Abstract
Using a long-term prospective longitudinal study of U.S. men and their fathers, the present study examined the extent to which the quantity (i.e., shared activities between fathers and sons) and the quality (i.e., assessors’ ratings of fathers’ positive behaviors toward sons and the relationship quality between fathers and sons) of father involvement during childhood influenced sons’ diurnal patterns of salivary cortisol in adulthood (late 30s) directly and indirectly through substance use across the 20s. Findings indicated that the quantity of father involvement during childhood was directly associated with sons’ diurnal cortisol patterns assessed almost 30 years later. Specifically, the quantity of father involvement in childhood significantly increased the intercept (i.e., upon awakening) and also led to a greater reduction in cortisol across the day, suggesting a well-regulated diurnal cortisol pattern. The quantity of father involvement significantly reduced the amount of sons’ illicit drug and tobacco use across the 20s. Tobacco use across the 20s was associated with a lower cortisol intercept level (upon awakening), although the mediating path was not significant. The present study provided empirical evidence demonstrating long-term physiological and behavioral consequences of father involvement in childhood and its potency as a crucial early caregiving environment for sons.
Keywords: father involvement, stress regulation system, diurnal cortisol patterns, substance use, longitudinal association
Father involvement with children is significantly associated with children’s positive mental, cognitive, social, and physical outcomes (Allen & Daly, 2007; Sarkadi, Kristiansson, Oberklaid, & Bremberg, 2008). However, studies often focused on childhood or adolescent outcomes at a behavioral level and longer-term effects of father involvement on offspring’s adjustment extended into adulthood remain largely underexplored (Parke, 2000). In particular, little is known about whether father involvement during childhood exerts an influence on offspring’s physiological functioning such as the stress regulation system that is thought to have significant implications for a wide range of individuals’ functioning, including cognitive, behavior, mental, and physical outcomes (Adam et al., 2017; Mills-Koonce et al., 2011). The present study sought to extend prior work by examining the extent to which the quantity and quality of father involvement during childhood would affect their son’s physiological stress regulation system as reflected in the diurnal patterns of salivary cortisol in adulthood (late 30s). We specifically focused on whether father involvement influenced their son’s diurnal patterns of cortisol directly or indirectly through problem behaviors (i.e., substance use) during early adulthood (the 20s); the Oregon Youth Study (OYS), a long-term longitudinal study of fathers and sons with at-risk family backgrounds, was used.
The Effects of Father Involvement
With a growing interest in the role of fathering in shaping offspring’s development, studies have shown that father involvement through direct interactions with the child positively influences a wide range of child’s outcomes (Cabrera, Volling, & Barr, 2018; Nettle, 2008; Pleck, 2007). Such beneficial effects appear to be true even when fathers do not live with the children (Adamsons, 2018; Cabrera, Tamis-LeMonda, Bradley, Hofferth, & Lamb, 2000) and controlling for socioeconomic status (SES) and mothers’ influence (Flouri & Buchannan, 2004; Mills-Konnce et al., 2011). A meta-analysis showed that fathers’ positive parenting (e.g., democratic or stimulating parenting style) was predictive of increases in children’s cognitive, prosocial, and self-regulation skills; whereas negative parenting (e.g., coercive discipline, physical punishment, and authoritarian or permissive behaviors) was predictive of children’s increased externalizing problems and reduced cognitive skills (McWayne, Downer, Campos, & Harris, 2013). In a more recent meta-analytic review, Rodrigues et al. (2021) found small to moderate associations between paternal sensitivity (defined as “reading the subtle affective and cognitive cues of children and in turn, responding in a manner that is sensitive, stimulating, or both” [p. 554]) and children’s better cognitive functioning and emotional regulation, and less externalizing problems. On the other hand, findings on the effects of quantity of father involvement are inconsistent; some showing the quantity of involvement being unrelated to children’s well-being (refer to a meta-analysis on nonresident fathers; Adamsons & Johnson, 2013) and others showing the quantity being a prerequisite for the quality of involvement (Fabricius, Sokol, Diaz, & Braver, 2012). Alternatively, some researchers have argued that both components are conceptually distinct and each contributes to children’s adjustment (Adamsons, 2018; Halme, Tarkka, Paavilainen, Nummi, & Åstedt-Kurki, 2010). A meta-analysis indeed showed that both quantity (e.g., frequency of positive engagement activities) and quality (e.g., warmth, nurturance, or responsiveness) of father involvement during early childhood influenced children’s social and cognitive development (McWayne et al., 2013).
Father involvement also works to prevent or reduce offspring’s involvement in health-risking behaviors during adolescence (Flouri & Buchanan, 2002). For instance, a composite measure of the quantity and quality of father involvement during adolescence (e.g., shared activities, parent–child closeness) was concurrently associated with lower levels of alcohol use in adolescent children (Cookston & Finlay, 2006). Greater father involvement (i.e., closeness and warmth, and supportive behaviors) and less authoritarian fathers’ parenting style predicted a reduced risk for the onset of delinquent behaviors and substance use in adolescents (Bronte-Tinkew, Moore, Capps, & Zaff, 2006). Similarly, Goncy and van Dulmen (2010) found that the quality of father involvement during adolescence (i.e., emotional closeness to fathers) was significantly associated with reduced levels of alcohol use, alcohol related problems, and risk behaviors co-occurring with alcohol use, while controlling for mothers’ involvement.
However, whether such positive effects of father involvement have lasting implications for offspring’s outcomes in adulthood have not been investigated with prospective longitudinal data (Parke, 2000), as many studies relied on cross-sectional, short-term longitudinal (e.g., Goncy & van Dulmen, 2010, Mills-Koonce et al., 2011), or retrospective designs (e.g., Finley & Schwartz, 2007). A few exceptions include Flouri and Buchannan (2004)’s work based on the National Child Development Study. They found that father involvement (e.g., reads, takes outings, and interested in child’s education) at age 7 years was strongly predictive of offspring’s educational attainment at age 20 years, independent of mother involvement with the offspring and the family structure (i.e., whether two-parent families or not). Based on the same National Child Development Study data, Nettle (2008) further found that father involvement in childhood (assessed at age 11 years) led to offspring’s upward social mobility (assessed by comparing father’s and adult offspring’s SES) by age 42 years. These findings suggest that father involvement is likely to have a lasting impact on the offspring’s adjustment in adulthood.
Although few studies have considered developmental aspects of father involvement, the aspects and amount of father involvement are likely to change in response to children’s developmental needs; the level of father involvement tends to increase from infancy to preschool age and decrease during adolescence when peer influence increases (Bruce & Fox, 1999; Jeynes 2015). Father involvement tends to be higher in childhood than in infancy because more shared activities and interactions (e.g., playing sports together and reading books) become possible as children get older (e.g., Planalp & Braungart-Rieker, 2016). Some studies suggested that the effect size of parent involvement on children’s outcome (i.e., academic achievement) was larger for elementary school students than secondary school students (Jeynes, 2005; 2007), implying the significance of father involvement during childhood. Furthermore, limited evidence suggested that fathers with low education levels generally showed lower levels of involvement in childcare than fathers with high education levels (Jones & Mosher, 2013) but that positive effects of father involvement on child academic outcomes were larger for low SES families than high SES families (Miller, Thomas, Waller, Nepomnyaschy, & Emory, 2020). However, many studies have focused on middle class or nonresidential fathers, thus family processes among residential fathers with socioeconomic at-risk background remains largely unknown.
