Abstract
Background
Tennis elbow is a common painful enthesopathy of the lateral elbow that limits upper limb function and frequently results in lost time at work. Surgeons often recommend surgery if symptoms persist despite nonsurgical management, but operations for tennis elbow are inconsistent in their efficacy, and what we know about those operations often derives from observational studies that assume the condition does not continue to improve over time. This assumption is largely untested, and it may not be true; meta-analyzing results from the control arms of tennis elbow studies can help us to evaluate this premise, but to our knowledge, this has not been done.
Questions/purposes
The aims of this systematic review were to describe the course of (1) global improvement, (2) pain, and (3) disability in participants who received no active treatment (placebo or no treatment) in published randomized controlled trials (RCTs) on tennis elbow. We also assessed (4) whether the duration of symptoms or placebo effect is associated with differences in symptom trajectories.
Methods
We searched MEDLINE, Embase, and CENTRAL from database inception to August 12, 2019, for trials including participants with tennis elbow and a placebo or a no-treatment arm and a minimum follow-up duration of 6 months. There were no language restrictions or exclusion criteria. We extracted global improvement, pain, and disability outcomes. We used the Cochrane Risk of Bias tool to assess the risk of bias of included trials. To estimate the typical course of tennis elbow without active treatment, we pooled global improvement (the proportion of participants who reported feeling much better or completely recovered), mean pain, and mean disability using baseline, 1-month, 3-month, 6-month, and 12-month follow-up data. We transformed pain and disability data from the original papers so that at each timepoint the relevant outcome was expressed as change relative to baseline to account for different baseline values. We used meta-regression to assess whether the placebo effect or duration of symptoms before enrollment was associated with differences in symptom trajectories. We included 24 trials with 1085 participants who received no active treatment.
Results
The number of patients who were not improved decreased exponentially over time. The half-life of global improvement was between 2.5 and 3 months (that is, every 2.5 to 3 months, 50% of the remaining symptomatic patients reported complete recovery or greatly improved symptoms). At 1 year, 89% (189 of 213; 95% CI 80% to 97%) of patients experienced global improvement. The mean pain and disability followed a similar pattern, halving every 3 to 4 months. Eighty-eight percent of pain (95% CI 70% to 100%) and 85% of disability (95% CI 60% to 100%) had resolved by 1 year. The mean duration of symptoms before trial enrollment was not associated with differences in symptom trajectories. The trajectories of the no-treatment and placebo arms were similar, indicating that the placebo effect of the studied active treatments likely is negligible.
Conclusion
Based on the placebo or no-treatment control arms of randomized trials, about 90% of people with untreated tennis elbow achieve symptom resolution at 1 year. The probability of resolution appears to remain constant throughout the first year of follow-up and does not depend on previous symptom duration, undermining the rationale that surgery is appropriate if symptoms persist beyond a certain point of time. We recommend that clinicians inform people who are frustrated with persisting symptoms that this is not a cause for apprehension, given that spontaneous improvement is about as likely during the subsequent few months as it was early after the symptoms first appeared. Because of the high likelihood of spontaneous recovery, any active intervention needs to be justified by high levels of early efficacy and little or no risk to outperform watchful waiting.
Level of Evidence
Level I, therapeutic study.
Introduction
Tennis elbow, also known as lateral elbow pain or lateral epicondylitis, is a common painful degenerative enthesopathy of the lateral elbow affecting up to 3% of people who are 40 to 55 years of age [37]. Current treatment strategies consume considerable resources, and the condition is associated with absence from work [38]. Although understanding of the histology and underlying pathomechanisms has improved substantially, the exact cause for pain is still unclear [11, 33] because asymptomatic people also show tendinopathic changes in imaging studies [24]. Despite many attempts to find a cure, there is no specific treatment for tennis elbow that has been proven effective [35]. Based on clinical observations, tennis elbow has been considered a self-limiting condition [11], but there is no systematic evidence of its course over time without medical treatment. Therefore, it is unclear what exactly self-limiting means in this context. A common treatment approach is to offer advice and education about the condition, simple analgesia or NSAIDs, and/or home-based exercises initially and glucocorticoid injections for people with more severe inflammatory symptoms [9, 10, 39].
Surgery may be considered for people whose symptoms persist [20]. The rationale for treating patients who have persistent symptoms with surgery appears to be that these patients have a condition that is somehow different from those whose symptoms resolve more quickly. This reasoning is undermined by some studies suggesting that people with long symptom duration may have similar prognosis as those with short duration [4, 5, 13], but systematic data on the prognostic value of symptom duration are lacking.
Previous trials that have included either placebo or no-treatment controls have found a rapid decline in the proportion of people with symptoms within the first 3 to 6 months after commencing the trial, regardless of the treatment received [9, 39]. After the early phase, the symptom trajectory seems to plateau. This has also been observed in other self-limiting conditions such as low back pain [2]. However, plateauing does not mean that a patient’s probability of spontaneous recovery decreases over time. In fact, if the probability stays constant irrespective of the time passed, we should observe recovery rates similar to the aforementioned studies [2, 9, 39]. This would mean that continuing follow-up when symptoms have persisted for months is as reasonable as it was when the symptoms first appeared. If we simply follow the patients with persistent symptoms long enough, a large proportion of them might experience symptom resolution without intervention, although the absence of pooled data on this point leaves clinicians with considerable uncertainty. If this proves true, this should cause us to recommend surgery less frequently, which would result in helping more patients avoid surgical risk. We therefore wished to ascertain the likely symptom-resolution patterns of tennis elbow without specific active treatment, and whether the probability of spontaneous recovery remains constant or changes during follow-up.
The specific aims of this systematic review were to describe the course of (1) global improvement, (2) pain, and (3) disability in participants who received no active treatment (placebo or no treatment) in published randomized controlled trials (RCTs) on tennis elbow. We also assessed (4) whether the duration of symptoms or placebo effect is associated with differences in symptom trajectories.
Materials and Methods
Protocol and Registration
The protocol was developed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses and registered in the PROSPERO database (ID: CRD42019127303).
