Abstract
Objective
Although the effect of coronary revascularization on clinical outcomes before and after transcatheter valve implantation (TAVI) is debatable, there is currently insufficient data to determine the most appropriate revascularization strategy. In this study, we present our single-center experience of percutaneous coronary intervention (PCI) and its effect on clinical outcomes and mortality in patients undergoing TAVI.
Methods
We performed a retrospective analysis of 526 consecutive patients at our center, and 127 patients with obstructive coronary artery disease were included in the study. Patients were divided into two groups: the revascularization group (group 1) and the non-revascularization group (group 2). Procedural complications and long-term all-cause mortality rates were compared between the two groups.
Results
Of the 526 patients, group 1 comprised 65 patients (12.3%) who underwent PCI, and group 2 comprised 62 patients (11.7%) who did not undergo revascularization. According to Valve Academic Research Consortium 2 criteria, post-procedural complications, including pericardial effusion, stroke, major vascular complications, major bleeding, and emerging arrhythmias, were similar between the groups. A Kaplan–Meier survival curve analysis showed no significant difference between the revascularization and non-revascularization groups (Overall: 40.0±2.8 month; 95% CI 34.4–45.6 month, p=0.959). After adjustment for basal SYNTAX score, chronic kidney disease stage, previous myocardial infarction, and baseline troponin levels, the long-term survival of group 1 was significantly longer when compared with group 2 (p=0.036). In 75.4% of cases, PCI was performed within 11.0±14.7 days before or after TAVI as a staged procedure. In 13.8% of cases, PCI was performed simultaneously with TAVI. While there was no significant difference in in-hospital, 6-month, and 1-year mortality rates between the simultaneous and staged PCI groups, there was a significant difference in 30-day mortality (11.1% vs. 0%, respectively; p=0.016).
Conclusion
Peri-procedural and long-term safety outcomes and mortality rates are not significantly different between revascularized and non-revascularized patients, and neither staged nor simultaneous PCI have adverse outcomes in patients undergoing TAVI.
Keywords: TAVI, aortic stenosis, coronary artery disease, percutaneous coronary intervention, structural heart disease
Introduction
The risk factors for aortic stenosis (AS) and atherosclerosis overlap, and these two conditions are prevalent (1). Before transcatheter aortic valve implantation (TAVI), all patients undergo routine invasive angiography to examine coronary anatomy and detect coronary artery disease (CAD). While the incidence of CAD reaches 50% (2) in the early stages of TAVI, this rate decreases with implementation of TAVI in intermediate–low-risk patients over time (3, 4). Although the effect of CAD on clinical outcomes before and after TAVI is debatable, there is currently insufficient data to determine the most appropriate revascularization strategy. European Society of Cardiology and American Heart Association guidelines recommend complete revascularization in patients with severe symptomatic AS who are scheduled for surgical valve replacement. However, generalizing this recommendation to TAVI raises questions about timing and the optimal revascularization strategy (5).
The first question is whether to implement revascularization in patients with severe symptomatic AS. Existing data favors revascularization in patients undergoing TAVI with an intermediate-to-high SYNTAX score. It is thought that survival in this patient group is reduced with possible comorbidities (6). However, despite a number of meta-analyses, it is still unclear that the management of CAD in TAVI patients as there is insufficient large-scale randomized data.
Once the decision has been made to perform revascularization, the second important question is the timing of revascularization. Because the structures of transcatheter heart valves (THVs) differ, coronary intervention after TAVI can be problematic. The decision to perform revascularization and the strategy adopted for TAVI are so complex; thus, this patient group is heterogeneous. Furthermore, the annulo-aortic localization of THVs and their anatomical relationship with coronary arteries also vary.
In this study, we present our single-center experience of CAD and the effect of percutaneous coronary intervention (PCI) on clinical outcomes and mortality in patients undergoing TAVI.
Methods
Study design, patient population, and pre-procedural planning
Five hundred twenty-six high- and intermediate-risk inoperable patients with severe symptomatic AS (aortic valve area <1.0 cm2, mean gradient >40 mm Hg, maximum jet velocity >4.0 m/s) who underwent TAVI at our tertiary care center between July 2011 and December 2019 were retrospectively analyzed, and 127 patients with obstructive CAD were included in the study. The SAPIEN XT valve (Edwards Lifesciences, Irvine, CA, USA), SAPIEN 3 THV System (Edwards Lifesciences), or the Lotus Edge™ Aortic Valve System (Boston Scientific, Marlborough, Massachusetts, USA) was used. The transfemoral route was used predominantly in most patients. The cardiology team discussed all patients and decided on treatment options, and patients who did not undergo invasive angiography were excluded from the study. We retrospectively collected baseline characteristics, laboratory results, echocardiograms, coronary angiograms, cardiac catheterization results, and outcome data. The distance between the coronary take-offs and the annulus, aortic annulus, and peripheral arteries was evaluated using a multimodal approach with multislice computed tomography and echocardiography. Post-procedural follow-up was performed after 30 days, 6 months, 1 year, and annually thereafter. Informed consent was obtained from all patients before the procedure, and our hospital Ethics Committee approved the study.
Procedure
CAD was defined as presence of one or more lesions >70% in epicardial coronary arteries, vessels with a diameter of >1.5 mm [>50% for left main coronary artery (LMCA)] (7). Patients were divided into two groups: the obstructive CAD and revascularization group (group 1) and the obstructive CAD without revascularization group (group 2). The decision to perform PCI was made according to the presence and severity of angina, lesion characteristics, comorbidities, and the profit–loss ratio of PCI. Patients with non-obstructive CAD (n=230; 43.7%) and normal coronary arteries (n=169; 32.1%) were excluded from the study. The decision to perform PCI was made after an individualized assessment of each patient. The majority of patients underwent staged PCI, and a small number of patients underwent simultaneous PCI. An experienced interventional cardiologist decided on the timing of PCI based on the symptoms of the patient, whether the lesion was localized to major epicardial arteries, basal renal function, and technical complexity. Simultaneous PCI was defined as concurrent PCI and TAVI. Since balloon expandable and mechanically expandable THVs were implanted, we did not hesitate to perform PCI after TAVI.