Father Involvement and Offspring’s Stress Regulation System
Another area that is relatively unknown is whether father’s involvement with children has implications for offspring’s adjustment at a psychobiological level (Mills-Koonce et al., 2011). There is ample evidence documenting a significant association between early caregiving environments (e.g., child maltreatment, poor parenting) and functioning of the hypothalamic-pituitary-adrenocortical (HPA) axis system, a key neurobiological system involved in stress reactivity and regulation (e.g., Adam et al., 2017; Gunnar & Vazquez, 2001; Luecken, Kraft, & Hagan, 2009). In the face of physical or psychological stressors, the HPA axis system releases the adrenocortical steroid hormone cortisol, which activates various systems throughout the body and brain (e.g., immune and inflammatory function, learning, and memory) to manage challenges (McEwen, 2007; Sapolsky, Romero, & Munck, 2000). In addition to its role as the stress reactivity system, cortisol also shows a diurnal pattern in humans (Adam et al., 2017). A well-regulated cortisol system is characterized by a strong circadian rhythm with levels relatively high upon waking, peaking 30–40 minutes after waking, followed by rapid declines in the next few hours, and more gradual declines until reaching a low point around bedtime (Adam et al., 2017; Saxbe, Repetti, & Nishina, 2008). In contrast, dysregulated diurnal cortisol patterns exhibit a flatter or blunted cortisol rhythm due to lower morning and higher evening cortisol level (Adam et al., 2017; Gunnar & Vazquez, 2006). Such flat or blunted patterns are often observed among individuals who were exposed to chronic stress (e.g., Bruce, Fisher, Pears, & Levine, 2009; Saxbe et al., 2008), which is in turn linked to a range of poor health outcomes such as depression (Doane et al., 2013) and cardiovascular disease (Matthews, Schwartz, Cohen, & Seeman, 2006).
Social experiences, especially poor early caregiving environments alter the development of the stress regulation system as reflected in dysregulated diurnal cortisol patterns, which subsequently contributes to maladjustment across the life span (Gunnar & Vazquez, 2001; Luecken et al., 2009). People with adverse experiences in childhood show chronic HPA dysregulation in adolescence (Essex et al., 2011) and adulthood (Kalmakis, Meyer, Chiodo, & Leung, 2015). HPA dysregulation is also related to exposure to maternal withdrawal in the first year of life (elevated morning cortisol in offspring at age 13 years; Murray, Halligan, Goodyer, & Herbert, 2010), and to higher levels of maternal insensitivity in the first 3 years (lower awakening cortisol levels at age 15 years, regardless of later caregiving experiences, early demographic characteristics, child sex, or child difficult temperament; Roisman et al., 2009). Alternatively, positive maternal parenting (e.g., sensitivity, stimulating) during the free and structured play was significantly associated with better HPA regulation (child’s lower cortisol level at ages 7, 15, and 24 months), leading to greater executive function at age 3 years (Blair et al., 2011).
Similarly, limited evidence suggests that early father involvement influences offspring’s stress regulation system. For example, based on data from cortisol reactivity to challenging tasks, Mills-Koonce et al. (2011) found that fathers’ negative interactions were associated with greater increases in cortisol at age 7 months, as well as higher baseline and overall levels in cortisol at age 24 months, indicating exacerbated acute stress reactivity and diminished ability in regulating physiologically arousing situations. Ibrahim and colleagues (2017) found that the quantity of father involvement in adolescence predicted lower amounts of total cortisol output in response to a challenging task assessed at ages 19–22 years, regardless of whether the fathers were biological or not (Ibrahim, Somers, Luecken, Fabricius, & Cookston, 2017). These findings suggest that the quantity and quality of father involvement are likely to have influences on the HPA axis system in offspring. Although these studies focused on offspring’s cortisol reactivity to acute stress, it is possible that father involvement in childhood, as significant emotional and psychosocial environment, is directly related to their children’s diurnal cortisol profiles in adulthood.
Father involvement in the child’s early years may also influence the child’s diurnal patterns of cortisol in adulthood indirectly through problem behaviors in early adulthood. As mentioned earlier, father involvement has been found to be consistently associated with less engagement in delinquent behaviors in offspring, including poly drug use, alcohol use, and cigarette smoking (Aho, Koivisto, Paavilainen, & Joronen, 2018; Ali & Dean, 2015; Bronte-Tinkew et al., 2006; Jones & Benda, 2004), which is closely related to dysregulated cortisol functioning (Badrick, Kirschbaum, & Kumari, 2007; Manetti, Cavagnini, Martino, & Ambrogio, 2014; Muehlhan et al., 2020). Alcohol, for example, is known to induce the release of corticotropin releasing hormone within the hypothalamic nuclei, which in turn leads to elevated adrenocorticotropic hormone and cortisol levels (Muehlhan et al., 2020). Similarly, illicit drugs such as cocaine leads to increased plasma levels of adrenocorticotropic hormone and subsequently cortisol levels (Manetti et al., 2014). Cigarette smoking also appears to alter the HPA axis system, although findings are somewhat inconsistent with some reporting increased cortisol levels throughout the day (Steptoe & Ussher, 2006) and some others reporting no relationship (Anthenelli & Maxwell, 2002). However, few studies have examined whether a lack of father involvement in childhood is predictive of greater substance use in offspring’s early adulthood, which in turn leads to dysregulated diurnal cortisol patterns in adulthood.
The Present Study
The present study sought to investigate whether the quantity and quality of father involvement measured in childhood would affect sons’ diurnal patterns of cortisol measured in adulthood, at approximately age 37 years, using a long-term prospective longitudinal study of boys (later men) and their fathers from the Oregon Youth Study (OYS), over 30 years. We hypothesized that there would be a significant direct link between a higher quantity and quality of father involvement and the son’s later cortisol regulation. It was also expected that the son’s substance use (i.e., drug, alcohol, and tobacco use) in their 20s would partially, but not completely, explain or mediate this association. This study extends the existing literature (a) by examining independent effects of both the quantity (the number of shared activities between fathers and sons) and the quality of father involvement (fathers’ positive behaviors toward their sons and the relationship quality between fathers and sons); (b) by focusing on father involvement within families with at-risk family backgrounds; and (c) by leveraging a long-term longitudinal data applying a multimethod, multi-informant design and controlling for early and concurrent economic status and relationships with fathers in adulthood.
Method
Data and Participants
Data for the present study were drawn from the OYS, a long-term multimethod, multi-informant longitudinal study of sons (N = 206) at risk for delinquency and their parents. The sons were recruited in 1984–1985 through fourth-grade classes (ages 9–10 years) in higher crime areas of a midsized city in the Pacific Northwest in the United States (participation rate = 74%), and they have been regularly assessed over 30 years. At Wave 29 (W29), 176 sons (85.44%) participated in the assessment, indicating very high retention rates over the years. The sons were approximately age 10 years (SD = 0.58) at Wave 1 (W1) and predominantly European American (89.81%). The median annual household income was between $10,000–14,999, and 38.35% of the sample reported having received at least one type of financial aid (e.g., food stamps, aid to families with dependent children) at W1. All participating fathers were living with their sons. The fathers were on average age 36.43 years (SD = 6.65) and their median education level was high school graduation at W1. At W29, 69.32% of sons were living with their partners, 63.64% were engaged in paid work or education, and their median household monthly income was $4,117. Approximately 50% of them had juvenile arrest records and only 52% graduated from high school with their class, indicating the at-risk nature of the sample (Kim et al., 2015). In the present study, we assessed father involvement in childrearing when the sons were ages 9 (W1) and 11 years (Wave 3 [W3]), sons’ substance use in their 20s at Waves 13, 15, 17, 19, and 21 (ages 21 to 29 years), and their diurnal cortisol patterns assessed at age 37 years (W29).
Procedures
Parents and sons were each interviewed separately at Oregon Social Learning Center (OSLC) or sometimes in later study years in their homes (if they had moved out of the area). In addition, they also completed parent–child problem-solving interaction tasks that were videotaped. The assessment lasted about 2–1/2 hours in total. The sons participated in the OYS–Couples Study with their intimate partners and completed nine couples’ assessment from late adolescence (ages 17–19 years) through adulthood (ages 37–38 years). The diurnal cortisol samples used in the present study were collected as part of this OYS–Couples Study. All participants were financially compensated for participation. The studies were approved by the OSLC Institutional Review Board. The data that support the findings of this study are openly available in ICPSR at https://www.icpsr.umich.edu/web/pages/ICPSR/index.html, except for the cortisol data and the observational data due to privacy or ethical restrictions.
