Abstract
Objective:
To test if the addition of chlorhexidine digluconate (CHD) might influence the mechanical properties and antibacterial properties of two different conventional glass ionomer cements (GICs) used for band cementation.
Materials and Methods:
Two commercial brands of conventional GICs were used: Ketac Cem Easymix (3M/ESPE, St Paul, Minn) and Meron (Voco, Cuxhaven, Germany). The cements were manipulated in their original composition and also with 10% and 18% CHD in the liquid to create a total of six groups. Diametral tensile strength, compressive strength, microhardness, shear bond strength, and antibacterial effects in 5, 45, and 65 days against Streptococcus mutans were tested in all groups, and the data were submitted to statistical analyses.
Results:
There were no significant differences between the groups of the same material in diametral tensile, compressive strength, and shear bond strength (P > .05). There was significant improvement in the microhardness to the Ketac Cem Easymix (P < .001). GICs with the addition of CHD showed significant inhibition of S. mutans growth in comparison with the control groups at the three time points evaluated (P < .001). The addition of 18% CHD resulted in higher bacterial inhibition (P < .001).
Conclusions:
The addition of chlorhexidine digluconate to conventional GICs does not negatively modify the mechanical properties and may increase the antibacterial effects around the GICs even for relatively long periods of time.
Keywords: Glass ionomer cement, Chlorhexidine digluconate, Mechanical properties, Antibacterial effects
INTRODUCTION
Orthodontic treatment predisposes plaque accumulation around the appliances, mainly around brackets and at the cervical margins of the bands due to difficulty in maintaining hygiene and the large number of sites available for colonization. This problem is one of the greatest concerns to orthodontists because it can lead to the development of enamel decalcification and hyperplasic gingivitis.1–7 Moreover, transient bacteremia during the procedures of banding and debanding has been demonstrated in clinical investigations, and this condition can be a risk for the small number of orthodontic patients who are predisposed to a potential endocarditis.8–11
Glass ionomer cements (GICs) remain the most commonly used material for band cementation.4,12,13 This material exhibits a continuous release and uptake of fluoride, which has certain antibacterial activities.3,4,12–17 However, conventional GICs have an antibacterial effect against a small spectrum of microorganisms and a low bactericide potential. Therefore, GICs may not avoid the plaque proliferation and development of caries and periodontal disease in some patients.4,17–19
Chlorhexidine digluconate (CHD) has been successfully used in dentistry as a component of mouthwashes and varnishes to chemically control plaque formation.1,17–21 Moreover, recent studies have shown that the addition of chlorhexidine digluconate, chlorhexidine dihydrochloride, or chlorhexidine diacetate to resin composites and glass ionomer cements for restoration and cementation can significantly improve the antibacterial effect.5,6,17,19,22
Based on the possibility of obtaining a high antibacterial control around orthodontic bands, the aim of the present study was to assess the incorporation of CHD in two commercial brands of conventional GICs through their mechanical properties and antibacterial effects.
MATERIALS AND METHODS
Two conventional glass ionomer cements used for band cementation were tested in this study: Ketac Cem Easymix (3M/ESPE, St Paul, Minn) and Meron (Voco, Cuxhaven, Germany). Aqueous solutions containing 10% tartaric acid and 10% or 18% of chlorhexidine digluconate were used to manipulate the powder of both cements instead of the regular liquid provided by the manufacturers. In addition, the two cements were also manipulated with conventional liquid which also contain 10% tartaric acid; therefore, four experimental and two control groups were tested.
