Abstract
Objective:
To compare the effects of fluoride and casein phosphopeptide–amorphous calcium phosphate (CPP-ACP) containing topical agents on shear bond strength (SBS) and fracture mode of orthodontic brackets bonded to demineralized enamel.
Materials and Methods:
Eighty freshly extracted human premolar teeth were randomly divided into four equal groups. The first group was the control, and no pretreatment was performed on this group. In the remaining three groups, demineralization process was performed and teeth were stored in artificial saliva. In group II, bonding was performed after demineralization. Pretreatment with fluoride and CPP-ACP gels was performed in groups III and IV, respectively. Brackets were bonded using a conventional system. The SBS of these brackets was measured and recorded in MPa. Adhesive remnant index (ARI) scores were determined after the brackets failed. Data were analyzed with analysis of variance (ANOVA), Tukey, and G-tests at the P < .05 level.
Results:
ANOVA comparison of four groups revealed statistically significant differences. No significant differences were found between control and CPP-ACP–treated groups. However, lower SBS values were recorded for group II (6.6 ± 3.9 MPa) and group III (17.1 ± 2.9 MPa). ARI scores were significantly different among the four groups (P < .001). No enamel detachment was found in the control group, and enamel detachment measured 75% for group II.
Conclusions:
Fluoride and CPP-ACP gel applications showed higher debonding forces compared to bonding in untreated demineralized samples. CPP-ACP pretreatment resulted in comparable SBS values compared with the control group.
Keywords: Phosphopeptide–amorphous calcium phosphate, Fluoride, Shear bond strength, Demineralization
INTRODUCTION
Orthodontic treatment complicates oral hygiene maintenance and increases the risk of subsequent enamel demineralization.1–4 Gorelick et al.3 reported that half of the patients undergoing fixed orthodontic treatment had nondevelopmental white spot lesions. Fluoride and casein phosphopeptide–amorphous calcium phosphate (CPP-ACP) applications, enamel microabrasion, and bleaching have been suggested as mechanisms with which to manage the white spot lesions.5 Gorelick et al.3 also reported the existence of white spot lesions in 24% of patients before orthodontic therapy. Boersma et al.6 found white spot lesions in 11% of orthodontically untreated subjects. This finding raised another concern, namely that orthodontic treatment may be performed in individuals with existing white spot lesions. In light of the contemporary orthodontic literature, fluoride and CPP-ACP applications have been accepted as a means by which to remineralize previously demineralized enamel. Fluoride ions in plaque immediately promote the remineralization by formation of fluorapatite.7 In addition, remineralization of previously demineralized enamel can be achieved with fluoride application in cases in which adequate amounts of calcium and phosphate ions are available.8 Anticariogenic activity of CPP-ACP has been demonstrated9–11 in laboratory, animal, and human experiments. CPP-ACP also has been demonstrated12 to increase the levels of calcium and phosphate ions significantly in supragingival plaque and to promote the remineralization of enamel subsurface lesions in situ.
The effects of these agents on the shear bond strength (SBS) of brackets have been investigated by several authors.13–24 Controversial results were reported in terms of fluoride's effect on bracket bond strength. Some authors13–16 reported decreases in SBS of brackets, while others had no such findings.17–20 Keçik et al.21 compared the effects of CPP-ACP and acidulated phosphate fluoride on SBS values and found higher SBS values for all test groups. Xiaojun et al.22 reported higher SBS in the CPP-ACP–applied group when light-cure adhesives were used. Moule et al.23 showed decreased SBS values after the combined use of carbamide peroxide and CPP-ACP applications. Tabrizi and Cakirer24 reported decreased SBS values with the use of fluoride. On the other hand, they also found that CPP-ACP and the combination of this agent and fluoride did not affect SBS values.
In all of these studies, CPP-ACP and fluoride were used in order to prevent demineralization, so these agents were applied to undemineralized enamel. Thus, the aim of this study was to evaluate the effects of CPP-ACP and fluoride on the SBS of orthodontic brackets bonded to pretreated demineralized enamel. The null hypothesis to be tested was that there is no statistically significant difference in (1) SBS and (2) fracture mode of brackets bonded to pretreated demineralized enamel after application of fluoride and CPP-ACP agents.