Measures
Father Involvement in Childhood
Quantity of Father Involvement.
To measure the quantity of father involvement in son’s activities during childhood (ages 9 and 11 years), we used the Family Activity List (Patterson, 1982) assessed at W1 and W3. Fathers were asked to respond whether they had participated in each of 25 types of activities with their son in the past week (e.g., play an indoor game with him and talk for l0 minutes or more about your activities). Fathers were also asked whether they participated in three types of activities with their son in the past month (e.g., go on a picnic together and take a day trip together, fishing, driving, or boating). If the response was affirmative, it was coded as 1; if the response was negative, it was coded as 0. We summed the responses, generating the number of activity scores ranging from 0 to 28 for each wave. The two scores from W1 (Cronbach’s α = .71) and W3 (Cronbach’s α = .77) were significantly associated (r = .31; p < .01) and thus were averaged to create an index value to represent the quantity of father involvement in childrearing. On average, fathers reported that they shared 11.54 activities with their study son (SD = 3.29) during childhood.
Quality of Father Involvement.
The quality of father involvement in childrearing was assessed using coders’ ratings from the videotaped Family Interaction Task (OSLC, 1984) completed at W1 and W3. The family discussion (including the parent[s] and the study son), included 5 minutes of planning a fun family activity and two 10-minute segments where the parent(s) and son discussed topics they had respectively picked from a list of family issues and rated as relatively hot topics in their family (e.g., son not doing his homework, his room not being picked up). The team of coders received 3 months of training to achieve acceptable reliability on the real-time Family Process Code (Dishion et al., 1983) of positive and negative interaction behaviors. Following coding the task, the coders completed a rating form of more global impressions of each parent and the boy. Of the 34 coder rating items, 9 items measured father’s positive parenting behaviors toward their sons (e.g., verbally affectionate with boy; showed empathy, support, and genuine concern for son; friendly and pleasant to boy), 3 items measured son’s behaviors that reflected the quality of father–child relationships (i.e., child was friendly to dad; boy seemed aloof, distant, or unattached to dad; boy seemed to have a hostile, arrogant, or noncompliant set to dad), and 1 item measured the overall impression of the father–child relationship.
The responses ranged from no basis to judge (0), doesn’t fit at all (1), rarely fits (2), sometimes fits (3), fits most of the time (4), and definitely, perfect fit (5). The only exception was the item on the overall quality of father–child relationships, which was measured on a 5-point Likert scale, ranging from very poor (1) to very good (5). The no basis responses were treated as missing data and some of the items were reverse scored such that high scores indicated higher quality paternal involvement. The responses were averaged for W1 (Cronbach’s α = .90) and W3 (Cronbach’s α = .92). The two scores were significantly associated (r = .28, p < .01) and thus were averaged for the present analysis (M = 4.06, SD = 0.59).
Men’s Substance Use in Adulthood
Illicit Drug Use.
To assess the son’s illicit drug use in their 20s, the number of times the men used marijuana, cocaine/crack, hallucinogens, and inhalants were summed. Men were asked whether they used each of these illicit drugs in the last 12 months; if the answer was affirmative, an open-ended question queried regarding the number of times they used the given drug in the last year. If the initial answer to the use was negative, the amount of use for the given drug was coded as 0. The amount of use for the four substances was summed for each wave. Values from Waves 13, 15, 17, 19, and 21 (ages 21 to 29 years) were significantly interrelated (rs ranging from .15 to .70; all ps < .05) and were thus averaged to create an indicator to represent men’s illicit drug use in their 20s (M = 59.31, SD = 106.76). Approximately 64% of the men reported having used at least one type of illicit drugs during their 20s.
Alcohol Use.
For son’s alcohol use in their 20s, indicators of three types of alcohol consumption (i.e., beer, wine, and hard liquor) were used. The men were asked whether they had beer in the last year and if the answer was affirmative, open-ended questions queried regarding how many times they had beer in the last year and how much beer they generally drank at one time (one occasion). The number of times they had beer in last year and the amount of beer drunk at one time were multiplied to create an index score representing the volume of beer consumed (e.g., Capaldi, Feingold, Kim, Yoerger, & Washburn, 2013). If the initial answer was negative, the volume was coded as 0. Wine and hard liquor use were computed in the same manner. The three indicators were summed to represent the total volume of the men’s alcohol use in each year. Values from Waves 13, 15, 17, 19, and 21 (ages 21 to 29 years) were significantly interrelated (rs ranging from .18 to .63; all ps < .05), except for correlations between the early 20s (Wave 13) and late 20s (Wave 19 and Wave 21). Such weaker correlations between the early and late 20s in alcohol use may be due to decreases in alcohol use as men reached their late 20s. The values across the men’s 20s were averaged to create a composite for the men’s alcohol use in their 20s (M = 489.08, SD = 826.39). Almost all of the men (97.6%) reported having consumed at least one type of alcohol, with 48.3% engaging in binge drinking (>= 5 drinks at one time for at least one type of alcohol) at age 21 years (Wave 13), which was gradually decreased to 18.8% at age 29 years (Wave 21).
Tobacco Use.
For son’s tobacco use in their 20s, the men were asked whether they smoked cigarettes or chewed tobacco in the last year; if the answer was affirmative, they were asked the number of times they had smoked or chewed tobacco in the last year. If the initial answer was negative, the men’s tobacco use was coded as 0. Values from Waves 13, 15, 17, 19, and 21 (ages 21 to 29 years) were significantly interrelated (rs ranging from .62 to .77; all ps < .01) and were thus averaged to create an indicator to represent men’s tobacco use in their 20s (M = 1829.66, SD = 2211.96). Approximately 76% of the men reported having used tobacco. Men who used 20 or more cigarettes in a day ranged from 8.4% to 13.1% across their 20s.
In the present sample, approximately 54% of the men reported having used all three substances, 32% reported having used two substances, and 12% reported having used only one type of substance. Around 2% of them reported no use of any substances. For the subsequent analysis, the scores for illicit drug, alcohol, and tobacco use were square root transformed to approximate the normal distribution.
Men’s Stress Regulation in Adulthood
The son’s stress regulation system in adulthood was assessed using diurnal salivary cortisol data collected at W29 when the men were approximately age 37 years. The men were asked to take saliva collection kits home, along with a copy of the written protocol and daily diary to record information about the collections. Prior to being asked to participate in diurnal salivary cortisol collections, men and their intimate partners completed problem-solving tasks (as part of the OYS–Couples Study, a companion study of men’s relationships with intimate partners) where saliva samples were collected; thus, the men were already familiar with the salivary cortisol collection procedures. Nonetheless, trained assessors reviewed the collection protocol with the men in person. The men were instructed to provide four saliva samples per day across 4 consecutive days (i.e., upon awakening, 30 minutes post awakening, in the mid to late afternoon, and at bedtime), using passive drool into 1.7ml Eppendorf tubes. They were instructed (a) not to eat or drink anything, brush their teeth, or smoke before collecting saliva samples and (b) to record additional information about each collection and any deviations from the protocol on the daily diary each day (e.g., general health, medication use, wake and bed time, and saliva collection times). Assessors called the men the evening before sampling began and reviewed key aspects of the saliva collection protocol. All saliva samples were refrigerated at each men’s home until the collection was completed and then were either mailed in prepaid envelops or dropped off at OSLC. The saliva samples were refrigerated onsite and then were sent to the Global Health Biomarker Laboratory at the University of Oregon and frozen at −80°C until assayed. Saliva collections were assessed in duplicate using an enzyme immunoassay kit (1–3002; Salimetrics, State College, PA), which was validated as the quantitative measurement of salivary cortisol and has been widely used in the field (Kim et al., 2015). The average intra-assay coefficients and inter-assay coefficients across 4 days were 10.57% and 10%, respectively. The average of the duplicate tests was used in the analyses.
