Abstract
Objective:
To test the hypothesis that short curing times using a high-intensity light-emitting diode (LED) or high-power halogen are not associated with compromised shear bond strength (SBS) of metal brackets before and after thermocycling.
Materials and Methods:
Two hundred forty extracted human premolar teeth were divided into six groups of 40 each. Metal brackets were bonded using a light-cured composite (Transbond XT). In group 1 a conventional halogen light (Hilux) was used for 40 seconds. In groups 2, 3, and 4 a high-power halogen light (Swiss Master) was used for 2, 3, and 6 seconds, respectively. In groups 5 and 6 a high-intensity LED (Bluephase) was used for 10 and 20 seconds, respectively. After bonding, half of the specimens in each group were thermocycled, and all specimens were tested for SBS. After debonding, the bracket bases and the enamel surfaces were scored according to the Adhesive Remnant Index.
Results:
Two-way analysis of variance detected significant differences in SBS values with respect to curing method (type of light-curing unit and curing time) (P = .0001) and thermocycling (P = .01). Tukey post hoc analysis showed that with or without thermocycling the mean SBS values of groups 1, 4, 5, and 6 were not significantly different, whereas group 2 showed the lowest SBS values. The predominant failure site for groups 2 and 3 was between the bracket and the adhesive and for groups 4, 5, 6 it was at the tooth/adhesive interface.
Conclusion:
Curing time can be reduced to 6 seconds with high-power halogen light and to 10 seconds with high-intensity LED without compromising in vitro SBS of metal brackets.
Keywords: Light-emitting diode, Halogen
INTRODUCTION
Stability of bonded brackets and the clinical time spent during bracket bonding are main concerns for every orthodontist. When orthodontic brackets are bonded by light-polymerizing composites, working time is increased, brackets may be positioned more correctly, curing depth is maximized, porosity is less, and the clinician has enough time to clean the composite around the base of the bracket, decreasing the possibility of enamel demineralization through plaque accumulation.1
Although the most common method of delivering blue light to cure light-activated composite material is through a quartz-tungsten-halogen light-curing unit (LCU),2 these devices have several disadvantages,2–4 including relatively longer curing times (40 to 60 seconds).5 Light-emitting diode (LED) technology has been proposed to overcome the shortcomings of standard quartz-tungsten-halogen–visible LCUs.2,5 Previous research, which evaluated LEDs with relatively low power densities, suggested irradiation of 20 to 40 seconds for bonding orthodontic brackets.6–8 However, high-intensity LED lamps, with shorter curing times (10 seconds) and an increased performance (over 1000 mW/cm2), are also currently available.7
The time needed for photopolymerization of brackets has been a major concern in clinical orthodontics. Manufacturers of LCUs for bonding of orthodontic brackets aim at reducing exposure time.9 Advances in technology have made it possible to increase light power density and thus reduce the necessary duration of exposure without compromising bonding efficacy. Recently, a high-power water-cooled halogen light with shorter curing times and an increased performance of 3000 mW/cm2 was introduced (Swiss Master Light, EMS Electromedical Systems, Nyon, Switzerland).10 Staudt et al.9,11 suggested that a 6-second cure time, and even—with caution—a 3-second cure time might be adequate for bonding orthodontic brackets to tooth enamel using this new high-power halogen light.
Bond strength of light-cured composites is affected not only by the efficiency of the polymerization but also by temperature variations in the oral cavity. Although testing is difficult, it is important to determine whether they introduce stresses in the adhesive that might influence its bond strength. The most commonly used artificial aging technique is thermocycling.12
The viability of different light sources in bonding brackets has been tested; however, conflicting results have been published with regard to the effects of short-term exposure on bracket bonding.11,13–15 Also, to our knowledge, there are no published comparisons of high-intensity LEDs and high-power halogen lights. Therefore, the purpose of this study was to test the hypothesis that short curing times using a high-intensity LED or high-power halogen are not associated with compromised shear bond strength (SBS) of metal brackets before and after thermocycling, which was used to mimic the temperature variations in the oral cavity.