For the diametral tensile (DTS) and compressive strength (CS) tests, 12 specimens of each material were prepared by inserting the material in cylindrical Teflon molds (6 mm diameter × 3 mm height for DTS, and 3 mm diameter × 6 mm height for CS). The molds were placed over Mylar strips, and after the insertion of the material, another Mylar strip was placed on the top surface followed by another glass slab that was manually pressed to obtain a regular surface. After 5 minutes (the initial curing time), specimens were stored at 37°C in 100% humidity for 60 minutes. Then, they were immersed in distilled water for 24 hours at 37°C. Before the mechanical tests, all specimens were measured with a digital caliper (Mitutoyo Corp, Aurora, Ill). The mechanical tests were performed in an EMIC DL 2000 (EMIC, São José dos Pinhais, PR, Brazil) universal testing machine at a crosshead speed of 1 mm/min. A compressive load was applied along the diameter of the specimen for the DTS test and along the long axis for the CS test. The maximum strength before rupture was recorded, and then the following equations were applied to each specimen to obtain the results of the DTS and CS tests in megapascals (MPa): DTS = 2F/πdt and CS = 4F/πd2, where F is the failure load, d is the diameter, and t the height of the specimens.
For the microhardness tests, five specimens of each material were prepared. The same mold that was used for the DTS test (6 mm diameter × 3 mm height) was used, and the same procedures for manipulation, insertion, and curing previously described were again followed. Vickers measurements were performed using a microhardness tester HMV (Shimadzu Corp, Kyoto, Japan) with 200 g of load over 15 seconds. In each specimen, three equidistant indentations were made, and therefore 15 measurements were obtained.
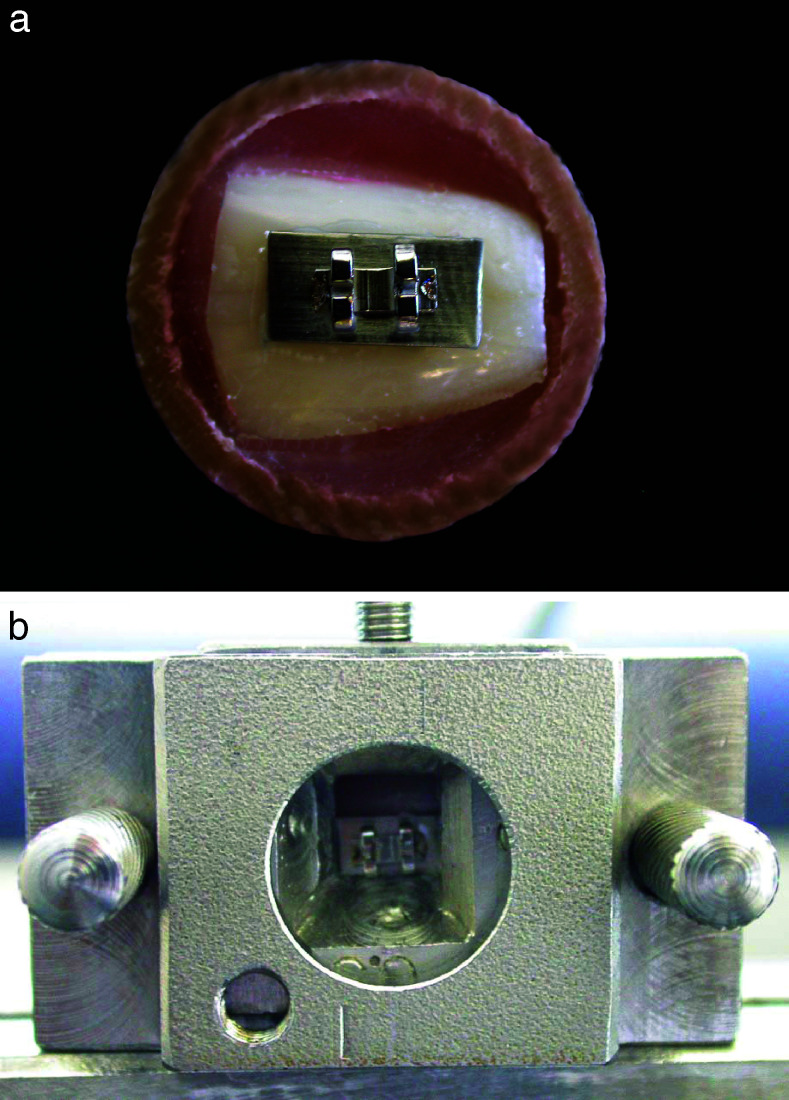
Eighty-four permanent bovine incisors were selected for the shear bond strength tests. The criteria for teeth selection were absence of fractures, deep grooves, and stains on the enamel surface. After 1 week in a 0.1% thymol solution, the teeth were segmented using a diamond disc with a low speed rotation hand piece around the cervical third of the roots and in the incisal third of the crown. Then, each bonding surface was mounted horizontally in a self-cured acrylic resin in plastic cylinders (25 × 20 mm). The surfaces were polished with pumice and a rubber cup with a low-speed rotation hand piece, washed, dried, and equally assigned to the different groups. Metallic matrices (n = 84) for orthodontic bands (Morelli, Sorocaba, SP, Brazil), 10 mm length × 4.5 mm height × 0.15 mm width, were cut, and metallic brackets (Morelli) were welded over them. The GICs were manipulated and each matrix was cemented to the center of the crown surface (Figure 1a). After 5 minutes of initial setting time, the specimens were stored at 37°C in 100% relative humidity for 60 minutes and immersed in distilled water for 24 hours at 37°C before the tests were performed. Shear bond strength tests were carried out in the same universal testing machine described previously using a matrix with a loading chisel (Figure 1b) at a crosshead speed of 1 mm/min and registered in MPa.