As has been typically done in prior studies, cortisol values exceeding 2 ug/dL (n = 7) were excluded (e.g., Kim et al., 2015) and values that deviated more than 3SDs from the mean of each collection were replaced by the value of 3SDs above the mean, resulting in a total of 1,829 cortisol samples for the analysis. Bivariate correlations of cortisol values that were collected at the same time across 4 days were all statistically significant: for upon awakening, r = .46 ~ .60 (all ps < .01), for 30 minutes post awakening, r = .39 ~ .58 (all ps < .01), for the mid to late afternoon, r = .21 ~ .44 (all ps < .05), and for bedtime, r = .39 ~ .61 (all ps < .01). For the present analysis, cortisol values were averaged across 4 days for each collection time. The mean values of cortisol were 0.32 ug/dl (SD = 0.17), 0.37 ug/dl (SD = 0.19), 0.14 ug/dl (SD = 0.10), and 0.11 ug/dl (SD = 0.12) for upon awakening, 30 minutes post awakening, mid to late afternoon, and bedtime, respectively.
Control Variables
To adjust potential effects of economic status on men’s diurnal cortisol pattern in adulthood, we included annual household income at W1 (1 = less than $4,999 to 8 = $40,000 or more) and the men’s annual personal income at W29 (an open-ended question). Additionally, to examine the unique contribution of father involvement in childhood, relationships with the father in adulthood (W29) were also controlled. The sons were asked, “How well do you get along with your dad (dad figure)?”. The responses ranged from not at all well (1) to very well (5).
Three additional variables that have been known to influence individuals’ diurnal cortisol patterns were also included in the model. First, if men recorded any deviation from the instruction for salivary cortisol collections (i.e., exercised, drank alcohol, used tobacco, or used medication), it was coded as 1. No deviation from the instruction was coded as 0. Second, a variable was included to reflect the cortisol collection day (1 = weekend, 0 = weekdays). Lastly, men’s total sleep time averaged across 4 collection days was included.
Analysis Procedures
The analyses were performed in two steps. First, men’s diurnal cortisol patterns in adulthood were examined using latent growth modeling, while controlling for covariates, and factor scores for the intercept and the slope were obtained. In the next step, the long-term direct effects of father involvement in childhood on men’s diurnal cortisol patterns in adulthood—as represented with the intercept and slope factor scores from the first step—and indirect effects through the men’s substance use in their 20s were examined using path modeling in Mplus 8 (Muthén & Muthén, 1998–2019). The proposed model fit was evaluated using Chi-square, Root-Mean Square Error of Approximation (RMSEA), Comparative Fit Index (CFI), and Tucker-Lewis Index (TLI). The significance of mediating paths was examined using the bias-corrected bootstrapping with sample iterations set to 5,000. If the 95% confidence interval does not include zero, the mediating path is considered significant (Nakagawa & Cuthill, 2007).
In the first step, the cortisol values from each collection time (averaged across 4 consecutive days: upon awakening, 30 minutes post awakening, in the mid to late afternoon, and at bedtime) were used as four observed variables in latent growth modeling. The typical diurnal cortisol rhythm starts with high levels of cortisol on waking, which peaks in the 30–40 minutes after waking, followed by rapidly declines during the day until reaching a nadir around bedtime (Adam et al., 2017). As such, the linear model would not properly reflect the diurnal cortisol patterns across the day; thus, we chose the linear spline model, in which the factor loading for the first cortisol collection (i.e., upon awakening) for the slope factor was fixed as 0 and the factor loading for the last collection (i.e., bedtime) was fixed as 1, while the remaining factor loadings were freely estimated. The linear spline model allows to compensate for nonlinearity in estimating trajectories and has been widely used in previous studies (e.g., Graham, Kim, & Fisher, 2012; Kim, Pears, Fisher, Connelly, & Landsverk, 2010). The unconditional linear spline model fit the data relatively well (X2[3] = 5.809, p > .05, RMSEA = 0.089, CFI = 0.985, TLI = 0.969). The mean of the intercept factor was 0.32 (p < .001) and the variance was 0.02 (p < .001). The mean of the slope factor was −0.20 (p < .001) and the variance was 0.02 (p < .001). The negative slope mean indicated a decreasing pattern of salivary cortisol during the day by 0.20 ug/dl.
We then analyzed the conditional model including three covariates (i.e., deviations from the cortisol collection instruction, collection on weekends, and the total amount of sleep) to take into account potential effects of these confounders. In this conditional model, the residual variance of the intercept and the slope were fixed to zero because they were negative and statistically nonsignificant (p > .05). The conditional model also fit the data well (X2[12] = 17.336, p > .05, RMSEA = 0.061, CFI = 0.972, TLI = 0.957). Of the covariates, collection on weekends was negatively associated with the intercept factor and positively associated with the slope factor, indicating decreased morning cortisol levels and slower decreases across the day on weekends. The other two covariates (i.e., deviations from the instruction and sleep time) were not significantly associated with the intercept and slope factors of men’s diurnal cortisol patterns. The adjusted intercept (i.e., upon awakening) mean was 0.32 ug/dl (SD = 0.03) and cortisol values decreased by 0.21 ug/dl (SD = 0.04) on average across the day. The scores for the intercept and slope factor in this conditional model were used in the following step.
In the second step, the quantity and quality of father involvement in childhood were included as independent variables and the intercept and slope factor scores of diurnal cortisol patterns that were obtained from the first step were included as dependent variables in the model. Men’s use of illicit drugs, alcohol, and tobacco in their 20s were included in the model as potential mediators of the link between father involvement during childhood and the HPA axis functioning during adulthood for the men. The three substance use variables were allowed to covary. Household income at W1, men’s income at W29, and men’s perceived relationships with fathers at W29 were included as control variables to account for their potential influences on men’s HPA axis system in adulthood.
To accommodate missing data, full information maximum likelihood was used, so all 206 cases were included in the analysis. Full information maximum likelihood provides less biased estimates compared to traditional approaches and has been used even when there is no information about the missing data mechanism (Enders & Bandalos, 2001). Men’s cortisol data at age 37 years was approximately 43% missing, but this was mainly due to the study design (i.e., only those men with an intimate partner were asked to participate in the cortisol collection) and there were no significant associations between the presence of cortisol data and sociodemographic characteristics assessed at W1 (i.e., parents’ education level, household income, and ethnicity [all ps > .05]). Additionally, the presence of cortisol data was not significantly correlated with the father involvement measures used in the study, substance use in 20s, and demographics and psychopathology (i.e., work status, income, depressive symptoms, and substance use) at W29.
Results
Descriptive Statistics of Study Variables
Table 1 presents bivariate correlations among the study variables, along with means and standard deviations. The quantity of father involvement with their study sons in childhood was positively associated with the men’s adult cortisol intercept level and negatively associated with the cortisol slope. On the other hand, the quality of father involvement with their sons was not significantly associated with the men’s cortisol intercept and slope. The quantity of father involvement was negatively associated with men’s illicit drug and tobacco use in their 20s. The quality of father involvement was negatively associated with men’s tobacco use. Men’s alcohol use and tobacco use were negatively associated with their cortisol intercept level at age 37 years.
Table 1.
