.9 years after surgery, in the other 2 (56 and 62 years), degeneration occurred within 5 years after implantation, at 4.9 and 2.1 years respectively. Among patients with SVD, the 56- and 64-year-old patients were in therapy with statins. Neither of these 3 patients had diagnosis of diabetes. The linear rate of SVD was 1.02% per valve-years and the overall freedom from the degeneration of 95%. Freedom from SVD at 1, 5 and 10 years was 100%, 94 ± 4% and 71 ± 21%, respectively (Fig. 2).
Figure 2:
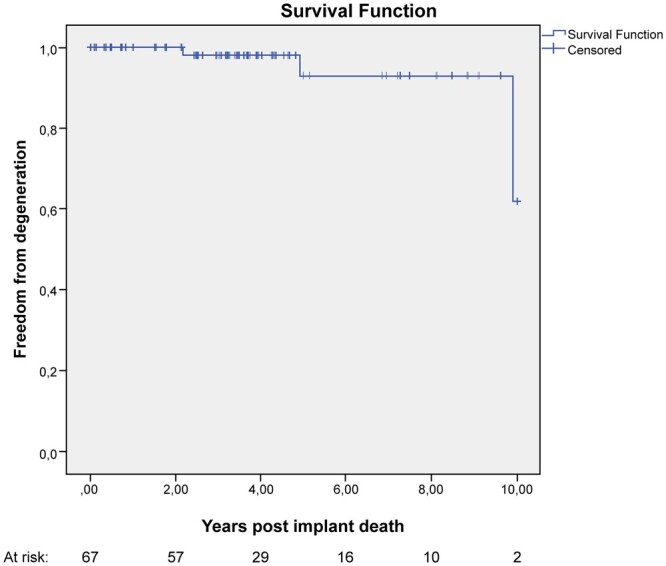
Kaplan–Meier curve showing freedom from structural valve degeneration after Mosaic bioprosthesis.
Endocarditis
Prosthetic infective endocarditis was observed in 5 patients (7%) for a linearized rate of 1.7% per valve-years. Two patients underwent reoperation with implantation of a new bioprosthesis, 1 patient was successfully treated with antibiotics and 2 patients died from septic shock. The mean interval between surgery and diagnosis of endocarditis was 34.5 ± 32 months. Overall freedom from endocarditis was 92%; 1-, 5- and 10-year freedom from endocarditis was 95 ± 3%, 90 ± 6% and 84 ± 8%, respectively (Fig. 3).
Figure 3:
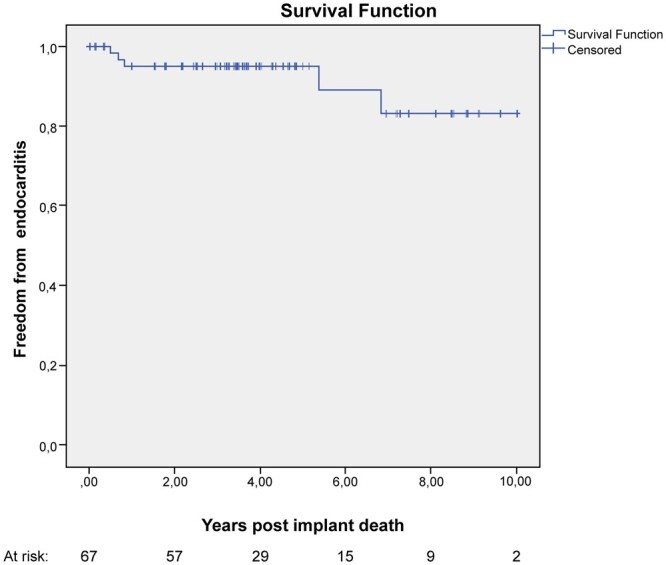
Kaplan–Meier curve showing freedom from prosthetic endocarditis.
Thromboembolic events
Five (7%) neurological thromboembolic events occurred at an average of 18.2 ± 6.7 months from surgery with a linearized event rate of 1.72% per valve-years. The patients were all anticoagulated for atrial fibrillation. One patient, presenting also gastrointestinal bleeding, underwent reoperation for valve thrombosis. Freedom from thromboembolic events at 1, 5 and 10 years was 94 ± 3%, 90 ± 5% and 90 ± 5%, respectively (Fig. 4).