Figure 1.
Shear bond strength test. Specimen prepared for the test (a), and specimen positioned into the matrix with a loading chisel in the universal testing machine (b).
For the agar plate diffusion tests, 12 specimens (6 mm diameter × 3 mm height) of each group were prepared, which were stored in distilled water changed every day. Prior to their placement on the agar plate, all specimens were sterilized with ethylene oxide gas for 5 hours and subsequently degassed for 48 hours. After the sterilizing process, specimens were also kept in sterilized water. The bacterial strains of Streptococcus mutans ATCC 25175 from stock culture were cultivated in brain heart infusion (BHI) (Merck & Co, Whitehouse Station, NJ). It was used with a dilution of 10−1, containing 1.2 × 10−8 CFU/mL, which was determined through the serial dilution in saline solution at 0.85%. After incubation at 37°C for 48 hours, the bacterial strains were spread on the BHI agar plates and left for 30 minutes at room temperature. Thereafter, three wells with 5.5-mm diameters were made with a punch in each bacterium-inoculated agar plate, and three specimens (control, 10%, and 18% CHD) of the same material were placed into them with full contact with the medium (Figure 2a). Then, plates were incubated at 37°C for 48 hours in a microaerophilic environment, and the diameter of the zones of inhibition were measured with a digital caliper (Mitutoyo) at two points, horizontally and vertically, at 5, 45, and 65 days (Figure 2b,c). The tests were made in triplicate for all groups.
Figure 2.
Agar plates with specimens positioned before incubation (a) and after incubation period (b) and the two lines of measure used in each inhibition zone (c).
Statistical Analyses
The data of mechanical tests were analyzed by one-way analyses of variance (ANOVA) and for the comparison among groups, Tukey multiple comparison test was used. To compare the antibacterial properties of three groups (control, 10%, and 18% of CHD) of a same material in the three different periods, the Kruskal-Wallis test was chosen, and to compare the changes between the periods for a same material and composition, the Friedman test was used. The significance level was set at P = .05 for all tests.