Means, Standard Deviations, and Bivariate Correlations of the Study Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Mean | Standard deviation | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Quantity of father involvement | 11.54 | 3.29 | |||||||||
| 2. Quality of father involvement | .14 | 4.06 | 0.59 | ||||||||
| 3. Men’s illicit drug use | −.21* | −.11 | 59.31 | 106.76 | |||||||
| 4. Men’s alcohol use | .10 | .04 | .12 | 489.08 | 826.39 | ||||||
| 5. Men’s tobacco use | −.27** | −.19* | .22** | .20** | 1829.66 | 2211.96 | |||||
| 6. Men’s diurnal cortisol intercept | .23* | .19 | −.01 | −.19* | −.26** | 0.32 | 0.03 | ||||
| 7. Men’s diurnal cortisol slope | −.22* | −.14 | −.02 | .18 | .18 | −.87** | −0.21 | 0.04 | |||
| 8. Household income (1=less than $4,999, 8=$4,000 or more) | .13 | .28** | −.08 | −.09 | −.06 | .15 | −.14 | 3.73 | 1.99 | ||
| 9. Personal income ($1,000) | −.02 | .13 | −.08 | −.10 | −.16* | .04 | −.08 | .10 | 48.11 | 78.54 | |
| 10. Relationships with father | .22* | .26** | −.21* | .05 | −.15 | .01 | −.01 | .21* | .00 | 4.15 | 0.85 |
Note. The quantity and quality of father involvement assessed at age 9 and 11 years. Men’s substance use assessed at ages 21–29 years. Men’s cortisol level assessed at age 37 years. Household income assessed at age 9 years. Men’s personal income and relationships with father assessed at age 37 years.
p < .05.
p < .01.
Effects of Father Involvement on Sons’ Substance Use and the Stress Regulation System
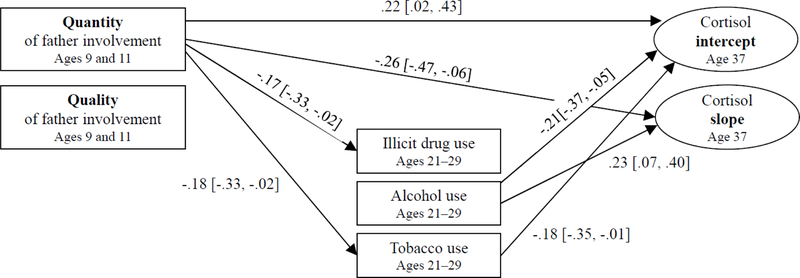
The proposed model fit the data well (X2[9] = 13.485, p > .05, RMSEA = 0.049, CFI = 0.981, TLI = 0.926). As shown in Figure 1, the quantity of father involvement with their sons in childhood was associated with a significantly increased intercept (i.e., upon awakening) of the son’s diurnal cortisol in adulthood, almost 30 years later. The quantity of father involvement also was also associated with a greater reduction in the son’s cortisol across the day, suggesting a steeper downward slope. In contrast, the quality of father involvement with their sons in childhood was not associated with the son’s diurnal cortisol intercept or slope in adulthood. For the son’s substance use in their 20s, the quantity of father involvement with their sons in childhood was associated with a reduced amount of men’s illicit drug and tobacco use. The amount of men’s alcohol use and tobacco use in their 20s was associated with a lower cortisol intercept level (i.e., upon awakening) at age 37 years. In addition, men’s alcohol use was associated with slower reductions in men’s cortisol across the day. However, the bias-corrected bootstrapping results showed that none of the mediating paths were statistically significant.
Figure 1:
Direct and Indirect Effects of Father Involvement in Childhood on Men’s Diurnal Cortisol Patterns in Adulthood
Note. Values are standardized coefficients and values in brackets represent 95% confidence intervals. Only significant paths (95% confident intervals not including zero) are presented in the figure. Nonsignificant paths and the control variables (household income at age 9 years, personal income at age 37 years, and relationships with father at age 37 years) are not presented.
Discussion and Conclusion
Although studies have shown positive effects of father involvement on children’s development, its long-term influence on physiological function has remained largely unknown. To fill this gap, we utilized a longitudinal study of fathers and their sons over 30 years and examined the extent to which the quantity and quality of father involvement in childhood (ages 9–11 years) influenced the son’s HPA axis functioning in adulthood (their late 30s) directly or indirectly through the son’s substance use in early adulthood (across their 20s). One of the most interesting findings is that father involvement with their sons in childhood, especially the quantity of involvement, was directly associated with the men’s diurnal cortisol patterns in adulthood assessed almost 30 years later, even after controlling for SES and concurrent relationships with fathers. Men who shared higher levels of activities with their fathers as children showed higher levels of cortisol upon awakening and greater reductions in cortisol across the day in their late 30s, suggesting a strong circadian rhythm. Our finding is in line with Ibrahim and colleagues’ (2017) work in which young adults (ages 19–22 years) who reported having shared activities with fathers more frequently during adolescence (ages 11–16 years) showed lower levels of cortisol reactivity (assessed as area under the curve) to an interpersonal challenge task, independent of effects of mother involvement during adolescence and concurrent father– and mother–young adult engagement.
The present findings also add to the existing literature evincing the broad association between adverse early caregiving environments (e.g., child maltreatment, institutionalization, and exposure to insensitive maternal care) and the development of dysregulation in the HPA axis system (Essex et al., 2011; Kalmakis et al., 2015; Luecken et al., 2009) by showing that high levels of father involvement, indicative of positive childhood experiences, can also help facilitate the offspring’s HPA axis functioning. Previous studies have shown that relatively common problems in early caregiving environments—maternal withdrawal behaviors (Murray et al., 2010) and maternal insensitivity during early childhood (Roisman et al., 2009)—predicted HPA axis functioning in adolescence. Our finding supports the argument that individuals’ HPA axis function is sensitive to childhood experiences and that father involvement, especially positive activities (e.g., playing games, talking for 10 minutes or more, going on a picnic together), is a potent aspect in early caregiving environments.
Another finding that is worth noting is that the quantity of father involvement in childhood was associated with lower levels of substance use by their sons, especially illicit drug and tobacco use in their 20s. Tobacco use was also related to the son’s well-regulated diurnal cortisol patterns in the late 30s (although the indirect effect as a whole was not statistically significant). This is somewhat consistent with previous findings in which father involvement was predictive of lower levels of substance use in adolescent children (Bronte-Tinkew et al., 2006; Cookston & Finlay, 2006). A recent meta-analysis has found that fathers’ direct involvement (both the quantity and quality) has a profound influence on various cognitive and social outcomes in childhood (e.g., general cognitive abilities, prosocial skills, internalizing symptoms, and externalizing symptoms) but is most strongly associated with children’s self-regulation abilities (McWayne et al., 2013). Another meta-analysis has also confirmed significant associations between paternal sensitivity and children’s executive function and emotional regulation from infancy to childhood (Rodrigues et al., 2021). Children’s self-regulatory abilities, a set of skills involved in attention, persistence, motivation, and effortful control, are closely associated with later substance use (Crockett, Raffaelli, & Shen, 2006; Fosco, Frank, Stormshak, & Dishion, 2013). Given the at-risk nature of the present sample with high prevalence rates of substance use among the sons (e.g., Washburn, Capaldi, Kim, & Feingold, 2014), we expected that men’s substance use in their 20s would work as additional life stressors and lead to dysregulated diurnal cortisol patterns in their 30s. Although the mediating role of substance use was not statistically significant in the present study, whether exposure to additional life stressors (e.g., mental health problems such as substance use and depression, or relationship conflict) during adolescence and emerging adulthood influences the men’s diurnal cortisol patterns in adulthood warrants further research before drawing any conclusion. The weak associations between father involvement and alcohol use in the present study might be due to limited variability in men’s alcohol use in their 20s (almost all men reported having used alcohol across their 20s). However, potentially differential effects of father involvement on different types of substance use in the offspring cannot be ruled out and await further research.
