Abstract
A new rifampin resistance gene, arr-2, has been found in Pseudomonas aeruginosa. The ARR-2 protein shows 54% amino acid identity to the rifampin ADP-ribosylating transferase encoded by the arr gene from Mycobacterium smegmatis. This arr-2 gene is located on a gene cassette within a class I integron.
Rifampin has been used in the treatment of tuberculosis and leprosy for several decades, and the length of antibiotic treatment may be 6 months or more. This creates selective pressure favoring bacteria that acquire resistance by mutation or gene transfer. Rifampin is derived from rifamycin, a product of Amycolatopsis mediterranei (11) that has been in natural environments for a long time. Resistance genes may have evolved in response to rifampins in natural environments, as well as in clinical situations.
Rifampin resistance in clinical isolates of Mycobacterium tuberculosis is predominantly due to missense mutations in the rpoB gene which decrease binding of RNA polymerase to rifampin. However, a considerable percentage of resistant strains do not contain mutations in rpoB (16). In nonclinical isolates, rifampin resistance of bacteria such as Nocardia, Bacillus, and Pseudomonas spp. and nontubercle species of mycobacteria is not usually due to mutations in the rpoB gene. Several other resistance mechanisms have been identified, including a rifampin efflux gene that has been identified in Pseudomonas fluorescens (4); inactivation of rifampin by decomposition (6), glucosylation (13, 17), phosphorylation (18), and ribosylation (5, 8) has also been reported.
Integrons are specialized genetic elements that allow genes to move by site-specific recombination (for reviews, see references 7, 9, and 10). An integron consists of an integrase gene with adjacent gene cassettes that commonly contain antibiotic resistance genes. The cassettes are bounded by integrase recombination core sites and have conserved features at the 3′ ends of the cassettes with an inverse core site and a 59-base element. The core site sequence is GTTRRRY (R is purine, and Y is pyrimidine). Integrons have been found in a variety of gram-negative species and are common in Pseudomonas aeruginosa (20).
Here we report a multiply resistant strain of P. aeruginosa, PaTh2, isolated from a patient in Bangkok, Thailand. It is highly resistant to many antibiotics, including rifampin, expanded-spectrum cephalosporins, many other β-lactam antibiotics, many aminoglycosides, chloramphenicol, tetracycline, and quinolone compounds. The MIC of rifampin for this strain is >256 μg/ml, while most P. aeruginosa strains are less resistant to rifampin and the MIC is around 32 μg/ml (19).
Cloning of arr-2 and nearby genes.
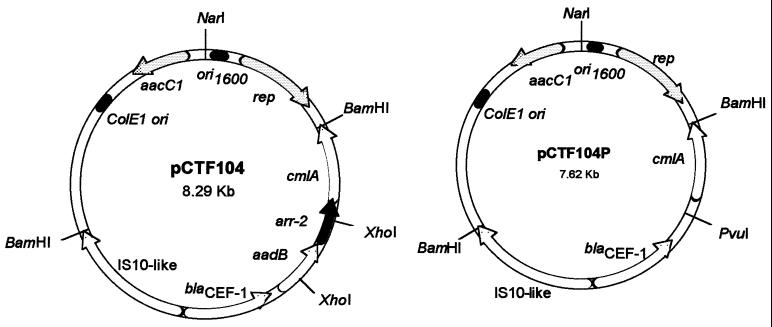
The DNA was isolated from PaTh2 by using the Sarkosyl-proteinase K method (12). One hundred micrograms of DNA was digested with BamHI and ligated with 5 μg of pUCP24 (15) digested with BamHI. The ligated library then was used to transform Escherichia coli XL1-Blue, and transformants were selected on Luria-Bertani (LB) agar plates containing 100 μg/ml ampicillin or ceftazidime (1). The clone pCTF104 was originally derived from an XL1-Blue transformant selected on LB with 100 μg/ml ampicillin. The DNA insert of this clone was sequenced with AmpliTaq DNA polymerase and dideoxynucleotide fluorescent terminators on an ABI Prism 377XL DNA sequencer (Cancer Research Center, University of Chicago). In order to inactivate what appears by sequence analysis to possibly be a rifampin resistance gene, pCTF104 was digested with XhoI, and then the recessed ends of the plasmid were filled in and religated to create pCTF104P, from which the 3′ end of the aadB gene and the 5′ end of the arr-2 gene have been deleted (Fig. 1).
FIG. 1.
Plasmid maps of two resistance constructs with intact arr-2 and disrupted arr-2 and aadB. pCTF104 is a recombinant plasmid created by insertion of a BamHI fragment from the total DNA of PaTh2 into the BamHI site of pUCP24. ori1600 is the origin of replication used by Pseudomonas, rep is the gene coding for replication protein, ColE1 ori is the origin of replication used by E. coli, and aacC1 is a gentamicin resistance gene in pUCP24. IS10-like is an insertion element, blaCEF-1 is a CEF-1 β-lactamase gene, aadB is a gentamicin resistance gene, arr-2 is a rifampin resistance gene, and cmlA is a chloramphenicol resistance gene in an insert from PaTh2. pCTF104P is a recombinant plasmid derived from pCTF104.