MATERIALS AND METHODS
A power analysis established by G*Power Ver 3.0.10 software (Franz Faul, Universität Kiel, Germany), based on 1∶1 ratio among groups, with a sample size of 20 teeth would give more than 80% power to detect significant differences with 0.35 effect size and at the α = .05 significance level.
Eighty caries-free human maxillary premolar teeth extracted with orthodontic indications were used (from patients aged between 12 and 16 years). Teeth with hypoplastic areas, cracks, or irregularities of the enamel structure were excluded. The criteria for tooth selection dictated that the tooth had undergone no pretreatment with a chemical agent such as alcohol, formalin, or hydrogen peroxide. Freshly extracted teeth were stored in 0.1% thymol solution for no more than 1 month. Soft tissue remnants and callus were removed with a scaler. Each tooth was mounted vertically in a self-cure acrylic block so that the crown was exposed. The buccal enamel surface of the teeth were cleaned and polished with nonfluoridated pumice and rubber prophylactic cups, washed with water, and dried. The teeth were randomly divided into four groups of 20 teeth each, as described in the following sections.
Group I (Control)
No enamel pretreatment was performed in this group. The buccal surface of each tooth was etched with 37% ortho-phosphoric acid (3M Dental Products, St Paul, Minn) for 15 seconds, rinsed with water for 15 seconds, and dried with oil-free air for 10 seconds until a frosty-white appearance was obtained. Transbond XT primer (3M Unitek, Monrovia, Calif) was applied to the etched surface as a thin uniform coat. Stainless-steel premolar brackets (G&H Wire Company, Greenwood, Ind), with a base surface area of 10 mm2 (according to the manufacturer's specifications), were bonded to the teeth using the standard protocols according to the manufacturer's instructions. Transbond XT composite (3M Unitek) was applied to the base of the brackets, and the brackets were positioned on the center of the buccal surfaces, pressed firmly against the tooth. The excess adhesive around the bracket was removed with a scaler, and the adhesive was light cured from the mesial and distal directions for 10 seconds each direction (total time = 20 seconds). A light-emitting diode unit (Elipar Freelight 2®, 3M-Espe, St Paul, Minn) was used for curing the specimens.
The other three groups were the test groups, and the enamel demineralization process was performed on these groups for 3 weeks. In groups III and IV remineralization agents (fluoride and CPP-ACP) were applied to demineralized enamel sequentially before brackets were bonded.
Group II
In this group, brackets were directly bonded to the demineralized enamel. The same bracket bonding procedure was used that was described for group I.
Group III
In this group, Fluoridin N5® (Fluoride gel; Voco-GmbH, Cuxhaven, Germany) was applied to the demineralized enamel before bonding. The fluoride agent was left undisturbed on the tooth surface for 5 minutes and was then rinsed with deionized water. After 6 hours the topical agent was reapplied to the tooth surface using the same method. This procedure was repeated 10 times for remineralization. During these cycles all teeth were stored in artificial saliva. After this step, brackets were bonded using the standard protocol.
Group IV
In this group, Tooth Mousse® (CPP-ACP gel; GC-Corp, Tokyo, Japan) was used instead of Fluoridin N5® according to the same protocol as described for group III.
Demineralization
The demineralization procedure was adopted from that of Hu and Featherstone.25 With this procedure sequential demineralization and remineralization were performed in order to mimic the remineralizing stage of the caries process. Each tooth was immersed in demineralization solution (pH 4.3) for 6 hours at 37°C, then teeth were removed from the solution, rinsed with deionized water, and immersed in remineralization solution (pH 7.0) for 18 hours at 37°C. The cycling procedure was repeated daily for 3 weeks. Compositions of demineralization and remineralization solutions are presented in Table 1.
Table 1.