Additionally, it should be noted that the present study did not examine men’s diurnal cortisol patterns before the later 30s; thus, whether the beneficial effects of the quantity of father involvement emerged early and remained stable into adulthood or the benefits accrued across transitions from childhood to adulthood should be examined. Albeit limited, recent studies shed important light on the possibility that the effects of early caregiving environments on HPA axis functioning may change over time (e.g., Miller, Chen, & Zhou, 2007). That is, maltreated children tend to exhibit hypercortisolism, whereas adults who experienced child maltreatment show hypocortisolism, suggesting that dysregulated HPA axis systems may be down regulated over time, especially when the stressor persists (Essex et al., 2011). The long-term processes, whereby the effects of father involvement on HPA axis functioning unfolds across childhood through adulthood, definitely warrant further investigation.
It is interesting to note that the qualitative aspect of father involvement was not significantly predictive of son’s diurnal patterns of cortisol in adulthood. This is unexpected given the previous findings demonstrating the significance of qualitative dimensions of father involvement in predicting children’s outcomes (e.g., Goncy & van Dulmen, 2010; Mills-Koonce et al., 2011). Fabricius et al. (2012) have suggested a conceptual model in which parenting time leads to the quantity of father–child interaction, which in turn influences the quality of father–child relationships (i.e., emotional security). Thus, it is possible that, for the present sample with at-risk backgrounds, the quantity of father involvement is an important condition that shapes the quality of father involvement, resulting in the nonsignificance of the quality of father involvement in the model. It is also possible that childhood data in the present study were assessed in 1980’s—when there was limited recognition of the importance of father involvement, especially among families with at-risk backgrounds (Alwin, 1984)—rendering limited variability in the quality measure to have significant effects. Finally, our measurement of the quality of father involvement was based on observational data by assessors, whereas the quantity measurement was from fathers’ self-reports focusing on mainly enjoyable activities, which might be responsible for the relatively weaker associations between the quality measurement and other variables in the present study.
Findings of the present study should be interpreted within the context of several limitations. First, potential effects of relevant factors such as biological status of fathers were not included in the model in the interest of parsimony. Although previous research showed that effects of father involvement on children’s adjustment were significant regardless of whether the father was biological (e.g., Ibrahim et al., 2017), future research should examine the potential roles of such factor in the relationships between father involvement and offspring’s diurnal cortisol patterns. Second, although we examined the quantity and quality of father involvement, future research should examine differential effects of other dimensions of father involvement (e.g., engagement, accessibility, and responsibility; Lamb, 2000) across development to increase specificity in our understanding of father involvement. It should also be noted that our quantity measure mainly focused on positive activities; thus, it is possible that both quantity and quality components are not conceptually distinctive. Although both were not significantly intercorrelated in the present study, findings should be interpreted with caution. Additionally, there might be potential effects of maternal involvement as well as indirect father involvement in which fathers exert influence on the offspring’s development through positive relationships with mothers or mother–child relationships (Cabrera et al., 2018). Future research should examine whether maternal and paternal involvement contribute independently or jointly to the offspring’s HPA axis functioning. In particular, it would be important to examine whether maternal and paternal involvement would show additive effects or buffering effects on the offspring’s HPA axis functioning.
Third, the present sample included an at-risk group of residential fathers and sons by virtue of living in areas with low SES and higher delinquency rates in childhood; therefore, findings from this study may have limited generalizability. In addition, the sample size was relatively small, and the sample consisted of sons from predominantly European American backgrounds. Thus, testing the hypotheses for samples for daughters and samples with other ethic characteristics is called for. Additionally, parental warmth or proximity—qualitative and quantitative aspects of parenting in childhood—can buffer the long-term detrimental effects of childhood stressors such as poverty on emotional, behavioral, and psychological responses that, in turn, affect biological pathways and health in adulthood (Chen, Brody, & Miller, 2017). Future work should examine buffering roles of father involvement in the association between low SES and a range of adulthood outcomes.
Despite these limitations, the present study provided empirical evidence demonstrating long-term physiological and behavioral consequences of father involvement with sons in childhood, while controlling for the concurrent father–son relationships in adulthood, using a prospective multimethod, multi-informant longitudinal study that spanned almost 30 years. This is one of the first studies to establish the association between early socioemotional experiences with fathers and their sons’ diurnal cortisol patterns in adulthood that, in turn, are likely to have significant repercussions for subsequent health outcomes for the men. Furthermore, considering that many of these men have started to have their own family by late 30s, their dysregulated diurnal cortisol patterns can also have significant implications for their offspring’s adjustment (Kane & Garber, 2004). The study also contributes to the literature by focusing on fathers and sons with at-risk backgrounds, a subgroup that has been vastly understudied (McWayne et al., 2013). Overall, our finding highlights the potency of father involvement within the early caregiving environment and its long-term influence on their offspring’s neurobiological functioning. This suggests the importance of supporting fathers through prevention and intervention efforts if we are to effectively promote individuals’ healthy development throughout the life span.
Acknowledgments:
Funding for this work was supported by the National Institutes of Health (NIH) grant number R01 DA015485 from the National Institute of Drug Abuse (NIDA) awarded to Drs. Capaldi and Kerr. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or NIDA. NIH or NIDA had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
This work was also supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2020S1A5B5A17090447) awarded to Jieun Choi.
Footnotes
Conflict of interest: None declared.
Data Availability statement:
The data that support the findings of this study are openly available in ICPSR at https://www.icpsr.umich.edu/web/pages/ICPSR/index.html, except for the cortisol data and the observational data due to privacy or ethical restrictions.
References
- Adam EK, Quinn ME, Tavernier R, McQuillan MT, Dahlke KA, & Gilbert KE (2017). Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology, 83, 25–41. 10.1016/j.psyneuen.2017.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamsons K (2018). Quantity versus quality of nonresident father involvement: Deconstructing the argument that quantity doesn’t matter. Journal of Child Custody, 15(1), 26–34. 10.1080/15379418.2018.1437002 [DOI] [Google Scholar]
- Adamsons K, & Johnson SK (2013). An updated and expanded meta-analysis of nonresident fathering and child well-being. Journal of Family Psychology, 27(4), 589–599. 10.1037/a0033786. [DOI] [PubMed] [Google Scholar]