Baseline SYNTAX score (bSS) and residual SYNTAX score (rSS) were evaluated by two experienced interventional cardiologists using an online calculator (www.syntaxscore.com, version 2.1). Both cardiologists were blinded to patients’ data. To determine the proportion of the CAD burden treated by PCI, we calculated the SYNTAX Revascularization Index (SRI) according to the following formula: SRI=(1–[rSS÷bSS])×100. If there was a discrepancy between the two interventionists, a third decision was accepted as the final decision.
Dual antiplatelet therapy consisting of 100 mg aspirin and 75 mg clopidogrel was sustained 6 months after bare metal stent (BMS) and 12 months after drug-eluting stent (DES) implantation. In patients with atrial fibrillation, considering the risk of bleeding, all patients were administered dual antiplatelet therapy and oral anticoagulants for 4 weeks, followed by 1 month (BMS) or 12 months (DES) of dual antiplatelet therapy according to stent type. Mortality, stroke, bleeding, vascular complications, device success, renal failure (Acute Kidney Injury Network), and adverse events were defined due to the consensus document of the Valve Academic Research Consortium (VARC)-2 (8). Unsuccessful PCI was defined as a final diameter stenosis of >30% or a post-dilatation thrombolysis in myocardial infarction score of ≤2.
Statistical analysis
All statistical analyses were performed using SPSS version 22.0 for Windows (SPSS Inc., Chicago, IL, USA). Categorical variables are presented as frequencies and percentages. Continuous variables are presented as mean ± standard deviation or median (interquartile range) where applicable. Categorical variables were analyzed using the Chi-squared test and expressed as percentages. For continuous variables, an independent-samples t-test (for normally distributed data) or a Mann–Whitney U test (for non-normally distributed data), as appropriate, was performed to compared the two groups. A two-tailed p value of <0.05 was considered statistically significant. The Kaplan–Meier method and the log-rank test were performed to estimate the cumulative incidence of mortality. The Cox proportional hazards survival model with covariate adjustments was used to pre-specify covariates in the multiple model, including bSS, chronic kidney disease stage, previous myocardial infarction, and baseline troponin levels.
Results
Table 1 describes baseline clinical characteristics, surgical risk scores, and laboratory values. In this retrospective study, 526 patients were analyzed retrospectively (24.1% of patients had obstructive CAD). Sixty-five patients (12.3%) with obstructive CAD who underwent PCI were in group 1 and 62 patients (11.7%) with obstructive CAD who did not undergo PCI were in group 2. Groups 1 and 2 were well matched with similar perioperative risk scores. A greater number of patients had undergone previous PCI in group 1 compared with group 2 (69.2% vs. 17.7%, respectively; p<0.001) and baseline renal function. There was a statistically significant difference between the two groups when functional capacities were compared (p=0.035). Except for baseline troponin levels, there were no statistically significant differences in laboratory parameters between the two groups. Baseline echocardiographic parameters were also similar between the two groups.
Table 1.
Baseline clinical features
| Parameters | Revascularization n=65 |
No revascularization n=62 |
P value |
|---|---|---|---|
| Age (years) | 78.4±7.4 | 79.5±7.5 | 0.416 |
| Female n (%) | 32 (49.2) | 39 (62.9) | 0.121 |
| BMI (kg/m2) | 27.0±4.6 | 29.9±10.3 | 0.076 |
| NYHA n (%) | |||
| 2 | 21 (32.3) | 13 (21.0) | 0.035 |
| 3 | 39 | (60.0) | 33 (53.2) |
| 4 | 5 (7.7) | 14 (22.6) | |
| Pulmonary edema | 0 (0.0) | 2 (3.2) | |
| DM n (%) | 18 (27.7) | 25 (40.3) | 0.133 |
| HT n (%) | 58 (89.2) | 56 (90.3) | 0.839 |
| HL n (%) | 44 (67.6) | 41 (66.1) | 0.852 |
| Previous PCI n (%) | 45 (69.2) | 11 (17.7) | <0.001 |
| Previous CABG n (%) | 14 (21.5) | 9 (14.5) | 0.304 |
| Previous MI n (%) | 13 (20.0) | 12 (19.4) | 0.927 |
| Moderate to severe COPD n (%) | 23 (35.4) | 25 (40.3) | 0.848 |
| AF n (%) | 17 (26.2) | 17 (27.4) | 0.872 |
| Stroke n (%) | 6 (9.2) | 5 (8.1) | 0.815 |
| STS score n (%) | 6.2±3.0 | 6.4±3.0 | 0.785 |
| EuroSCORE II (%) median (IQR) | 7.4 (4.7–12.2) | 8.6 (5.2–13.2) | 0.681 |
| logisticEUROSCORE (%) median (IQR) | 15.4 (9.0–36.0) | 26.3 (12.6–41.2) | 0.465 |
| Serum Glucose | 125.2±42.3 | 133.6±51.1 | 0.314 |
| Total cholesterol | 171.1±39.4 | 167.5±44.1 | 0.642 |
| Triglyceride | 117.3±50.2 | 115.8±50.6 | 0.876 |
| LDL | 105.4±31.4 | 101.0±39.2 | 0.491 |
| HDL | 43.0±12.3 | 43.3±12.2 | 0.860 |