Composition of Demineralization and Remineralization Solutions
At day 21, teeth were dried and evaluated for frosty-white appearance of enamel. Presence of demineralization of the teeth was confirmed by a portable battery-powered laser fluorescence device (DIAGNOdent Pen; KaVo, Germany). Next, all teeth were removed from the solutions. At this stage, the allocation of teeth into groups II through IV after demineralization was randomized. The DIAGNOdent Pen scores of each group were determined and recorded. Statistical comparison of these scores showed no significant differences in demineralization among these groups. After demineralization, teeth were stored in artificial saliva (20 mmol/L NaHCO3; 3 mmol/L NaH2PO4; and 1 mmol/L CaCl2, neutral pH) for 30 days.
Debonding Procedure
After completion of the procedures, the embedded specimens were secured in a jig attached to the base plate of an Instron Testing Machine (Instron Corp, Norwood, Mass). A chisel-edge plunger was mounted in the movable crosshead of the testing machine and positioned so that the leading edge was aimed at the enamel-adhesive interface. A crosshead speed of 0.5 mm/min was used, and the maximum load necessary to debond the bracket was recorded. The force required to remove the brackets was measured in Newtons (N), and the SBS (1 MPa = 1 N/mm2) was then calculated by dividing the force values by the bracket base area (10 mm2).
Fracture Analysis
Fracture analyses were performed using an optical stereomicroscope (SZ 40; Olympus, Tokyo, Japan) at 20× magnification. The remnant adhesive on the enamel surface was coded by one investigator who was blinded to group allocations. Any adhesive remaining after bracket removal was assessed with the ARI.26,27 Failures were ranked from zero to three, as follows: 0 = no adhesive remaining on the enamel surface; 1 = less than 50% adhesive remaining on tooth; 2 = more than 50% adhesive remaining on tooth; and 3 = all adhesive remaining on tooth surface.
Statistical Methods
All statistical analyses were performed with the Statistical Package for the Social Sciences software package (SPSS for Windows 13.0, SPSS, Chicago, Ill) and Applet “Frequency Matrix Applet,” Version 3.1. The normality test of Shapiro-Wilks and the Levene's variance homogeneity test were applied to the data. The data were found to be normally distributed, and there was homogeneity of variance among the groups. Thus, the statistical evaluation of SBS values among test groups was performed using parametric tests.
Descriptive statistics, including the mean, standard deviation, and minimum and maximum values, were calculated for the four groups of teeth tested. Comparisons of means of SBS values were made with an analysis of variance (ANOVA). Post hoc multiple comparisons were done with the Tukey Honestly Significance Difference test. The G-test was used to determine significant differences in the ARI scores among the groups. The statistical significance level was set at P < .05.
RESULTS
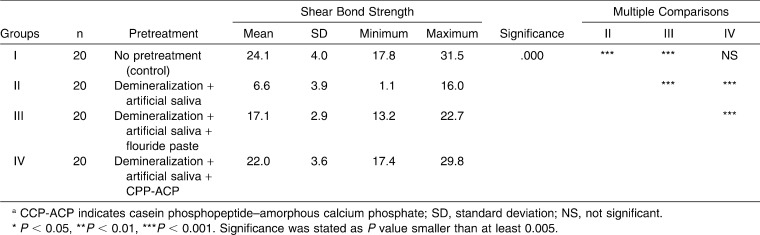
The descriptive statistics and statistical comparison of the groups are presented in Table 2. The results of the ANOVA indicated statistically significant differences among the four groups (P < .001). Thus, the first part of the null hypothesis of this study was rejected. The mean SBS of the control group (group I: 24.1 ± 4.0 MPa) was greater than that of the test groups. Group IV (22.0 ± 3.6 MPa) showed the greatest mean SBS value among test groups, and this difference was found to be statistically significant (P = .000). The fluoride-pretreated group (group III: 17.1 ± 2.9 MPa) showed higher SBS values when compared to group II (6.6 ± 3.9 MPa) (P = .000). The differences between control and CPP-ACP–pretreated groups were not statistically significant.
Table 2.