Determination of MICs for the transformants.
We determined the MICs by using the agar dilution method. An inoculum of 104 CFU per spot was delivered onto LB plates containing antibiotics in twofold dilutions. The MIC was determined as the lowest concentration of an antibiotic at which no visible growth or the growth of two or fewer colonies was observed after 20 h of incubation at 37°C. We determined the MICs for both E. coli and P. aeruginosa strains carrying pCTF104, pCTF104P, or no plasmid.
We first obtained the rifampin resistance gene while cloning the nearby ceftazidime resistance gene. The results from the DNA sequencing showed several potential resistance genes, including genes for rifampin, aminoglycoside, chloramphenicol, and ceftazidime resistance (Fig. 1). The DNA sequence around the rifampin resistance gene revealed characteristic integron 5′ and 3′ elements (Fig. 2). We also found an integrase gene for a class I integron adjacent to the IS10-like element (data not shown). The aadB and the cmlA gene cassettes have already been identified (2, 3). The blaCEF-1 gene responsible for ceftazidime resistance was also on a gene cassette in this class I integron element. The arr-2 gene putatively coded for a 150-amino-acid protein. We searched for the closest homologue in the GenBank database and found the ADP-ribosylating transferase encoded by the arr gene in Mycobacterium smegmatis (8). There is no other homologue in the databases. When we used the CLUSTAL W program (14), ARR-2 showed 54% identity, 68% similarity, and only two gaps in the alignment with ARR (Fig. 3). The mechanism of resistance from arr is inactivation of rifampin by ribosylation (5, 8).
FIG. 2.
DNA sequence of the arr-2 cassette and its boundary. The underlined portions are 59-base elements. The double-underlined portions are the core site and the inverse core site of the arr-2 cassette. The boxed codons are the start and stop codons of the arr-2 gene. The vertical bars indicate the boundaries of the cassettes.
FIG. 3.
CLUSTAL W protein alignment of a putative amino acid sequence of ARR-2 and ARR. The shaded areas indicate identical amino acids, and dashes indicate gaps inserted into the sequence to maximize the alignment.
The MICs of rifampin increased from 8 to >256 μg/ml for E. coli with pCTF104 and from 16 to >512 μg/ml for P. aeruginosa with pCTF104; the transformants with pCTF104P show the same susceptibility to rifampin as the wild type. Ceftazidime resistance was not affected by deletion of a 665-bp XhoI fragment (Table 1). The finding that arr-2 conferred rifampin resistance along with the homology between arr and arr-2 strongly suggests that the protein from arr-2 is an ADP-ribosylating transferase.
TABLE 1.
MICs for E. coli and P. aeruginosa strains with various cloned resistance genes
| Straina | MIC (μg/ml) of:
|
|||
|---|---|---|---|---|
| Ampicillin | Carbenicillin | Ceftazidime | Rifampin | |
| WT E. coli DH5α | 4 | 4 | 1 | 8 |
| DH5α(pCTF104) | >2048 | >2048 | 2048 | >256 |
| DH5α(pCTF104P) | >2048 | >2048 | 2048 | 8 |
| WT P. aeruginosa PAK | 2048 | 64 | 8 | 16 |
| PAK(pCTF104) | >2048 | >2048 | 2048 | >512 |
| PAK(pCTF104P) | >2048 | >2048 | 2048 | 16 |
WT, wild type.
Our findings on arr-2, along with the findings on arr from M. smegmatis, raise the questions of how these two genes evolved and if they have been transferred between mycobacteria and pseudomonads. Both mycobacteria and pseudomonads are found in soil, and some species of each are also found in human lung infections. The arr genes could have arisen in response to rifampin in soil or to rifampin used in the treatment of infections. The available data do not allow us to select between these two possibilities. The second question is how these genes might have been transferred between mycobacteria and pseudomonads. One possibility is that the location of arr-2 on an integron allows it to move around to various DNA molecules, such as plasmids or transposons, inside the cell, that could then be transferred to other bacterial species.
Nucleotide sequence accession number.
The sequence in Fig. 2 has been deposited in GenBank under accession no. AF078527.