- Aho H, Koivisto AM, Paavilainen E, & Joronen K (2018). Parental involvement and adolescent smoking in vocational setting in Finland. Health Promotion International, 33(5), 846–857. 10.1093/heapro/dax027 [DOI] [PubMed] [Google Scholar]
- Ali MM, & Dean D Jr (2015). The influence of nonresident fathers on adolescent and young adult cigarette smoking. Families, Systems, & Health, 33(3), 314–323. 10.1037/fsh0000137 [DOI] [PubMed] [Google Scholar]
- Allen S & Daly K (2007). The effects of father involvement: An updated research summary of the evidence. Ontario: Centre for Families, Work & Well-Being, University of Guelph. [Google Scholar]
- Alwin DF (1984). Trends in parental socialization values: Detroit, 1958–1983. American Journal of Sociology, 90(2), 359–382. 10.1086/228083 [DOI] [Google Scholar]
- Anthenelli RM, & Maxwell RA (2002). Independent alcohol and tobacco effects on stress axis function. Alcoholism, Clinical and Experimental research, 26(12), 1932–1933. http://doi.org.ssl.access.yonsei.ac.kr:8080/10.1111/j.1530-0277.2002.tb02508.x [DOI] [PubMed] [Google Scholar]
- Badrick E, Kirschbaum C, & Kumari M (2007). The relationship between smoking status and cortisol secretion. The Journal of Clinical Endocrinology & Metabolism, 92(3), 819–824. 10.1210/jc.2006-2155 [DOI] [PubMed] [Google Scholar]
- Blair C, Granger DA, Willoughby M, Mills-Koonce R, Cox M, Greenberg MT, Kivlighan KT, & Fortunato CK (2011). Salivary cortisol mediates effects of poverty and parenting on executive functions in early childhood. Child Development, 82(6), 1970–1984. 10.1111/j.1467-8624.2011.01643.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte-Tinkew J, Moore KA, Capps RC, & Zaff J (2006). The influence of father involvement on youth risk behaviors among adolescents: A comparison of native-born and immigrant families. Social Science Research, 35(1), 181–209. 10.1016/j.ssresearch.2004.08.002 [DOI] [Google Scholar]
- Bruce C, & Fox GL (1999). Accounting for patterns of father involvement: Age of child, father-child coresidence, and father role salience. Sociological Inquiry, 69(3), 458–476. 10.1111/j.1475-682X.1999.tb00881.x [DOI] [Google Scholar]
- Bruce J, Fisher PA, Pears KC, & Levine S (2009). Morning cortisol levels in preschool-aged foster children: Differential effects of maltreatment type. Developmental Psychobiology, 51(1), 14–23. 10.1002/dev.20333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera NJ, Tamis-LeMonda CS, Bradley RH, Hofferth S, & Lamb ME (2000). Fatherhood in the twenty-first century. Child Development, 71(1), 127–136. 10.1111/1467-8624.00126 [DOI] [PubMed] [Google Scholar]
- Cabrera NJ, Volling BL, & Barr R (2018). Fathers are parents, too! Widening the lens on parenting for children’s development. Child Development Perspectives, 12(3), 152–157. 10.1111/cdep.12275 [DOI] [Google Scholar]
- Capaldi DM, Feingold A, Kim HK, Yoerger K, & Washburn IJ (2013). Heterogeneity in growth and desistance of alcohol use for men in their 20s: Prediction from early risk factors and association with treatment. Alcoholism: Clinical and Experimental Research, 37, E347–E355. 10.1111/j.1530-0277.2012.01876.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Brody GH, & Miller GE (2017). Childhood close family relationships and health. American Psychologist, 72(6), 555–566. 10.1037/amp0000067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookston JT, & Finlay AK (2006). Father involvement and adolescent adjustment: longitudinal findings from Add Health. Fathering: A Journal of Theory, Research & Practice about Men as Fathers, 4(2), 137–158. 10.3149/fth.0402.137 [DOI] [Google Scholar]
- Crockett LJ, Raffaelli M, & Shen YL (2006). Linking self-regulation and risk proneness to risky sexual behavior: Pathways through peer pressure and early substance use. Journal of Research on Adolescence, 16(4), 503–525. 10.1111/j.1532-7795.2006.00505.x [DOI] [Google Scholar]
- Dishion TJ, Gardner K, Patterson GR, Reid JB, Spyrou S, & Thibodeaux S (1983). The Family Process Code: A multidimensional system for observing family interaction. Available from the Oregon Social Learning Center, 160 E. 4th Ave., Eugene, OR 97401. [Google Scholar]
- Doane LD, Mineka S, Zinbarg RE, Craske M, Griffith JW, & Adam EK (2013). Are flatter diurnal cortisol rhythms associated with major depression and anxiety disorders in late adolescence? The role of life stress and daily negative emotion. Development and Psychopathology, 25(3), 629–642. 10.1017/S0954579413000060 [DOI] [PubMed] [Google Scholar]
- Enders CK, & Bandalos DF (2001). The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling: A Multidisciplinary Journal, 8(3), 430–457. 10.1207/S15328007SEM0803_5 [DOI] [Google Scholar]
- Essex MJ, Shirtcliff EA, Burk LR, Ruttle PL, Klein MH, Slattery MJ, Kalin NH, & Armstrong JM (2011). Influence of early life stress on later hypothalamic–pituitary–adrenal axis functioning and its covariation with mental health symptoms: A study of the allostatic process from childhood into adolescence. Development and Psychopathology, 23(4), 1039–1058. 10.1017/S0954579411000484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricius WV, Sokol KR, Diaz P, & Braver SL (2012). Parenting time, parent conflict, parent-child relationships, and children’s physical health. In Kuehnle K & Drozd L (Eds.), Parenting plan evaluations: Applied research for the family court (pp. 188–213). Oxford: Oxford University Press. 10.1093/med:psych/9780199754021.003.0007 [DOI] [Google Scholar]
- Finley GE, & Schwartz SJ (2007). Father involvement and long-term young adult outcomes: The differential contributions of divorce and gender. Family Court Review, 45(4), 573–587. 10.1111/j.1744-1617.2007.00172.x [DOI] [Google Scholar]
- Flouri E, & Buchanan A (2002). Father involvement in childhood and trouble with the police in adolescence: Findings from the 1958 British cohort. Journal of Interpersonal Violence, 17(6), 689–701. 10.1177/0886260502017006006 [DOI] [Google Scholar]
- Flouri E, & Buchanan A (2004). Early father’s and mother’s involvement and child’s later educational outcomes. British Journal of Educational Psychology, 74(2), 141–153. 10.1348/000709904773839806 [DOI] [PubMed] [Google Scholar]
- Fosco GM, Frank JL, Stormshak EA, & Dishion TJ (2013). Opening the “Black Box”: Family Check-Up intervention effects on self-regulation that prevents growth in problem behavior and substance use. Journal of School Psychology, 51(4), 455–468. 10.1016/j.jsp.2013.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncy EA, & van Dulmen MH (2010). Fathers do make a difference: Parental involvement and adolescent alcohol use. Fathering, 8(1), 93–108. 10.3149/fth.0801.93 [DOI] [Google Scholar]
- Graham AM, Kim HK, & Fisher PA (2012). Partner aggression in high-risk families from birth to age 3 years: Associations with harsh parenting and child maladjustment. Journal of Family Psychology, 26(1), 105–114. 10.1037/a0026722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, & Vazquez DM (2001). Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Development and Psychopathology, 13(3), 515–538. http://doi.org.ssl.access.yonsei.ac.kr:8080/10.1017/S0954579401003066 [DOI] [PubMed] [Google Scholar]
- Gunnar MR, & Vazquez DM (2006). Stress neurobiology and developmental psychopathology. In Cicchetti D & Cohen D (Eds.), Developmental psychopathology: developmental neuroscience (2nd ed., vol 2, pp. 533–577). New Jersey: Wiley. [Google Scholar]
- Halme N, Tarkka MT, Paavilainen E, Nummi T, & Åstedt-Kurki P (2010). The design and development of the father-child instrument (FCI) for assessing the characteristics of fathers’ availability and engagement with their preschool children. American Journal of Men’s Health, 4(2), 145–156. 10.1177/1557988309331825 [DOI] [PubMed] [Google Scholar]