Eligibility Criteria
We included studies if they were randomized or quasirandomized controlled trials, included participants with a diagnosis of tennis elbow, had a nonactive treatment control, had a follow-up duration of at least 6 months, and reported at least one of the following: global improvement of symptoms, elbow pain intensity, or disability. Nonactive treatment controls could include a wait-and-see protocol, nonspecific exercises, over-the-counter medication, provision of patient information or reassurance, and placebo and sham treatments (for example, placebo or sham devices, injections, or surgery). Although RCTs are not the same as usual care, we chose to focus on RCTs for investigating the course of tennis elbow because they systematically collect longitudinal outcome data and usually account for a high proportion of patients they enroll at follow-up, and thus are a source of the most reliable data available. There were no exclusion criteria.
Information Sources
We searched MEDLINE, Embase, and CENTRAL from database inception to August 12, 2019, for relevant trials without language restrictions (Supplementary Table 1; http://links.lww.com/CORR/A667). Two investigators (JI, TK) assessed eligibility independently. Any discrepancies in the screening process were resolved by consensus. We also searched the reference lists of the included studies.
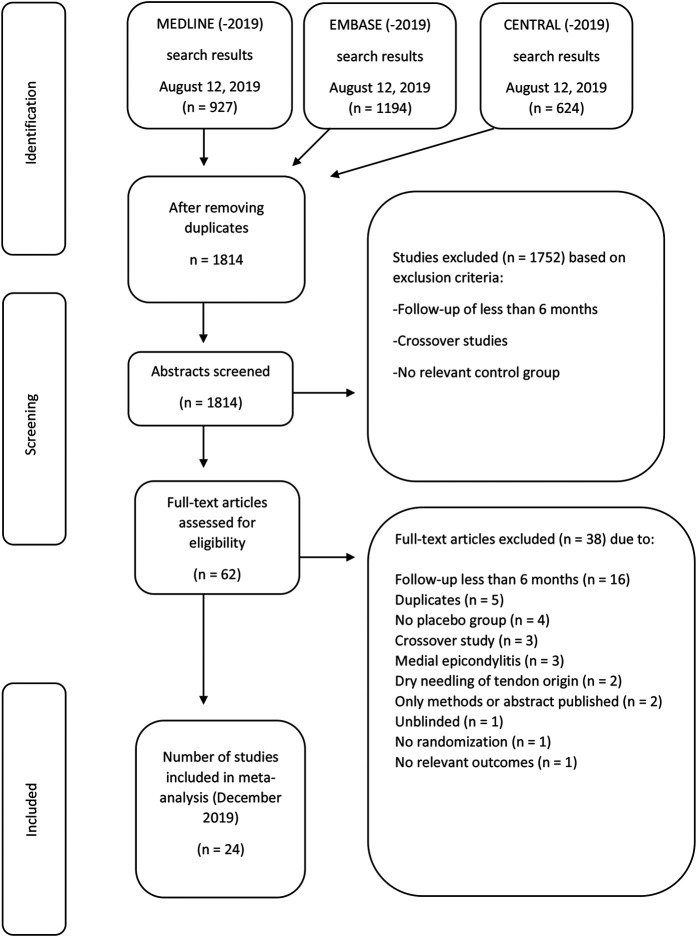
The search returned 1814 records; 24 RCTs were included [1, 4, 8, 9, 12, 14-16, 21-23, 25-27, 29-32, 34, 39, 40-42, 44], with 1085 participants receiving no active treatment (Fig. 1). The trials were performed in 11 countries and were published between 1990 and 2019. The 20 placebo-controlled trials included 25 active treatment groups (Table 1). The most common active treatment group was glucocorticoid injection (seven trials), followed by extracorporeal shockwave therapy (four trials), platelet-rich plasma injection (two trials), laser (two trials), and glycosaminoglycan or hyaluronate (two trials). The four trials with a no-treatment control included seven active treatment groups. The most common active treatments were glucocorticoid injections (three trials) and physical therapy (three trials).
Fig. 1.
This flowchart shows article selection according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines [28].
Table 1.
Characteristics of the included studies (n = 24)
| Author [ref] | Placebo/no treatment | Active intervention | Additional interventions or therapy | Outcomes used in the analyses | Number of patients in control arm | Mean age ± SD or mean age (range) in years | Mean ± SD duration of symptoms or mean (range or IQR) in months | Follow-up periods in months | Difference between active intervention and placebo/no treatment? |
| Åkermark [1] | Placebo | Glycosaminoglycan injection | Paracetamol, activity avoidance, PT, sick leave | Pain with activity VAS, patient-reported overall elbow function | 31 | 42 (27-58) | 9 (3-30) | 1, 3, 6 | Pain in favor of active intervention at 6 and 12 weeks |
| Bisset [4] | No treatment | Glucocorticoid injection or PT | Activity avoidance, analgesics, heat/cold, braces as needed | Pain VAS, pain-free function questionnaire | 67 | 47 ± 8.1 | 6.5 (IQR 2.5-10.5) | 1, 6, 12 | Pain in favor of active intervention at 6 weeks, pain in favor of placebo at 12 months |
| Chesterton [8] | No treatment | Transcutaneous electrical nerve stimulation | Activity avoidance, exercise | Pain, PRTEE | 120 | 50 ± 9.1 | NR | 1, 6, 12 | None |
| Coombes [9] | Placebo | Glucocorticoid injection, glucocorticoid injection + PT, or placebo + PT | Activity avoidance, analgesics, heat/cold, braces as needed | Worst pain VAS, patient-reported global rating | 40 | 50 ± 7.4 | 16 (IQR 8-24) | 1, 6, 12 | Global improvement in favor of placebo at 26, 52 weeks |
| Fink [12] | Placebo | Acupuncture | Medication and PT was not allowed | Pain at rest 0-6 | 20 | 52 ± 10 | 10.5 | 3, 12 | Pain in favor of active intervention at 2 weeks |
| Haker [14] | Placebo | Laser | NR | None | 26 | 47 (24-70) | 6 (1-36) | 3, 12 | None |
| Haker [15] | Placebo | Laser | Activity avoidance, no other medication or therapy allowed | None | 29 | 45 (33-65) | 4 (1-24) | 3, 12 | None |
| Hay [16] | Placebo | Glucocorticoid injection or naproxen | Co-codamol, information leaflet | Pain, self-assessed impairment of function | 57 | NR | NR | 1, 6, 12 | Pain, global improvement in favor of active intervention at 4 weeks, pain and global improvement in favor placebo at 6, 12 months |
| Kroslak [21] | Placebo | ECRB excision | Acetaminophen, postop rehabilitation, ice stretching | Patient-reported level of pain with activity, patient-reported overall elbow function | 13 | 51 (41-77) | 74 (10-348) | 3, 6, 12+ | None |