MATERIALS AND METHODS
Two hundred forty extracted human premolar teeth were collected from patients whose teeth had to be extracted for orthodontic treatment. The extracted teeth were cleansed of soft tissue, polished with no fluoridated pumice and rubber prophylactic cups at low speed for 10 seconds, and immersed in distilled water in a sealed container for 1 to 3 months until testing. The water was changed weekly to prevent the growth of bacteria and fungi. The criteria for tooth selection included: intact buccal enamel with no cracks, hypoplastic areas, or gross irregularities; no caries; and buccal enamel surfaces that had not been treated with hydrogen peroxide, formalin, alcohol, or other chemical agents after extraction.
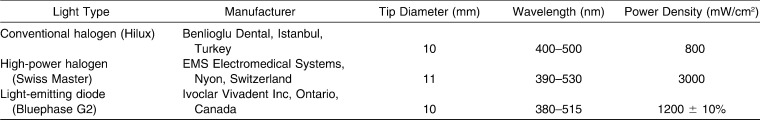
The polymerization sources used in this study were a conventional halogen lamp (Hilux, Benlioglu Dental, Istanbul, Turkey), a high-power halogen lamp (Swiss Master Light, EMS Electromedical Systems), and a high-intensity LED curing light (Bluephase G2, Ivoclar Vivadent Inc, Ontario, Canada). The technical characteristics of the polymerization units under investigation were summarized in Table 1.
Table 1.
Characteristics of the Light-Curing Units Used in the Study
The teeth were randomly divided into six groups of 40 teeth each. After the buccal surface of each tooth was etched with a 37% phosphoric acid gel for 30 seconds, the teeth were rinsed with a water spray for 30 seconds and dried with an oil-free air source for 10 seconds. Foil mesh–based stainless steel (upper premolar) Gemini MBT brackets (3M/Unitek, St Paul, Minn, USA) were bonded with the same adhesive (Transbond XT, 3M Unitek, St Paul, Minn, USA). The brackets were positioned on the teeth near the center of the buccal surfaces with sufficient pressure to express excess adhesive, which was removed before polymerization.
A conventional halogen light source (Hilux, Benlioglu Dental, Istanbul, Turkey) was used for polymerization for a total of 40 seconds (20 seconds from each of the mesial and distal sides) in group 1. A high-power halogen light (Swiss Master Light, E.M.S.) in the fast-cure mode was used for 2 seconds in group 2, for 3 seconds in group 3, and for 6 seconds in group 4. The light tip was positioned on the middle of the bracket in the second and third groups. For the fourth group, the time of 6 seconds was divided into 3 seconds on the mesial side and 3 seconds on the distal side of the bracket. In groups 5 and 6, a high-intensity LED (Bluephase G2, Ivoclar Vivadent Inc) was used for 10 and 20 seconds, respectively (total curing time was halved to provide equal curing times on each of the mesial and distal sides). The distance of the light guiding tip was kept standard for all specimens and the tip was angulated 90 degrees to the bracket on each tooth. Each tooth with the bracket already bonded was mounted in a 2-cm-diameter circular mould using chemically cured acrylic resin (Vertex, Zeist, Netherlands). A specially constructed metal paralleling device was used to position the tooth in the acrylic resin so that the base of the bracket was parallel to the shear force vector.
After bonding, half of the specimens in each group were stored in distilled water at 37°C for 24 hours, and the other half were thermocycled between 5°C and 55°C for 7500 cycles after 24 hours of storage in distilled water at 37°C.