| Creatinine mg/dL | 0.9±0.3 | 1.0±0.4 | 0.542 |
| Hemoglobin mg/dL | 11.6±1.8 | 11.2±1.6 | 0.203 |
| Platelets 109/L | 239.3±84.5 | 241.8±79.4 | 0.867 |
| Troponin (pg/mL) median (IQR) | 33.9 (21.5–76.8) | 85.1 (26.7–203.7) | 0.019 |
| CK-MB (ng/mL) median (IQR) | 2.6 (1.5–4.6) | 2.5 (1.7–4.0) | 0.330 |
| LVEF (%) median (IQR) | 55.9 (45.0–63.5) | 55.0 (40.0–65.0) | 0.571 |
| LA (cm) | 4.6±0.6 | 4.7±0.6 | 0.249 |
| Aortic velocity (cm/s) | 4.4±0.5 | 4.3±0.7 | 0.788 |
| Aortic max gradient (mm Hg) | 78.9±17.6 | 79.4±24.9 | 0.890 |
| Aortic mean gradient (mm Hg) | 49.4±13.0 | 50.2±16.1 | 0.751 |
| AVA (cm2) | 0.69±0.15 | 0.68±0.16 | 0.745 |
| Aortic Annulus (cm) | 2.15±0.2 | 2.14±0.1 | 0.917 |
| sPAP (mm Hg) | 33.8±11.2 | 34.4±14.1 | 0.647 |
| Aortic regurgitation-moderate to severe n (%) | 1 (1.5) | 4 (6.4) | 0.132 |
| Mitral regurgitation-moderate to severe n (%) | 7 (10.7) | 13 (21.0) | 0.201 |
BMI - body mass index; NYHA - New York Heart Association; DM - diabetes mellitus; HT - hypertension; PCI - percutaneous coronary intervention; CABG - coronary artery bypass grafting; MI - myocardial infarction; COPD - chronic obstructive pulmonary disease; AF - atrial fibrillation; STS - Society of Thoracic Surgeons; LVEF - left ventricular ejection fraction; LA - left atrium; AVA - aortic valve area; sPAP - systolic pulmonary artery pressure; MSCT - multislice computed tomography
PCI-related features are shown in Table 2. The mean bSS was 10.1±6.6 in group 1 and 9.4±4.9 in group 2. The rSS was 0.7±3.1 in group 1. According to the SRI, 87.6% of patients were completely revascularized. A total of 60% of patients in group 1 had single-vessel disease, 32.3% had double-vessel disease, and 7.7% had triple-vessel disease; however, no significant differences were observed in the number of affected vessels between groups 1 and 2. In 75.4% of cases, PCI was performed within 11.0±14.7 days before or after TAVI as a staged procedure. In 13.8% of cases, PCI was performed as a simultaneous procedure on the day of TAVI immediately before THV implantation. The revascularized target vessel was the LMCA in 4.6% of cases and the left anterior descending artery in 24.6% of cases, and a DES was implanted in 75% of patients. One of the patients in group 1 developed contrast-induced nephropathy; this patient underwent pre-TAVI PCI. In 6.1% of patients, PCI failed due to anatomical complexity.
Table 2.
Percutaneous coronary intervention procedural features
| Parameters | Revascularization n=65 |
No revascularization n=62 |
P value |
|---|---|---|---|
| Basal SS median (IQR) | 8.0 (5.0–14.0) | 8.0 (6.0–13.0) | 0.563 |
| Residual SS | 0.7±3.1 | NA | NA |
| SRI n (%) | |||
| Complete | 56 (87.6) | NA | NA |
| Incomplete | 4 (12.4) | ||
| Syntax II | 39.3±8.9 | 36.6±11.6 | 0.170 |
| CAD n (%) | |||
| 1 Vessel disease | 39 (60.0) | 30 (50.0) | 0.317 |
| 2 Vessel disease | 21 (32.3) | 27 (45.0) | |
| 3 Vessel disease | 5 (7.7) | 3 (5.0) | |
| Chronic Kidney Disease n (%) | |||
| Stage 1 | 10 (15.4) | 5 (8.2) | 0.031 |
| Stage 2 | 29 (44.6) | 32 (52.5) | |
| Stage 3a | 20 (30.8) | 10 (16.4) | |
| Stage 3b | 3 (4.6) | 12 (19.7) | |
| Stage 4 | 3 (4.6) | 2 (3.3) | |
| Timing of PCI n (%) | |||
| Pre-TAVI | 49 (75.4) | NA | NA |
| Simultaneous TAVI | 9 (13.8) | ||
| Post-TAVI | 7 (10.8) | ||
| Target vessel n (%) | |||
| LMCA | 3 (4.6) | NA | NA |
| LAD | 16 (24.6) | ||
| LCx | 12 (18.5) | ||
| OM | 1 (1.5) | ||
| RCA | 31 (47.7) | ||
| Greft | 2 (3.1) | ||
| Additional Target vessel n (%) | |||
| LAD | 4 (6.2) | NA | NA |
| LCx | 6 (9.2) | ||
| OM | 3 (4.6) | ||
| RCA | 3 (4.6) | ||
| D1 | 2 (3.1) | ||
| Total stent length (mm) | 29.2±17.0 | NA | NA |
| Number of stents | 1.45±0.7 | NA | NA |
| Drug-Eluting Stent n (%) | 48 (75.0) | NA | NA |
| PCI associated complication n (%) | |||
| CIN | 1 (1.5) | NA | NA |
| Vascular | 2 (3.1) | ||
| Unsuccessful | 4 (6.1) | ||
SS - SYNTAX score; SRI - SYNTAX revascularization index; CAD - coronary artery disease; LAD - left anterior descending; LCx - left circumflex; RCA - right coronary artery; D1 - diagonal 1 artery; CIN - contrast-induced nephropathy
Procedural characteristics and clinical outcomes are shown in Table 3. There were no significant differences between the vascular access site, device size, type of THV, post-dilatation, or device success. Pre-dilatation was more frequently performed in group 1 compared with group 2 (p=0.024). The device success rate was 100% in the revascularized group. According to VARC-2 criteria, post-procedural complications, including pericardial effusion, stroke, major vascular complications, major bleeding, and emerging arrhythmias, were similar.