REFERENCES
- 1.Ausubel F, Brent R, Kingston R, Moore D, Seidman J, Smith J, Struhl K E, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1989. [Google Scholar]
- 2.Bissonnette L, Champetier S, Buisson J-P, Roy P H. Characterization of the nonenzymatic chloramphenicol resistance (cmlA) gene of the In4 integron of Tn1696: similarity of the product to transmembrane transport proteins. J Bacteriol. 1991;173:4493–4502. doi: 10.1128/jb.173.14.4493-4502.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron F H, Groot Obbink D J, Ackerman V P, Hall R M. Nucleotide sequence of the AAD(2") aminoglycoside adenylyltransferase determinant aadB. Evolutionary relationship of this region with those surrounding aadA in R538-1 and dhfrII in R388. Nucleic Acids Res. 1986;14:8625–8635. doi: 10.1093/nar/14.21.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandrasekaran S, Lalithakumari D. Plasmid-mediated rifampicin resistance in Pseudomonas fluorescens. J Med Microbiol. 1998;47:197–200. doi: 10.1099/00222615-47-3-197. [DOI] [PubMed] [Google Scholar]
- 5.Dabbs E R, Yazawa K, Mikami Y, Miyaji M, Morisaki N, Iwasaki S, Furihata K. Ribosylation by mycobacterial strains as a new mechanism of rifampin inactivation. Antimicrob Agents Chemother. 1995;39:1007–1009. doi: 10.1128/aac.39.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dabbs E R, Yazawa K, Tanaka Y, Mikami Y, Miyaji M, Andersen S J, Morisaki N, Iwasaki S, Shida O, Takagi H, et al. Rifampicin inactivation by Bacillus species. J Antibiot (Tokyo) 1995;48:815–819. doi: 10.7164/antibiotics.48.815. [DOI] [PubMed] [Google Scholar]
- 7.Hall R M, Collis C M. Mobile gene cassettes and integrons: capture and spread of genes by site-specific recombination. Mol Microbiol. 1995;15:593–600. doi: 10.1111/j.1365-2958.1995.tb02368.x. [DOI] [PubMed] [Google Scholar]
- 8.Quan S, Venter H, Dabbs E R. Ribosylative inactivation of rifampin by Mycobacterium smegmatis is a principal contributor to its low susceptibility to this antibiotic. Antimicrob Agents Chemother. 1997;41:2456–2460. doi: 10.1128/aac.41.11.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Recchia G D, Hall R M. Gene cassettes: a new class of mobile element. Microbiology. 1995;141:3015–3027. doi: 10.1099/13500872-141-12-3015. [DOI] [PubMed] [Google Scholar]
- 10.Recchia G D, Hall R M. Origins of the mobile gene cassettes found in integrons. Trends Microbiol. 1997;5:389–394. doi: 10.1016/S0966-842X(97)01123-2. [DOI] [PubMed] [Google Scholar]
- 11.Sensi P. History of the development of rifampin. Rev Infect Dis. 1983;5 (Suppl 3):S402–S406. doi: 10.1093/clinids/5.supplement_3.s402. [DOI] [PubMed] [Google Scholar]
- 12.Strom M S, Lory S. Cloning and expression of the pilin gene of Pseudomonas aeruginosa PAK in Escherichia coli. J Bacteriol. 1986;165:367–372. doi: 10.1128/jb.165.2.367-372.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka Y, Yazawa K, Dabbs E R, Nishikawa K, Komaki H, Mikami Y, Miyaji M, Morisaki N, Iwasaki S. Different rifampicin inactivation mechanisms in Nocardia and related taxa. Microbiol Immunol. 1996;40:1–4. doi: 10.1111/j.1348-0421.1996.tb03303.x. [DOI] [PubMed] [Google Scholar]
- 14.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.West S E, Schweizer H P, Dall C, Sample A K, Runyen-Janecky L J. Construction of improved Escherichia-Pseudomonas shuttle vectors derived from pUC18/19 and sequence of the region required for their replication in Pseudomonas aeruginosa. Gene. 1994;148:81–86. doi: 10.1016/0378-1119(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 16.Williams D L, Waguespack C, Eisenach K, Crawford J T, Portaels F, Salfinger M, Nolan C M, Abe C, Sticht-Groh V, Gillis T P. Characterization of rifampin-resistance in pathogenic mycobacteria. Antimicrob Agents Chemother. 1994;38:2380–2386. doi: 10.1128/aac.38.10.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yazawa K, Mikami Y, Maeda A, Akao M, Morisaki N, Iwasaki S. Inactivation of rifampin by Nocardia brasiliensis. Antimicrob Agents Chemother. 1993;37:1313–1317. doi: 10.1128/aac.37.6.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yazawa K, Mikami Y, Maeda A, Morisaki N, Iwasaki S. Phosphorylative inactivation of rifampicin by Nocardia otitidiscaviarum. J Antimicrob Chemother. 1994;33:1127–1135. doi: 10.1093/jac/33.6.1127. [DOI] [PubMed] [Google Scholar]
- 19.Yee Y C, Kisslinger B, Yu V L, Jin D J. A mechanism of rifamycin inhibition and resistance in Pseudomonas aeruginosa. J Antimicrob Chemother. 1996;38:133–137. doi: 10.1093/jac/38.1.133. [DOI] [PubMed] [Google Scholar]
- 20.Zühlsdorf M T, Wiedemann B. Tn21-specific structures in gram-negative bacteria from clinical isolates. Antimicrob Agents Chemother. 1992;36:1915–1921. doi: 10.1128/aac.36.9.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]