Abstract
Background
Dedifferentiated chondrosarcoma is a chondrosarcoma subtype associated with high rates of recurrence and a poor prognosis. Others have proposed treatment of dedifferentiated chondrosarcoma using osteosarcoma protocols, including perioperative chemotherapy. However, the rarity of this condition poses difficulties in undertaking single- institution studies of sufficient sample size.
Question/purpose
Is perioperative chemotherapy associated with improved overall survival in patients with dedifferentiated chondrosarcoma?
Methods
We queried the Surveillance, Epidemiology, and End Results (SEER) 1973 to 2016 database for patients with a diagnosis of dedifferentiated chondrosarcoma (n = 308). As dedifferentiated chondrosarcoma was only classified as a distinct entity in SEER starting in 2000, only patients treated in 2000 and later were included. We excluded from our analyses those patients with distant disease at diagnosis, a primary site of disease other than bone or joints, and those who did not receive cancer-directed surgery. These criteria yielded 185 dedifferentiated chondrosarcoma patients for inclusion. We used Kaplan-Meier analyses and Cox proportional hazards models to assess the association of clinical, demographic, and treatment characteristics on overall survival (OS).
Results
After controlling for confounding variables, including age, sex, tumor size, stage, grade, location, and radiation treatment status, and after adjusting for missing data, no overall survival benefit was associated with receipt of chemotherapy in patients with dedifferentiated chondrosarcoma (hazard ratio 0.75 [95% confidence interval 0.49 to 1.12]; p = 0.16).
Conclusion
Chemotherapy treatment of dedifferentiated chondrosarcoma was not associated with improved OS. These results must be viewed cautiously, given the limited granularity of information on chemotherapy treatment, the concerns regarding chemotherapy misclassification in SEER data, and the small sample of patients with dedifferentiated chondrosarcoma, all of which limit the power to detect a difference. Our findings are nevertheless consistent with those of prior reports in which no benefit of chemotherapy could be detected. Lack of clear benefit from perioperative chemotherapy in dedifferentiated chondrosarcoma argues that it should be used only after careful consideration, and ideally in the context of a clinical trial.
Level of Evidence
Level III, therapeutic study.
Introduction
Chondrosarcomas represent approximately 25% of bone sarcomas [12]. Conventional chondrosarcoma is the most common subtype [43]. Dedifferentiated chondrosarcomas (DDCS) represent approximately 10% to 15% of chondrosarcomas [8, 38]. DDCS is characterized histologically by the presence of a conventional, lower-grade chondrosarcoma, with an abrupt change to a high-grade, nonchondrogenic sarcoma component [12]. DDCS is aggressive, with high rates of recurrence and metastasis and a poorer prognosis than conventional chondrosarcoma [10, 13, 20, 23, 26, 28, 34]
The primary treatment of chondrosarcomas is surgical resection [3, 11]. Low-grade lesions of the extremities may be managed with more conservative forms of local therapy, including intralesional curettage [5, 17, 40]. Radiotherapy is used in the management of central lesions not amenable to surgery, and proton therapy is an area of investigation in this radioresistant tumor [1, 21].
As distant disease is the dominant mode of treatment failure in DDCS, perioperative systemic therapy might be considered to mitigate this risk. Chondrosarcomas are considered relatively resistant to conventional cytotoxic agents, although this view is based on limited data [32]. Systemic therapy may have meaningful activity in some chondrosarcoma subtypes, including DDCS [22].
Perioperative chemotherapy has been considered in patients with DDCS. A recently published report of an observational assessment of aggressive perioperative chemotherapy in DDCS indicated that administration was feasible and exhibited favorable outcomes versus prior reports [18]. However, this study was noncomparative and enrolled those with both localized and metastatic DDCS. Achievement of a surgical complete remission was the only factor associated with prolonged overall survival (OS). Previous studies assessing perioperative chemotherapy have demonstrated mixed results regarding improvements in relapse-free survival and OS [10, 24, 27, 28, 34]. These retrospective studies all had small sample sizes due to the rarity of the condition.
A much larger study assessed outcomes in 337 DDCS patients treated at nine European referral centers [13]. In a univariable Cox analysis, chemotherapy treatment was not associated with improved OS (HR 1.317 [95% CI 0.931 to 1.86]; p = 0.12). In a subanalysis limited to patients with potentially curable disease treated with surgery, chemotherapy was also not associated with improved OS.
Despite this limited evidence, patients receive primary treatment for DDCS according to osteosarcoma protocols, including chemotherapy [43]. The National Comprehensive Cancer Network guidelines recommend that primary DDCS treatment follow osteosarcoma protocols [32]. This is a Category 2B recommendation (“Based upon lower-level evidence ...”). However, treatment of another high-risk bone sarcoma, osteosarcoma, was revolutionized by the addition of perioperative chemotherapy [2, 25, 36]. No such evidence base exists to support the use of perioperative chemotherapy in DDCS treatment.
We therefore asked: Is perioperative chemotherapy associated with improved overall survival in patients with dedifferentiated chondrosarcoma?
Patients and Methods
Study Population
This study conformed to the Strengthening the Reporting of Observational Studies in Epidemiology criteria for cohort studies [42]. The target study population was patients with DDCS of osseous origin who received surgical treatment with curative intent. A similar population of patients with osteosarcoma was assembled for comparison. This parallel analysis was intended to confirm that the known benefit of perioperative chemotherapy in osteosarcoma could be detected among the patients derived from the Surveillance, Epidemiology, and End Results (SEER) database used for this retrospective study. SEER is a population-based collection of individual-level data on patients with cancer, representing approximately 35% of the US population [31]. We queried the SEER 1973 to 2016 database for patients with sarcoma using SEER*Stat [30]. This identified 308 patients with a diagnosis of DDCS and 6844 with osteosarcoma.
