Abstract
Objective:
To test the null hypotheses that there are no significant differences in craniofacial structures and orofacial airway dimensions in subjects with Class I malocclusion and different growth patterns.
Materials and Methods:
Lateral cephalometric radiographs of 31 low angle (mean age, 14.0 ± 2.0 years; range, 10.3–16.5 years), 40 high angle (mean age, 12.7 ± 1.6 years; range, 10.1–16.2 years), and 33 normal growth (mean age, 13.9 ± 1.3 years; range, 11.2–16.8 years) subjects with Class I malocclusion were examined. In total, 34 measurements (27 craniofacial and 7 orofacial airways) were evaluated. Groups were constituted according to the SN-MP angle. Group differences were analyzed with analysis of variance (ANOVA) and the Tukey test, at the P < .05 level.
Results:
According to ANOVA, only 5 of the 27 craniofacial measurements showed no statistically significant differences among different growth patterns. For orofacial airway measurements, statistically significant differences were found in nasopharyngeal airway space (P < .01), palatal tongue space (P < .05), upper posterior airway space (PAS) (P < .05), and tongue gap (P < .001). No statistically significant orofacial airway differences were determined between low angle and normal growth subjects. High angle subjects had a larger tongue gap than those with normal and low angles (P < .01). Additionally, nasopharyngeal airway space (P < .01) and upper PAS (P < .05) measurements were larger and palatal tongue space (P < .05) was narrower in low angle than in high angle subjects.
Conclusions:
The null hypotheses were rejected. Significant differences in craniofacial morphology and orofacial airway dimensions of Class I subjects with different growth patterns were identified.
Keywords: Airway measurements, Growth patterns, Tongue
INTRODUCTION
Vertical malocclusions can be sourced as predominantly skeletal or dentoalveolar. Various etiologic factors, including dentoalveolar development, growth of the maxilla and mandible, function of the tongue and lips, and eruption of the teeth, may cause vertical malocclusion during the growth period.1
Sagittal facial growth is seen as downward and forward growth.2,3 Isaacson et al.3 and Schudy4 indicated that vertical growth of condyles is lesser than vertical growth of facial sutures and alveolar processes, resulting in backward mandibular rotation and bite opening. On the contrary, if vertical growth of condyles is greater than vertical growth of facial sutures and molar areas, forward mandibular rotation and bite closing are seen. Therefore, the ultimate vector of mandibular growth is a consequence of the competition between horizontal and vertical growth.5 An interaction occurs between respiratory function and the maxillary6 and mandibular growth pattern.7
Pharyngeal space size is determined primarily by relative growth and size of the soft tissues surrounding the dentofacial skeleton. From adulthood to older age (20–50 years of age), the nasopharyngeal skeleton may change.8
Craniofacial anomalies, including mandibular or maxillary retrognathism, short mandibular body, and backward and downward rotation of the mandible, may lead to reduction of the pharyngeal airway passage.9 Decreased space between the mandibular corpus and the cervical column may lead to changes in posture of the tongue and soft palate posteriorly, may impair respiratory function during the day, and may cause possible nocturnal problems such as snoring, upper airway resistance syndrome, and obstructive sleep apnea.10
Some authors11,12 have reported associations between vertical growth pattern and obstruction of the upper and lower pharyngeal airways and mouth breathing. If this relationship presents, vertical growth patterns and Class II malocclusions are required to reveal anatomic predisposing factors.11
A significant relationship exists between airway space and facial morphology13; also, airway space may be affected by conditions such as functional anterior shifting,14 head posture,15 sagittal skeletal relation,16 and maxillary protraction.17 Consequently, healthy subjects with Class I skeletal malocclusions and vertical growth patterns might have narrower airway passages than healthy subjects with horizontal growth patterns. To investigate this assumption, the main aim of this study was to compare craniofacial dimensions and widths of the orofacial airways and tongue in healthy Class I subjects with different vertical growth patterns (low, normal, and high angle). For these purposes, the null hypothesis assumed that no significant differences were present in craniofacial measurements or orofacial airway dimensions of Class I subjects with different vertical growth patterns.
MATERIALS AND METHODS
This study was approved by the Regional Ethical Committee on Research of the Erciyes University, Faculty of Dentistry.
A power analysis was established by G*Power, version 3.0.10 (Franz Faul Universität, Kiel, Germany); based on a 1∶1 ratio between groups, a sample size of 31 subjects would yield more than 80% power to detect significant differences, with 0.30 effect size (to detect a clinically meaningful difference of 1 mm [±1.5 mm] in the distance of the Pog to N perp) among groups and at the α = .05 significance level.