RESULTS
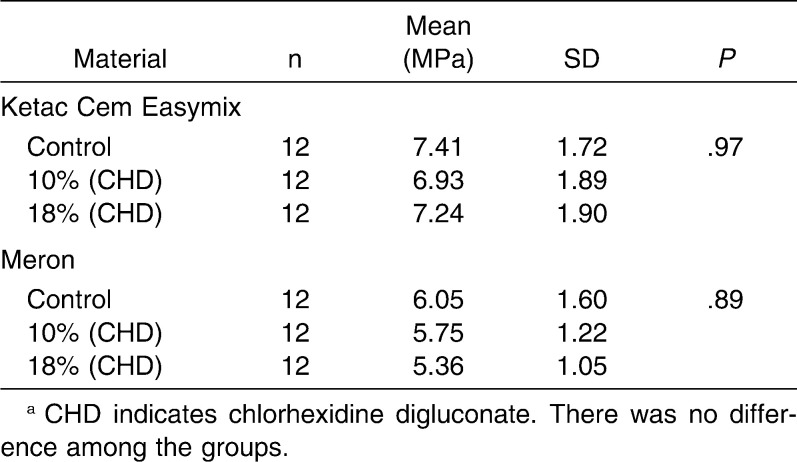
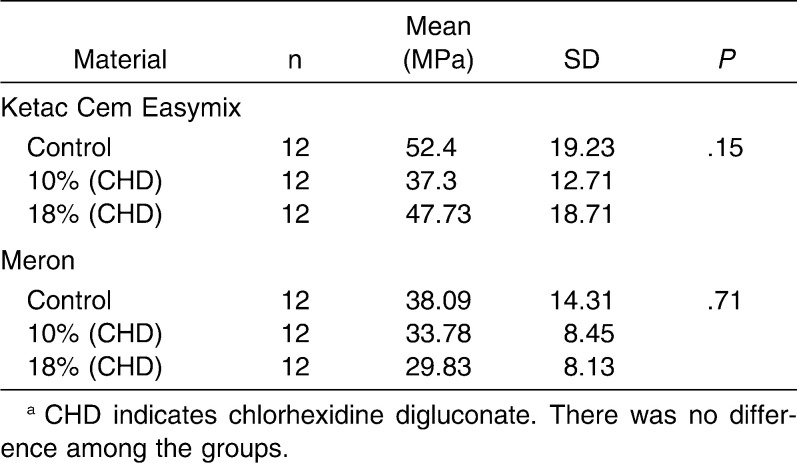
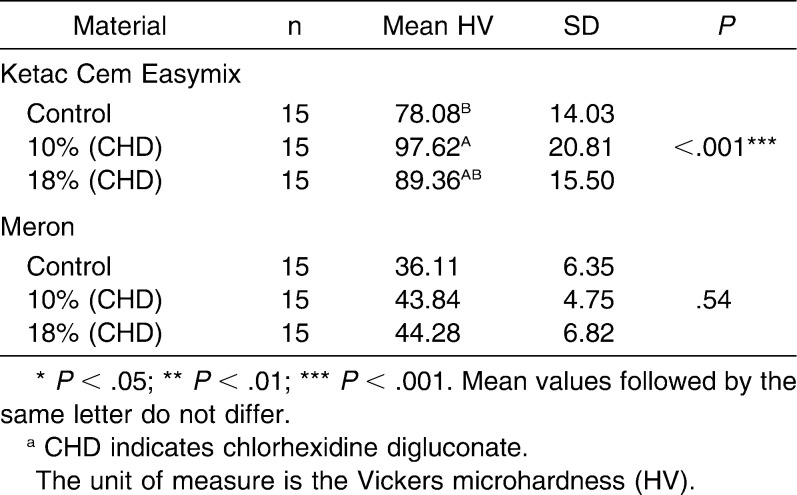
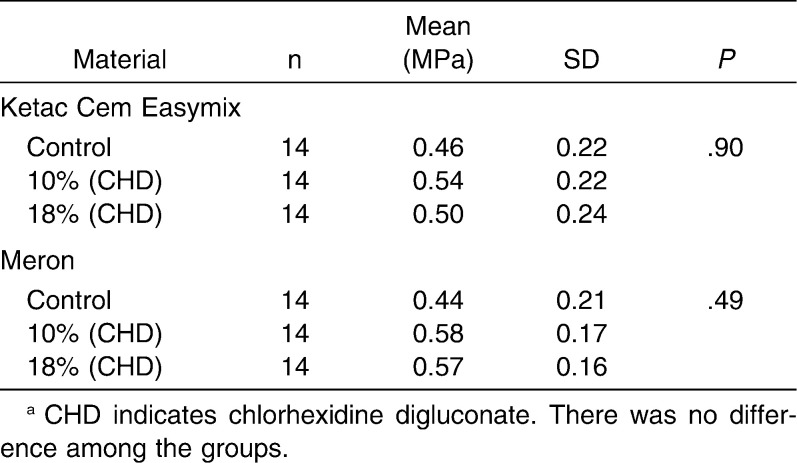
The analyses of the results revealed that there was no significant difference (P > .05) between conventional and chlorhexidine-added GICs in a same material with regard to diametral tensile strength (Ketac Cem Easymix, P = .97; Meron, P = .89) and compressive strength (Ketac Cem Easymix, P = .15; Meron, P = .71) (Tables 1 and 2). The data obtained for the microhardness tests (Table 3) showed that there were no differences among all groups of Meron (P = .54). Experimental groups of the Ketac Cem Easymix did not show difference between them (P = .49). Nevertheless, Ketac Cem Easymix with 10% CHD displayed a significantly higher microhardness than the control group (P < .001). The shear bond strength results did not present significant differences between all groups (P > .05) (Table 4).