We used histologic-type International Classification of Diseases for Oncology, 3rd edition (ICD-O-3), code 9243 to identify patients with DDCS. The following subtypes were included in the parallel analysis of osteosarcoma patients: osteosarcoma, not otherwise specified (ICD-O-3 code 9180); chondroblastic osteosarcoma (ICD-O-3 code 9181); fibroblastic osteosarcoma or osteofibrosarcoma (ICD-O-3 code 9182); telangiectatic osteosarcoma (ICD-O-3 code 9183); osteosarcoma in Paget disease of bone (ICD-O-3 code 9184); small cell osteosarcoma or round cell osteosarcoma (ICD-O-3 code 9185); central osteosarcoma, conventional central osteosarcoma, or medullary osteosarcoma (ICD-O-3 code 9186); high-grade surface osteosarcoma (ICD-O-3 code 9194); and intracortical osteosarcoma (ICD-O-3 code 9195). Only 14 patients with high-grade surface osteosarcoma met eligibility criteria out of 2261 eligible patients. No eligible patients with intracortical osteosarcoma were identified (Supplementary Table 1; http://links.lww.com/CORR/A639). Although the latter two entities are exceedingly rare, they do possess metastatic capacity, and chemotherapy use is reported in their management [15]. Patients (n = 357) with the following osteosarcoma subtypes were excluded because they displayed low- to intermediate-grade, indolent behavior, and distinct biology: intraosseous well-differentiated osteosarcoma or intraosseous low-grade osteosarcoma, parosteal osteosarcoma or juxtacortical osteosarcoma, and periosteal osteosarcoma [4, 14-16, 46].
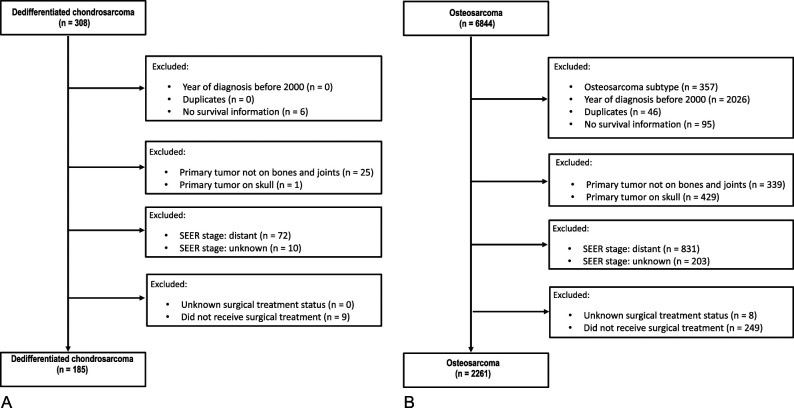
Except for histologic subtype, all other inclusion and exclusion criteria were applied identically to the chondrosarcoma and osteosarcoma datasets (Fig. 1). The SEER database did not specifically classify the diagnosis of DDCS until the year 2000. Therefore, all patients with a diagnosis before 2000 were excluded. SEER patient identification numbers were used to identify duplicate entries; these patients (DDCS = 0, osteosarcoma = 46) were excluded due to inability to reconcile the duplicate records. As OS was the primary endpoint, six DDCS and 95 osteosarcoma patients without OS data were excluded. This study also focused on tumors of osseous origin; patients with primary tumors not involving the bones and joints (SEER variable “site recode ICD-O-3/WHO 2008” for “bones and joints”) were excluded (DDCS = 25, osteosarcoma = 339). One DDCS and 429 osteosarcoma patients with tumor location of the skull (SEER variable “primary site-labeled” for “C41.0-bones of skull and face and associated joints” or “C41.1-mandible”) were also excluded. As stage information was important to identify those patients potentially eligible for curative surgical treatment, 82 DDCS and 1034 osteosarcoma patients with distant or unknown stage were excluded. As curative therapy in DDCS and osteosarcoma is generally considered to rely on surgery, those patients classified as either not receiving cancer-directed surgery or with unknown surgical status were excluded (DDCS = 9, osteosarcoma = 249).
Fig. 1.
A-B These flowcharts show selection of patients in the (A) dedifferentiated chondrosarcoma and (B) osteosarcoma groups.
Variables
The following demographic and clinicopathologic characteristics were abstracted from the SEER database: sex, race, age at diagnosis, primary tumor size, SEER stage (local versus regional), tumor grade, histologic subtype (for osteosarcoma), and primary tumor location. Age at the time of diagnosis was dichotomized according to the median age for the respective disease (DDCS or osteosarcoma). Tumor grade was classified as either low (Grades I and II) or high (Grades III and IV). The primary tumor location was categorized as involving either the extremities (SEER variable “primary site-labeled” for “C40.0-long bones: upper limb, scapula, and associated joints,” “C40.1-short bones of upper limb and associated joints,” “C40.2-long bones of lower limb and associated joints,” “C40.3-short bones of lower limbs and associated joints,” “C40.8-overlap of bones, joints, and cartilage of limbs,” or “C40.9-bones of limbs, not otherwise specified”) or nonextremities (SEER variable “primary site-labeled” for “C41.2-vertebral column,” “C41.3-rib, sternum, clavicle, and associated joints,” “C41.4-pelvic bones, sacrum, coccyx, and associated joints,” or “C41.9-bone, not otherwise specified”). Treatment modalities evaluated included surgery, radiation therapy, and chemotherapy. For radiation therapy and chemotherapy, SEER classifies treatment as either “yes” versus “no/unknown.” SEER does not make a distinction between nonreceipt and unknown treatment status for these modalities.