In the present study, 95 low angle, 185 high angle, and 50 normal vertical growth Class I subjects were evaluated, and 104 subjects were selected by the sample selection criteria presented in Table 1. Pretreatment cephalometric radiographs of 104 subjects taken by a standard technique were collected. Subjects were divided into three groups according to growth pattern: Group I, low angle; Group II, high angle; and Group III, normal growth.
Table 1.
Adopted Criteria for Sample Selection
The sample included 31 low angle, 40 high angle, and 33 normal vertical growth subjects admitted to the Department of Orthodontics, University of Erciyes, who needed orthodontic treatment. All subjects had a Class I skeletal relationship (ANB: 2.8 ± 1.4 degrees, 3.0 ± 1.2 degrees, and 3.09 ± 1.09 degrees in low angle, high angle, and normal growth subjects, respectively). Before participation in the study, written informed consents were given by the parents of all subjects.
Different vertical growth patterns were categorized according to the SN-MP angle (low angle, <26 degrees; normal angle, 26–38 degrees; and high angle, >38 degrees). These factors were considered for the diagnosis of vertical growth pattern according to Isaacson et al.3 Group I (low angle) comprised 14 boys and 17 girls (mean, 14.0 ± 2.0 years; age range, 10.3–16.5 years), Group II (high angle) included 14 boys and 26 girls (mean, 12.7 ± 1.6 years; age range, 10.1–16.2 years), and Group III (normal) consisted of 8 boys and 25 girls (mean, 13.9 ± 1.3 years; age range, 11.2–16.8 years) (Table 2). This group was chosen at random from a group of children who had varied orthodontic problems according to inclusion criteria. Orofacial airway dimensions were evaluated according to Jung et al.13
Table 2.
Sex and Number Distribution of Subjects by Vertical Growth Pattern and Agea
Cephalometric Measurements
Lateral cephalometric radiographs were taken with the Instrumentarum Cephalometer (Ortoceph OC100, Tuusula, Finland). All subjects were positioned in the cephalostat with the sagittal plane at a right angle to the path of the x-rays; the Frankfort plane was parallel to the horizontal plane, the teeth were in centric occlusion, and the lips were lightly closed.
All radiographs were traced manually, and whole angular and linear measurements were recorded by a single author (FIU) and were reviewed twice by other investigators for accurate landmark identification.
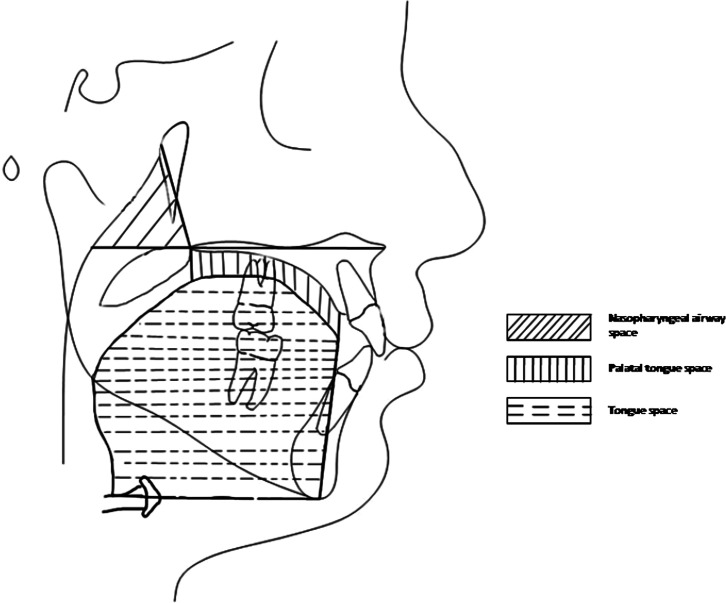
Landmarks and reference lines used for craniofacial measurements are shown in Table 3, and orofacial airway dimensions are shown in Figure 1. Fifteen angular (Figures 2 and 3) and 12 linear (Figure 4) measurements were used for craniofacial evaluation. Additionally, seven measurements were used for orofacial airway dimensions (Figures 5 and 6).
Table 3.