The SBS was measured with a universal testing machine (Z010, Zwick Testing Machines Ltd, Ulm, Germany). The acrylic resin block with the bonded tooth was secured into the lower jaw of the testing machine. The shear bond force was applied in an occlusogingival direction with a crosshead speed of 0.5 mm/min. The force necessary to debond the brackets was recorded automatically in Newtons and then converted into megapascals (MPa) by dividing the Newtons by the surface area of the bracket base (7.9 ± 0.3 mm2).11
Tooth and bracket specimens were collected and examined under a light microscope at 50× magnification to evaluate the amount of adhesive remaining on the tooth. The adhesive remnant index (ARI) was used to evaluate the amount of resin remaining on tooth surfaces. The ARI score in our study was the same as the one used by Årtun and Bergland.16 The criteria for scoring were as follows: 0, no adhesive remained on the tooth; 1, less than half of the adhesive remained on the tooth; 2, more than half of the adhesive remained on the tooth; and 3, all the adhesive remained on the tooth.
NCSS 2007 software was used for the statistical analysis of the data. Data were subjected to two-way analysis of variance (ANOVA) to identify differences in mean SBS with respect to curing method (type of LCU and curing time) and thermocycling, including any interaction. Tukey post hoc tests were used to identify where differences occurred. The unpaired t-test was performed to compare groups with and without thermocycling. The chi-square test was used to evaluate differences in the ARI scores between groups with different SBS. The level of significance was set at P < .05.
RESULTS
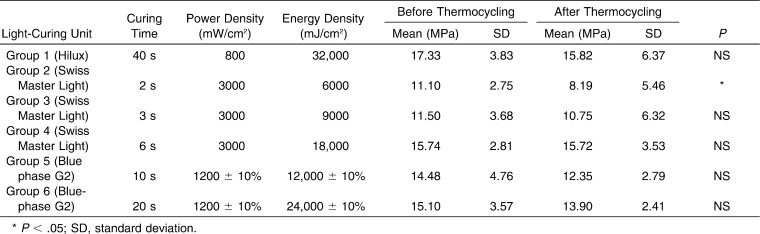
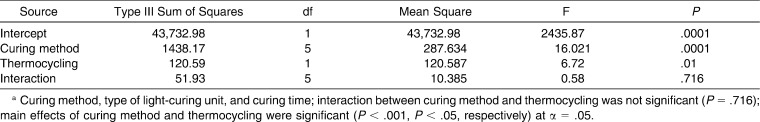
The means and standard deviations of SBS values of the six groups before and after thermocycling and the results of the unpaired t-test are given in Table 2. Although thermocycling caused decreases in the SBS values of all groups, the decrease was significant only for group 2. The results of two-way ANOVA are shown in Table 3. The two-way ANOVA detected statistically significant differences in SBS values with respect to curing method (type of LCU and curing time) (P = .0001) and thermocycling (P = .01). The interaction was not significant (P = .716).
Table 2.
Mean Shear Bond Strength (SBS) Values of the Groups in MPa and the Result of Unpaired t-Test Demonstrating the Effects of Thermocycling on SBS Values of the Groups*
Table 3.
Two-Way ANOVAa
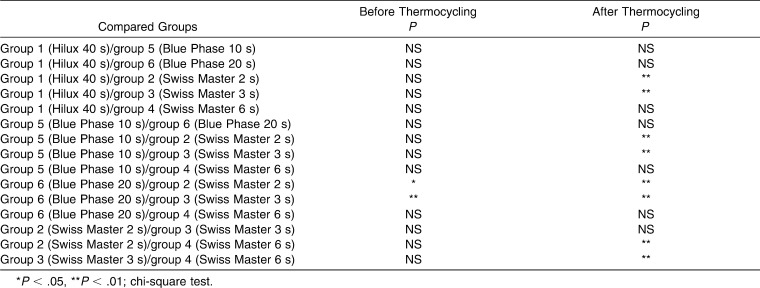
Tukey post hoc analysis was performed to determine which groups were significantly different. The test showed that, before thermocycling, the mean SBS value of group 2 was significantly lower than those of groups 1 (P = .0001), 4 (P = .001), 5 (P = .044), and 6 (P = .009). The mean SBS value of group 3 was significantly lower than those of groups 1 (P = .0001), 4 (P = .004), and 6 (P = .026). After thermocycling, the mean SBS value of group 2 was significantly lower than those of groups 1 (P = .0001), 4 (P = .0001), and 6 (P = .003). The mean SBS value of group 3 was significantly lower than those of groups 1 (P = .013) and 4 (P = .016).