Baseline Characteristics
A total of 185 patients with DDCS and 2261 patients with osteosarcoma met the criteria for study inclusion (Fig. 1). Patients in the DDCS cohort were older (median age 66 versus 17 years; p < 0.001) and more likely to be white (94% [173 of 185] versus 75% [1703 of 2261]; p < 0.001) than those with osteosarcoma (Table 1). Seventy-two percent (134 of 185) of patients with DDCS had primary tumors located on extremities compared with 89% (2008 of 2261) of patients with osteosarcomas. DDCS patients had a higher proportion of those with high grade tumors (78% [144 of 185]) than did osteosarcoma patients (73% [1641 of 2261]; p = 0.002). There were no differences between the groups in sex, primary tumor size, or stage. Patients with DDCS were more likely to receive radiation therapy (20% [37 of 185] versus 4% [101 of 2261]; p < 0.001) and less likely to receive chemotherapy (32% [60 of 185] versus 89% [2002 of 2261]; p < 0.001) as part of their primary treatment than those in the osteosarcoma cohort. Among the eight osteosarcoma histologic subtypes included, three subtypes (osteosarcoma not otherwise specified, chondroblastic osteosarcoma, and fibroblastic osteosarcoma) comprised 90% (2026 of 2261) of this group.
Table 1.
Baseline demographic and clinicopathologic characteristics
| Characteristic | Dedifferentiated chondrosarcoma (n = 185) | Osteosarcoma (n = 2261) | p value |
| Sex | |||
| Male | 57 (106) | 55 (1252) | 0.65 |
| Racea | < 0.001 | ||
| White or unknown | 94 (173) | 75 (1703) | |
| Black | 2 (4) | 15 (331) | |
| Other (Native American, Alaska native, Asian, Pacific Islander) | 4 (8) | 10 (227) | |
| Age in years | < 0.001 | ||
| Median (range) | 66 (18-95) | 17 (3-91) | |
| 0-66 | 51 (94) | ||
| > 66 | 49 (91) | ||
| 0-17 | 52 (1179) | ||
| > 17 | 48 (1082) | ||
| Size in mm | 0.280 | ||
| Median (range) | 90 (7-420) | 90 (4-888) | |
| ≤ 8 cm | 36 (67) | 35 (781) | |
| > 8 cm | 50 (92) | 47 (1056) | |
| Missing | 14 (26) | 19 (424) | |
| SEER stage | 0.053 | ||
| Local | 36 (67) | 44 (988) | |
| Regional | 64 (118) | 56 (1273) | |
| Tumor grade | 0.002 | ||
| I and II | 10 (19) | 6 (141) | |
| III and IV | 78 (144) | 73 (1641) | |
| Missing | 12 (22) | 21 (479) | |
| Primary tumor location | < 0.001 | ||
| Extremities | 72 (134) | 89 (2008) | |
| Trunk or bone, NOS | 28 (51) | 11 (253) | |
| Subtype | N/A | ||
| Osteosarcoma, NOS | 68 (1541) | ||
| Chondroblastic osteosarcoma | 16 (356) | ||
| Fibroblastic osteosarcoma | 6 (129) | ||
| Telangiectatic osteosarcoma | 4 (95) | ||
| Osteosarcoma in Paget disease of bone | 1 (16) | ||
| Small cell osteosarcoma | 1 (18) | ||
| Central osteosarcoma | 4 (92) | ||
| High-grade surface osteosarcoma | 1 (14) | ||
| Radiation | < 0.001 | ||
| No/unknown | 80 (148) | 96 (2160) | |
| Yes | 20 (37) | 4 (101) | |
| Chemotherapy | < 0.001 | ||
| No/unknown | 68 (125) | 11 (259) | |
| Yes | 32 (60) | 89 (2002) |
Data presented as % (n), unless otherwise indicated.
Self-reported; NOS = not otherwise specified.
In the DDCS group, the median (range) follow-up time was 13 months (1 to 170), and 70% (130 of 185) of patients died. In the osteosarcoma group, the median follow-up time was 53 months (1 to 203), and 32% (715 of 2261) of patients died.
The available baseline variables were assessed by logistic regression for their association with receipt versus nonreceipt of chemotherapy/unknown chemotherapy treatment (Supplementary Table 2; http://links.lww.com/CORR/A640). For DDCS, age older than 66 years was associated with a lesser likelihood of chemotherapy treatment (odds ratio [OR] 0.34; p < 0.01); size greater than 8 cm was associated with an increased likelihood of chemotherapy treatment (OR 2.13; p = 0.04). For osteosarcoma, age older than 17 years (OR 0.11; p < 0.01), low tumor grade (OR 0.07; p < 0.01), primary location on the trunk or not otherwise specified (OR 0.22; p < 0.01), and receipt of radiation therapy (OR 0.33; p < 0.01) were associated with a lesser likelihood of chemotherapy treatment. Size greater than 8 cm (OR 1.47; p = 0.01) and the presence of regional disease at the initial diagnosis (OR 1.81; p < 0.01) were associated with an increased likelihood of chemotherapy treatment.
The only missing data were in primary tumor size and tumor grade (Supplementary Table 3; http://links.lww.com/CORR/A641). For the DDCS cohort, 77% (142 of 185) had complete tumor size and grade data. For the osteosarcoma cohort, 66% (1501 of 2261) had complete data. Missing data with respect to these variables were not correlated with one another for DDCS but were positively correlated for osteosarcoma (ρ = 0.0917, p = 0.21 and ρ = 0.1474, p < 0.01, respectively).
Ethical Approval
Because the SEER database is a publicly available database of deidentified patient data, no ethics committee review was required for its use in this project.
Statistical Analyses
Demographic and clinical characteristics were compared using a Fisher exact test for categorical variables and Wilcoxon rank sum test for continuous variables. Univariable logistic regression analysis compared the association between baseline clinicopathologic variables and receipt or nonreceipt of chemotherapy/unknown status (see description under Baseline Characteristics).
