Abstract
The incidence rates of hepatocellular carcinoma (HCC) worldwide are increasing, and the role of radiotherapy is currently under discussion. Radioresistance is one of the most important challenges in the therapy of HCC compared with other local advanced, recurrent and metastatic cancers. The mechanisms of radioresistance are complex and remain to be fully understood; however, extracellular vesicles have been investigated in recent studies. Exosomes, which are 40- to 150-nm extracellular vesicles released by cancer cells, contain multiple pathogenic components, including proteins, nucleic acids and lipids, and play critical functions in cancer progression. Emerging data indicate a diagnosis potential for exosomes in HCC, since radiation-derived exosomes promote radioresistance. Radiation-based therapy alters the contents and components of exosomes, suggesting that exosomes and their components may serve as prognostic and predictive biomarkers to monitor radiation response. Therefore, understanding the roles and mechanisms of exosomes in HCC progression and radiation response during HCC therapy may increase our knowledge concerning the roles of exosomes in radioresistance, and may lead to novel approaches for HCC prognosis and treatment. The current review summarizes recent studies on exosome involvement in HCC and the molecular changes in exosome components during HCC progression. It also discusses the functions of exosomes in HCC therapy, and highlights the importance of exosomes in HCC progression and resistance for the development of novel therapies.
Keywords: hepatocellular carcinoma, radioresistance, exosomes, radiotherapy, ncRNA
1. Introduction
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer (1,2) and the second leading cause of cancer-associated mortality worldwide (3). HCC occurs most frequently in patients with chronic liver diseases, including cirrhosis caused by hepatitis B or C infection, which accounts for ~50% of cases (4,5). The risk of HCC, which is the most common type of liver cancer, is higher in patients with long-term liver diseases as well as in patients infected with hepatitis B or C (6,7). HCC is also more common in individuals who consume large quantities of alcohol and in those with accumulated fat in the liver (8). Other factors, such as tobacco smoke inhalation and intake of aflatoxin B1, are also well-described contributors to HCC (8).
As HCC is one of the most common malignant tumors in clinical practice, its diagnosis, treatment and prognosis attract significantly considerable attention. Surgical interventions, including resection and transplantation, are the most common methods for early HCC therapy (9). However, since HCC is difficult to diagnose at an early stage, and the majority of patients tend to be diagnosed at advanced stages and are not ideal for resection, conservative treatment strategies are generally useful for advanced HCC (2). However, radiotherapy, including stereotactic body radiation therapy and radiofrequency ablation, is affected by increased radioresistance, indicating that HCC therapy should be improved due to the high morbidity, mortality and recurrence of this disease (10,11). Radioresistance is a complex biological process associated with multiple factors, such as abnormal DNA damage response, gene mutations, cell autophagy, apoptosis, cell cycle checkpoint and other dysregulated signaling pathways (12). These mechanisms lead to poor prognosis in patients and cause major clinical obstacles for radiotherapy, ultimately leading to tumor metastasis and relapse (13). Radioresistance is also highly associated with the tumor microenvironment (14,15), which is an important factor associated with tumor progression and therapeutic response (16,17) and is actively achieved by exosome-regulated cell-cell communication.
Exosomes are single-membrane secreted organelles 40-150 nm in diameter. Exosomes have similar topologies with cells, and are enriched in nucleic acids, selected proteins, lipids and glycoconjugates. The biogenesis of exosomes relies on a mechanism of protein quality control, and exosomes display activities in remodeling the extracellular matrix and transmitting signals and molecules from the contributor cells to other cells. This pathway plays important functions in numerous aspects of human homeostasis and disease, such as development, tissue homeostasis, immunity, neurodegenerative diseases and cancer (18,19). Increasing evidence suggests that cancer-derived exosomes play multiple and critical functions in cancer. Exosomes, as well as their components, may serve as cancer prognostic markers, therapeutic targets or anticancer drug carriers (20,21). Regarding the advantages of exosomes in cancer diagnosis and their newly suggested application, previous studies have indicated that radiation-derived extracellular vesicles, particularly exosomes, decrease survival, increase tumor burden, cause radiation-induced bystander effects and promote radioresistance. The present study reviews recent reports on radiation-induced changes in extracellular vesicles (particularly exosomes) in HCC, and discusses the molecular mechanisms of exosome-regulated HCC progression and radioresistance for the development of novel therapeutic strategies.