Description of Measurements Used in the Study
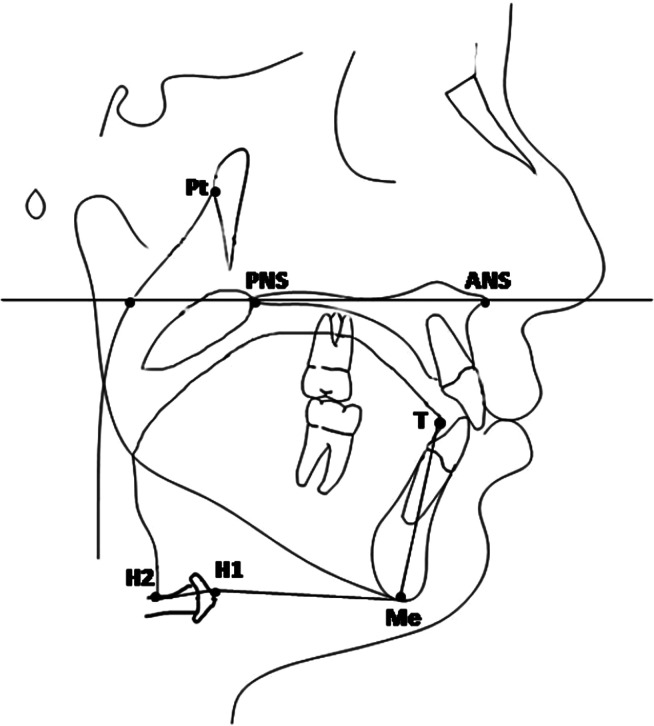
Figure 1.
Landmarks and reference lines used for orofacial airway space: Pt (pterygoid point), the posterior point of the pterygopalatine fossa; ANS (anterior nasal spine), anterior point of the maxilla; PNS (posterior nasal spine), posterior point of the palatine bone; Me (Menton), the inferior point of the symphysis; H1, intersection between the posterior border of the tongue and the hyoid bone; H2, the most anterior point of the hyoid bone; T, the most anterior point of the outline of the tongue; palatal plane, a line passing through the ANS and PNS.
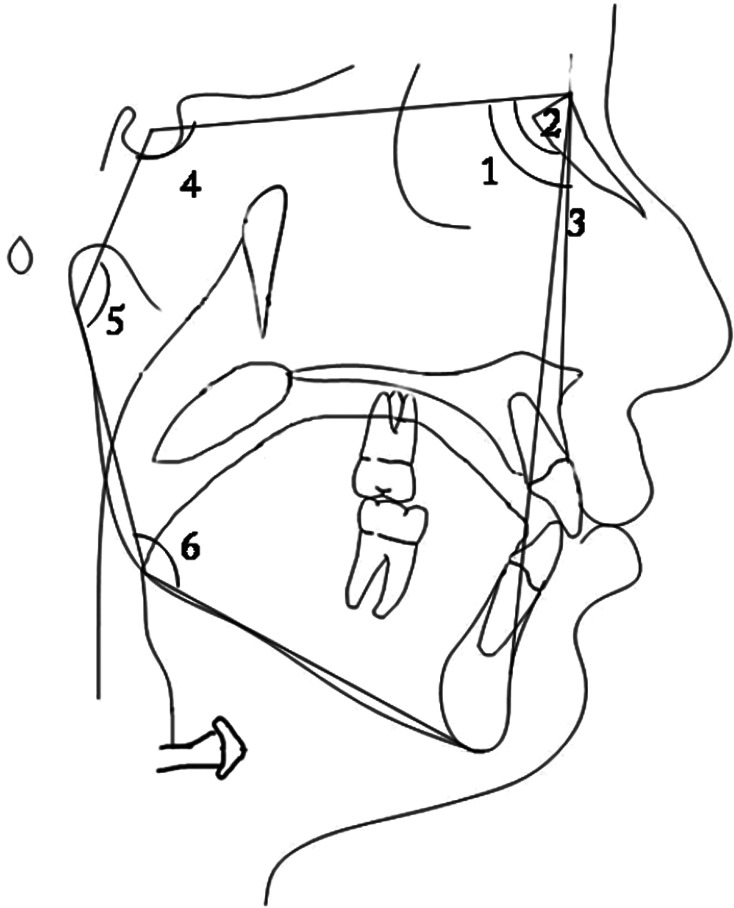
Figure 2.
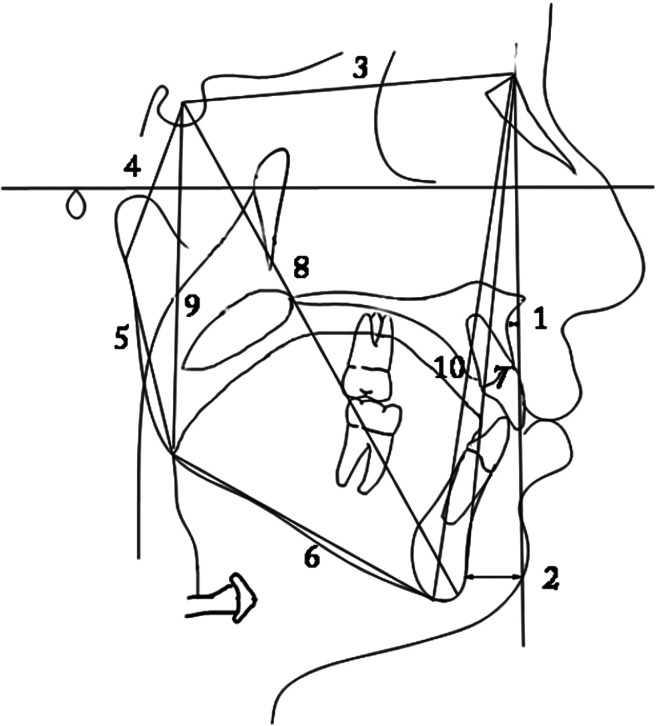
(1) SNA; (2) SNB; (3) ANB; (4) SN-Ar; (5) articular angle; and (6) Ar-Go/MP.