| Kroslak [22] | Placebo | Counterforce bracing | Rehabilitation protocol | Patient-reported level of pain with activity, patient-reported overall elbow function | 22 | 51 (40-73) | 4 (1-8) | 1, 3, 6, 12+ | Elbow function in favor of active intervention at 6 months |
| Lindenhovius [23] | Placebo | Dexamethasone injection | All additional treatment allowed | Pain VAS, DASH |
30 | 51 ± 10 | 2 ± 1 | 1, 6 | None |
| Mehra [25] | Placebo | Lithotripsy | Lignocaine injection before treatment | Pain VAS | 11 | NR | 11 | 3, 6 | Pain in favor of active intervention at 6 months |
| Melikyan [26] | Placebo | Extracorporeal shockwave therapy | No restrictions on activities | Pain VAS, DASH |
37 | 43 (35-71) | NR | 1, 3, 12 | None |
| Mishra [27] | Placebo | Platelet-rich plasma injection | Bupivacaine local anesthesia | Pain, PRTEE |
113 | 47 | NR | 1, 2, 3, 6 | Pain in favor of active intervention at 2, 6 months |
| Montalvan [29] | Placebo | Autologous conditioned plasma | Paracetamol and ice recommended; corticosteroid injections/rehabilitation not allowed | Global pain | 25 | 46 ± 8.6 | NR | 1, 3, 6, 12 | None |
| Olaussen [30] | No treatment | Glucocorticoid injection or PT | Naproxen, paracetamol, activity avoidance, sick leave | Pain at rest, affected function VAS |
60 | 44 ± 9.7 | 1.9 (IQR 1-2.5) | 1, 3, 6, 12 | Global improvement in favor of active intervention at 6 weeks and in favor of control at 6 months |
| Paoloni [31] | Placebo | Topical nitric oxide application | Rehabilitation program, activity avoidance, brace | Pain at activity | 43 | 46 (30-74) | 17 (3-232) | 1, 3, 6 | Function and pain in favor of active intervention at 2, 6, 12, 24 weeks |
| Petrella [32] | Placebo | Sodium hyaluronate injection | RICE and ASA allowed; other analgesics, corticosteroids and topical analgesics not allowed | Pain after grip test, patient assessment of normal function | 47 | 47 ± 11 | 22 ± 18 | 1, 3, 12 | Pain in favor of active intervention at 1, 3, 12 months |
| Runeson [34] | Placebo | Cortisone iontophoresis | NR | Pain at function VAS, patient- reported outcome | 31 | 45 (22-64) | 4 (1-36) | 3, 6 | None |
| Smidt [39] | No treatment | Glucocorticoid injection or PT | Activity avoidance, paracetamol, naproxen, NSAIDs, information | Pain during the day, elbow disability | 59 | 46 (IQR 42-54) | 2.8 (2-5.25) | 1, 3, 6, 12 | Global improvement in favor of active intervention at 6 weeks Global improvement in favor of no treatment at 52 weeks |
| Spacca [40] | Placebo | Radial shockwave therapy | NR | Pain at rest VAS, DASH |
31 | 47 ± 9.15 | 13 ± 5 | 6 | Pain and DASH in favor of active intervention at 6 months |
| Staples [41] | Placebo | Extracorporeal shockwave therapy | Stretching exercises, paracetamol allowed; no other forms of therapy allowed | Overall pain VAS, DASH |
32 | 49 ± 8.8 | 17 ± 24.7 | 1, 3, 6 | None |
| Tahririan [42] | Placebo | Glucocorticoid injection or splinting | NR | General pain VAS, OES | 19 | 49 ± 6.02 | NR | 1, 6 | Pain in favor of active intervention at 2, 4 weeks Pain in favor of placebo at 6 months OES in favor of placebo at 6 months |
| Wolf [44] | Placebo | Autologous blood injection or glucocorticoid injection | Stretching exercises | Pain VAS, DASH |
9 | 49 (34-64) | NR | 3, 6 | None |
PT = physiotherapy; IQR = interquartile range; PRTEE = Patient-Rated Tennis Elbow Evaluation; NR = not reported; ECRB = extensor carpi radialis brevis; RICE = rest, ice, compression, and elevation; ASA = acetylsalicylic acid; OES = Oxford elbow score.
The criteria for making the diagnosis of tennis elbow varied across studies, and we accepted studies regardless of their inclusion criteria. The diagnosis in the included studies was almost uniformly based on history and clinical examination, but one study [40] required both clinical diagnosis and either an ultrasound or MRI of the lateral epicondyle. Four studies [25, 31, 32, 44] did not specify diagnostic criteria (“clinical diagnosis of tennis elbow”). Other studies described the clinical criteria and based on a combination of at least two of the following: pain around the lateral epicondyle; pain on palpation of lateral epicondyle; or positive Cozen, Mills, Maudsley, or chair-lift test.
Data Management
We extracted the following data: name of the first author, year of publication, title, type of intervention in the control group (placebo or no treatment), intervention in the active group, allowed cointerventions in the control group, allowed or received treatments before enrollment (including glucocorticoid injections), number of participants at each timepoint, outcomes at each timepoint, longest follow-up duration, mean age, and mean duration of symptoms (Table 1).
For studies that did not report the mean or median duration of symptoms, we imputed the median value of all studies that reported this variable.