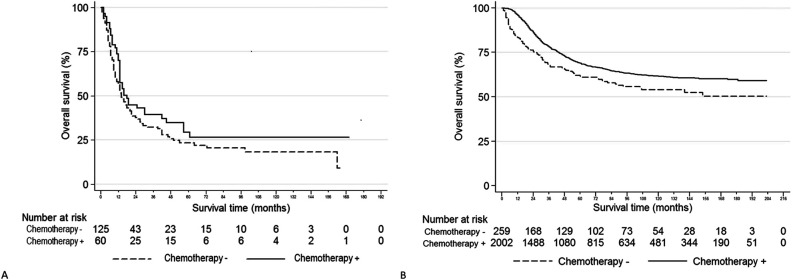
The association of chemotherapy treatment with OS was explored preliminarily with Kaplan-Meier analyses, with the log-rank test used for statistical comparison (Fig. 2) and with univariable Cox proportional hazards models (Supplementary Table 4; http://links.lww.com/CORR/A642). When OS in DDCS was compared with respect to chemotherapy treatment, there was no difference in OS (chemotherapy median OS: 18 months [95% CI 13 to 42] versus no/unknown: 14 months [95% CI 11 to 21]; hazard ratio = 0.77 [95% CI 0.53 to 1.13]; p = 0.18) (Fig. 2A). In contrast to DDCS, chemotherapy was associated with improved OS among the osteosarcoma study population (chemotherapy median OS: not reached [95% CI not calculable] versus chemotherapy no/unknown: not reached [95% CI 89 m-not calculable]; HR 0.70 [95% CI 0.57 to 0.86]; p = 0.001).
Fig. 2.
A-B Kaplan-Meier curves representing overall survival by (+) receipt or (-) nonreceipt of chemotherapy or unknown treatment status. (A) This figure shows dedifferentiated chondrosarcoma (log-rank p = 0.17). (B) This figure shows osteosarcoma (log-rank p < 0.01).
The primary objective of this study was to assess the association between chemotherapy treatment and OS in patients with DDCS, using adjusted multivariable Cox models. We analyzed SEER patients with osteosarcoma as a parallel analysis because concerns have been raised regarding misclassification of chemotherapy and radiation treatment in the SEER database [33] and because osteosarcoma treatment protocols are proposed as a model for DDCS treatment [32]. Univariable Cox models were used to select variables for inclusion in the multivariable models (Supplementary Table 4; http://links.lww.com/CORR/A642). To allow comparison, identical models were developed for the DDCS and osteosarcoma cohorts. The only exception was for the variable age, which was dichotomized at a median of 66 years for patients with DDCS and 17 years for those with osteosarcoma, reflecting the differing age distributions of the two conditions. Variables were included as adjustment variables in the multivariable Cox models for each cohort if the 95% CI of the univariable HR did not include 1.0 for either cohort. Histologic subtypes of osteosarcoma were not included as a variable in multivariable modeling because the DDCS cohort included only one histologic subtype. Chemotherapy treatment status was included as the variable of primary interest.
Age was the only variable associated with OS in the DDCS cohort. In the osteosarcoma cohort, sex, age, tumor size, SEER stage, tumor grade, primary tumor location, and radiation therapy treatment status were associated with OS. Race was not associated with OS in either cohort, and therefore, it was not included in multivariable analyses. The selected covariates included all variables statistically associated with receipt or nonreceipt/unknown chemotherapy treatment status (Supplementary Table 2; http://links.lww.com/CORR/A640).
To confirm the suitability of the selected covariates for the multivariable models, they were assessed for proportional hazard assumption violations using Schöenfeld residuals and examination of log-log plots. For the DDCS cohort, only the primary tumor location term violated the proportional hazard assumptions (p < 0.01). Examination of the corresponding log-log plot indicated that this was because of a limited number of patients with prolonged survival in the nonextremity site (data not shown). Because this was an adjustment variable and not of primary interest, we did not undertake a specific adjustment.
For the osteosarcoma cohort, age, primary tumor location and chemotherapy covariates violated the proportional hazard assumptions (p = 0.02, p = 0.04, and p < 0.01, respectively). The log-log plots for these variables indicated that this was related to a limited number of patients with shorter survival (data not shown). Because the log-log normal graph indicated that only a limited number of patients explained the divergence, and there was no crossing of the curves (data not shown), no specific adjustment was made. To assess the significance of adjustment variables in the multivariable models, we conducted the likelihood ratio test to compare the full models with models reduced by excluding chemotherapy treatment. The results of this test did not change the conclusions of the primary analyses (data not shown).
Multivariable Cox models assessing the association between chemotherapy treatment and OS for both cohorts included sex, age, tumor size, SEER stage, tumor grade, tumor location, and receipt of radiation as adjustment covariates. There were missing data for tumor size (11% [21 of 185] of DDCS and 12% [281 of 2261] of osteosarcoma) and tumor grade (9% [17 of 185] for DDCS and 15% [336 of 2261] for osteosarcoma). In the primary study analysis, missing data for these two variables were considered as a separate classification.
As a sensitivity analysis to explore the impact of these missing data, we performed multivariable Cox analyses using multiple imputation by chain equation to generate 50 individual datasets based on the covariates using the STATA function (mi) (StataCorp) [19, 41, 44, 45]. Tumor grade and size were treated as binary categorical variables. The calculations were based on sex, age, stage, tumor location, receipt of radiation, receipt of chemotherapy, Nelson-Aalen survival estimates, and a survival indicator variable.
A nominal p value of ≤ 0.05 was designated for statistical significance. Stata Version 12.1 (StataCorp) was used for all statistical analyses.
Results
Is Perioperative Chemotherapy Associated with Improved OS in Patients with DDCS?