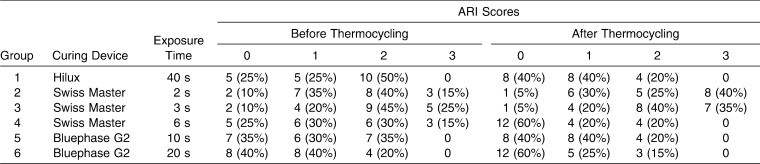
The frequency distribution of the ARI score before and after thermocycling is given in Table 4. Before thermocycling, most of the specimens in groups 2 and 3 failed at the bracket/adhesive interface (ARI of 2 or 3), with most of the adhesive remaining on the tooth; however, for groups 4, 5, and 6, the predominant failure site was found to be the tooth/adhesive interface (ARI of 0 or 1), where most of the adhesive remained on the bracket. On the other hand, in group 1, while half of the specimens failed at the bracket/adhesive interface, the other half failed at the tooth/adhesive interface. When thermocycling was performed, the predominant failure site of the specimens in groups 2, 3, 4, 5, and 6 did not change; however, in group 1 more specimens failed at the tooth/adhesive interface (ARI of 0 or 1). The chi-square test detected significant differences in ARI scores between the groups with different SBS values (Table 5). The ARI scores of the groups with the smallest SBS values (groups 1 and 2) were found to be significantly higher (ARI of 2 or 3) than the ARI scores (ARI of 0 or 1) of the groups with higher SBS values (groups 1, 4, 5, and 6) (Table 5).
Table 4.
Frequency Distribution of the ARI Scores of the Groups Before and After Thermocycling
Table 5.
Differences in ARI Between Groups with Different SBS Before and After Thermocycling
DISCUSSION
The results of this study showed that high-power halogen curing light decreases curing time dramatically without compromising the SBS of orthodontic brackets, agreeing with the results of Staudt et al.11 Exposure to the high-power halogen curing light for 6 seconds led to SBS values equivalent to those achieved after 40 seconds of exposure to the conventional halogen light and 10 seconds of exposure to the light-emitting diode. The mean SBS value achieved after a 3-second exposure to high-power halogen light was not significantly different than the SBS value achieved after 10 seconds of exposure to high-intensity LED; therefore it can be speculated that even 3 seconds of exposure to a high-power halogen LCU may be effective for orthodontic needs. Even the SBS values achieved after an exposure time of only 2 seconds with the high-power halogen LCU were above the minimal requirement of 6 to 8 MPa that was suggested by Reynolds.17 However, after the thermocycling, the decrease in SBS of group 2 was significant, showing that curing for only 2 seconds with high-power halogen light may lead to premature failure of brackets during orthodontic treatment as a result of insufficient bond strength. After thermocycling, the mean SBS values of groups 2, 3, and 5 were not found to be significantly different from each other. Therefore, curing for 3 seconds with high-power halogen light and for 10 seconds with high-intensity LED may not result in sufficient SBS to overcome the effects of composite aging, and components may be lost prematurely during a normal 24-month orthodontic treatment18–20; thus, these approaches should be used with caution.