Table 3.
Procedural characteristics and follow-up outcomes after TAVI
| Parameters | Revascularization n=65 |
No revascularization n=62 |
P value |
|---|---|---|---|
| Access site n (%) | |||
| - Trans-axillary | 2 (3.1) | 3 (4.9) | 0.547 |
| - Cut-down | 2 (3.1) | 6 (9.8) | 0.072 |
| Valve size mm n (%) | |||
| 23 | 27 (41.5) | 27 (43.5) | 0.590 |
| 25 | 1 (1.5) | 1 (1.6) | |
| 26 | 24 (36.9) | 26 (41.9) | |
| 27 | - | 1 (1.6) | |
| 29 | 13 (20.0) | 7 (11.3) | |
| Edwards Sapien XT n (%) | 58 (89.2) | 53 (83.9) | 0.375 |
| Sapien 3 n (%) | 4 (6.2) | 4 (6.5) | 0.945 |
| Lotus n (%) | 3 (4.6) | 5 (8.1) | 0.424 |
| Pre-dilatation n (%) | 54 (83.1) | 40 (65.6) | 0.024 |
| Post-dilatation n (%) | 2 (3.1) | 2 (3.3) | 0.949 |
| Device success n (%) | 65 (100.0) | 60 (96.8) | 0.144 |
| Pace maker (%) | 1 (1.5) | 4 (6.5) | 0.155 |
| Stroke | - | - | - |
| Pericardial effusion | - | 2 (3.2) | 0.144 |
| Emerging AF (%) | 1 (1.5) | - | 0.356 |
| PostTAVI CKD n (%) | |||
| Stage 1 | 13 (21.3) | 10 (16.7) | 0.334 |
| Stage 2 | 30 (49.2) | 28 (46.7) | |
| Stage 3a | 13 (21.3) | 13 (21.7) | |
| Stage 3b | 3 (4.9) | 8 (13.3) | |
| Stage 4 | 2 (3.3) | - | |
| Stage 5 | - | 1 (1.7) | |
| Acute renal failure n (%) | - | - | - |
| Major bleeding n (%) | 1 (1.5) | - | 0.509 |
| Major vascular complication n (%) | 4 (6.1) | 4 (6.4) | 0.934 |
| Discharge time (day) | 4.4±2.2 | 4.8±2.4 | 0.316 |
| In-hospital mortality n (%) | 1 (1.5) | 3 (4.8) | 0.287 |
| 30-Day mortality n (%) | 1 (1.6) | 4 (7.4) | 0.125 |
| 6th Month mortality n (%) | - | 1 (2.3) | 0.245 |
| 1st Year mortality n (%) | 7 (12.3) | 3 (7.0) | 0.381 |
| Total Mortality n (%) | 21 (32.3) | 19 (30.6) | 0.840 |
| 30-Day NYHA n (%) | |||
| 1 | 19 (41.3) | 10 (30.3) | 0.333 |
| 2 | 25 (54.3) | 19 (57.6) | |
| 3 | 2 (4.3) | 4 (12.1) | |
| 6th Month NYHA n (%) | |||
| 1 | 16 (72.7) | 4 (44.4) | 0.189 |
| 2 | 5 (22.7) | 5 (55.6) | |
| 3 | 1 (4.5) | 0 (0.0) | |
| 1st Year NYHA n (%) | |||
| 1 | 13 (92.9) | 3 (60.0) | 0.084 |
| 2 | 1 (7.1) | 2 (40.0) | |
| 3 | 0 (0.0) | 0 (0.0) | |
| Mean follow-up time (month) | 18.1±14.9 | 15.6±16.9 | 0.371 |
| PostTAVI LVEF (%) | 53.7±12.0 | 51.4±13.7 | 0.330 |
| PostTAVI mean gradient (mm Hg) | 9.7±2.6 | 9.5±3.2 | 0.794 |
| PostTAVI sPAP (mm Hg) | 33.8±11.2 | 34.4±14.1 | 0.803 |
| PostTAVI PVL n (%) | |||
| Mild | 5 (7.8) | 11 (19.3) | 0.177 |
| Moderate | - | - | |
| 30-Day LVEF (%) | 54.7±10.8 | 55.5±12.3 | 0.768 |
| 30-Day mean gradient (mm Hg) | 10.0±2.8 | 10.1±3.1 | 0.852 |
| 30-Day sPAP (mm Hg) | 36.1±13.0 | 33.2±11.5 | 0.312 |
| 30-Day PVL n (%) | |||
| Mild | 11 (23.9) | 3 (9.4) | 0.238 |
| Moderate | - | - | |
| 1st year LVEF (%) | 57.0±7.8 | 58.7±12.1 | 0.684 |
| 1st year mean gradient (mm Hg) | 9.7±2.8 | 11.0±3.7 | 0.368 |
| 1st year sPAP (mm Hg) | 32.1±11.6 | 37.5±16.9 | 0.375 |
NYHA - New York Heart Association; AF - atrial fibrillation; TAVI - transcatheter aortic valve implantation; PVL - paravalvular leak; MR - mitral regurgitation; LVEF - left ventricular ejection fraction; sPAP - systolic pulmonary artery pressure
At a median follow-up of 15.2±14.9 months, there was no significant difference in in-hospital, 30-day, 6-month, or 12-month mortality in patients who underwent PCI and those who did not. When the groups were compared, although there was a trend toward higher mortality after 6 months in group 2, it was not statistically significant (p=0.245). In contrast, the rates of in-hospital, 6-month, and 1-year mortality were similar between the two groups. There was no difference in functional capacity during the follow-up period. Excellent performance of the THV was observed with a similar final mean gradient in all patients. Table 3 shows follow-up echocardiographic parameters; however, there was no difference between the follow-up echocardiographic parameters with between the two strategies.