We fit a multivariable Cox model assessing the association of chemotherapy treatment with OS among patients with DDCS, adjusting for sex, age, tumor size, SEER stage, tumor grade, tumor location, and receipt of radiation. Perioperative chemotherapy was not associated with improved OS (HR 0.75 [95% CI 0.49 to 1.12]; p = 0.16) (Table 2). To account for missing data in tumor size and grade, we repeated the adjusted analysis using multiple imputation (Supplementary Table 5; http://links.lww.com/CORR/A643). Again, there was no OS benefit associated with chemotherapy treatment in DDCS.
Table 2.
Multivariable Cox proportional hazards analyses of overall survival
| Characteristics | Dedifferentiated chondrosarcoma (n = 185) HR (95% CI) | p value | Osteosarcoma (n = 2261) HR (95% CI) | p value |
| Sex | 0.19 | 0.002 | ||
| Male | Referent | Referent | ||
| Female | 0.78 (0.53-1.13) | 0.79 (0.68-0.92) | ||
| Age in years | 0.09 | < 0.001 | ||
| 0-66 | Referent | |||
| > 66 | 1.40 (0.95-2.06) | |||
| 0-17 | Referent | |||
| < 17 | 1.58 (1.35-1.85) | |||
| Tumor size | ||||
| ≤ 8 cm | Referent | Referent | ||
| > 8 cm | 1.26 (0.84-1.88) | 0.26 | 1.22 (1.03-1.45) | 0.02 |
| Missing | 1.82 (1.08-3.07) | 0.03 | 1.15 (0.93-1.42) | 0.19 |
| SEER stage | 0.57 | < 0.001 | ||
| Local | Referent | Referent | ||
| Regional | 1.12 (0.76-1.64) | 1.64 (1.40-1.93) | ||
| Tumor grade | ||||
| Low (I and II) | 0.86 (0.46-1.58) | 0.62 | 0.36 (0.24-0.55) | < 0.001 |
| High (III and IV) | Referent | Referent | ||
| Missing | 1.22 (0.73-2.03) | 0.44 | 0.96 (0.79-1.15) | 0.64 |
| Primary tumor location | 0.06 | < 0.001 | ||
| Extremities | Referent | Referent | ||
| Trunk or bone, NOS | 0.66 (0.43-1.01) | 1.97 (1.59-2.43) | ||
| Radiation | 0.54 | < 0.001 | ||
| No/unknown | Referent | Referent | ||
| Yes | 1.15 (0.74-1.79) | 1.85 (1.41-2.42) | ||
| Chemotherapy | 0.16 | 0.002 | ||
| No/unknown | Referent | Referent | ||
| Yes | 0.75 (0.49-1.12) | 0.69 (0.55-0.88) |
NOS = not otherwise specified.
Given that it is well known that chemotherapy is associated with improved survivorship of patients with osteosarcoma, we wished to run an analysis among patients with osteosarcoma similar to the one we ran in patients with DDCS to confirm the face validity of our analysis on chemotherapy in patients with DDCS. After adjusting for sex, age, tumor size, SEER stage, tumor grade, tumor location, and receipt of radiation, receipt of chemotherapy was associated with improved OS in osteosarcoma (HR 0.69 [95% CI 0.55 to 0.88]; p < 0.01). Chemotherapy remained associated with improved OS among osteosarcoma patients, after accounting for missing data using multiple imputation (Supplementary Table 5; http://links.lww.com/CORR/A643).
Discussion
Primary management of DDCS remains controversial, and the development of distant metastatic disease is the major obstacle to improving long-term outcomes [27]. With the success of perioperative chemotherapy in other high-grade bone sarcomas, it is reasonable to consider such therapy in patients with DDCS [25], particularly given the frequent finding of an osteosarcomatous component in DDCS [34]. In this study, we found no improvement in OS for patients with DDCS who received chemotherapy versus those who did not. As expected, chemotherapy was associated with improved OS in osteosarcoma. The latter analysis was performed to verify the face validity of our analytic approach using the SEER database.
Limitations
This study is a retrospective analysis, a limitation imposed by the rarity of DDCS, which has prevented the conduct of adequately powered prospective studies of the question posed herein. Retrospective studies are subject to several biases, for which we have attempted to control. Selection bias could play a role in our results. For example, the population identified from SEER with DDCS may not reflect the DDCS population as a whole. However, SEER is a population-based registry, as opposed to patients identified at a single or small number of institutions, which is typically the case in other DDCS studies. We would anticipate that DDCS patients identified in SEER better reflect the actual diversity of DDCS than in institution-based studies.
This is also a study that compares outcomes of those receiving chemotherapy for DDCS with those not doing so. We have controlled for a variety of variables that might be associated with OS in this condition. The DDCS cohort was limited to 185 patients. As this is a negative study, a post hoc power calculation can be undertaken for illustrative purposes. A prospective, randomized clinical study using a similar DDCS cohort to detect an OS benefit for chemotherapy as observed in the osteosarcoma group (HR 0.65) would have 74% power to detect such a benefit, if such a benefit existed (two-sided alpha = 0.05, sample size = 185, chemotherapy/no chemotherapy allocation ratio = 0.48). Although not statistically rigorous, this calculation suggests that the DDCS group under study could reasonably have detected an OS benefit for chemotherapy of a magnitude similar to that in the osteosarcoma group. If the actual OS benefit of chemotherapy was less than that for osteosarcoma, the power of this DDCS cohort to detect that benefit would be correspondingly less, but the actual clinical significance of such a benefit could be criticized.
Parenthetically, a sample size estimate also illustrates the great difficulties facing any randomized clinical trial to assess perioperative chemotherapy in DDCS. A randomized clinical trial to assess a treatment regimen for the same HR would require 178 patients (two-sided alpha = 0.05, power = 80%, chemotherapy/no chemotherapy allocation ratio = 1). Because our study using the SEER database, which represents one-third of the US population over a 16-year period, could only identify 185 patients, it suggests that such a study could be accomplished only with great difficulty. Development of more active agents in DDCS or identification of patient subsets with particular sensitivity to chemotherapy treatment would be prerequisites to conducting successful studies.