Descriptive Statistics and Results of Analysis of Variance (ANOVA) Comparing Shear Bond Strengthsa
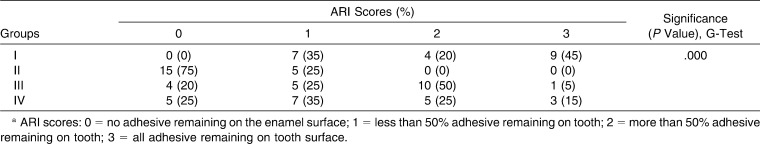
The G-test results indicated significant differences among groups regarding the fracture mode (Table 3). Therefore, the second part of the null hypothesis of this study was also rejected. No enamel detachment was found in the control group, and the control group showed a higher percentage of ARI scores of 3. Enamel detachment was 75% for group II.
Table 3.
Frequency Distrubution of Adhesive Remnant Index (ARI) Scores of the Groupsa
DISCUSSION
Several investigators have evaluated the effects of fluoride and CPP-ACP products21–24,28 on the SBS of orthodontic brackets. But in all but one of the studies,28 these agents were used for prophylactic purposes; in the study of Keles,28 CPP-ACP solutions were used after the demineralization process. In the present study we used fluoride and CPP-ACP for remineralization and evaluated the bond strength of orthodontic brackets with SBS testing and ARI scores.
In this study, the demineralization process was adopted from that of Hu and Featherstone.25 The authors advocated their pH cycling method as a well-established laboratory cycling. The process included both remineralization and demineralization stages, similar to those that occur in the intraoral environment. In this way, acid attacks were simulated with demineralization solutions, and remineralization by saliva was performed with remineralization solutions. Although they performed 14 days of pH cycling, in our study we used 21 days, as the frosty-white appearance of enamel could be obtained after 21 days.
According to Arends and Christoffersen,29 artificial caries lesions may not be identical to natural caries lesions, but they are quite similar. They also suggested that the advantage of artificial caries lesions studies was the possibility of testing mechanisms and parameters.
A routine etching removes 3–10 µm of surface enamel; another 25 µm reveals subtle histological alterations creating the necessary mechanical interlocks. After removal of enamel by demineralization, adhesive resin application led to evenly distributed rows of tags.30 In this study, the SBSs of demineralized specimens were lower than those of the other groups. This may be attributed to the poor quality of the enamel surface and the lack of resin tags that form the mechanical interlock. Keles28 evaluated the SBS values in control, demineralization, remineralization, and prophylaxis subgroups. In study groups, sequential remineralization and demineralization were performed for the formation of enamel lesions. Similar to the present findings, they found the highest SBS values for the untreated control group. Prophylaxis and remineralization groups showed similar SBS values. The only statistical difference occurred between the demineralization and other groups. In the demineralization group, lower SBS scores were recorded.
We determined that the SBSs of CPP-ACP–treated samples were higher compared to the demineralization group, and there were no significant differences between the control and CPP-ACP–treated groups. It would be reasonable to think that CPP-ACP led to an identical enamel surface composition as observed in the teeth in the control group. The current results were in accordance with those of the Keles study,28 while Keçik et al.21 and Xianojun et al.22 found that the SBS values of CPP-ACP–treated samples were higher than that of the control group. This was the opposite of our study's findings. These differences may be the result of the different bonding materials used in the above-mentioned studies.
It has been suggested24,31–33 that topical fluoride application interferes with the etching effect of phosphoric acid on enamel and results in reduced bond strength of dental resins. Gwinnet and Smith34 explained the reduction in bond strengths by the disrupting formation of enamel tags. Moreover, teeth with high fluoride compositions are considered to be more resistant to acid etching.24 Fluoride-pretreated teeth demonstrated a 40% reduction in bond strengths.35 Tabrizi and Cakirer24 found lower SBS values in the fluoride-pretreated group compared to the control. On the other hand, Keçik et al.21 found contrary results. In our study, the SBS values of fluoride-pretreated teeth were lower than those of the control and CPP-ACP groups. But the SBS values for the fluoride-treated group were higher than the critical limit suggested by Reynolds.36 The highly variable results regarding the use of fluoride and SBS values may be attributed to the different concentrations and different fluoride applications used in the studies.