A Kaplan–Meier survival curve of patients with revascularization versus patients without revascularization is shown in Figure 1. Overall survival probability was not significantly different between the two groups (Overall: 40.0±2.8 month; 95% CI 34.4–45.6 month, p=0.959; Revascularization: 37.9±4.0 month; 95% CI 30.1–45.8 month; No Revascularization: 42.2±3.7 month; 95% CI 34.8–49.7 month). After adjustment for bSS, chronic kidney disease stage, previous myocardial infarction, and baseline troponin levels, long-term survival in group 1 was longer when compared with group 2 (p=0.036; Fig. 2).
Figure 1.

Kaplan–Meier survival curve analysis in patients with and without revascularization. Overall survival probability was not significantly different between groups (overall: 40.0±2.8 months, 95% CI 34.4–45.6 months, p=0.959; revascularization: 37.9±4.0 months, 95% CI 30.1–45.8 months; no revascularization: 42.2±3.7 months, 95% CI 34.8–49.7 months)
Figure 2.
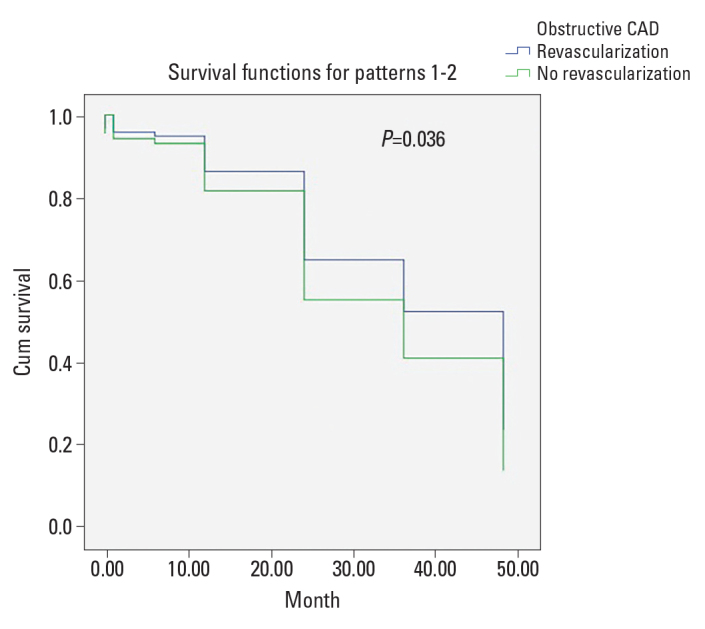
Cox proportional hazards model adjusted for bSS, previous myocardial infarction, chronic kidney disease stage, and troponin level in patients with and without revascularization. Overall survival probability was significantly different between groups (p=0.036, hazard ratio=0.728, 95% CI; 0.251–2.112)
Table 4 demonstrates the characteristics and outcomes of patients undergoing simultaneous and staged PCI. When basal characteristics were compared, the previous PCI rate was higher in the staged PCI group despite the basal troponin level being higher in the simultaneous PCI group. In the simultaneous PCI group, one patient had a permanent pacemaker (p=0.014), but there was no statistically significant difference between the two groups in terms of other peri-procedural complications according to VARC-2. There were no statistically significant differences between the two groups in terms of in-hospital, 6-month, and 1-year mortality rates. Moreover, in the 30-day follow-up, one patient died in the simultaneous PCI group, but there were no deaths in the staged group (11.1% vs. 0%, respectively; p=0.016).
Table 4.
Timing of percutaneous coronary intervention
| Parameters | Staged PCI group n=56 |
Simultaneous PCI group n=9 |
P value |
|---|---|---|---|
| Age (years) | 78.3±7.5 | 79.1±7.0 | 0.614 |
| Female gender (%) | 50.0 | 44.4 | 0.757 |
| BMI (kg/m2) | 27.3±4.8 | 25.2±3.5 | 0.314 |
| NYHA (%) | |||
| 2 | 28.6 | 55.6 | 0.226 |
| 3 | 62.5 | 44.4 | |
| 4 | 8.9 | - | |
| 30-Day NYHA (%) | |||
| 1 | 39.5 | 66.7 | 0.638 |
| 2 | 55.8 | 33.3 | |
| 3 | 4.7 | - | |
| 6th Month NYHA (%) | |||
| 1 | 70.0 | 80.0 | 0.662 |
| 2 | 25.0 | 20.0 | |
| 3 | 5.0 | - | |
| 1st Year NYHA (%) | |||
| 1 | 92.3 | 90.0 | 0.773 |
| 2 | 7.7 | 10.0 | |
| 3 | - | - | |
| DM (%) | 26.8 | 33.3 | 0.684 |
| HT (%) | 87.5 | 100 | 0.262 |
| Previous PCI (%) | 78.6 | 11.1 | <0.001 |
| Previous CABG (%) | 23.2 | 11.1 | 0.412 |
| Previous MI (%) | 23.2 | - | 0.106 |
| Moderate to severe COPD (%) | 30.7 | 33.3 | 0.942 |
| CAD | |||
| 1 Vessel disease | 60.7 | 55.6 | 0.906 |
| 2 Vessel disease | 32.1 | 33.3 | |
| 3 Vessel disease | 7.1 | 11.1 | |
| Stroke (%) | 8.9 | 11.1 | 0.834 |
| Basal SS | 10.0±6.9 | 10.4±4.6 | 0.482 |
| Residual SS | 0.8±3.3 | - | 0.407 |
| SRI (%) | |||
| Complete | 92.3 | 100.0 | 0.417 |
| Incomplete | 7.7 | - | |
| Syntax II | 39.7±8.9 | 36.8±9.1 | 0.604 |
| LVEF (%) | 50.5±14.3 | 51.8±7.9 | |
| Aortic mean gradient (mm Hg) | 50.9±13.0 | 56.6±17.8 | |
| AVA (cm2) | 0.6±0.1 | 0.6±0.2 | 0.6±0.1 |
| Target vessel | |||
| LMCA | 5.4 | - | 0.706 |
| LAD | 21.4 | 44.4 | |
| LCx | 19.6 | 11.1 | |
| OM | 1.8 | - | |
| RCA | 48.2 | 44.4 | |
| Greft | 3.6 | - | |
| Additional Target vessel | |||
| LAD | 5.4 | 11.1 | 0.812 |
| LCx | 8.9 | 11.1 | |
| OM | 5.4 | - | |
| RCA | 3.6 | 11.1 | |
| D1 | 3.6 | - | |