Two authors (JI, TL) extracted the data in duplicate using a standard form, which we pilot-tested before extracting data. A third arbiter (TK) checked the extracted data and resolved inconsistencies. When the trial authors did not report any measure of variance or sufficient data to estimate this measure, we imputed the SD using the median value of the corresponding measurement instrument across all included studies. If the study was the only one that used a specific measurement, we imputed the median value of all measures at that timepoint. When numeric data were not available, we extracted data from graphs, where available, using the WebPlotDigitizer application (https://automeris.io/WebPlotDigitizer/).
Risk of Bias in Individual Studies
The risk of bias of each included study was assessed by two independent reviewers (TK, JI). We used the Cochrane Risk of Bias tool, version 1.0, to assess the risk of bias in six domains: selection bias, participant and personnel blinding (performance bias), participant blinding in self-reported outcomes (detection bias), incomplete outcome data (attrition bias), selective reporting, and other bias [17]. Because our analysis did not focus on comparative efficacy but the change relative to baseline, we focused on selection bias, blinding, and attrition bias as important sources of bias. Studies with an unclear or high risk of bias in any of those three domains were judged as having a high risk of bias in the analyses and excluded in sensitivity analysis assessing the robustness of our findings
Primary and Secondary Outcomes
Our primary goal was to describe the resolution of global symptoms in tennis elbow. For this analysis, we pooled data from studies that had defined global improvement of symptoms or treatment success as an outcome. The definition of treatment success in the original papers was the proportion of patients who experienced either much improved symptoms or total recovery. We pooled the number of patients who had experienced treatment success closest to predefined timepoints at 1 month, 3 months, 6 months, and 12 months of follow-up. We then calculated the proportion of patients who did not have successful treatment and plotted these data on a graph with 95% confidence intervals.
Our secondary goals were to describe the improvement in continuous outcomes of pain and disability. Because we were primarily interested in the rate of improvement, all pain and disability data were transformed so that a higher score indicated a worse outcome, and then the baseline value was scaled to a value of 100 and follow-up points relative to that value ([value at follow-up] x 100 / [value at baseline]). Using relative-to-baseline values also decreased heterogeneity between studies and allowed us to pool different outcome measures using different scales. We extracted data on the outcomes closest to the predefined timepoints of 1 month, 3 months, 6 months, and 12 months.
Pain intensity was included in the analysis in the following order of preference: pain with exertion/use, overall pain, pain at rest, or worst pain. Disability or function was included in the analysis in the following order of preference: condition-specific patient-reported outcome measure (PROM), elbow-specific PROM, upper limb–specific PROM, globally perceived disability, or disability as defined in any other way by original authors.
We also extracted pain and disability data from the active intervention arms to plot the trajectories of the active treatment arms; however, we did not use these data in meta-analyses because the interventions differed, and we were not interested in the treatment effects.
For each meta-analysis with a continuous outcome, we also performed a meta-regression analysis to assess whether symptom duration or control group type (placebo versus no treatment) was associated with differences in the trajectory of pain or disability. The mean or median duration of symptoms at the time of enrollment was entered into the model as a continuous variable, imputing median value across all studies when the authors did not report the duration of symptoms.
Synthesis of Results
To account for the longitudinal dependence of the outcomes, we used a multivariate meta-analysis for continuous outcomes. We used a general positive-definite covariance matrix and drew the correlation matrix from patient-level data from our ongoing Finnish Tennis Elbow Trial pilot study (ClinicalTrials.gov identifier NCT02425982) (Supplementary Table 2; http://links.lww.com/CORR/A668).
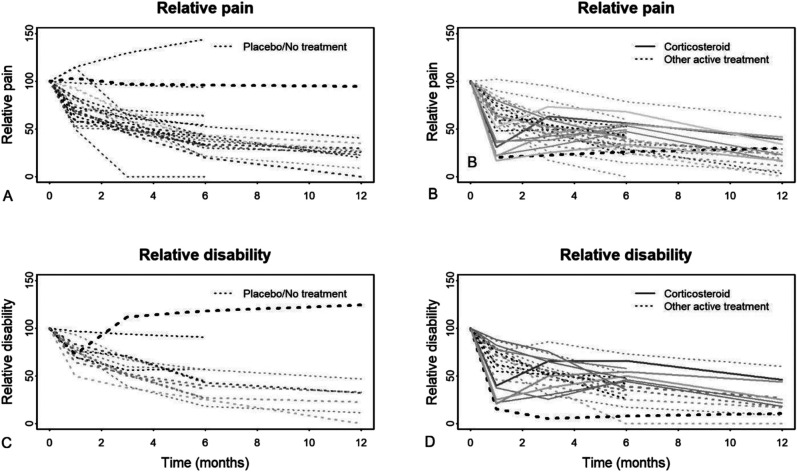
In addition to performing a multivariate analysis, we pooled each timepoint separately in a univariate meta-analysis to assess statistical heterogeneity using visual inspection and I2 statistics. Three trials substantially contributed to heterogeneity in pain and disability outcomes [25, 32, 40] (Supplementary Table 3; http://links.lww.com/CORR/A669). The trajectory for participants in the placebo arms in these trials appeared different from most because these participants had symptoms that either did not improve or became worse over time (Fig. 2). We excluded these outlier trials from the main analyses.
Fig. 2.
A-D These graphs show the relative pain in the (A) placebo or no-treatment groups and (B) the active treatment groups plotted over time. Additionally, disability in the (C) placebo or no-treatment groups and (D) the active treatment arms is plotted over time. The thickness of the lines is proportional to the number of participants in the trials, and the darkness of the lines is proportional to symptom duration at enrollment. Only the glucocorticoid injection arms (in active treatment groups) and the outlier studies (in controls) deviate from the general pattern of an exponential curve of improvement.
We explored the robustness of our estimates by excluding studies with a high or unclear risk of selection, detection, or attrition bias. All analyses were performed with R with the packages meta v 5.0 (https://CRAN.R-project.org/package=meta), metafor v 3.0-2 (https://CRAN.R-project.org/package=metafor), and mvmeta v. 1.0.3 (https://CRAN.R-project.org/package=mvmeta).