Despite the small DDCS group size, this is the second-largest study regarding the use of chemotherapy in this condition [13]. Extending the period of eligibility earlier could have increased patient numbers to some degree. However, DDCS was not specified in the SEER database before 2000, and a histologic diagnosis of DDCS would have required imputation from a diagnosis of chondrosarcoma and high histologic grade. This would have introduced unacceptable uncertainty into the analysis. In addition, treatment in the selected time interval (2000 to 2016) likely reflects current treatment paradigms. Another group analyzing DDCS using the SEER database similarly limited the time period for eligibility, although this earlier analysis did not address the impact of chemotherapy [37].
The detail of chemotherapy treatment provided by the SEER database is limited to yes versus no/unknown. Patients who did not receive chemotherapy and those with unknown treatment status are combined into one group and are indistinguishable. Patients in the no/unknown status group who, in fact, received chemotherapy would tend to minimize differences versus the group receiving chemotherapy treatment. In this respect, the osteosarcoma comparison is important: The finding of a difference in treatment effect when comparing the chemotherapy-treated and the untreated/unknown groups suggests that the conflation of untreated and unknown status patients does not prevent detection of a chemotherapy benefit.
Furthermore, SEER does not provide information regarding regimens administered, dose intensity, duration of therapy, timing of other treatments (such as surgery), or other data that might present a more granular characterization of treatment in these patients. Other retrospective studies possessing more detailed chemotherapy treatment information suggest significant variation in DDCS treatment regimens; it is reasonable to assume that this cohort is similar [9, 13, 22, 24, 27, 34, 39]. This study assessed the benefit of chemotherapy in DDCS, as administered for this condition to the SEER population. One might presume that treatment regimens administered favored those with activity against osteosarcoma, but this cannot be ascertained. No OS benefit from chemotherapy in DDCS could be detected. Whether there exists some form of chemotherapy treatment from which DDCS patients could benefit is beyond the capability of this dataset to assess.
Finally, this analysis cannot control for every possible confounding factor. In comparing the outcomes of the multivariable analyses, all variables included in the multivariable analysis remained significant in the osteosarcoma group, but only age appeared to be significant in those with DDCS. The choice of covariates for adjustment is limited to those available in SEER. Another SEER analysis of DDCS examined the entire DDCS population, including those with metastatic disease [37]. Although race, stage, tumor size, and the presence of distant disease at diagnosis were identified as factors in a univariable analysis of disease-specific survival, only metastasis at diagnosis remained significant in a multivariable analysis. Thus, our findings in DDCS patients are consistent with the prior analysis, given the differences in patient selection, covariate selection, and endpoints.
Is Perioperative Chemotherapy Associated with Improved OS in Patients with DDCS?
With the numbers available, we found no benefit in survival associated with the administration of chemotherapy in the primary treatment of DDCS. The study population was limited to those with local or regional disease who received surgical treatment for their disease. This group was selected to reflect patients whose disease was considered potentially curable at the time of initial diagnosis. Some studies have suggested that perioperative chemotherapy might be beneficial in DDCS [9, 24, 27, 28, 35]. These studies identify specific clinicopathologic or treatment factors, such as a large osteosarcomatous component [9] or primary disease arising in the context of osteochondroma [35], as potentially helpful in selecting patients for perioperative chemotherapy. Other studies, including the two largest to date [13, 22], have found no benefit [10, 13, 23, 34].
The largest of these, Grimer et al. [13], analyzed 242 potentially curable patients; 76 received perioperative chemotherapy and 166 did not. Their cohort was recruited from nine European referral centers between 1975 and 2005. Our DDCS data were accrued between 2000 and 2016, reflecting a large, more recent pool of patients from numerous centers assessable via SEER. Both Grimer et al. [13] and our study reached similar conclusions: Chemotherapy was not associated with improved OS in primary DDCS treatment.
Misclassification of chemotherapy and radiation therapy treatment assignment, a recognized limitation of the SEER database, could be one explanation for lack of a detectable benefit for chemotherapy in DDCS [33]. The use of SEER data for analyses such as that in our study has been questioned [29]. Given the limited number of data sources to address the question, and the ability to account for misclassification in interpreting the analytical results, we believe the use of SEER data to explore the role of chemotherapy in primary DDCS treatment is reasonable [6, 7].
Our parallel analysis of chemotherapy treatment of osteosarcoma patients was conducted, in part, to address this limitation. Misclassification would minimize or eliminate differences in OS outcomes when comparing those receiving chemotherapy with those who did not or those in whom treatment receipt was unknown. This is not what we observed. For osteosarcoma, the two groups are clearly different in their OS outcome, and those classified as receiving chemotherapy exhibited the anticipated improvement in OS. However, the magnitude of difference between the two groups is markedly less than might be predicted, given historical osteosarcoma cure rates of about 15% with surgery alone [25]. Although unknown factors could explain this discrepancy, the simplest explanation is that a substantial proportion of patients with osteosarcoma whose therapy was classified as no or unknown with respect to chemotherapy did, in fact, receive perioperative chemotherapy. The results in the osteosarcoma cohort suggest that some degree of misclassification might also be present in the DDCS cohort. If so, there could still be differences in the magnitude of misclassification in the DDCS and osteosarcoma groups.