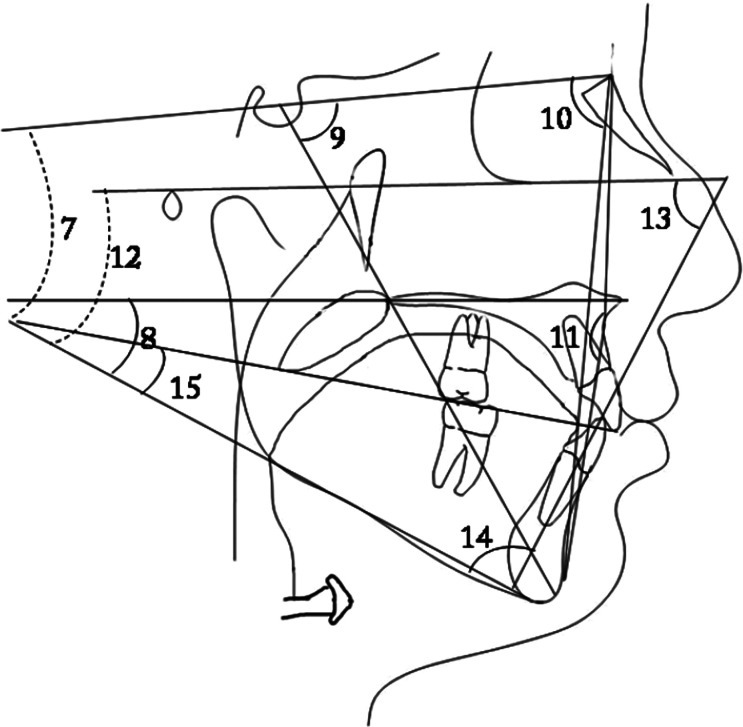
Figure 3.
(7) SN-MP; (8) PP-GoGn; (9) Y-Axis; (10) SN-Npog; (11) NA-Apog; (12) FMA; (13) FMIA; (14) IMPA; and (15) MP-OP.
Figure 4.
(1) A to N perp; (2) Pog to N perp; (3) S-N; (4) S-Ar; (5) Ar-Go; (6) Go-Gn; (7) NGo; (8) S-Gn; (9) posterior facial height; and (10) anterior facial height.
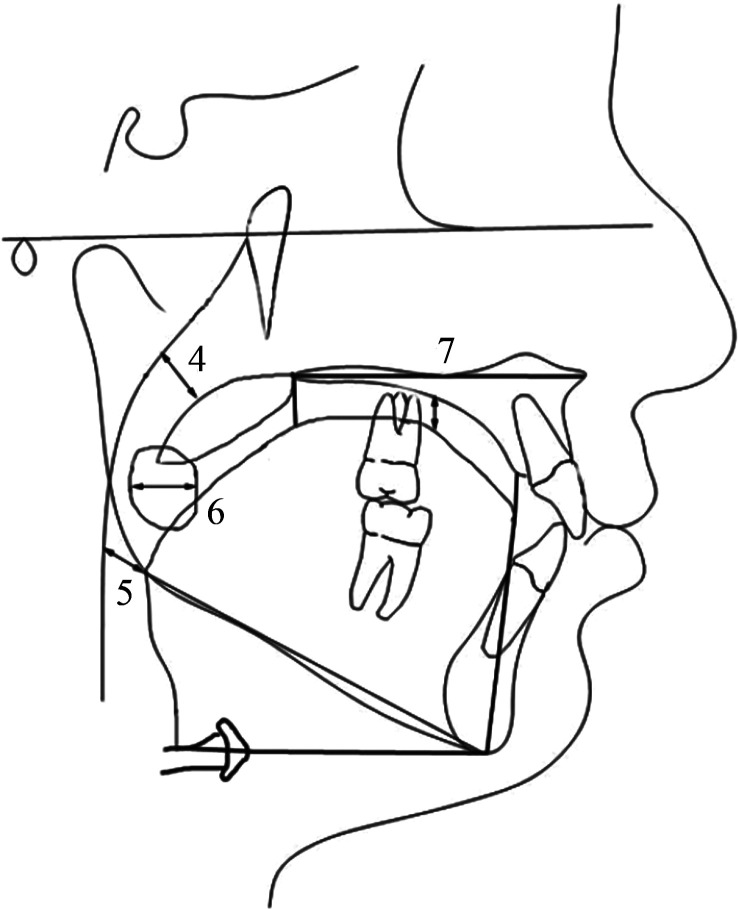
Figure 5.