| Total stent length (mm) | 28.8±16.6 | 31.7±20.0 | 0.624 |
| Number of stents | 1.46±0.7 | 1.44±0.8 | 0.636 |
| Drug-Eluting stent (%) | 72.7 | 88.9 | 0.550 |
| Creatinine mg/dL | 1.0±0.3 | 0.8±0.2 | 0.171 |
| PostTAVI creatinine mg/dL | 0.9±0.2 | 0.9±0.3 | 0.486 |
| Troponin | 68.8±107.7 | 111.0±74.3 | 0.049 |
| CK-MB | 3.0±2.4 | 5.3±3.8 | 0.060 |
| PostTAVI troponin | 216.2±235.4 | 196.3±110.3 | 0.487 |
| PostTAVI CK-MB | 7.1±6.6 | 8.6±7.4 | 0.407 |
| 2nd PostTAVI troponin | 203.0±195.4 | 229.9±273.9 | 0.987 |
| 2nd PostTAVI CK-MB | 4.5±2.7 | 2.3±1.5 | 0.186 |
| Pace maker (%) | - | 11.1 | 0.014 |
| Acute renal failure (%) | 1.7 | - | 0.862 |
| Major bleeding (%) | - | - | - |
| Major vascular complication (%) | 8.4 | - | 0.925 |
| Stroke | - | - | - |
| Pericardial effusion | - | - | - |
| Emerging AF (%) | 1.7 | - | 0.089 |
| In-hospital mortality (%) | 1.7 | - | 0.681 |
| 30-Day mortality (%) | - | 11.1 | 0.016 |
| 6th Month mortality (%) | - | - | - |
| 1st Year mortality (%) | 10.0 | 25.0 | 0.227 |
BMI - body mass index; NYHA - New York Heart Association; DM - diabetes mellitus; HT - hypertension; PCI - percutaneous coronary intervention; CABG - coronary artery bypass grafting; MI - myocardial infarction; COPD - chronic obstructive pulmonary disease; AF - atrial fibrillation; STS - Society of Thoracic Surgeons, SS - SYNTAX score; SRI - SYNTAX revascularization index; CAD - coronary artery disease; LAD - left anterior descending; LCx - left circumflex; RCA - right coronary artery; D1 - diagonal 1 artery; CIN - contrast-induced nephropathy
Discussion
This study aimed to evaluate the effect of revascularization in patients undergoing TAVI. The main findings of the present study are as follows. First, in this patient population, 24.1% of patients undergoing TAVI have obstructive CAD. Second, although there was a strong trend toward higher 30-day mortality for patients with non-revascularized obstructive CAD, there was no difference in mortality between the two groups. Third, after multiple adjustments (bSS, chronic kidney disease stage, previous myocardial infarction, and baseline troponin levels), long-term survival was better for revascularized patients. Finally, this data demonstrates comparable outcomes and in-hospital, 6-month, and 1-year mortality rates between staged and simultaneous PCI.
The ultimate treatment for CAD in patients with TAVI remains to be clarified. The results of studies investigating the effect of TAVI for CAD on mortality are controversial. There are a limited number of reports indicating increased mortality in patients with obstructive CAD undergoing TAVI. Although Dewey et al. (9) showed that CAD is an independent predictor of short- and long-term mortality, this data is not supported by other studies. Two recent meta-analyses showed inconsistent results concerning the association between CAD and TAVI outcomes. D’Ascenzo et al. (10) showed that CAD complexity was strictly related to post-TAVI mortality, and mortality was higher in patients with a bSS >22. Sankaramangalam et al. (11) showed that CAD accompanying TAVI does not impact 30-day mortality, but it does affect 1-year mortality. This was the first meta-analysis with more than 5,000 patients to examine the impact of CAD on TAVI outcomes. The second important finding was that procedural complications were no different based on CAD status. Inconsistencies may be due to lack of a uniform definition of CAD; TAVI outcomes were not stratified by CAD and may be attributed to the heterogeneous nature of the disease. According to our data, although obstructive CAD causes numerically increased 30-day mortality, this increase in mortality is not statistically significant. In our study, similar to other studies, co-existing CAD and TAVI did not cause a significant difference in 1-year mortality, overall mortality, or TAVI outcomes.