Keçik et al.,21 Xianojun et al.,22 Tabrizi and Cakirer24 and Keles28 reported no significant differences between debonding sites. Keles28 emphasized that in the demineralization group, SBS testing lead to cracks in enamel, and enamel particles' were seen at the bracket base. In this study, statistically significant differences were found between groups with regard to the ARI scores. In the control group, no detachment was found at the enamel-composite interface, whereas in group II (demineralization), 75% of total detachments were of this type. The weak bonding between enamel and composite in the demineralization group may explain the poor bond quality of demineralized enamel.
According to Reynolds,36 5.9–7.8-MPa SBS values are adequate for orthodontic purposes. In our study, both CPP-ACP and fluoride applications led to SBS values that were greater than this. Thus, the results of the present study indicated that pretreatment of demineralized enamel with CPP-ACP increases the SBS of orthodontic brackets. In addition, fluoride application after demineralization leads to clinically acceptable SBS values.
CONCLUSIONS
Demineralization significantly reduces the SBS of orthodontic brackets.
Remineralization with fluoride or CPP-ACP improved bonding to demineralized enamel.
CPP-ACP pretreatment is more efficient than fluoride pretreatment for bonding orthodontic brackets.
Higher failures were found in the composite-enamel interface in untreated demineralized samples.
REFERENCES
- 1.Zachrisson B. U, Zachrisson S. Caries incidence and oral hygiene during orthodontic treatment. Scand J Dent Res. 1971;79:394–401. doi: 10.1111/j.1600-0722.1971.tb02028.x. [DOI] [PubMed] [Google Scholar]
- 2.Mizrahi E. Enamel demineralization following orthodontic treatment. Am J Orthod. 1982;82:62–67. doi: 10.1016/0002-9416(82)90548-6. [DOI] [PubMed] [Google Scholar]
- 3.Gorelick L, Geiger A. M, Gwinnett A. J. Incidence of white spot formation after bonding and banding. Am J Orthod. 1982;81:93–98. doi: 10.1016/0002-9416(82)90032-x. [DOI] [PubMed] [Google Scholar]
- 4.O'Reilly M. M, Featherstone J. D. Demineralization and remineralization around orthodontic appliances: an in vivo study. Am J Orthod Dentofacial Orthop. 1987;92:33–40. doi: 10.1016/0889-5406(87)90293-9. [DOI] [PubMed] [Google Scholar]
- 5.Donly K, Sasa I. Potential remineralization of postorthodontic demineralized enamel and the use of enamel microabrasion and bleaching for esthetics. Semin Orthod. 2008;14:220–225. [Google Scholar]
- 6.Boersma J. G, van der Veen M. H, Lagerweij M. D, Bokhout B, Prahl-Andersen B. Caries prevalence measured with QLF after treatment with fixed orthodontic appliances: influencing factors. Caries Res. 2005;39:41–47. doi: 10.1159/000081655. [DOI] [PubMed] [Google Scholar]
- 7.Ten Cate J. M. Current concepts on the theories of the mechanism of action of fluoride. Acta Odontol Scand. 1999;57:325–329. doi: 10.1080/000163599428562. [DOI] [PubMed] [Google Scholar]
- 8.Reynolds E. C, Cai F, Cochrane N. J, Shen P, Walker G. D, Morgan M. V, Reynolds C. Fluoride and casein phosphopeptide–amorphous calcium phosphate. J Dent Res. 2008;87:344–348. doi: 10.1177/154405910808700420. [DOI] [PubMed] [Google Scholar]