Results
Global Improvement
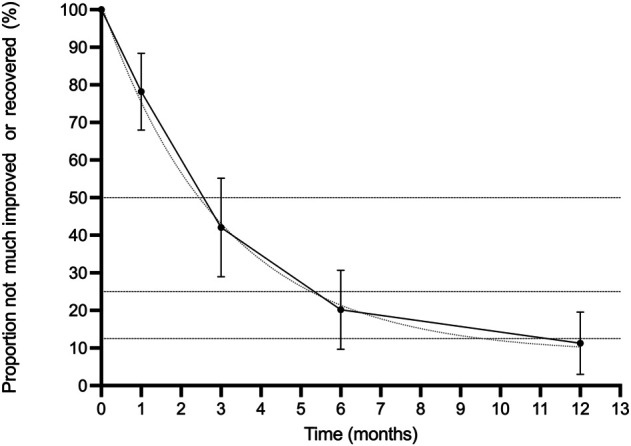
Symptom resolution occurred quickly in the absence of treatment. The half-life of the persistence of global symptoms was 2.5 to 3 months; meaning every 2.5 to 3 months, 50% of patients had symptom improvement. By 12 months, 89% (189 of 213 [95% CI 80% to 97%]) of patients had experienced improvement in their symptoms and were defined as treatment successes in the original studies (Fig. 3). Five trials (n = 257 participants) included a global improvement outcome [4, 9, 30, 34, 39].
Fig. 3.
This graph shows the proportion of patients who did not experience global improvement (five trials; 257 participants). The dashed line represents the optimal line of the half-life fit.
Pain
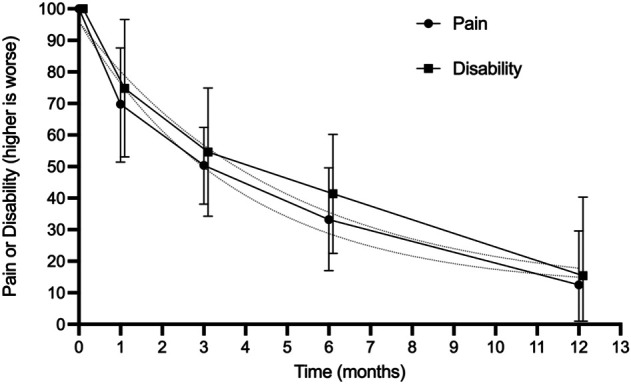
The mean pain scores followed a similar pattern, halving every 3 to 4 months and approaching 88% (95% CI 70% to 100%) resolution from the baseline values at 1 year (Fig. 4). The trajectories of the active treatment groups were generally similar to those of the placebo or no-treatment groups. Most of the glucocorticoid injection groups did not follow the same exponential pattern. Participants in glucocorticoid injection groups appeared to have rapid improvement at 6 to 8 weeks, followed by a partial rebound, and finally improvement (Fig. 2). The only control groups that did not report recovery in pain were the three outlier studies excluded from the meta-analysis [25, 32, 40] (Fig. 2A).
Fig. 4.
The trajectories of pain and disability (the percentage of remaining symptoms compared with baseline) over time are shown in this graph. The dashed lines represent the optimal half-life fit curve. Twenty trials reported pain at baseline, dropping to 10 trials at 1 year. Disability was reported by 17 trials at baseline, dropping to seven trials at 1 year.
Disability
Disability scores decreased over time, halving every 3 to 4 months and approaching 85% (95% CI 60% to 100%) improvement relative to baseline values by 1 year (Fig. 4). The only control groups that did not show recovery of disability were the two outlier studies that reported a disability outcome [32, 40] (Fig. 2C).
Differences in Trajectories Associated with Symptom Duration Before Enrollment or Placebo Effect
Differences Associated with Symptom Duration Before Enrollment
The duration of symptoms before trial enrollment was not associated with the course of pain or disability after enrollment. We found that studies in which the patients had a longer pretrial symptom duration were more likely to have a slightly larger improvement at 3 months (0.2% better improvement for each additional month of symptom duration [95% CI 0.1% to 0.3%]; p < 0.001) (Supplementary Table 4; http://links.lww.com/CORR/A670). However, this is likely a spurious finding related to one study [21] with a particularly long symptom duration (mean 74 months). Other timepoints did not show any difference, and the association at 3 months disappeared once the study with very long symptom duration was removed (Supplementary Fig. 1; http://links.lww.com/CORR/A671).
Differences Associated with Placebo Effect
Participants improved similarly after a placebo intervention compared with a no-treatment intervention with respect to pain and disability at 1 month, 3 months, and 6 months of follow-up (Fig. 5). However, at 12 months, the placebo groups had lower mean disability (pain did not differ) than the no-treatment groups did (24% mean difference [95% CI 12% to 35%] in six trials with 345 participants; p < 0.001) (Supplementary Table 4; http://links.lww.com/CORR/A670).
Fig. 5.
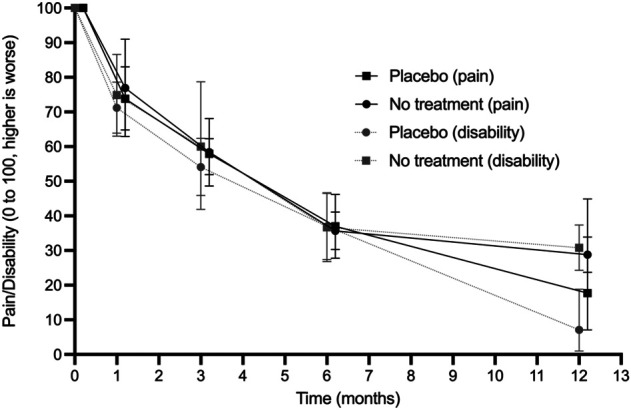
This graph shows that the relative pain and disability in participants receiving a placebo and those receiving no treatment had similar mean trajectories, except for disability at 12 months.
Sensitivity Analysis
In the sensitivity analysis, we removed studies with high risk of bias, and this analysis showed slightly lower values for the mean pain and disability but did not change the exponential decay pattern (Supplementary Fig. 2; http://links.lww.com/CORR/A672). For the global improvement outcome, only one of five trials had a low risk of bias [9] (Supplementary Table 5; http://links.lww.com/CORR/A673).