Symptoms of angina are problematic because when severe symptomatic AS co-exists with CAD, it is difficult to distinguish which condition causes the symptoms of angina. Therefore, in clinical practice, we initially treated patients with obstructive CAD who could not tolerate short-term hemodynamic instability (rapid pacing, balloon inflation, hypotension) during TAVI. The second issue was the functionally significant lesions or proximal lesions affecting major epicardial arteries. We evaluated angina symptoms in patients with side-branch stenosis or complete revascularization after TAVI.
In our study, we used the SYNTAX score to evaluate the complexity and severity of CAD. We did not observe any differences in TAVI complications and mortality according to bSS and rSS. Conversely, Shamekhi et al. (12) observed an association between bSS and all-cause mortality. However, they did not observe an association between CAD severity and rates of myocardial infarction, stroke, or major vascular complications 30 days after TAVI.
Current guidelines recommend coronary artery bypass graft for obstructive CAD in patients undergoing surgical aortic valve replacement. However, randomized trials examining whether PCI is suitable for patients with CAD undergoing TAVI are limited. In the TAVI group, which was older and had a shorter life expectancy, the expected long-term advantages of revascularization may not be seen. However, the most crucial advantage expected in the short term is facilitation of procedural safety. The ongoing PCI previous to TAVI (ACTIVATION) study was the first randomized controlled trial designed to interpret the non-inferiority of PCI compared with non-revascularization (13). Several small studies showed the feasibility and safety of PCI with TAVI. In a meta-analysis including 3,858 patients, an increase in 30-day mortality and major vascular complications was detected in patients undergoing PCI before or concomitant with TAVI (14). However, they stated that this association did not persist until the first year. According to this study, concomitant PCI with TAVI may increase major vascular complications and lead to adverse outcomes. In contrast to this study, 30-day mortality tended to be higher in the non-revascularized group in our study. This trend may be due to the similar major vascular complication rates between groups 1 and 2. The reason for the lower major complication rate was the rigorous patient selection for PCI. To add, staged PCI allows peripheral access sites to heal.
The timing of PCI in patients with severe symptomatic AS undergoing TAVI is still a matter of debate. Since routine invasive angiography is performed in many centers before TAVI, PCI is performed prior to TAVI to reduce the risk of peri-procedural myocardial infarction. Staged PCI is associated with less contrast agent and a shorter fluoroscopy time in a single setting (15). Staged PCI may reduce the risk of acute kidney failure whether performed before or after TAVI. However, it should be considered that performing PCI before TAVI may require repeat revascularization after TAVI. Abdel-Wahab et al. demonstrated the safety of PCI before TAVI; the outcomes were similar between patients who did and did not undergo PCI in 30-day and 6-month follow ups (16). Another essential question is how many days the optimal delay between PCI and TAVI should be. In a study investigating the optimal delay period, no difference was observed between short- and long-term survey outcomes with delays of >30 days and <30 days. However, a delay of <30 days was associated with minor vascular complications and bleeding (17). Simultaneous PCI increases contrast agent use and procedure time; nevertheless, it may be preferred in patients with an inappropriate access site and bleeding risk. Although not preferred, PCI may be performed after TAVI in some cases, especially where the risk of bleeding is high or in cases where it is unclear whether anginal symptoms are related to CAD or severe AS. However, the extension of THVs into aortic sinuses results in difficult cannulation, and accessing the coronary ostia is the most crucial reason that this technique is not preferred. Short THVs, such as the SAPIEN 3 THV System (Edwards Lifesciences), Direct Flow Medical, and the Lotus Edge Aortic Valve System (Boston Scientific, Marlborough, Massachusetts, USA), do not routinely cover the coronary ostium. However, other THVs, such as the CoreValve (Medtronic Inc., Minneapolis, MN, USA) (Medtronic), the Portico (St. Jude Medical, Inc, St. Paul, MN, USA) transcatheter aortic valve, and ACURATE neo THV (Boston Scientific, Marlborough, MA, USA), systematically jail the coronary arteries; therefore, if these THVs are implanted, it is appropriate to perform complete revascularization with a DES before TAVI. Jeroudi et al. (18) showed that coronary interventions are feasible for the majority of patients who have undergone previous TAVI using the CoreValve; furthermore, selective engagement of the right coronary artery ostium is more challenging to achieve compared with the LMCA.
We used staged PCI to prevent acute kidney failure and allow the peripheral access site to heal. However, simultaneous PCI was performed for simple lesions, which was thought not to increase fluoroscopy time and the amount of contrast agent used. Our findings suggest that neither simultaneous nor staged PCI confer a clinical advantage. However, there was a statistically significant increase in 30-day mortality in the simultaneous PCI group; this difference was due to the small size of the study population.
Study limitations
There are some limitations of our study that should be highlighted. First, the single-center, observational, and retrospective nature of our study may have introduced bias. The number of patients was relatively small in both the revascularized and simultaneous PCI patient groups. The timing of PCI and TAVI and the procedural approach varied depending on the center’s choice and patients’ clinical situations. Additionally, most THVs were balloon expandable valves. Consequently, it may be difficult to generalize these results to all patients who undergo PCI and TAVI.
Conclusion
In conclusion, this study demonstrates no significant difference in peri-procedural and long-term safety outcomes and mortality between revascularized and non-revascularized patients. However, neither staged nor simultaneous PCI cause adverse outcomes in patients undergoing TAVI. Randomized prospective trials are needed to establish the role and timing of routine revascularization in patients with significant CAD undergoing TAVI.
HIGHLIGHTS .
Coronary artery disease (CAD) and aortic stenosis (AS) commonly coexist. The impact of CAD's treatment on prognosis is not manifest in patients who underwent transcatheter aortic valve implantation (TAVI). This study aimed to evaluate the influence of the prognostic value of revascularization with regard to short-and long-term outcome in patients undergoing TAVI.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – B.D.K., H.A., E.B.; Design – B.D.K., H.A.; Supervision – T.K., E.B.; Fundings – T.K., E.B.; Materials – B.D.K., H.A., E.B.; Data collection and/or processing – B.D.K., H.A., T.K.; Analysis and/or interpretation – B.D.K., H.A.; Literature search – B.D.K., H.A., E.B.; Writing – B.D.K., H.A.; Critical review – T.K., E.B.