According to the total energy concept, the light-curing process depends on the energy, which is determined by the multiplication of light intensity and time.21 However, Mavropoulos et al.15 reported that this concept was not valid for orthodontic light-curing bracket bonding; an exposure time of less than 4 seconds, irrespective of the power density, could not guarantee sufficient bracket bond strength. Power density seemed to have an advantage over exposure duration in the context of metallic bracket bonding. For efficient light-cured bracket bonding, there was an absolute lower limit of exposure duration (4 seconds) and an upper limit of useful power density (3000 mW/cm2).15 This principle agrees well with another study,22 suggesting that there may be no benefit to polymerization of resin composite when power density is increased above a value of approximately 1000 mW/cm2. Since direct composite exposure is impossible for metallic bracket light-curing bonding, part of the energy emitted by the light is simply lost, reflected on the metallic bracket. The results of the current study corroborate the results of Mavropoulos et al.15 In the current study 6 seconds of 3000 mW/cm2 seemed to result in similar SBS values as 40 seconds of 800 mW/cm2 or 20 seconds of 1200 mW/cm2. On the other hand, 2 seconds of 3000 mW/cm2 resulted in lower SBS values than the rest of the groups. While an energy density between 9000 and 12,000 mJ/cm2 could only be used with caution, 6000 mJ/cm2 was not found to be sufficient.
Recently, Staudt et al.11 reported that the innovations of the high-power halogen light are its high power density and the unique size of its light-guiding tip (diameter of 11 mm). In the current study, a one-shot application of 3 seconds with the light-guiding tip covering the entire bracket was found to result in acceptable SBS values, even after thermocycling. However, the power increase that is made possible by the device's innovative water cooling system instead of fan cooling is accompanied by a bulky (270 × 205 × 256 mm) and heavy (7 kg) device.11 Although its cost is higher than that of other halogen lights, the high-power halogen light is not as expensive as the plasma lamp, which also has a fast-curing innovation.11 Only the LED lamps are less expensive. The recently increased intensity, relatively moderate cost, portability, and the long life of its diodes make last-generation LEDs attractive to clinicians.
SBS values of orthodontic brackets bonded with LED-curing units at various polymerization times have been evaluated7,8 to find the shortest polymerization time possible that still allows sufficient polymerization. Swanson et al.7 found clinically satisfactory SBS, even with a 10-second cure, but recommended longer periods of curing as in the manufacturer's instructions. Usumez et al.8 suggested that 20 seconds of LED exposure might yield SBS values comparable to those obtained with halogen-based units in 40 seconds.
Recently, manufacturers have turned their attention to the high-intensity LED light source (≥ 1000 mW/cm2). However, the high temperatures generated by these high-intensity LEDs (900 to 1000 mW/cm2) may damage the device, requiring constant cooling.14 Türkkahraman and Küçükeşmen23 suggested that 20 seconds of high-intensity LED (1250 mW/cm2) exposure in the fast mode may yield SBS values comparable with those obtained with halogen-based units in 40 seconds. In the current study, 10 seconds of exposure to a high-intensity LED was found to be sufficient to achieve acceptable SBS values, even after thermocycling.
The predominant failure site of the specimens in all groups did not change before and after thermocycling. However, when the ARIs of the groups with different SBS values were compared, it was found that the differences in bond strength were associated with differences in ARI scores. While most specimens in groups 2 and 3 failed at the bracket/adhesive interface (ARI of 2 or 3), the predominant failure site for groups 4, 5, and 6 was the tooth/adhesive interface (ARI of 0 or 1). The ARIs indicated that most of the composite remained on the tooth after bracket debonding in groups 2 and 3, and when the ARIs of the groups with different SBS values were compared it was found that the differences in bond strength were associated with differences in ARI scores. The ARI scores of the groups with the lowest SBS values (groups 1 and 2) were found to be significantly higher (ARI of 2 or 3) than the ARI scores (0 or 1) of the groups with higher SBS values (groups 1, 4, 5, and 6). It can be speculated that incomplete curing with high-power halogen for 2 and 3 seconds might have caused lower bond strength as a result of weaker adhesion to the bracket base and thus be associated with a higher ARI score. This finding is consistent with the findings of Thind et al.24 and Mavropoulos et al.15
A decrease in bonding effectiveness is believed to be caused by degradation of the interface components by hydrolysis.19 In addition, water can also infiltrate and weaken the mechanical properties of the polymer matrix.25 The teeth undergo significant fluctuations in temperature, varying from hot to cold in liquid and solid forms, resulting in further thermodynamic stresses on the bonded brackets. In the current study, this was duplicated in vitro with thermocycling.