- 9.Reynolds E. C, Cain C. J, Webber F. L, et al. Anticariogenicity of calcium phosphate complexes of tryptic casein phosphopeptides in the rat. J Dent Res. 1995;74:1272–1279. doi: 10.1177/00220345950740060601. [DOI] [PubMed] [Google Scholar]
- 10.Shen P, Cai F, Nowicki A, Vincent J, Reynolds E. C. Remineralization of enamel subsurface lesions by sugar-free chewing gum containing casein phosphopeptide–amorphous calcium phosphate. J Dent Res. 2001;80:2066–2070. doi: 10.1177/00220345010800120801. [DOI] [PubMed] [Google Scholar]
- 11.Iijima Y, Cai F, Shen P, Walker G, Reynolds C, Reynolds E. C. Acid resistance of enamel subsurface lesions remineralized by a sugar-free chewing gum containing casein phosphopeptide–amorphous calcium phosphate. Caries Res. 2004;38:551–556. doi: 10.1159/000080585. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds E. C, Cai F, Shen P, Walker G. D. Retention in plaque and remineralization of enamel lesions by various forms of calcium in a mouth rinse or sugar-free chewing gum. J Dent Res. 2003;82:206–211. doi: 10.1177/154405910308200311. [DOI] [PubMed] [Google Scholar]
- 13.Low T, von Fraunhofer J. A, Winter G. B. The bonding of a polimeric fissure sealant to topical fluoride-treated teeth. J Oral Rehabil. 1975;2:303–307. doi: 10.1111/j.1365-2842.1975.tb00922.x. [DOI] [PubMed] [Google Scholar]
- 14.Gwinnett A. J, Buonocore M. G, Sheykholeslam Z. Effect of fluoride on etched human and bovine tooth enamel surfaces as demonstrated by scanning electron microscopy. Arch Oral Biol. 1972;117:271–278. doi: 10.1016/0003-9969(72)90210-5. [DOI] [PubMed] [Google Scholar]
- 15.Merrill J. M, Shannon I. L. Effect of pretreatment with fluoride solutions on tensile strength between bonding resin and acid-etched enamel. Int J Orthod. 1980;18:7–14. [PubMed] [Google Scholar]
- 16.Sheykholeslam Z, Buonocore M. G, Gwinnett A. J. Effect of fluorides on the bonding of resins to phosphoric acid–etched bovine enamel. Arch Oral Biol. 1972;17:1037–1045. doi: 10.1016/0003-9969(72)90178-1. [DOI] [PubMed] [Google Scholar]
- 17.Hirce J. D, Sather A. H, Chao E. Y. S. The effect of topical fluorides, after acid etching of enamel, on the bond strength of directly bonded orthodontic brackets. Am J Orthod. 1980;78:444–452. doi: 10.1016/0002-9416(80)90025-1. [DOI] [PubMed] [Google Scholar]
- 18.Thornton J. B, Retief D. H, Bradley E. L, Denys F. R. The effect of fluoride in phosphoric acid on enamel uptake and the tensile bond strength of an orthodontic bonding resin. Am J Orthod Dentofacial Orthop. 1986;90:91–101. doi: 10.1016/0889-5406(86)90039-9. [DOI] [PubMed] [Google Scholar]
- 19.Buyukyilmaz T, Ogaard B, Dahm S. The effect on the tensile bond strength of orthodontic brackets of titanium tetrafluoride (TiF4) application after acid etching. Am J Orthod Dentofacial Orthop. 1995;108:256–261. doi: 10.1016/s0889-5406(95)70018-8. [DOI] [PubMed] [Google Scholar]
- 20.Damon P. L, Bishara S. E, Olsen M. E, Jakobsen J. R. Effects of fluoride application on shear bond strength of orthodontic brackets. Angle Orthod. 1996;1:61–64. doi: 10.1043/0003-3219(1996)066<0061:EOFAOS>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 21.Keçik D, Çehreli S. B, Şar Ç, Ünver B. Effect of acidulated phosphate fluoride and casein phosphopeptide–amorphous calcium phosphate application on shear bond strengths of orthodontic brackets. Angle Orthod. 2008;78:129–133. doi: 10.2319/122506-529.1. [DOI] [PubMed] [Google Scholar]