Conclusion
We did not identify evidence to support the routine use of chemotherapy in primary DDCS management. Although chemotherapy was not associated with superior OS, we cannot definitively state that perioperative chemotherapy does not have some beneficial association with survival because of the limitations of the SEER database. Directions for future progress include the identification of patient subsets more likely to benefit from chemotherapy and development of more active systemic therapies. Currently, we believe that patients who are determined to be candidates for chemotherapy should ideally receive it in the context of a clinical trial. If chemotherapy is to be offered outside of the experimental setting, a frank discussion should be conducted with the patient regarding the very limited data available to support its use in DDCS.
Supplementary Material
Footnotes
One of the authors (LDC) certifies receipt of personal payments or benefits, during the study period, in an amount of USD 10,000 to USD 100,000 from Daiichi Sanyko. One of the authors (SMP) certifies receipt of personal payments or benefits, during the study period, in an amount of less than USD 10,000 from Daiichi Sanyko.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Ethical approval was not sought for this study.
This work was conducted at the University of Washington, Seattle, WA, USA.
Contributor Information
Bonny Chau, Email: chaub@uw.edu.
Jose G. Mantilla, Email: mantilla@uw.edu.
Elizabeth T. Loggers, Email: eloggers@fredhutch.org.
Seth M. Pollack, Email: spollack@seattlecca.org.
Teresa S. Kim, Email: tkim5@uw.edu.
Edward Y. Kim, Email: edykim@uw.edu.
Gabrielle M. Kane, Email: kaneg@uw.edu.
Matthew J. Thompson, Email: mthomp2@uw.edu.
Jared L. Harwood, Email: harwoodj@uw.edu.
Michael J. Wagner, Email: mjwagner@seattlecca.org.
References
- 1.Amichetti M, Amelio D, Cianchetti M, et al. A systematic review of proton therapy in the treatment of chondrosarcoma of the skull base. Neurosurg Rev. 2010;33:155-165. [DOI] [PubMed] [Google Scholar]
- 2.Anderson ME. Update on survival in osteosarcoma. Orthop Clin North Am. 2016;47:283-292. [DOI] [PubMed] [Google Scholar]
- 3.Andreou D, Ruppin S, Fehlberg S, et al. Survival and prognostic factors in chondrosarcoma: results in 115 patients with long-term follow-up. Acta Orthop. 2011;82:749-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan CM, Lindsay AD, Spiguel ARV, et al. Periosteal osteosarcoma: a single-institutional study of factors related to oncologic outcomes. Sarcoma. 2018;2018:8631237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X, Yu LJ, Peng HM, et al. Is intralesional resection suitable for central grade 1 chondrosarcoma: a systematic review and updated meta-analysis. Eur J Surg Oncol. 2017;43:1718-1726. [DOI] [PubMed] [Google Scholar]
- 6.Cranmer LD, Chau B, Rockhill JK, et al. Chemotherapy in esthesioneuroblastoma/olfactory neuroblastoma: an analysis of the Surveillance Epidemiology and End Results (SEER) 1973-2015 Database. Am J Clin Oncol. 2020;43:203-209. [DOI] [PubMed] [Google Scholar]
- 7.Cranmer LD, Chau B, Thompson MJ, et al. Impact of nodal involvement on survival outcomes in chondrosarcoma: retrospective cohort analysis of Surveillance, Epidemiology, and End Results (SEER) database (2004–2015). Int J Surg Oncol (N.Y.). 2020;5. [Google Scholar]
- 8.Dahlin DC, Beabout JW. Dedifferentiation of low-grade chondrosarcomas. Cancer. 1971;28:461-466. [DOI] [PubMed] [Google Scholar]
- 9.Dhinsa BS, DeLisa M, Pollock R, et al. Dedifferentiated chondrosarcoma demonstrating osteosarcomatous differentiation. Oncol Res Treat. 2018;41:456-460. [DOI] [PubMed] [Google Scholar]
- 10.Dickey ID, Rose PS, Fuchs B, et al. Dedifferentiated chondrosarcoma: the role of chemotherapy with updated outcomes. J Bone Joint Surg Am. 2004;86:2412-2418. [PubMed] [Google Scholar]
- 11.Fiorenza F, Abudu A, Grimer RJ, et al. Risk factors for survival and local control in chondrosarcoma of bone. J Bone Joint Surg Br. 2002;84:93-99. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher CDM. World Health Organization, International Agency for Resarch on Cancer. WHO Classification of Tumours of Soft Tissue and Bone, 4th ed. IARC Press; 2013. [Google Scholar]
- 13.Grimer RJ, Gosheger G, Taminiau A, et al. Dedifferentiated chondrosarcoma: prognostic factors and outcome from a European group. Eur J Cancer. 2007;43:2060-2065. [DOI] [PubMed] [Google Scholar]
- 14.Hang J-F, Chen PC-H. Parosteal osteosarcoma. Arch Pathol Lab Med. 2014;138:694-699. [DOI] [PubMed] [Google Scholar]
- 15.Harper K, Sathiadoss P, Saifuddin A, et al. A review of imaging of surface sarcomas of bone. Skeletal Radiol. 2021;50:9-28. [DOI] [PubMed] [Google Scholar]
- 16.He X, Pang Z, Zhang X, et al. Consistent amplification of FRS2 and MDM2 in low-grade osteosarcoma: a genetic study of 22 cases with clinicopathologic analysis. Am J Surg Pathol. 2018;42:1143-1155. [DOI] [PubMed] [Google Scholar]
- 17.Hickey M, Farrokhyar F, Deheshi B, et al. A systematic review and meta-analysis of intralesional versus wide resection for intramedullary grade I chondrosarcoma of the extremities. Ann Surg Oncol. 2011;18:1705-1709. [DOI] [PubMed] [Google Scholar]
- 18.Hompland I, Ferrari S, Bielack S, et al. Outcome in dedifferentiated chondrosarcoma for patients treated with multimodal therapy: results from the EUROpean Bone Over 40 Sarcoma Study. Eur J Cancer. 2021;151:150-158. [DOI] [PubMed] [Google Scholar]