Conclusions drawn from the results of any properly constructed laboratory investigation will provide a sound basis for the clinical introduction of new products and techniques. It is possible to simulate conditions that are close to those in clinical use, but the potential for unrecognized factors to influence the outcome should always be kept in mind.26 The results of the current study are based on in vitro laboratory conditions, and the clinical relevance of the findings should be confirmed in vivo.
CONCLUSIONS
Curing time can be reduced to 6 seconds with high-power halogen light (3000 mW/cm2) and to 10 seconds with high-intensity LED (1200 ± 10% mW/cm2) without compromising in vitro SBS of metal brackets.
Differences in bond strength are associated with differences in ARI score. Incomplete curing with high-power halogen for 2 and 3 seconds might have caused lower bond strength as a result of weaker adhesion to the bracket base and thus be associated with a higher ARI score.
Acknowledgments
This study was supported by the Scientific Research Projects Commission of Marmara University (BAPKO), project number SAG-BGS-060907-0175.
REFERENCES
- 1.Armas Galindo H. R, Sadowsky P. L, Vlachos C, Jacobson A, Wallace D. An in vivo comparison between a visible light-cured bonding system and a chemically cured bonding system. Am J Orthod Dentofacial Orthop. 1998;113:271–275. doi: 10.1016/s0889-5406(98)70296-3. [DOI] [PubMed] [Google Scholar]
- 2.Mills R. W, Jandt K. D, Ashworth S. H. Dental composite depth of cure with halogen and blue light emitting diode technology. Br Dent J. 1999;186:388–391. doi: 10.1038/sj.bdj.4800120. [DOI] [PubMed] [Google Scholar]
- 3.Stahl F, Ashworth S. H, Jandt K. D, Mills R. W. Light emitting diode (LED) polymerization of dental composites: flexural properties and polymerization potential. Biomaterials. 2000;21:1379–1385. doi: 10.1016/s0142-9612(00)00029-6. [DOI] [PubMed] [Google Scholar]
- 4.Miyazaki M, Hattori T, Ichiishi Y, et al. Evaluation of curing units used in private dental offices. Oper Dent. 1998;23:50–54. [PubMed] [Google Scholar]
- 5.Wendl B, Droschl H. A comparative in vitro study of the strength of directly bonded brackets using different curing techniques. Eur J Orthod. 2004;26:535–544. doi: 10.1093/ejo/26.5.535. [DOI] [PubMed] [Google Scholar]
- 6.Bishara S. E, Ajlouni R, Oonsombat C. Evaluation of a new curing light on the shear bond strength of orthodontic brackets. Angle Orthod. 2003a;73:431–435. doi: 10.1043/0003-3219(2003)073<0431:EOANCL>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Swanson T, Dunn W. J, Childers D. E, Taloumis L. J. Shear bond strength of orthodontic brackets bonded with light-emitting diode curing units at various polymerization times. Am J Orthod Dentofacial Orthop. 2004;125:337–341. doi: 10.1016/j.ajodo.2003.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Usumez S, Buyukyilmaz T, Karaman A. I. Effect of light-emitting diode on bond strength of orthodontic brackets. Angle Orthod. 2004;74:259–263. doi: 10.1043/0003-3219(2004)074<0259:EOLDOB>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Staudt C. B, Krejci I, Mavropoulos A. Bracket bond strength dependence on light power density. J Dent. 2006;34:498–502. doi: 10.1016/j.jdent.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Gordon C. J. The light-curing mania. J Am Dent Assoc. 2004;135:461–463. doi: 10.14219/jada.archive.2004.0211. [DOI] [PubMed] [Google Scholar]