Table 1.
Mean, Standard Deviation (SD), and One-Way Analysis of Variance With Tukey Multiple Comparison Test Within Groups for the Diametral Tensile Strength Testsa
Table 2.
Mean, Standard Deviation (SD), and One-Way Analysis of Variance With Tukey Multiple Comparison Test Within Groups for the Compressive Strength Testsa
Table 3.
Mean, Standard Deviation (SD), and One-Way Analysis of Variance With Tukey Multiple Comparison Test Within Groups for the Microhardness Testsa
Table 4.
Mean, Standard Deviation (SD), and One-Way Analysis of Variance With Tukey Multiple Comparison Test Within Groups for the Shear Bond Strength Testsa
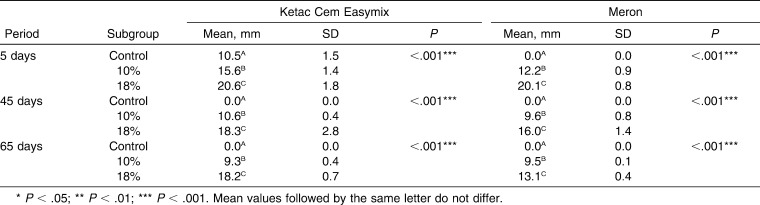
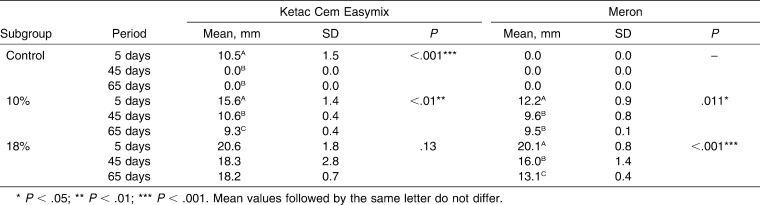
There was a significant difference in the inhibition zones in agar plates between the control group (without CHD) and both experimental groups (10% and 18% CHD) (P < .001) for both GICs at all of the periods evaluated (Table 5). There was also a significant difference (P < .001) between experimental groups with the addition of 10% and 18% CHD for the two materials and at the three periods (Table 5). All of the subgroups showed some decrease in the antibacterial effects along the three periods of analysis (P < .05). Only the subgroup Ketac Cem Easymix with 18% CHD did not show significant decrease (P = .13) in inhibition zones (Table 6).
Table 5.
Mean, Standard Deviation (SD), and Kruskal-Wallis Test Comparing the Three Subgroups of the Composition of the Two Glass Ionomer Cements According to the Periods for the Inhibition Zones Measures
Table 6.
Mean, Standard Deviation (SD), and Friedman Test Comparing the Three Subgroups of the Periods of the Two Glass Ionomer Cements According to Their Three Compositions for the Inhibition Zones Measures
DISCUSSION
Currently, researchers have proposed the addition of CHD, chlorhexidine dihydrochloride, and chlorhexidine diacetate in some restorative, luting, and filling materials with the intent to improve bacterial control.4–6,16,22 According to Ribeiro and Ericson16 and Hoszek and Ericson,19 the addition of CHD at a concentration of at least 10% of the liquid material was efficient for protection against S. mutans. Moreover, those authors observed that the gradual increase of CHD into the composition of cements also increased the antibacterial effects. The maximum amount possible of CHD in the liquid with 10% tartaric acid is 18%; therefore, concentrations of 10% and 18% CHD were chosen to verify if those amounts can affect the mechanical properties and antibacterial properties of conventional GICs for band cementation.