References
- 1.Goel SS, Ige M, Tuzcu EM, Ellis SG, Stewart WJ, Svensson LG, et al. Severe aortic stenosis and coronary artery disease--implications for management in the transcatheter aortic valve replacement era: a comprehensive review. J Am Coll Cardiol. 2013;62:1–10. doi: 10.1016/j.jacc.2013.01.096. [DOI] [PubMed] [Google Scholar]
- 2.Walther T, Hamm CW, Schuler G, Berkowitsch A, Kötting J, Mangner N, et al. GARY Executive Board. Perioperative Results and Complications in 15,964 Transcatheter Aortic Valve Replacements: Prospective Data From the GARY Registry. J Am Coll Cardiol. 2015;65:2173–80. doi: 10.1016/j.jacc.2015.03.034. [DOI] [PubMed] [Google Scholar]
- 3.Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, et al. PARTNER 3 Investigators. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med. 2019;380:1695–705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 4.Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D, et al. Evolut Low Risk Trial Investigators. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med. 2019;380:1706–15. doi: 10.1056/NEJMoa1816885. [DOI] [PubMed] [Google Scholar]
- 5.Paradis JM, Fried J, Nazif T, Kirtane A, Harjai K, Khalique O, et al. Aortic stenosis and coronary artery disease: what do we know? What don't we know? A comprehensive review of the literature with proposed treatment algorithms. Eur Heart J. 2014;35:2069–82. doi: 10.1093/eurheartj/ehu247. [DOI] [PubMed] [Google Scholar]
- 6.Witberg G, Regev E, Chen S, Assali A, Barbash IM, Planer D, et al. The Prognostic Effects of Coronary Disease Severity and Completeness of Revascularization on Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2017;10:1428–35. doi: 10.1016/j.jcin.2017.04.035. [DOI] [PubMed] [Google Scholar]
- 7.Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1:219–27. [PubMed] [Google Scholar]
- 8.Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J. 2012;33:2403–18. doi: 10.1093/eurheartj/ehs255. [DOI] [PubMed] [Google Scholar]
- 9.Dewey TM, Brown DL, Herbert MA, Culica D, Smith CR, Leon MB, et al. Effect of concomitant coronary artery disease on procedural and late outcomes of transcatheter aortic valve implantation. Ann Thorac Surg. 2010;89:758–67. doi: 10.1016/j.athoracsur.2009.12.033. [DOI] [PubMed] [Google Scholar]
- 10.D'Ascenzo F, Verardi R, Visconti M, Conrotto F, Scacciatella P, Dziewierz A, et al. Independent impact of extent of coronary artery disease and percutaneous revascularisation on 30-day and one-year mortality after TAVI: a meta-analysis of adjusted observational results. EuroIntervention. 2018;14:e1169–77. doi: 10.4244/EIJ-D-18-00098. [DOI] [PubMed] [Google Scholar]
- 11.Sankaramangalam K, Banerjee K, Kandregula K, Mohananey D, Parashar A, Jones BM, et al. Impact of Coronary Artery Disease on 30-Day and 1-Year Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement: A Meta-Analysis. J Am Heart Assoc. 2017;6:e006092. doi: 10.1161/JAHA.117.006092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shamekhi J, Stundl A, Weber M, Mellert F, Welz A, Grube E, et al. Impact of coronary artery disease in patients undergoing transfemoral transcatheter aortic valve implantation. Int J Cardiol. 2017;245:215–21. doi: 10.1016/j.ijcard.2017.07.082. [DOI] [PubMed] [Google Scholar]
- 13.Khawaja MZ, Wang D, Pocock S, Redwood SR, Thomas MR. The percutaneous coronary intervention prior to transcatheter aortic valve implantation (ACTIVATION) trial: study protocol for a randomized controlled trial. Trials. 2014;15:300. doi: 10.1186/1745-6215-15-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotronias RA, Kwok CS, George S, Capodanno D, Ludman PF, Townend JN, et al. Transcatheter Aortic Valve Implantation With or Without Percutaneous Coronary Artery Revascularization Strategy: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2017;6:e005960. doi: 10.1161/JAHA.117.005960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conradi L, Seiffert M, Franzen O, Baldus S, Schirmer J, Meinertz T, et al. First experience with transcatheter aortic valve implantation and concomitant percutaneous coronary intervention. Clin Res Cardiol. 2011;100:311–6. doi: 10.1007/s00392-010-0243-6. [DOI] [PubMed] [Google Scholar]
- 16.Abdel-Wahab M, Mostafa AE, Geist V, Stöcker B, Gordian K, Merten C, et al. Comparison of outcomes in patients having isolated transcatheter aortic valve implantation versus combined with preprocedural percutaneous coronary intervention. Am J Cardiol. 2012;109:581–6. doi: 10.1016/j.amjcard.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 17.van Rosendael PJ, van der Kley F, Kamperidis V, Katsanos S, Al Amri I, Regeer M, et al. Timing of staged percutaneous coronary intervention before transcatheter aortic valve implantation. Am J Cardiol. 2015;115:1726–32. doi: 10.1016/j.amjcard.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Jeroudi OM, Rehman H, Barker MB. Technical Considerations and Feasibility of Coronary Angiography and Percutaneous Coronary Intervention after CoreValve® Transcatheter Aortic Valve Implantation. Structural Heart. 2018;2:297–302. [Google Scholar]