- 22.Xiaojun D, Xuehua G, Jing L, Hong R, Youcheng Y, Zhangyu G, Sun J. Effects of CPP-ACP paste on the shear bond strength of orthodontic brackets. Angle Orthod. 2009;79:945–950. doi: 10.2319/101108-573.1. [DOI] [PubMed] [Google Scholar]
- 23.Moule C. A, Angelis F, Kim G. H, Le S, Malipatil S, Foo M. S, Burrow M. F, Thomas D. Resin bonding using an all-etch or self-etch adhesive to enamel after carbamide peroxide and/or CPP-ACP treatment. Aust Dent J. 2007;52:133–137. doi: 10.1111/j.1834-7819.2007.tb00478.x. [DOI] [PubMed] [Google Scholar]
- 24.Tabrizi A, Cakirer B. A comparative evaluation of casein phosphopeptide–amorphous calcium phosphate and fluoride on the shear bond strength of orthodontic brackets. Eur J Orthod. 2010 doi: 10.1093/ejo/cjq062. doi:10.1093/ejo/cjq062. [DOI] [PubMed] [Google Scholar]
- 25.Hu W, Featherstone J. D. Prevention of enamel demineralization: an in-vitro study using light-cured filled sealant. Am J Orthod Dentofacial Orthop. 2005;128:592–600. doi: 10.1016/j.ajodo.2004.07.046. [DOI] [PubMed] [Google Scholar]
- 26.Årtun J, Bergland S. Clinical trials with crystal growth conditioning as an alternative to acid-etch enamel pretreatment. Am J Orthod. 1984;85:333–340. doi: 10.1016/0002-9416(84)90190-8. [DOI] [PubMed] [Google Scholar]
- 27.Oliver R. G. The effect of different methods of bracket removal on the amount of residual adhesive. Am J Orthod Dentofacial Orthop. 1988;93:196–200. doi: 10.1016/s0889-5406(88)80003-9. [DOI] [PubMed] [Google Scholar]
- 28.Keles K. Evaluation of Shear Bond Strength After Remineralization of Enamel Subsurface Lesion by CPPACP In Vitro Study [PhD thesis] Adana, Turkey: University of Cukurova. 2010; [Google Scholar]
- 29.Arends J, Christoffersen J. Invited review article: the nature of early caries lesions in enamel. J Dent Res. 1986;65:2–11. doi: 10.1177/00220345860650010201. [DOI] [PubMed] [Google Scholar]
- 30.Zachrisson B. U. Bonding in orthodontics. In: Graber T. M, Swain B. F, editors. Orthodontics Current Principles and Techniques. St Louis, Mo: Mosby; 1985. pp. 485–563. [Google Scholar]
- 31.Cacciafesta V, Sfondrini M. F, Calvi D, Scribante A. Effect of fluoride application on shear bond strength of brackets bonded with a resin-modified glass-ionomer. Am J Orthod Dentofacial Orthop. 2005;127:580–583. doi: 10.1016/j.ajodo.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 32.Meng C. L, Li C. H, Wang W. N. Bond strength with APF applied after acid etching. Am J Orthod Dentofacial Orthop. 1998;114:510–513. doi: 10.1016/s0889-5406(98)70170-2. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Godoy F, Hubbard G. W, Storey A. T. Effect of fluoridated etching gel on enamel morphology and shear bond strength of orthodontic brackets. Am J Orthod Dentofacial Orthop. 1991;100:163–170. doi: 10.1016/S0889-5406(05)81523-9. [DOI] [PubMed] [Google Scholar]
- 34.Gwinnett A. J, Smith D. C. Fissure sealant. In: Smith D. C, Williams D. F, editors. Biocompatibility of Dental Materials Vol 2. Boca Raton, Fla: CRC Press; 1982. pp. 15–49. [Google Scholar]
- 35.Opinya G. N, Parmeijer C. H. Tensile bond strength of fluorosed Kenyan teeth using the acid etch technique. Int Dent J. 1986;36:225–229. [PubMed] [Google Scholar]
- 36.Reynolds I. R. A review of direct orthodontic bonding. Br J Orthod. 1975;2:171–178. [Google Scholar]