Nasopharyngeal airway space: formed by palatal plane and the Pt-PNS posterior nasopharyngeal line. Palate-tongue space: space between tongue and palate from the line perpendicular to the palatal plane at the incisive foramen to the line perpendicular to the palatal plane at the PNS. Tongue space: area formed by superior and posterior borders of the tongue and T, Me, H1, and H2.
Figure 6.
(5) Upper posterior airway space; (6) lower posterior airway space; (7) tonsil size; and (8) tongue gap.
Statistical Analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS), version 13.0 (SPSS Inc., Chicago, Ill). The normality test of Shapiro-Wilks and Levene's variance homogeneity test were applied to the data, which were found to be normally distributed, and homogeneity of variance was noted among groups. Thus, statistical evaluation of cephalometric values between test groups was performed using parametric tests.
To keep the distribution of sex and age in balance among the three different vertical growth patterns, a chi-square test was performed. Arithmetic mean and standard deviation values were calculated for each measurement. Group differences were analyzed with one-way analysis of variance (ANOVA). For multiple comparisons, a post hoc Tukey honestly significant difference (HSD) test was used. When the P value was less than .05, the statistical test was regarded as significant.
To identify errors associated with radiographic measurements, 15 radiographs were selected randomly. Their tracings and measurements were repeated 8 weeks after the first measurements were taken. A paired sample t-test was applied to the first and second measurements, and the differences between measurements were insignificant. Correlation analysis applied to the same measurements showed the highest r value (0.988) for the overbite and the lowest r value (0.867) for NA-APog and IMPA measurements.
RESULTS
According to ANOVA, only 5 of the 27 craniofacial measurements showed no statistically significant differences among three vertical growth patterns. Thus, this part of the null hypothesis was rejected.
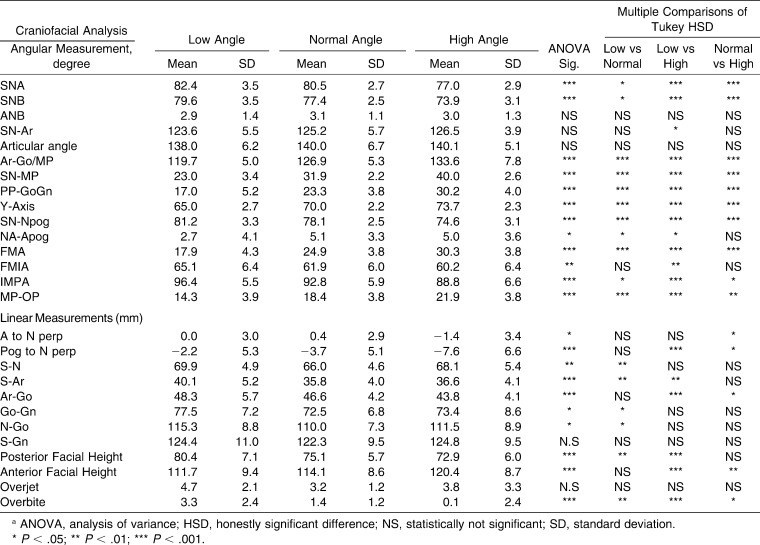
Significant differences among groups were observed in SNA, SNB, Ar-Go/MP, SN-MP, PP-GoGn, Y-Axis, SN-Npog, FMA, IMPA, and MP-OP (P < .001) and in FMIA (P < .01) and NA-Apog (P < .05) (Table 4). In addition, all linear measurements were found to be statistically different among groups, except S-Gn and overjet (Table 4).
Table 4.