Figure 4:
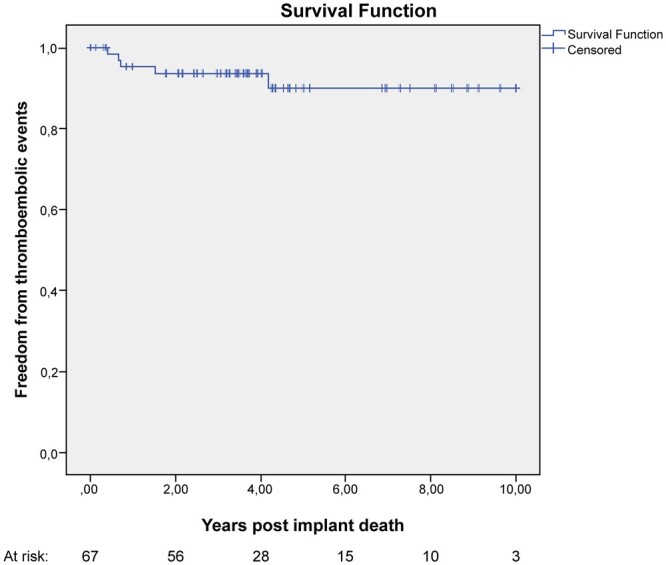
Kaplan–Meier curve showing freedom from thromboembolic events.
Reoperation
Eight patients (12%) underwent valve-related reoperation with a linearized rate of 2.73% per valve-years. Three patients needed reoperation for SVD, 2 for infective endocarditis, 1 for valve thrombosis and 2 for late paravalvular leak. Freedom from reoperation at 1, 5 and 10 years was 94 ± 3%, 87 ± 5% and 65 ± 19%, respectively (Fig. 5).
Figure 5:
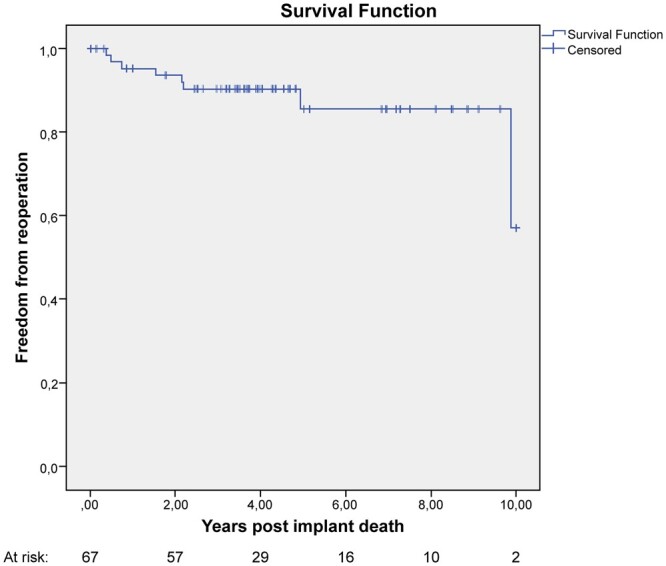
Kaplan–Meier curve showing freedom from reoperation.
DISCUSSION
Although the use of biological valve prostheses markedly increased in the recent years, the implantation of bioprosthesis in young patients still remains controversial. For MVR, the 2017 European Society of Cardiology and European Association of Cardiothoracic Surgery Guidelines recommend a biological valve for patients aged >70 years (class IIa) and consider reasonable either a mechanical or a biological prosthesis in patients aged between 65 and 70 years [17]. The 2017 American College of Cardiology/American Heart Association statements do not consider age as a class I recommendation. Patients aged >70 years are candidates for biological valve, and patients <50 years with no contraindications for anticoagulation should have a mechanical valve; patients between 50 and 70 years may receive either a biological or a mechanical prosthesis (class IIa recommendation) [18].