The results revealed no significant difference in diametral tensile and compressive strength when comparing the conventional and the experimental groups (CHD) of the two materials. These results are in accordance with those of Sanders et al.5 who analyzed only the diametral tensile strength properties of a resin-modified glass-ionomer (used for fillings) that had chlorhexidine diacetate added in. Nevertheless, Jedrychowsky et al.23 found that more than 5% CHD in the total composition of the conventional GICs could significantly influence their compressive strength. Furthermore, Takahashi et al.22 found that more than 2% chlorhexidine added to the GIC can also reduce compressive strength; however, those authors used chlorhexidine diacetate and dihydrochloride for the incorporation.
There are no studies in the literature that have tested the microhardness of conventional GICs with antibacterial agents. Interestingly, the microhardness increased for both cements and showed a significant difference to the Ketac Cem Easymix. Meron demonstrated a gradual increase in microhardness from the conventional composition with up to 18% CHD. Ketac Cem Easymix showed a great increase from the conventional composition with 10% CHD, and this result was significant. CHD addition from 10% to 18% resulted in only a slight reduction of the microhardness for this material. Sanders et al.5 found no differences in hardness between a resin-modified glass ionomer with or without the addition of chlorhexidine dihydrochloride after 24 hours of manipulation and according to them, a great amount of chlorhexidine stayed on the external surface of the specimens. This condition could create a layer which would increase the surface resistance against indentations. However, further studies are necessary before accepting this hypothesis.
The results of shear strength were low, probably due to the nonpolishing of the bovine enamel surfaces with grit sandpapers and because the metallic matrices did not receive sandblasting. There were no differences in either of the Ketac Cem Easymix groups or the Meron groups or among the two material groups to the shear bond tests. Millett et al.4 also observed similar results with bands cemented to human third molars with GICs modified by the addition of chlorhexidine digluconate. These findings led us to believe that the addition of CHD does not have a significant influence on bonding between teeth, cement, and metal, thus avoiding unwanted conditions such as accidental debanding, shrinkage, and consequently decalcification of the enamel or periodontal diseases.4,13,15
An initial antibacterial effect was measurable on the fifth day after the specimen's preparation due to the initial incubation for 24 hours at 37°C and the sterilization process with ethylene oxide gas. At this time point, the control Ketac Cem Easymix cement showed an inhibition zone that was significantly greater than the control Meron cement. Furthermore, the inhibition zones of the experimental GICs were significantly greater than the control GICs, and the experimental groups with addition of the CHD of 18% had significantly more inhibition than experimental groups with 10% of CHD. A similar behavior was also observed by Hoszek and Ericson,19 who analyzed conventional GICs with the inclusion of CHD immediately after manipulation and after 2 hours of immersion in water. Other studies also observed increased antibacterial effects in GICs at initial measurements; however, they used chlorhexidine diacetate added to the powder of the cement.5,22,24
In orthodontic treatment, patients have to remain with appliances for long periods. Therefore, it would be interesting if the GICs retained a higher antibacterial effect during this period. The results of the 65-day analysis after the specimen's preparation showed significant inhibition of S. mutans growth in GICs with CHD, even with the changing of the distilled water once a day. Hoszek and Ericson19 evaluated the antimicrobial effect until 60 days after manipulation and verified a decreased but measurable inhibition of bacterial growth at this time. However, those authors did not change the water once a day, which could facilitate the maintenance of the antimicrobial properties of the material. Moreover, some studies that analyzed the antimicrobial properties of resin-modified glass ionomers and orthodontic resin over long periods did not reveal significant inhibition after 25 days of the manipulation.5,6 According to Sanders et al.,5 a probable explanation for the results obtained in our study is the greater solubility shown by conventional GICs and CHD compared to resin-modified glass ionomers and resins as well as chlorhexidine diacetate. In addition, significant levels of chlorhexidine can remain on the surface of the specimen, and the direct contact between bacteria and materials could increase the antibacterial effect.