Descriptive Statistics and Statistical Comparisons (ANOVA) of Angular and Linear Craniofacial Measurements Among Different Growth Patternsa
Multiple comparisons of the groups in terms of angular measurements showed that SNA, SNB, SN-Npog, and IMPA angles were decreased from low angle to normal to high angle subjects (P < .001). In addition, Ar-Go/MP, SN-MP, PP-GoGn, Y-Axis, FMA, and MP-OP measurements were increased from low angle to normal to high angle (P < .001). The FMIA angle was statistically lower in high angle cases (P < .01) (Table 4). Multiple comparisons of the groups via Tukey HSD for linear measurements showed that Go-Gn, N-Go, S-N, and S-Ar values were increased in the high angle group compared with other groups (P < .01). Posterior facial height was greater in low angle subject, and anterior facial height was greater in the high angle group; these differences were found to be statistically significant (P < .01) (Table 4).
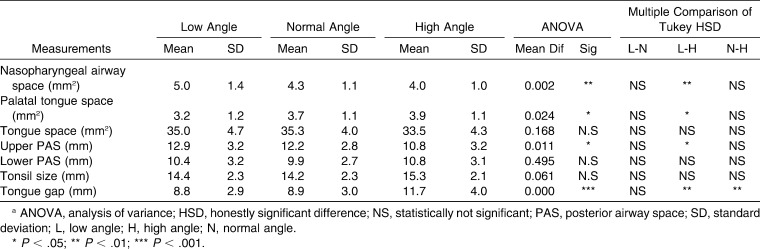
According to ANOVA results, statistically significant differences were found in nasopharyngeal airway space, palatal tongue space, upper posterior airway space (PAS), and tongue gap measurements (Table 5). As a result, this part of the null hypothesis was also rejected.
Table 5.
Descriptive Statistics and Results of ANOVA Comparisons of Airway Space Measurements in All Groupsa
Pairwise comparisons among groups of orofacial airway measurements were also done via the Tukey HSD test. The data demonstrated a significant difference between low angle and high angle groups at the level of the nasopharyngeal airway space, palatal tongue space, upper PAS, and tongue gap. Nasopharyngeal airway space decreased from low angle to normal to high angle (P < .01). In contrast, palatal tongue space increased from high angle to normal to low angle (P < .05). The sagittal dimension of the superior part of the upper airway (upper PAS) decreased from low angle to normal to high angle (P < .05). The most significant difference was noted from low angle to normal to high angle (P < .001). Tongue gap distance was greater in high angle subjects than in normal and low angle subjects (P < .01) (Table 5).
DISCUSSION
Good compatibility for age and sex was observed in the present cross-sectional study (Table 2). Because only healthy pharyngeal subjects with Class I malocclusion were selected, we estimated that the nasopharyngeal airway space would reflect only natural anatomic conditions without pathology. To eliminate the influences of growth and aging, postpubertal subjects were selected for the current study.
This study was performed with two-dimensional cephalometric films to evaluate only pharyngeal airway width—not airway flow capacity, which would have required a more complex three-dimensional cone beam computed tomography (CBCT) and dynamic estimation.12 Therefore, these results do not suggest that individuals with vertical growth patterns have smaller airway flow capacities than those with normal growth patterns. This should be further investigated.
Malkoc et al.18 has stated that cephalometric films are significantly reliable and reproducible in determining airway dimensions. When computed tomography (CT) and cephalometric films were compared in subjects with skeletal malocclusion, Cameron et al.19 found a significant positive relationship between nasopharyngeal airway size on cephalometric films and its true volumetric size as determined from CBCT scan in adolescents. We used lateral head films for airway measurement, according to the findings of Cameron et al.19 However, we did not measure the anteroposterior dimensions of the airway, so we cannot necessarily determine three-dimensional volumetric measurements with lateral measurements.
Ceylan and Oktay20 reported that changes in the ANB angle affected nasopharyngeal airway size, and that the oropharyngeal space was reduced in subjects with an enlarged ANB angle. Akcam et al.21 found a decrease in the upper airway dimensions of subjects who had posterior mandibular rotation. Similarly, Ucar et al.14 reported a decrease in upper airway space with functional anterior shifting. This reveals a close relationship between the upper airway passage and positioning of the jaws. Sample selection criteria were sensitive, and samples were classified as skeletal Class I, according to the ANB angle.
No statistically significant difference in the lower pharyngeal airways was noted among groups, and no association of the lower pharyngeal airway space was seen with a different vertical growth pattern. This confirms the findings of previous studies.20,22
Joseph et al.9 reported that the nasopharyngeal airway in hyperdivergent individuals was significantly narrower than that in normodivergent individuals. However, they suggested that this difference occurred because of the relative bimaxillary retrusion exhibited by the hyperdivergent group. Their conclusions were similar to those of the present study, in which investigators found a smaller nasopharyngeal airway space in high angle subjects when compared with low angle and normal growth subjects. However, selection criteria of the experimental group in the study reported here included no restriction of the sagittal skeletal pattern; however, classification as skeletal Class I was a requirement.