The Scientific Societies Guidelines reflect the general trend in the ‘real world’ to a more liberal use of biological valves also in younger patients. Goldstone et al. [1] in a large California statewide retrospective cohort study reported that in the period 1996 through 2016 the use of biological mitral prostheses increased from 16.8% to 53.7%. Many recent studies showed improved results in terms of durability and reduced incidence of complications observed with the new generation biological valves [1, 4–11, 19, 20]. The promising recent results of the percutaneous mitral valve-in-valve implant for SVD oriented surgeons and patients towards a more favourable attitude for biological valves [21–24]. Advantages of bioprostheses are well known and relevant. No need of life-long anticoagulation therapy and a low risk of thromboembolic and haemorrhagic events are appealing characteristics and justify continuous attempts to extend the use of biological valves to younger patients, despite the higher risk of reintervention.
As mentioned, in the Guidelines [17, 18], 70 years was considered the lower age limit for biological mitral valve indication. Improved durability of most recent bioprostheses induced many surgical groups to move such limit towards a younger age [25, 26].
To further explore the safety of this tendency, we intended to evaluate the results of MVR with a third-generation biological valve in patients 65 years old or younger.
The Mosaic valve is a third-generation stented porcine bioprosthesis, presenting several technical innovations such as a low-profile haemodynamic design with supra-annular configuration, a semiflexible stent, physiological tissue fixation with gluteraldehyde at zero pressure and antimineralization treatment with alpha-amino oleic acid to reduce calcification and structural deterioration, characteristics intended to improve its haemodynamics and durability [4–5].
Both mid-term and long-term results were promising. Thomson et al. [6] studied 242 MVR patients with MBP, mean age 68 years, and reported 79.2 ± 6.8% 4-year survival, 100% freedom from SVD, 97 ± 3.2% freedom from reoperation and 95.7 ± 3.8% from thromboembolic events. The Jamieson et al. [7] 2011 study, in 232 patients, mean age 67 years, at 7.3 ± 3.9 years, reported 43.9 ± 7.4% 12-year survival with a linearized mortality rate of 5.1% patients-years. Freedom from SVD was 91.8 ± 5.9%.
In 2018, Riess et al. [8] reported results of 232 patients undergone Mosaic MVR. Thirty-five of them were <60 years old (age 48.7 ± 12.9) and 197 patients were ≥60 years. Sixteen-year survival was 67.6% in patients <60 years and 20.6% in patients ≥60 years (P ≤ 0.01). Freedom from SVD was 65.2% for patients younger than 60 years and 83.8% for patients 60 years or older (P = 0.23).
In a recent cooperative study, Lorusso et al. [27] reported 14-year experience with Mosaic MVR in 805 patients aged 73.5 ± 7 years. The median follow-up was 44 months. Overall early mortality was 7.8% with 57.4% 10-year survival and cumulative incidence of cardiac-related and valve-related death 7.4% and 1.1%, respectively. In their elderly patient population, incidence of SVD was low (at 1, 5 and 10 years was 0.1%, 1.4% and 5.8% with reoperation rate 4.8% at 10 years). At 10 years, incidence of thromboembolic, haemorrhagic and endocarditis events was 6.6%, 3.9% and 3%, respectively.
In our series with younger patients, the hospital mortality rate was 3% (2 deaths). The 2 deceased patients had already undergone previous cardiac surgery; both had renal failure, COPD and cardiovascular risk factors. Both patients had a 25-mm Mosaic valve implanted and died from postoperative heart failure. One of them, a 62-year-old obese (body mass index 32) female died on the 8th postoperative day. She had been admitted in cardiogenic shock and pulmonary oedema; the previously implanted mechanical mitral valve had acutely thrombosed with blockage of 1 leaflet. A pericardiectomy was simultaneously performed for associated constrictive pericarditis. The second patient, a 57-year-old male, was operated for the failure of previous mitral valvuloplasty. He died on the 51th postoperative day, having never recovered from terminal congestive heart failure. Sixty-five patients (97%) were discharged in good clinical conditions.
Overall mortality rate was 12% for a linearized rate of 3% per valve-years. Late mortality rate was 9%, and valve-related death was 3%, with 2 deaths, 1 from infective endocarditis and the other one from heart failure. Long-term survival seems to compare favourably to that reported in most of previous studies. At 1, 5 and 10 years, survival was 94 ± 3%, 89 ± 4% and 77 ± 9%, respectively.