The findings of the present study proved that the addition of CHD does not significantly influence the mechanical properties tested and can lead to greater bacterial control around orthodontic appliances, such as bands. These results are encouraging for the use of these materials in clinical practice in patients who demonstrate a need for greater antibacterial control. To better understand the behavior of those materials further studies should be performed, ie, other mechanical tests, direct contact test, quantification of the bacterial colonies, analysis of chlorhexidine release, and long-term tests.
CONCLUSIONS
The addition of CHD does not significantly influence the diametral tensile, compressive, or shear bond strength and increase the microhardness of Ketac Cem Easymix.
Both GICs showed significantly high antibacterial effect with the addition of 10% and 18% chlorhexidine digluconate for a relatively long period of time. The addition of CHD in 18% showed higher inhibition than addition in 10% along the analyses.
REFERENCES
- 1.Cacciafesta V, Sfondrini M. F, Stifanelli P, Scribante A, Klersy C. Effect of chlorhexidine application on shear bond strength of brackets bonded with a resin-modified glass ionomer. Am J Orthod Dentofacial Orthop. 2006;129:273–276. doi: 10.1016/j.ajodo.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 2.Hallgren A, Oliveby A, Twetman S. Fluoride concentration in plaque adjacent to orthodontic appliances retained with glass ionomer cement. Caries Res. 1993;27:51–54. doi: 10.1159/000261515. [DOI] [PubMed] [Google Scholar]
- 3.Matalon S, Slutsky H, Weiss E. I. Antibacterial properties of 4 orthodontic cements. Am J Orthod Dentofacial Orthop. 2005;127:56–63. doi: 10.1016/j.ajodo.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 4.Millett D. T, Doubleday B, Alatsaris M, Love J. Chlorhexidine-modified glass ionomer for band cementation? An in vitro study. J Orthod. 2005;32:36–42. doi: 10.1179/14653120522502078. [DOI] [PubMed] [Google Scholar]
- 5.Sanders B. J, Gregory R. L, Moore K, Avery D. R. Antibacterial and physical properties of resin modified glass-ionomers combined with chlorhexidine. J Oral Rehabil. 2002;29:553–558. doi: 10.1046/j.1365-2842.2002.00876.x. [DOI] [PubMed] [Google Scholar]
- 6.Sehgal V. V, Shetty S, Mogra S, Bhat G, Eipe M, Jacob S, Prabu L. Evaluation of antimicrobial and physical properties of orthodontic composite resin modified by addition of antimicrobial agents—an in-vitro study. Am J Orthod Dentofacial Orthop. 2007;131:525–529. doi: 10.1016/j.ajodo.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 7.Sukontapatipark W, El-Agroudi M. A, Selliseth N. J, Thunold K, Selvig K. A. Bacterial colonization associated with fixed orthodontic appliances. A scanning electron microscopy study. Eur J Orthod. 2001;23:475–484. doi: 10.1093/ejo/23.5.475. [DOI] [PubMed] [Google Scholar]
- 8.Erverdi N, Acar A, Isguden B, Kadir T. Investigation of bacteremia after orthodontic banding and debanding following chlorhexidine mouth wash application. Angle Orthod. 2001;71:190–194. doi: 10.1043/0003-3219(2001)071<0190:IOBAOB>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Erverdi N, Biren S, Kadir T, Acar A. Investigation of bacteremia following orthodontic debanding. Angle Orthod. 2000;70:11–14. doi: 10.1043/0003-3219(2000)070<0011:IOBFOD>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 10.Erverdi N, Kadir T, Ozkan H, Acar A. Investigation of bacteremia after orthodontic banding. Am J Orthod Dentofacial Orthop. 1999;116:687–690. doi: 10.1016/s0889-5406(99)70205-2. [DOI] [PubMed] [Google Scholar]