2. Biological components of exosomes in HCC
The majority of commonly studied cells are characterized by the production of a set of lipid membrane-coated vesicles that can be secreted to the extracellular space. They are named extracellular vesicles, and the most well-known ones are exosomes (22). Exosomes, which were first identified in the late 1990s, are a set of membranous vesicles released into the extracellular space by contributor cells after multiple intracellular vesicles fuse into the cell membrane (23). The majority of known cell types, including normal and pathogenic cells, are capable of secreting exosomes, and numerous studies suggest that, compared with that of normal epithelial cells, the release of exosomes by tumor cells is more active (24). Exosomes are present in almost all types of body fluids, including urine, saliva, blood, breast milk, bile, synovial fluid, seminal fluid and amniotic liquid, indicating their critical functions in intercellular communication by transducing both genomic and proteomic materials among cells and subsequently modulating physiological responses (25). However, the mechanisms of the biological formation of exosomes remain to be clarified. The functions and characteristics of exosomes are mostly based on their sizes, expression of surface markers and composition, including DNA, RNA, lipids, metabolites and surface proteins (18,26). This variety of characteristic protein components on the outer membrane of exosomes, which serve as surface markers, includes CD9, CD81, CD63, CD53, CD82, CD37, TSG101, Hsp70 and Alix (26,27). These proteins are commonly used as markers to confirm the presence of exosomes, since they are detectable by western blotting. In addition, exosomes contain numerous lipid molecules, which participate in multiple biological processes and play critical roles in the morphological stability of exosomes in the extracellular fluid (28).
In the tumor microenvironment, cancer cells secret numerous exosomes, which are transferred from cancer cells to other cell types to participate in signaling transduction, and in the processes of tumor formation and progression (29). In addition, exosomes derived from both tumor and stromal cells have been reported to be implicated in all stages of cancer progression and to exhibit crucial functions in tumor therapy resistance. Due to their characteristics as mediators of cell-cell communication, exosomes are integral to tumor microenvironment-dependent therapy resistance (30). Furthermore, exosomes are also able to deliver oncogenes to normal epithelial cells under pathological conditions, which has been reported to be one of the mechanisms involved in tumor invasion and metastasis (31). Numerous previous studies have demonstrated that exosomes can deliver various proteins and ribonucleic acids to recipient cells (18). These studies are validated by the activation and expansion of exosome-producing immune cells in murine models of acute or chronic inflammation (32).