SVD occurred in 3 patients (4%) who required successful reintervention. At 1, 5 and 10 years, freedom from SVD was 100%, 94 ± 4% and 71 ± 21%, respectively. One valve degenerated at 9.9 years, in line with what reported by other authors [4, 8, 9]. This prosthesis was stenotic and insufficient, partially calcified and presented a leaflet tear. Two valves degenerated earlier, 2.1 and 4.9 years postoperatively. The first one presented tear of a leaflet, and the second valve was very calcified and presented a paravalvular leak. In the latter case, a year after surgery the patient had developed possible infective endocarditis successfully treated with antibiotics that may have accelerated the prosthesis degeneration process. Our results are in line with a recent meta-analysis of Malvindi et al. [13] who reported a 93% freedom from SVD at 10 years for patients having implanted an MBP for MVR, thus confirming the optimal reliability of porcine bioprostheses in the long term. Endocarditis rate was 7.4% with 10-year freedom from endocarditis 84 ± 8%. Of the 5 endocarditic patients, 2 died from septic shock before reoperation could have been attempted, thus confirming the high mortality of prosthetic endocarditis [28, 29].
Valve-related reoperation rate was 12% (8 patients). At 1, 5 and 10 years, freedom from reoperation was 94 ± 3%, 87 ± 5% and 65 ± 19%, respectively. At 1, 5 and 10 years, freedom from thromboembolic events was 94 ± 3%, 90 ± 5% and 90 ± 5%, respectively.
Our results obtained in a younger patient population appear gratifying and confirm that Mosaic valve is a reliable prosthesis even when employed in the ≤65-year-old patients. As mentioned, 1 patient presented a leaflet tear 2.1 years from surgery. Such an early structural valve deterioration is the only case in our experience and is rare in the literature. The valve was replaced with no complications, confirming that reoperation for SVD is associated with a low surgical risk [8, 11].
Despite encouraging results from biological prostheses, the choice between mechanical and biological prostheses in younger patients still remains controversial. Recently Goldstone et al. [1] reported a significantly lower mortality in patients with a mechanical valve compared to patients with a mitral bioprosthesis, in MVR patients aged up to 70 years. Previous studies in 50–69-year-old patients reported different results with similar mortality regardless of valve type or position, suggesting that biological valve could be safe in younger patients too [3, 11, 22, 23]. The Goldstone et al. [1] study is in contrast with these findings and invites to temper the current trend towards abandoning mechanical valves in younger mitral patients.
Our experience may contribute to add some insight in the delicate prosthesis selection process in MVR for younger patients. We are aware that larger series of patients for each age-group and very extended follow-up would be needed to establish the superiority and/or reliability of a biological prosthesis. However, satisfying outcome with the evidence of good performance of MBP also in 65-year-old or younger patients may confirm that MBP in mitral position could represent an acceptable solution, thus encouraging to extend the benefits of biological valve replacement also to younger patients.
Conflict of interest: none declared.