- 11.Staudt C. B, Mavropoulos A, Bouillaguet S, Kiliaridis S, Krejci I. Light-curing time reduction with a new high-power halogen lamp. Am J Orthod Dentofacial Orthop. 2005;128:749–754. doi: 10.1016/j.ajodo.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 12.Bishara S. E, Ajlouni R, Laffoon J. F. Effect of thermocycling on the shear bond strength of a cyanoacrylate orthodontic adhesive. Am J Orthod Dentofacial Orthop. 2003;123:21–24. doi: 10.1067/mod.2003.1. [DOI] [PubMed] [Google Scholar]
- 13.Silta Y. T, Dunn W. J, Peters C. B. Effect of shorter polymerization times when using the latest generation light-emitting diodes. Am J Orthod Dentofacial Orthop. 2005 Dec;128(6):744–748. doi: 10.1016/j.ajodo.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 14.Rego E. B, Romano F. L. Shear bond strength of metallic brackets photo-activated with light-emitting diode(LED) at different exposure times. J Appl Oral Sci. 2007;15:412–415. doi: 10.1590/S1678-77572007000500007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mavropoulos A, Cattani-Lorente M, Krejci I, Staudt C. B. Kinetics of light-cure bracket bonding: power density vs exposure duration. Am J Orthod Dentofacial Orthop. 2008;134:543–547. doi: 10.1016/j.ajodo.2006.09.068. [DOI] [PubMed] [Google Scholar]
- 16.Årtun J, Bergland S. Clinical trials with crystal growth conditioning as an alternative to acid etch enamel pretreatment. Am J Orthod. 1984;85:333–340. doi: 10.1016/0002-9416(84)90190-8. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds I. R. A review of direct orthodontic bonding. Br J Orthod. 1975;2:171–178. [Google Scholar]
- 18.Oesterle L. J, Shellhart W. C. Effect of aging on the shear bond strength of orthodontic brackets. Am J Orthod Dentofacial Orthop. 2008;133:716–720. doi: 10.1016/j.ajodo.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 19.De Munck J, Van Landuyt K, Peumans M, et al. A critical review of the durability of adhesion of tooth tissue: methods and results. J Dent. 2005;84:118–132. doi: 10.1177/154405910508400204. [DOI] [PubMed] [Google Scholar]
- 20.Ito S, Hashimoto M, Wadgaonkar B, et al. Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials. 2005;26:6449–6459. doi: 10.1016/j.biomaterials.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 21.Koran P, Kürschner R. Effect of sequential versus continuous irradiation of a light-cured resin composite on shrinkage, viscosity, adhesion, and degree of polymerization. Am J Dent. 1998;10:17–22. [PubMed] [Google Scholar]
- 22.Musanje L, Darvell B. W. Polymerization of resin composite restorative materials: exposure reciprocity. Dent Mater. 2003;19:531–541. doi: 10.1016/s0109-5641(02)00101-x. [DOI] [PubMed] [Google Scholar]
- 23.Türkkahraman H, Küçükeşmen H. C. Orthodontic bracket shear bond strengths produced by two high-power light-emitting diode modes and halogen light. Angle Orthod. 2005;75:854–857. doi: 10.1043/0003-3219(2005)75[854:OBSBSP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Thind B. S, Stirrups D. R, Lloyd C. H. A comparison of tungsten-quartz-halogen, plasma arc and light-emitting diode light sources for the polymerization of an orthodontic adhesive. Eur J Orthod. 2006;28:78–82. doi: 10.1093/ejo/cji076. [DOI] [PubMed] [Google Scholar]
- 25.Bishara S. E, Olsen M. E, Damon P, Jakobsen J. R. Evaluation of a new light-cured orthodontic bonding adhesive. Am J Orthod Dentofacial Orthop. 1998;114:80–87. doi: 10.1016/s0889-5406(98)70242-2. [DOI] [PubMed] [Google Scholar]
- 26.Katona T. R. A comparison of stresses developed in tension, shear peel, and torsion strength testing of direct bonded orthodontic brackets. Am J Orthod Dentofacial Orthop. 1997;112:244–251. doi: 10.1016/S0889-5406(97)70251-8. [DOI] [PubMed] [Google Scholar]