Discussion
Tennis elbow is a common cause for elbow pain and disability; it mainly affects patients in the fifth through the seventh decades of life. People with chronic musculoskeletal pain who are dissatisfied with the level of their symptoms after attempts at conservative treatment often are considered candidates for surgery despite the limited evidence to suggest that surgery is effective. The rationale behind trying surgery is that when symptoms persist despite nonsurgical interventions, the belief is that those symptoms probably will not disappear spontaneously. However, there is little evidence in support of this belief, and we felt that meta-analyzing results from the control arms of existing RCTs could help us determine to what degree it is valid, as this could help surgeons to determine whether persistence of symptoms is itself a sufficient justification to recommend surgery. Our findings, which are based on a meta-analysis of the best-available evidence, seem to undermine the persistence of symptoms as a basis to recommend surgery. We found that longer durations of symptoms were not associated with poorer prognosis, which suggests that persistent symptoms are no justification to recommend surgical interventions that have questionable efficacy profiles and known risks, such as surgery for tennis elbow.
Limitations
The main limitation of this meta-analysis is the substantial statistical heterogeneity between trials, causing uncertainty in our estimates (Supplementary Table 3; http://links.lww.com/CORR/A669). The heterogeneity was largely driven by the three outlier trials [25, 32, 40]. In one trial, the participants were competitive racket sport athletes [32], and in another [25], the number of participants was small (11 participants in the placebo group) and thus was at risk of having a large random error. These outlier studies were also the only studies where the control arm did not recover at all. Besides the three outlier studies, the pattern of recovery was consistent even if the level of symptoms differed; we observed this pattern in 21 studies including 877 patients.
There was some variation in the diagnostic criteria of tennis elbow in the included studies; most used generally accepted clinical tests, but some trials did not specify the criteria. This may be one source of heterogeneity; it is possible that those who did not recover actually did not have extensor carpi radialis brevis tendinopathy, but a clinical study would be required to test this hypothesis. We do not believe this to be a disqualifying problem; the means of diagnosis of this condition in the clinical research we evaluated generally mirrored what we have observed in typical clinical practice.
Furthermore, we did not perform meta-regression to see whether age or gender was associated with differences in symptom resolution. The demographics of the included studies represent typical tennis elbow cohorts with a mean age between 42 and 52 years and a balanced mixture of both men and women. To gauge whether age or gender is associated with differences in symptom resolution could be further investigated in another study.
Although the best fit for the curve for our primary outcome was found with an exponential decay curve, the authors may have overlooked mathematical models that could fit the data even better. We also cannot be sure whether the exponential decay pattern would stay constant if we continued follow-up for many years.
The trajectories of improvement observed in our review may not perfectly reflect the clinical course of tennis elbow, since other nonspecific effects such as regression to the mean and the Hawthorne effect (because of trial participation) may impact the reported symptom levels [3, 36]. Furthermore, volunteers in clinical trials could, in theory, be different from patients in routine care. However, patients in the included RCTs were recruited from typical clinical populations. We found a uniform pattern of symptom resolution in most trials, and we therefore assume that most patients will display similar patterns as the participants in the included trials.
Discussion of Key Findings
We found that approximately 90% of patients who received no active treatment for tennis elbow had either completely recovered or were much improved by 1 year, and they had little remaining pain and disability by that point. The global improvement followed exponential decay similar to the half-life of drugs, which means that the probability of spontaneous recovery remained constant for up to 1 year from the start of the trials despite the inclusion of participants with prolonged symptoms (months or even years) in many studies. Considering this, surgeons should not offer surgery based on an arbitrary symptom duration. A drug molecule that remains in the body after three half-lives is no different from a molecule that is eliminated first. Our study indicates that patients can expect spontaneous recovery whether their symptoms have been present for a few weeks or much longer. Conveying this prognostic information to patients may help them to cope with their symptoms and reduce the likelihood they will seek or be treated with ineffective or unproven treatments, such as platelet-rich plasma injections or surgery, respectively [6, 19].
Improvements in pain and disability (continuous measures) followed a similar trajectory as global improvement (binary measure) at the group level. Eighty-eight percent of pain (95% CI 70% to 100%) and 85% of disability (95% CI 60% to 100%) had resolved at the group level by 1 year. The trajectories of the means likely do not reflect individual patients’ symptoms—the resolution of pain or disability is not likely spread evenly among participants. Some may have had little or no change, whereas most have had complete recovery. This assumption was supported by the global improvement meta-analysis, and one trial that reported both median (0.5 of 100) and mean (5.3 of 100) values for pain at 12 months [9]. Therefore, clinicians need to underline the uncertainty for their patients: Although mean trajectories show constant and substantial improvement in all outcomes, we cannot accurately predict outcomes at the individual level.
We are not aware of previous meta-analyses or individual observational studies of the natural history or clinical course of tennis elbow or other tendinopathy, but the findings corroborate anecdotal observations [11]. Furthermore, placebo-controlled trial evidence in other degenerative conditions, such as symptomatic rotator cuff disease and degenerative knee pain [6, 18, 43], also show large improvements in the control arms. There are similar findings for low back pain [2].
Understanding the rate of symptom resolution at the population level is useful for determining whether trials of new or existing unproven interventions are likely to find worthwhile benefits. Because up to 90% of people have either completely recovered or are much improved by 1 year with little remaining pain and disability after this time, worthwhile surgical interventions would need to be justified by high levels of early efficacy and little or no risk to outperform watchful waiting. Our finding of a high likelihood of recovery irrespective of symptom duration means that observational studies of surgery for chronic tennis elbow are likely to have overestimated the benefit attributable to surgery. The basis for this claim is that such studies generally assume that the condition is not going to improve on its own; our findings suggest that this is not a valid assumption, and that some—perhaps much—of the benefit attributed to surgery was likely to have occurred in the absence of any intervention. In addition, our data suggest that providing early surgical care is unlikely to improve on the favorable natural history of symptoms. The only available placebo-controlled trial on the surgical treatment of tennis elbow [21] appears to support this.