Author contributions
Giovanni A. Chiariello: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Supervision; Validation; Visualization; Writing – original draft; Writing—review & editing. Anne-Sophie Beraud: Data curation; Funding acquisition; Methodology. Olivier Vahdat: Data curation; Supervision; Visualization. Jérôme Van Rothem: Data curation; Methodology. Olivier Garcia: Data curation; Investigation; Supervision. Philippe Soula: Investigation; Project administration; Supervision. Pierre Berthoumieu: Data curation; Formal analysis. Issam Abouliatim: Conceptualization; Formal analysis; Project administration; Supervision; Validation; Visualization.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Tirone E. David and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
ABBREVIATIONS
- MVR
Mitral valve replacement
- MBP
Mosaic bioprosthesis
- SVD
Structural valve degeneration
REFERENCES
- 1. Goldstone AB, Chiu P, Baiocchi M, Lingala B, Patrick WL, Fischbein MP. et al. Mechanical or biologic prostheses for aortic-valve and mitral-valve replacement. N Engl J Med 2017;377:1847–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Russo A, Grigioni F, Avierinos JF, Freeman WK, Suri R, Michelena H. et al. Thromboembolic complications after surgical correction of mitral regurgitation incidence, predictors, and clinical implications. J Am Coll Cardiol 2008;51:1203–11. [DOI] [PubMed] [Google Scholar]
- 3. Chiang YP, Chikwe J, Moskowitz AJ, Itagaki S, Adams DH, Egorova NN.. Survival and long-term outcomes following bioprosthetic vs mechanical aortic valve replacement in patients aged 50 to 69 years. JAMA 2014;312:1323–9. [DOI] [PubMed] [Google Scholar]
- 4. Rieß F-C, Fradet G, Lavoie A, Legget M.. Long-term outcomes of the mosaic bioprosthesis. Ann Thorac Surg 2018;105:763–9. [DOI] [PubMed] [Google Scholar]
- 5. David TE, Ivanov J, Armstrong S, Feindel CM, Cohen G.. Late results of heart valve replacement with the Hancock II bioprosthesis. J Thorac Cardiovasc Surg 2001;121:268–77. [DOI] [PubMed] [Google Scholar]
- 6. Thomson DJ, Jamieson WR, Dumesnil JG, Burgess JJ, Peniston CM, Métras J. et al. Medtronic Mosaic porcine bioprosthesis: midterm investigational trial results. Ann Thorac Surg 2001;71:S269–72. [DOI] [PubMed] [Google Scholar]
- 7. Jamieson WRE, Riess F-C, Raudkivi PJ, Metras J, Busse EFG, Goldstein J. et al. Medtronic Mosaic porcine bioprosthesis: assessment of 12-year performance. J Thorac Cardiovasc Surg 2011;142:302–7. [DOI] [PubMed] [Google Scholar]
- 8. Riess F-C, Cramer E, Hansen L, Schiffelers S, Wahl G, Wallrath J. et al. Clinical results of the Medtronic Mosaic porcine bioprosthesis up to 13 years. Eur J Cardiothorac Surg 2010;37:145–53. [DOI] [PubMed] [Google Scholar]
- 9. Matsumoto Y, Fujita T, Hata H, Shimahara Y, Sato S, Kobayashi J.. Hemodynamic performance and durability of mosaic bioprostheses for aortic valve replacement, up to 13 years. Circ J 2015;79:1044–51. [DOI] [PubMed] [Google Scholar]
- 10. Guiraudon GM, Ofiesh JG, Kaushik R.. Extended vertical transatrial septal approach to the mitral valve. Ann Thorac Surg 1991;52:1058–60. [DOI] [PubMed] [Google Scholar]
- 11. Bourguignon T, Espitalier F, Pantaleon C, Vermes E, El-Arid JM, Loardi C. et al. Bioprosthetic mitral valve replacement in patients aged 65 years or younger: long-term outcomes with the Carpentier–Edwards PERIMOUNT pericardial valve. Eur J Cardiothorac Surg 2018;54:302–9. [DOI] [PubMed] [Google Scholar]
- 12. Akins CW, Miller DC, Turina MI, Kouchoukos NT, Blackstone EH, Grunkemeier GL. et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. Ann Thorac Surg 2008;85:1490–5. [DOI] [PubMed] [Google Scholar]
- 13. Malvindi PG, Mastro F, Kowalewski M, Ringold M, Margari V, Suwalski P. et al. Durability of mitral valve bioprostheses: a meta-analysis of long-term follow-up studies. Ann Thorac Surg 2020;109:603–11. [DOI] [PubMed] [Google Scholar]