The relationship between the upper PAS and the vertical facial pattern might be the result of deficient development of the craniomaxillary complex.15 In the present study, analysis of the craniofacial skeleton demonstrated that reduced SNA, SNB, and posterior facial height may explain the lack of deficiency in high angle subjects, which may be caused by a decrease in dimensions of the superior part of the upper airway in high angle subjects. Clinically, we assumed that with bialveolar retrusion, the high angle individual may lack airway dimensions.
Facial growth changes not only are related to differences in the direction of condylar growth but also may result from differences in development of anterior facial height and posterior facial height.3 These differences in vertical development may lead to rotational growth or positional changes of the mandible.
Given that no significant difference in the ANB angle distribution was noted in the subgroups, the impact of a different sagittal skeletal pattern on the superior part of the upper airway was excluded from consideration because sagittal development of the mandible has a significant effect on the PAS.15,22 It is necessary to include all subjects with similar sagittal development of the mandible to eliminate any effect on PAS caused by changes in the sagittal plane, while pharyngeal airway dimensions are evaluated among subjects with different vertical growth patterns. In the current sample, although all subjects had a Class I sagittal relation, decreased SNB showed rotation of the mandible backward and downward in the high angle group and increased SNB in the low angle group, confirmed as forward and upward rotation.
Kerr23 reported that Class II malocclusion subjects showed narrow nasopharyngeal airway space compared with Class I and normal occlusion subjects. However, in his study, the vertical skeletal pattern was not emphasized. In the present study, vertical pattern affected the upper airway space, and greater upper PAS was found in low angle subjects than in high angle subjects.
Although the mandible has both retruded and rotated in downward and backward directions, the tongue base might be positioned more posteriorly and inferiorly; thus, the oropharyngeal airway space may have decreased.9 The present study confirms this result in that the lower pharyngeal airway space was decreased only in high angle subjects who had mandibular retrognathism.
It is known that the tongue position is more backward and that contact with the soft palate may result in a posterior location of the soft palate and narrowing of the oropharyngeal airway in subjects with mandibular retrognathism.24 In our study, the tongue gap between the hard palate and the tongue outline was identical among normal and low angle subjects; however, in high angle subjects who had mandibular retrognathism, the tongue gap was larger than in the others. This situation might be explained by the fact that tongue position may be parallel to downward and backward rotation of the mandible.
Tonsil size was related to the size of the mandible and the horizontal growth pattern in subjects with nasal obstruction.13 Tonsil size was not associated with the vertical growth pattern in this study, possibly because the tonsils are soft tissues and are not affected by skeletal morphology.
CONCLUSIONS
Statistically significant differences were identified in most of the craniofacial measurements among Class I subjects with three different vertical growth patterns.
Nasopharyngeal airway space and upper PAS in Class I subjects were found to be larger in low angle subjects than in high angle subjects.
Palatal tongue space and tongue gap were larger in high angle subjects than in low angle subjects.
Tongue gap was statistically greater in high angle than in normal angle subjects.
REFERENCES
- 1.Nielsen L. Vertical malocclusions: etiology, development, diagnosis and some aspects of treatment. Angle Orthod. 1991;61:247–260. doi: 10.1043/0003-3219(1991)061<0247:VMEDDA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 2.Creekmore T. D. Inhibition or stimulation of the vertical growth of the facial complex, its significance to treatment. Angle Orthod. 1967;37:285–297. doi: 10.1043/0003-3219(1967)037<0285:IOSOTV>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Isaacson J. R, Isaacson R. J, Speidel T. M, Worms F. W. Extreme variation in vertical facial growth and associated variation in skeletal and dental relations. Angle Orthod. 1971;41:219–229. doi: 10.1043/0003-3219(1971)041<0219:EVIVFG>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 4.Schudy F. F. The rotation of the mandible resulting from growth: its implications in orthodontic treatment. Angle Orthod. 1965;35:36–50. doi: 10.1043/0003-3219(1965)035<0036:TROTMR>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 5.Chung C. H, Mongiovi V. D. Craniofacial growth in untreated skeletal Class I subjects with low, average, and high MP-SN angles: a longitudinal study. Am J Orthod Dentofacial Orthop. 2003;124:670–678. doi: 10.1016/j.ajodo.2003.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Gungor A. Y, Turkkahraman H. Effects of airway problems on maxillary growth: a review. Eur J Dent. 2009;3:250–254. [PMC free article] [PubMed] [Google Scholar]