Some clinicians wonder, and perhaps hope, that even if the active component of an intervention (such as an injection of growth factors) is not efficacious, then perhaps the insertion of the needle used when delivering some product will have some effect. Our findings undermine this reasoning, at least for the interventions included in our meta-analysis. Participants in the studies generally showed comparable changes over time whether or not they were aware they received no active treatment. This implies that the placebo effect (as well as perhaps detection and performance bias) is small compared with other nonspecific effects in this population. Future studies might evaluate whether the observations of this meta-analysis apply to other tendinopathies as well.
Conclusion
Data from participants in the nonactive treatment arms in published randomized controlled trials confirm that tennis elbow is almost always a self-limiting condition: Roughly 90% of patients achieve resolution of symptoms at 1 year without any treatment, and this occurs regardless of symptom duration before trial enrollment. People who are frustrated with persistent symptoms should be informed that as far as we know, spontaneous improvement is about as likely during the subsequent few months as it was early after the symptoms first appeared. Because a long duration does not affect prognosis, it should not be used to justify interventions with questionable efficacy such as surgery.
Supplementary Material
Acknowledgments
We thank Mr. Andrew Barnett and Mr. Joona Juurakko with their help checking and extracting the data and preparing the figures for this manuscript.
Footnotes
Each author certifies that there are no funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article related to the author or any immediate family members.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
This work was performed at Hospital Nova of Central Finland, Finland.
Contributor Information
Joona Ikonen, Email: jooiko@utu.fi.
Tuomas Lähdeoja, Email: tuomas.lahdeoja@gmail.com.
Clare L. Ardern, Email: clare.ardern@liu.se.
Rachelle Buchbinder, Email: rachelle.buchbinder@monash.edu.
Aleksi Reito, Email: aleksi@reito.fi.
References
- 1.Åkermark C, Crone H, Elsasser U, Forsskahl B. Glycosaminoglycan polysulfate injections in lateral humeral epicondylalgia: a placebo-controlled double-blind trial. Int J Sports Med. 1995;16:196-200. [DOI] [PubMed] [Google Scholar]
- 2.Artus M, van der Windt DA, Jordan KP, Hay EM. Low back pain symptoms show a similar pattern of improvement following a wide range of primary care treatments: a systematic review of randomized clinical trials. Rheumatology (Oxford) . 2010;49:2346‐2356. [DOI] [PubMed] [Google Scholar]
- 3.Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Int J Epidemiol . 2005;34:215-220. [DOI] [PubMed] [Google Scholar]
- 4.Bisset L, Beller E, Jull G, Brooks P, Darnell R, Vicenzina B. Mobilisation with movement and exercise, corticosteroid injection, or wait and see for tennis elbow: randomised trial. BMJ . 2006;333:939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bisset L, Smidt N, Van Der Windt D, et al. Conservative treatments for tennis elbow do subgroups of patients respond differently? Rheumatology (Oxford) . 2007;46:1601-1605. [DOI] [PubMed] [Google Scholar]
- 6.Brignardello-Petersen R, Guyatt GH, Buchbinder R, et al. Knee arthroscopy versus conservative management in patients with degenerative knee disease: a systematic review. BMJ Open . 2017;7:e016114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchbinder R, Johnston RV, Barnsley L, Assendelft WJ, Bell SN, Smidt N. Surgery for lateral elbow pain. Cochrane Database Syst Rev. 2011;2011(3):CD003525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chesterton LS, Lewis AM, Sim J, et al. Transcutaneous electrical nerve stimulation as adjunct to primary care management for tennis elbow: pragmatic randomised controlled trial (TATE trial). BMJ . 2013;347:f5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coombes BK, Bisset L, Brooks P, Khan A, Vicenzino B. Effect of corticosteroid injection, physiotherapy, or both on clinical outcomes in patients with unilateral lateral epicondylalgia. JAMA . 2013;309:461-469. [DOI] [PubMed] [Google Scholar]
- 10.Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet . 2010;376:1751-1767. [DOI] [PubMed] [Google Scholar]
- 11.Cyriax JH. The pathology and treatment of tennis elbow. J Bone Joint Surg . 1936;18:921-940. [Google Scholar]
- 12.Fink M, Wolkenstein E, Karst M, Gehrke A. Acupuncture in chronic epicondylitis: a randomized controlled trial. Rheumatology (Oxford) . 2002;41:205-209. [DOI] [PubMed] [Google Scholar]
- 13.Haahr JP, Andersen JH. Prognostic factors in lateral epicondylitis: a randomized trial with one-year follow-up in 266 new cases treated with minimal occupational intervention or the usual approach in general practice. Rheumatology (Oxford) . 2003;42:1216-1225. [DOI] [PubMed] [Google Scholar]
- 14.Haker E, Lundeberg T. Laser treatment applied to acupuncture points in lateral humeral epicondylalgia. A double-blind study. Pain . 1990;43:243-247. [DOI] [PubMed] [Google Scholar]
- 15.Haker EH, Lundeberg TC. Lateral epicondylalgia: report of noneffective midlaser treatment. Arch Phys Med Rehabil. 1991;72:984-988. [PubMed] [Google Scholar]
- 16.Hay EM, Paterson SM, Lewis M, Hosie G, Croft P. Pragmatic randomised controlled trial of local corticosteroid injection and naproxen for treatment of lateral epicondylitis of elbow in primary care. BMJ. 1999;319:964-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ . 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karjalainen TV, Jain NB, Page CM, et al. Subacromial decompression surgery for rotator cuff disease. Cochrane Database Syst Rev . 2019;1:CD005619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karjalainen T, Silagy M, O’Bryan E, et al. Autologous blood and platelet-rich plasma injection therapy for lateral elbow pain. Cochrane Database Syst Rev . 2021;9:CD010951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keijsers R, de Vos RJ, Kuijer PPF, van den Bekerom MP, van der Woude HJ, Eygendaal D. Tennis elbow. Shoulder Elbow. 2019;11:384-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroslak M, Murrell GAC. Surgical treatment of lateral epicondylitis: a prospective, randomized, double-blinded, placebo-controlled clinical trial. Am J Sports Med . 2018;46:1106-1113. [DOI] [PubMed] [Google Scholar]