- 14. Edmunds LH Jr, Clark RE, Cohn LH, Grunkemeier GL, Miller DC, Weisel RD.. Guidelines for reporting morbidity and mortality after cardiac valvular operations. The American Association for Thoracic Surgery, Ad Hoc Liaison Committee for Standardizing Definitions of Prosthetic Heart Valve Morbidity. Ann Thorac Surg 1996;62:932–5. [DOI] [PubMed] [Google Scholar]
- 15. Eichinger WB, Botzenhardt F, Gunzinger R, Kemkes BW, Sosnowski A, Maïza D. et al. European experience with the Mosaic bioprosthesis. J Thorac Cardiovasc Surg 2002;124:333–9. [DOI] [PubMed] [Google Scholar]
- 16. Zoghbi WA, Chambers JB, Dumesnil JG, Foster E, Gottdiener JS, Grayburn PA. et al. Recommendations for evaluation of prosthetic valves with echocardiography and Doppler ultrasound. J Am Soc Echocardiogr 2009;22:975–1014. [DOI] [PubMed] [Google Scholar]
- 17. Falk V, Baumgartner H, Bax JJ, De Bonis M, Hamm C, Holm PJ. et al. ; ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg 2017;52:616–64. [DOI] [PubMed] [Google Scholar]
- 18. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Fleisher LA. et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC Guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;70:252–89. [DOI] [PubMed] [Google Scholar]
- 19. Bourguignon T, Bouquiaux-Stablo A-L, Loardi C, Mirza A, Candolfi P, Marchand M. et al. Very late outcomes for mitral valve replacement with the Carpentier-Edwards pericardial bioprosthesis: 25-year follow-up of 450 implantations. J Thorac Cardiovasc Surg 2014;148:2004–11. [DOI] [PubMed] [Google Scholar]
- 20. Mykén PSU, Bech-Hansen O.. A 20-year experience of 1712 patients with the Biocor porcine bioprosthesis. J Thorac Cardiovasc Surg 2009;137:76–81. [DOI] [PubMed] [Google Scholar]
- 21. Kamioka N, Babaliaros V, Morse MA, Frisoli T, Lerakis S, Iturbe JM. et al. Comparison of clinical and echocardiographic outcomes after surgical redo mitral valve replacement and transcatheter mitral valve-in-valve therapy. JACC Cardiovasc Interv 2018;11:1131–8. [DOI] [PubMed] [Google Scholar]
- 22. Yoon S-H, Whisenant BK, Bleiziffer S, Delgado V, Schofer N, Eschenbach L. et al. Transcatheter mitral valve replacement for degenerated bioprosthetic valves and failed annuloplasty rings. J Am Coll Cardiol 2017;70:1121–31. [DOI] [PubMed] [Google Scholar]
- 23. Yoon S-H, Whisenant BK, Bleiziffer S, Delgado V, Dhoble A, Schofer N. et al. Outcomes of transcatheter mitral valve replacement for degenerated bioprostheses, failed annuloplasty rings, and mitral annular calcification. Eur Heart J 2019;40:441–51. [DOI] [PubMed] [Google Scholar]
- 24. Modine T, Overtchouk P.. Catheter-based innovations in mitral valve surgery. Eur J Cardiothorac Surg 2019;56:429–32. [DOI] [PubMed] [Google Scholar]
- 25. Chikwe J, Chiang YP, Egorova NN, Itagaki S, Adams DH.. Survival and outcomes following bioprosthetic vs mechanical mitral valve replacement in patients aged 50 to 69 years. JAMA 2015;313:1435–42. [DOI] [PubMed] [Google Scholar]
- 26. Gammie JS, Sheng S, Griffith BP, Peterson ED, Rankin JS, O'Brien SM. et al. Trends in mitral valve surgery in the United States: results from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg 2009;87:1431–7. [DOI] [PubMed] [Google Scholar]
- 27. Lorusso R, Miceli A, Gelsomino S, Lio A, Parise O, Montisci A. et al. Mitral valve replacement with a third generation porcine valve: an Italian multicentered study. Ann Thorac Surg 2020;109:1865–72. [DOI] [PubMed] [Google Scholar]
- 28. Luciani N, Mossuto E, Ricci D, Luciani M, Russo M, Salsano A. et al. Prosthetic valve endocarditis: predictors of early outcome of surgical therapy. A multicentric study. Eur J Cardiothorac Surg 2017;52:768–74. [DOI] [PubMed] [Google Scholar]
- 29. Musci M, Hübler M, Amiri A, Stein J, Kosky S, Meyer R. et al. Surgical treatment for active infective prosthetic valve endocarditis: 22-year single-centre experience. Eur J Cardiothorac Surg 2010;38:528–38. [DOI] [PubMed] [Google Scholar]