- 19.Hosmer DW, Lemeshow S, May S. Applied Survival Analysis: Regression Modeling of Time-to-Event Data . John Wiley and Sons Inc; 2008. [Google Scholar]
- 20.Huang R, Sun Z, Zheng H, et al. Identifying the prognosis factors and predicting the survival probability in patients with non-metastatic chondrosarcoma from the SEER Database. Orthop Surg. 2019;11:801-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Indelicato DJ, Rotondo RL, Begosh-Mayne D, et al. A prospective outcomes study of proton therapy for chordomas and chondrosarcomas of the spine. Int J Radiat Oncol Biol Phys. 2016;95:297-303. [DOI] [PubMed] [Google Scholar]
- 22.Italiano A, Mir O, Cioffi A, et al. Advanced chondrosarcomas: role of chemotherapy and survival. Ann Oncol. 2013;24:2916-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson S, Têtu B, Ayala AG, et al. Chondrosarcoma with additional mesenchymal component (dedifferentiated chondrosarcoma). I. A clinicopathologic study of 26 cases. Cancer. 1986;58:278-286. [DOI] [PubMed] [Google Scholar]
- 24.Kawaguchi S, Sun T, Lin PP, et al. Does ifosfamide therapy improve survival of patients with dedifferentiated chondrosarcoma? Clin Orthop Relat Res. 2014;472:983-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maki RG. Pediatric sarcomas occurring in adults. J Surg Oncol. 2008;97:360-368. [DOI] [PubMed] [Google Scholar]
- 26.Mercuri M, Picci P, Campanacci L, et al. Dedifferentiated chondrosarcoma. Skeletal Radiol. 1995;24:409-416. [DOI] [PubMed] [Google Scholar]
- 27.Miao R, Choy E, Raskin KA, et al. Prognostic factors in dedifferentiated chondrosarcoma: a retrospective analysis of a large series treated at a single institution. Sarcoma. 2019;2019:9069272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell AD, Ayoub K, Mangham DC, et al. Experience in the treatment of dedifferentiated chondrosarcoma. J Bone Joint Surg Br. 2000;82:55-61. [DOI] [PubMed] [Google Scholar]
- 29.National Cancer Institute. SEER acknowledgment of treatment data limitations. Available at: https://seer.cancer.gov/data-software/documentation/seerstat/nov2020/treatment-limitations-nov2020.html. Accessed September 15, 2021.
- 30.National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov). SEER*Stat Database: Incidence - SEER 18 Regs Custom Data (with additional treatment fields), Nov 2018 Sub (1975-2016 varying) - Linked To County Attributes - Total U.S., 1969-2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2019, based on the November 2018 submission. Available at: https://seer.cancer.gov/data. Accessed September 15, 2021. [Google Scholar]
- 31.National Cancer Institute. Overview of the SEER Program. Available at: https://seer.cancer.gov/about/overview.html. Accessed December 16, 2020.
- 32.National Comprehensive Cancer Network. NCCN Guidelines Version 1.2020 Bone Cancer. 2020. Available at: https://www.nccn.org/professionals/physician_gls/pdf/bone.pdf. Accessed December 16, 2020. [Google Scholar]
- 33.Noone AM, Lund JL, Mariotto A, et al. Comparison of SEER treatment data with Medicare claims. Med Care. 2016;54:e55-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Staals EL, Bacchini P, Bertoni F. Dedifferentiated central chondrosarcoma. Cancer. 2006;106:2682-2691. [DOI] [PubMed] [Google Scholar]
- 35.Staals EL, Bacchini P, Mercuri M, et al. Dedifferentiated chondrosarcomas arising in preexisting osteochondromas. J Bone Joint Surg Am. 2007;89:987-993. [DOI] [PubMed] [Google Scholar]
- 36.Strauss SJ, Whelan JS. Current questions in bone sarcomas. Curr Opin Oncol. 2018;30:252-259. [DOI] [PubMed] [Google Scholar]
- 37.Strotman PK, Reif TJ, Kliethermes SA, et al. Dedifferentiated chondrosarcoma: a survival analysis of 159 cases from the SEER database (2001-2011). J Surg Oncol. 2017;116:252-257. [DOI] [PubMed] [Google Scholar]
- 38.Thorkildsen J, Taksdal I, Bjerkehagen B, et al. Chondrosarcoma in Norway 1990-2013: an epidemiological and prognostic observational study of a complete national cohort. Acta Oncol. 2019;58:273-282. [DOI] [PubMed] [Google Scholar]
- 39.van Maldegem A, Conley AP, Rutkowski P, et al. Outcome of first-line systemic treatment for unresectable conventional, dedifferentiated, mesenchymal, and clear cell chondrosarcoma. Oncologist. 2019;24:110-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veth R, Schreuder B, van Beem H, et al. Cryosurgery in aggressive, benign, and low-grade malignant bone tumours. Lancet Oncol. 2005;6:25-34. [DOI] [PubMed] [Google Scholar]
- 41.Vittinghoff E, Glidden DV, Shibowski SC, et al. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models . Springer; 2012. [Google Scholar]
- 42.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495-1499. [DOI] [PubMed] [Google Scholar]
- 43.Wagner MJ, Livingston JA, Patel SR, et al. Chemotherapy for bone sarcoma in adults. J Oncol Pract. 2016;12:208-216. [DOI] [PubMed] [Google Scholar]
- 44.White IR, Royston P. Imputing missing covariate values for the Cox model. Stat Med. 2009;28:1982-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377-399. [DOI] [PubMed] [Google Scholar]
- 46.Yoshida A, Ushiku T, Motoi T, et al. Immunohistochemical analysis of MDM2 and CDK4 distinguishes low-grade osteosarcoma from benign mimics. Mod Pathol. 2010;23:1279-1288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.