- 22.Kroslak M, Pirapakaran K, Murrell GAC. Counterforce bracing of lateral epicondylitis: a prospective, randomized, double-blinded, placebo-controlled clinical trial. J Shoulder Elbow Surg . 2019;28:288-295. [DOI] [PubMed] [Google Scholar]
- 23.Lindenhovius A, Henket M, Gilligan BP, Lozano-Calderon S, Jupiter JB, Ring D. Injection of dexamethasone versus placebo for lateral elbow pain: a prospective, double-blind, randomized clinical trial. J Hand Surg Am . 2008;33:909-919. [DOI] [PubMed] [Google Scholar]
- 24.Martin CE, Schweitzer ME. MR imaging of epicondylitis. Skeletal Radiol. 1998;27:133-138. [DOI] [PubMed] [Google Scholar]
- 25.Mehra A, Zaman T, Jenkin AIR. The use of a mobile lithotripter in the treatment of tennis elbow and plantar fasciitis. Surgeon. 2003;1:290-292. [DOI] [PubMed] [Google Scholar]
- 26.Melikyan EY, Shahin E, Miles J, Bainbridge LC. Extracorporeal shock-wave treatment for tennis elbow. A randomised double-blind study. J Bone Joint Surg Br . 2003;85:852-855. [PubMed] [Google Scholar]
- 27.Mishra AK, Skrepnik NV, Edwards SG, et al. Efficacy of platelet-rich plasma for chronic tennis elbow: a double-blind, prospective, multicenter, randomized controlled trial of 230 patients. Am J Sports Med. 2014;42:463-471. [DOI] [PubMed] [Google Scholar]
- 28.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med . 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montalvan B, Le Goux P, Klouche S, Borgel D, Hardy P, Breban M. Inefficacy of ultrasound-guided local injections of autologous conditioned plasma for recent epicondylitis: results of a double-blind placebo-controlled randomized clinical trial with one-year follow-up. Rheumatology (Oxford) . 2016;55:279-285. [DOI] [PubMed] [Google Scholar]
- 30.Olaussen M, Holmedal Ø, Mdala I, Brage S, Lindbæk M. Corticosteroid or placebo injection combined with deep transverse friction massage, Mills manipulation, stretching and eccentric exercise for acute lateral epicondylitis: a randomised, controlled trial. BMC Musculoskelet Disord . 2015;16:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paoloni JA, Appleyard RC, Nelson J, Murrell GA. Topical nitric oxide application in the treatment of chronic extensor tendinosis at the elbow: a randomized, double-blinded, placebo-controlled clinical trial. Am J Sports Med. 2003;31:915-920. [DOI] [PubMed] [Google Scholar]
- 32.Petrella RJ, Cogliano A, Decaria J, Mohamed N, Lee R. Management of tennis elbow with sodium hyaluronate periarticular injections. Sports Med Arthrosc Rehabil Ther Technol . 2010;2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rees J, Wilson AM, Wolman RL. Current concepts in the management of tendon disorders. Rheumatology (Oxford) . 2006;45:508-521. [DOI] [PubMed] [Google Scholar]
- 34.Runeson L, Haker E. Iontophoresis with cortisone in the treatment of lateral epicondylalgia (tennis elbow) - a double-blind study. Scand J Med Sci Sports . 2002;12:136-142. [DOI] [PubMed] [Google Scholar]
- 35.Sayegh ET, Strauch RJ. Does nonsurgical treatment improve longitudinal outcomes of lateral epicondylitis over no treatment? A meta-analysis. Clin Orthop Relat Res . 2015;473:1093-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sedgwick P, Greenwood N. Understanding the Hawthorne effect. BMJ. 2015;351:h4672. [DOI] [PubMed] [Google Scholar]
- 37.Shiri R, Viikari-Juntura E, Varonen H, Heliövaara M. Prevalence and determinants of lateral and medial epicondylitis: a population study. Am J Epidemiol . 2006;164:1065-1074. [DOI] [PubMed] [Google Scholar]
- 38.Silverstein B, Welp E, Nelson N, Kalat J. Claims incidence of work-related disorders of the upper extremities: Washington state, 1987 through 1995. Am J Public Health . 1998;88:1827-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smidt N, van der Windt DA, Assendelft WJ, Devillé WL, Korthals-de Bos IB, Bouter LM. Corticosteroid injections, physiotherapy, or a wait-and-see policy for lateral epicondylitis: a randomised controlled trial. Lancet. 2002;359:657-662. [DOI] [PubMed] [Google Scholar]
- 40.Spacca G, Necozione S, Cacchio A. Radial shock wave therapy for lateral epicondylitis: a prospective randomised controlled single-blind study. Eura Medicophys . 2005;41:17-25. [PubMed] [Google Scholar]
- 41.Staples MP, Forbes A, Ptasznik R, Gordon J, Buchbinder R. A randomized controlled trial of extracorporeal shock wave therapy for lateral epicondylitis (tennis elbow). J Rheumatol. 2008;35:2038-2046. [PubMed] [Google Scholar]
- 42.Tahririan MA, Moayednia A, Momeni A, Yousefi A, Vahdatpour B. A randomized clinical trial on comparison of corticosteroid injection with or without splinting versus saline injection with or without splinting in patients with lateral epicondylitis. J Res Med Sci . 2014;19:813-818. [PMC free article] [PubMed] [Google Scholar]
- 43.Wartolowska KA, Gerry S, Feakins BG, et al. A meta-analysis of temporal changes of response in the placebo arm of surgical randomized controlled trials: an update. Trials. 2017;18:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolf JM, Ozer K, Scott F, Gordon MJ, Williams AE. Comparison of autologous blood, corticosteroid, and saline injection in the treatment of lateral epicondylitis: a prospective, randomized, controlled multicenter study. J Hand Surg Am . 2011;36:1269-1272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.