- 7.Jena A. K, Singh S. P, Utreja A. K. Sagittal mandibular development effects on the dimensions of the awake pharyngeal airway passage. Angle Orthod. 2010;80:1061–1067. doi: 10.2319/030210-125.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnston C. D, Richardson A. Cephalometric changes in adult pharyngeal morphology. Eur J Orthod. 1999;21:357–362. doi: 10.1093/ejo/21.4.357. [DOI] [PubMed] [Google Scholar]
- 9.Joseph A. A, Elbaum J, Cisneros G. J, Eisig S. B. A cephalometric comparative study of the soft tissue airway dimensions in persons with hyperdivergent and normodivergent facial pattern. J Oral Maxillofac Surg. 1998;56:135–139. doi: 10.1016/s0278-2391(98)90850-3. [DOI] [PubMed] [Google Scholar]
- 10.Ozbek M. M, Miyamoto K, Lowe A. A, Fleetham J. A. Natural head posture, upper airway anatomy and obstructive sleep apnoea severity in adults. Eur J Orthod. 1998;20:133–143. doi: 10.1093/ejo/20.2.133. [DOI] [PubMed] [Google Scholar]
- 11.Tourne L. P. Growth of the pharynx and its physiologic implications. Am J Orthod Dentofacial Orthop. 1991;99:129–139. doi: 10.1016/0889-5406(91)70115-D. [DOI] [PubMed] [Google Scholar]
- 12.Vig K. W. Nasal obstruction and facial growth: the strength of evidence for clinical assumptions. Am J Orthod Dentofacial Orthop. 1998;113:603–611. doi: 10.1016/s0889-5406(98)70219-7. [DOI] [PubMed] [Google Scholar]
- 13.Jung H. L, Cha K. S, Chung D. H. A study on the correlation between airway space and facial morphology in Class III malocclusion children with nasal obstruction. Korean J Orthod. 2007;37:192–203. [Google Scholar]
- 14.Ucar F. I, Kurt G, Ekizer A, Ramoglu S. I. Effects of functional anterior shifting on skeletal and airway structures. Turkish J Orthod. 2009;22:218–227. [Google Scholar]
- 15.Zhong Z, Tang Z, Gao X, Zeng X. L. A comparison study of upper airway among different skeletal craniofacial patterns in nonsnoring Chinese children. Angle Orthod. 2010;80:267–274. doi: 10.2319/030809-130.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiyama S, Suda N, Suzuki M. I, Tsuiki S, Ogawa M, Suzuki S, Kuroda T. Effects of maxillary protraction on craniofacial structures and upper airway dimension. Angle Orthod. 2002;72:43–47. doi: 10.1043/0003-3219(2002)072<0043:EOMPOC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Oktay H, Ulukaya E. Maxillary protraction appliance effect on the size of the upper airway passage. Angle Orthod. 2008;78:209–214. doi: 10.2319/122806-535.1. [DOI] [PubMed] [Google Scholar]
- 18.Malkoc S, Usumez S, Nur M, Donaghy C. E. Reproducibility of airway dimensions and tongue and hyoid positions on lateral cephalograms. Am J Orthod Dentofacial Orthop. 2005;128:513–516. doi: 10.1016/j.ajodo.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Aboudara C, Nielsen I. B, Huang J. C, Maki K, Miller A. J, Hatcher D. Comparison of airway space with conventional lateral headfilms and 3-dimensional reconstruction from cone-beam computed tomography. Am J Orthod Dentofacial Orthop. 2009;135:468–479. doi: 10.1016/j.ajodo.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 20.Ceylan I, Oktay H. A study of the pharyngeal size in different skeletal patterns. Am J Orthod Dentofacial Orthop. 1995;108:69–75. doi: 10.1016/s0889-5406(95)70068-4. [DOI] [PubMed] [Google Scholar]
- 21.Akcam M. O, Toygar U, Wada T. Longitudinal investigation of soft palate and nasopharyngeal airway relations in different rotation types. Angle Orthod. 2002;72:521–526. doi: 10.1043/0003-3219(2002)072<0521:LIOSPA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 22.DeFreitas M. R, Alcazar N. M, Janson G, DeFreitas K. M. S, Henriques J. F. C. Upper and lower pharyngeal airways in subjects with Class I and Class II malocclusions and different growth patterns. Am J Orthod Dentofacial Orthop. 2006;130:742–745. doi: 10.1016/j.ajodo.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 23.Kerr W. J. The nasopharynx, face height, and overbite. Angle Orthod. 1985;55:31–36. doi: 10.1043/0003-3219(1985)055<0031:TNFHAO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Muto T, Yamazaki A, Takeda S. A cephalometric evaluation of the pharyngeal airway space in patients with mandibular retrognathia and prognathia, and normal subjects. Int J Oral Maxillofac Surg. 2008;37:228–231. doi: 10.1016/j.ijom.2007.06.020. [DOI] [PubMed] [Google Scholar]