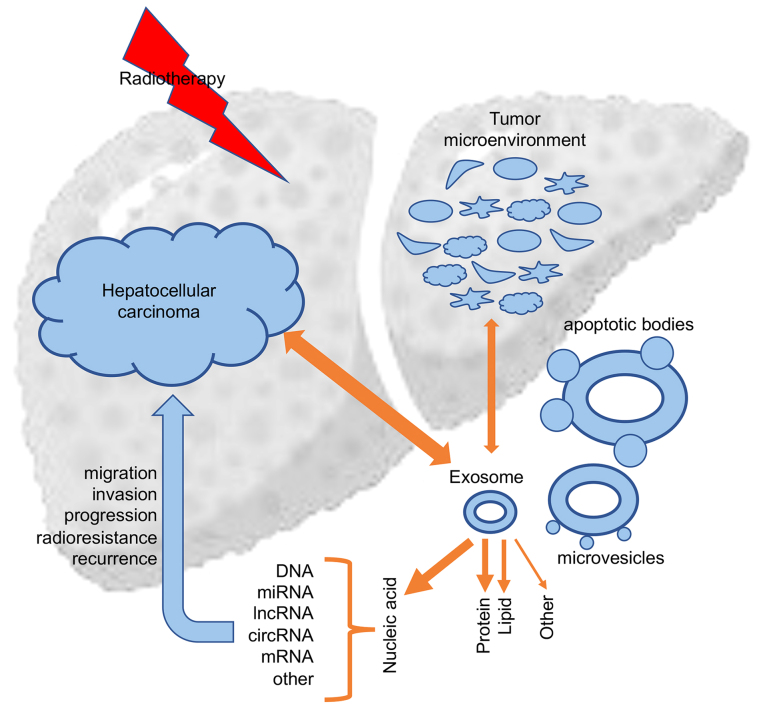
Exosomes are of different phenotypes in different body fluids and contain a variety of biologically active molecules, which leads to diverse and heterogenous exosome types (26). These molecules are divided into lipids (including sphingomyelin, phosphatidylserine and cholesterol) (33), proteins (including tumor-suppressor proteins, oncoproteins and transcription regulators) (34), DNA (including genomic DNA, single-strand DNA, and retrotransposon elements) (35,36) and RNAs [including long non-coding RNAs (lncRNAs), microRNAs (miRNAs), circular RNAs (circRNAs), messenger RNAs (mRNAs) and other non-coding RNAs (ncRNAs)] (37). Multiple studies have confirmed that these exosomal active components exhibit critical functions in the resistance of a variety of tumors, particularly in HCC radioresistance (18). Therapeutic interventions such as chemotherapy and radiotherapy, could affect exosome uptake and subsequent tumor biological responses, which in return could influence the secretion and components of exosomes (13) (Fig. 1).
Figure 1.
The involvement of exosomes and their contents in the development, progression and recurrence of hepatocellular carcinoma. mRNA, messenger RNA; miRNA, microRNA; lncRNA, long non-coding RNA; circRNA, circular RNA.
3. Protein transduction by HCC-derived exosomes
Exosomes contain abundant proteins, which include not only self-proteins, but also proteins derived from contributor cells.
Exosomal proteins as HCC biomarkers
Exosomal proteins derived from cancer cells are becoming novel biomarkers for cancer monitoring and efficacy evaluation, and are also becoming a popular research topic in studies on cancer radioresistance (38), particularly in HCC. Mass spectrometry (MS) analysis demonstrated that 213 proteins could be detected in exosomes secreted by an HCC cell line, and, among them, 158 proteins were specifically expressed in exosomes secreted by highly malignant HCC cells compared with the exosomal proteome of each cell line (39). Exosomes secreted by metastatic HCC cell lines were found to carry several protumorigenic RNAs and proteins, including MET proto-oncogene, caveolins and S100 family members. Notably, exosomes from motile HCC cell lines significantly enhanced the migratory and invasive abilities of non-motile MIHA cells. Uptake of exosomal molecules may trigger signaling pathways, including the PI3K/AKT and MAPK signaling pathways, in MIHA cells with increased secretion of active matrix metalloproteinases MMP-2 and MMP-9. These findings demonstrated that HCC-derived exosomes had the potential of mobilizing normal hepatocytes, which may have implications in promoting the protrusive activities of HCC cells through the liver parenchyma during the process of metastasis (39). Another study using MS investigated exosomes isolated from the HCC cell line HepG2 and identified a total of 1,428 proteins in HepG2 cell exosomes. These proteins were found to facilitate the activation of several kinases and the NF-κB signaling pathway in HCC cells (39). Eukaryotic translation initiation factor 3 subunit C (EIF3C), a protein associated with poor patient survival that is upregulated during HCC tumor progression, was found to significantly increase extracellular exosome secretion, exosome size and exosome marker expression diversity. EIF3C-overexpressing exosomes were oncogenic, and enhanced tumor angiogenesis and blood vessel growth. Subcutaneous inoculation of EIF3C-enriched exosomes together with Huh7 HCC cells increased the growth of vessels and upregulated the expression of EIF3C in tumors. Furthermore, EIF3C activated the expression of S100A11, which is involved in EIF3C-enriched exosome-mediated increased tube formation in angiogenesis (40). A previous study performed protein profiling of exosomes derived from different HCC cell types, and identified 