- 11.McLaughlin J. O, Coulter W. A, Coffey A, Burden D. J. The incidence of bacteremia after orthodontic banding. Am J Orthod Dentofacial Orthop. 1996;109:639–644. doi: 10.1016/s0889-5406(96)70076-8. [DOI] [PubMed] [Google Scholar]
- 12.Aguiar D. A, da Silveira M. R, Ritter D. E, Locks A, Calvo M. C. M. Avaliação das propriedades mecânicas de quatro cimentos de ionômero de vidro convencionais utilizados na cimentação de bandas ortodônticas. Rev Dent Press Ortod Ortop Facial. 2008;13:104–111. [Google Scholar]
- 13.Millett D. T, Duff S, Morrison L, Cummings A, Gilmour W. H. In vitro comparison of orthodontic band cements. Am J Orthod Dentofacial Orthop. 2003;123:15–20. doi: 10.1067/mod.2003.48. [DOI] [PubMed] [Google Scholar]
- 14.Bresciani E, Barata T. J. E, Fagundes T. C, Adachi A, Terrin M. M, Navarro M. F. L. Compressive and diametral tensile strength of glass ionomer cements. J Appl Oral Sci. 2004;12:344–348. doi: 10.1590/s1678-77572004000400017. [DOI] [PubMed] [Google Scholar]
- 15.Millett D. T, Cummings A, Letters S, Roger E, Love J. Resin-modified glass ionomer, modified composite or conventional glass ionomer for band cementation? An in vitro evaluation. Eur J Orthod. 2003;25:609–614. doi: 10.1093/ejo/25.6.609. [DOI] [PubMed] [Google Scholar]
- 16.Ribeiro J, Ericson D. In vitro antibacterial effect of chlorhexidine added to glass-ionomer cements. Scand J Dent Res. 1991;99:533–540. doi: 10.1111/j.1600-0722.1991.tb01066.x. [DOI] [PubMed] [Google Scholar]
- 17.Palmer G, Jones F. H, Billington R. W, Pearson G. J. Chlorhexidine release from an experimental glass ionomer cement. Biomaterials. 2004;25:5423–5431. doi: 10.1016/j.biomaterials.2003.12.051. [DOI] [PubMed] [Google Scholar]
- 18.Beyth N, Redlich M, Harari D, Friedman M, Steinberg D. Effect of sustained-release chlorhexidine varnish on Streptococcus mutans and Actinomyces viscosus in orthodontic patients. Am J Orthod Dentofacial Orthop. 2003;123:345–348. doi: 10.1067/mod.2003.19. [DOI] [PubMed] [Google Scholar]
- 19.Hoszek A, Ericson D. In vitro fluoride release and the antibacterial effect of glass ionomers containing chlorhexidine gluconate. Oper Dent. 2008;33:696–701. doi: 10.2341/08-20. [DOI] [PubMed] [Google Scholar]
- 20.Brightman L. J, Terezhalmy G. T, Greenwell H, Jacobs M, Enlow D. H. The effects of a 0.12% chlorhexidine gluconate mouthrinse on orthodontic patients aged 11 through 17 with established gingivitis. Am J Orthod Dentofacial Orthop. 1991;100:324–329. doi: 10.1016/0889-5406(91)70069-9. [DOI] [PubMed] [Google Scholar]
- 21.Heasman P. A, Seymour R. A. Pharmacological control of periodontal disease. I. Antiplaque agents. J Dent. 1994;22:323–335. doi: 10.1016/0300-5712(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi Y, Imazato S, Kaneshiro S. E, Ebisu S, Frencken J. E, Tay F. R. Antibacterial effects and physical properties of glass-ionomer cements containing chlorhexidine for the ART approach. Dent Mater. 2006;22:647–652. doi: 10.1016/j.dental.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Jedrychowski J. R, Caputo A. A, Kerper S. Antibacterial and mechanical properties of restorative materials combined with chlorhexidine. J Oral Rehabil. 1983;10:373–381. doi: 10.1111/j.1365-2842.1983.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 24.Frenckel J. E, Imazato S, Toi C, Mulder J, Mickenautsch S, Takahashi Y, Ebisu S. Antibacterial effect of chlorhexidine-containing glass ionomer cement in vivo: a pilot study. Caries Res. 2007;41:102–107. doi: 10.1159/000098042. [DOI] [PubMed] [Google Scholar]