- Ibrahim MH, Somers JA, Luecken LJ, Fabricius WV, & Cookston JT (2017). Father–adolescent engagement in shared activities: Effects on cortisol stress response in young adulthood. Journal of Family Psychology, 31(4), 485–494. 10.1037/fam0000259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeynes WH (2005). A meta-analysis of the relation of parental involvement to urban elementary school student academic achievement. Urban Education, 40(3), 237–269. 10.1177/0042085905274540 [DOI] [Google Scholar]
- Jeynes WH (2007). The relationship between parental involvement and urban secondary school student academic achievement: A meta-analysis. Urban Education, 42(1), 82–110. 10.1177/0042085906293818 [DOI] [Google Scholar]
- Jeynes WH (2015). A meta-analysis: The relationship between father involvement and student academic achievement. Urban Education, 50(4), 387–423. 10.1177/0042085914525789 [DOI] [Google Scholar]
- Jones J, & Mosher WD (2013). Fathers’ involvement with their children: United States, 2006–2010 (National Health Statistics Reports; no 71). National Center for Health Statistics. https://www.fatherly.com/wp-content/uploads/2015/08/nhsr071.pdf [PubMed] [Google Scholar]
- Jones KA, & Benda BB (2004). Alcohol use among adolescents with non-residential fathers: A study of assets and deficits. Alcoholism Treatment Quarterly, 22(1), 3–25. 10.1300/J020v22n01_0218607515 [DOI] [Google Scholar]
- Kalmakis KA, Meyer JS, Chiodo L, & Leung K (2015). Adverse childhood experiences and chronic hypothalamic–pituitary–adrenal activity. Stress, 18(4), 446–450. 10.3109/10253890.2015.1023791 [DOI] [PubMed] [Google Scholar]
- Kane P, & Garber J (2004). The relations among depression in fathers, children’s psychopathology, and father–child conflict: A meta-analysis. Clinical Psychology Review, 24(3), 339–360. 10.1016/j.cpr.2004.03.004 [DOI] [PubMed] [Google Scholar]
- Kim HK, Pears KC, Fisher PA, Connelly CD, & Landsverk JA (2010). Trajectories of maternal harsh parenting in the first 3 years of life. Child Abuse & Neglect, 34(12), 897–906. 10.1016/j.chiabu.2010.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HK, Tiberio SS, Capaldi DM, Shortt JW, Squires EC, & Snodgrass JJ (2015). Intimate partner violence and diurnal cortisol patterns in couples. Psychoneuroendocrinology, 51, 35–46. 10.1016/j.psyneuen.2014.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb ME (2000). The history of research on father involvement: An overview. Marriage & Family Review, 29(2–3), 23–42. 10.1300/J002v29n02_03 [DOI] [Google Scholar]
- Luecken LJ, Kraft A, & Hagan MJ (2009). Negative relationships in the family-of-origin predict attenuated cortisol in emerging adults. Hormones and Behavior, 55(3), 412–417. 10.1016/j.yhbeh.2008.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manetti L, Cavagnini F, Martino E, & Ambrogio A (2014). Effects of cocaine on the hypothalamic–pituitary–adrenal axis. Journal of Endocrinological Investigation, 37(8), 701–708. 10.1007/s40618-014-0091-8 [DOI] [PubMed] [Google Scholar]
- Matthews K, Schwartz J, Cohen S, & Seeman T (2006). Diurnal cortisol decline is related to coronary calcification: CARDIA study. Psychosomatic Medicine, 68(5), 657–661. 10.1097/01.psy.0000244071.42939.0e [DOI] [PubMed] [Google Scholar]
- McEwen BS (2007). Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiological Reviews, 87(3), 873–904. 10.1152/physrev.00041.2006 [DOI] [PubMed] [Google Scholar]
- McWayne C, Downer JT, Campos R, & Harris RD (2013). Father involvement during early childhood and its association with children’s early learning: A meta-analysis. Early Education & Development, 24(6), 898–922. 10.1080/10409289.2013.746932 [DOI] [Google Scholar]
- Miller DP, Thomas MM, Waller MR, Nepomnyaschy L, & Emory AD (2020). Father involvement and socioeconomic disparities in child academic outcomes. Journal of Marriage and Family, 82(2), 515–533. 10.1111/jomf.12666 [DOI] [Google Scholar]
- Miller GE, Chen E, & Zhou ES (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin, 133(1), 25–45. 10.1037/0033-2909.133.1.25 [DOI] [PubMed] [Google Scholar]
- Mills-Koonce WR, Garrett-Peters P, Barnett M, Granger DA, Blair C, & Cox MJ (2011). Father contributions to cortisol responses in infancy and toddlerhood. Developmental Psychology, 47(2), 388–395. 10.1037/a0021066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlhan M, Höcker A, Miller R, Trautmann S, Wiedemann K, Lotzin A, Barnow S & Schäfer I (2020). HPA axis stress reactivity and hair cortisol concentrations in recently detoxified alcoholics and healthy controls with and without childhood maltreatment. Addiction Biology, 25(1), e12681. 10.1111/adb.12681 [DOI] [PubMed] [Google Scholar]
- Murray L, Halligan SL, Goodyer I, & Herbert J (2010). Disturbances in early parenting of depressed mothers and cortisol secretion in offspring: A preliminary study. Journal of Affective Disorders, 122(3), 218–223. 10.1016/j.jad.2009.06.034 [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (1998–2019). Mplus user’s guide. 8th ed. Los Angeles: [Google Scholar]; Muthén & Muthén. [Google Scholar]
- Nakagawa S, & Cuthill IC (2007). Effect size, confidence interval and statistical significance: a practical guide for biologists. Biological Reviews, 82(4), 591–605. 10.1111/j.1469-185X.2007.00027.x [DOI] [PubMed] [Google Scholar]
- Nettle D (2008). Why do some dads get more involved than others? Evidence from a large British cohort. Evolution and Human Behavior, 29(6), 416–423. 10.1016/j.evolhumbehav.2008.06.002 [DOI] [Google Scholar]
- OSLC. (1984). Family interaction task FPC coder ratings. Available from the Oregon Social Learning Center, 160 E. 4th Ave., Eugene, OR 97401. [Google Scholar]
- Parke RD (2000). Father involvement: A developmental psychological perspective. Marriage & Family Review, 29(2–3), 43–58. 10.1300/J002v29n02_04 [DOI] [Google Scholar]
- Patterson GR (1982). Family activities list. Available from the Oregon Social Learning Center, 160 E. 4th Ave., Eugene, OR 97401. [Google Scholar]
- Planalp EM, & Braungart-Rieker JM (2016). Determinants of father involvement with young children: Evidence from the early childhood longitudinal study–birth cohort. Journal of Family Psychology, 30(1), 135–146. 10.1037/fam0000156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleck JH (2007). Why could father involvement benefit children? Theoretical perspectives. Applied Development Science, 11(4), 196–202. 10.1080/10888690701762068 [DOI] [Google Scholar]
- Rodrigues M, Sokolovic N, Madigan S, Luo Y, Silva V, Misra S, & Jenkins J (2021). Paternal sensitivity and children’s cognitive and socioemotional outcomes: A meta-analytic review. Child Development, 92(2), 554–577. 10.1111/cdev.13545 [DOI] [PubMed] [Google Scholar]
- Roisman GI, Susman E, Barnett-Walker K, Booth-LaForce C, Owen MT, Belsky J, Bradley RH, Houts R, & Steinberg L (2009). Early family and child-care antecedents of awakening cortisol levels in adolescence. Child Development, 80(3), 907–920. 10.1111/j.1467-8624.2009.01305.x [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, & Munck AU (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews, 21(1), 55–89. 10.1210/edrv.21.1.0389 [DOI] [PubMed] [Google Scholar]
- Sarkadi A, Kristiansson R, Oberklaid F, & Bremberg S (2008). Fathers’ involvement and children’s developmental outcomes: A systematic review of longitudinal studies. Acta Paediatrica, 97(2), 153–158. https://10.1111/j.1651-2227.2007.00572.x [DOI] [PubMed] [Google Scholar]
- Saxbe DE, Repetti RL, & Nishina A (2008). Marital satisfaction, recovery from work, and diurnal cortisol among men and women. Health Psychology, 27(1), 15–25. 10.1037/0278-6133.27.1.15 [DOI] [PubMed] [Google Scholar]
- Steptoe A, & Ussher M (2006). Smoking, cortisol and nicotine. International Journal of Psychophysiology, 59(3), 228–235. 10.1016/j.ijpsycho.2005.10.011 [DOI] [PubMed] [Google Scholar]
- Washburn IJ, Capaldi DM, Kim HK, & Feingold A (2014). Alcohol and marijuana use in early adulthood for at-risk men: Time-varying associations with peer and partner substance use. Drug and Alcohol Dependence, 140, 112–117. 10.1016/j.drugalcdep.2014.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in ICPSR at https://www.icpsr.umich.edu/web/pages/ICPSR/index.html, except for the cortisol data and the observational data due to privacy or ethical restrictions.