129 proteins, among which, adenylyl cyclase-associated protein 1 (CAP1), a protein implicated in HCC metastasis, was significantly enriched in exosomes secreted by HCC cells with high motile abilities (41). Similarly, super-stable isotope labeling by/with amino acids in cell culture-based MS analysis on exosomes secreted by three human HCC cell lines, non-motile Hep3B cells, and motile 97H and LM3 cells quantified >1,400 exosomal proteins in each MS analysis with highly biological reproducibility. In total, 469 and 443 exosomal proteins, respectively, represented differentially expressed proteins in LM3/Hep3B vs. 97H/Hep3B cells. These proteins were involved in sugar metabolism-centric canonical pathways according to signaling pathway analysis. Specifically, these pathways included the glycolysis I, gluconeogenesis I and pentose phosphate pathways, and the proteins enriched in these signaling pathways could form a tightly connected network. The above findings suggest that motile HCC cells have the potential to preferentially export more sugar metabolism-associated proteins by exosomes that facilitate the differentiation of motile HCC from non-motile HCC cells (42).
Exosomal proteins in HCC metastasis and therapy resistance
Another study investigated the proteome of macrovesicles present in urine samples acquired from liver injury experimental models as a method to identify potential biomarkers of hepatic diseases. The biochemical and proteomic characterization of highly purified exosomes identified 28 previously unreported proteins, a number of which were closely associated with liver diseases. This analysis suggested that urinary exosomes could potentially be a sample source for identifying biomarkers of liver injury, and certain proteins such as CD26, CD81, solute carrier family 3 member 1 (SLC3A1) and CD10 were differentially expressed in urinary exosomes derived from experimental models, thus serving as potential biomarkers for liver injury (43). HCC cells were reported to promote cancer cell proliferation and lung metastasis formation in a paracrine/endocrine manner, which was achieved by primary HCC-derived exosomes. In the presence of these malignant exosomes, enhanced cell adhesion was observed. Notably, attached HCC cells released exosomes containing both mothers against decapentaplegic homolog 3 (SMAD3) protein and mRNA, and delivered them to detached HCC cells to subsequently facilitate their adhesion. These exosomes contained activated SMAD3 signaling in the recipient HCC cells and improved their adhesive capacities. Clinically, SMAD3-containing exosomes were detected in the peripheral blood of patients with HCC, and their levels were closely associated with disease stage and SMAD3 expression in primary tumors. That study suggested a potential mechanism by which exosome SMAD3 mediated communication between primary and circulating HCC cells (44). Tumor-derived exosomes were found to activate B cells, which expressed high levels of T-cell immunoglobulin and mucin domain 1 (TIM-1) protein and suppressed the activities of CD8+ T cells in a similar manner to TIM-1+ regulatory B (Breg) cells isolated from HCC tissues. Enrichment of TIM-1+ Breg cells in HCC tissues was involved in advanced disease stage, and predicted early recurrence in HCC as well as reduced patient survival in HCC. High mobility group box protein 1 (HMGB1) derived from exosomes activated B cells and promoted the expansion of TIM-1+ Breg cells through the Toll-like receptor 2/4 (TLR2/4) and MAPK signaling pathways (45). T cells could uptake exosomes containing 14-3-3ζ secreted by HCC cells, which suggested that 14-3-3ζ is delivered from HCC cells to tumor-infiltrating T lymphocytes by exosomes. In tumorinfiltrating T cells of the HCC microenvironment, 14-3-3ζ was upregulated and inhibited their antitumor functions. Specifically, 14-3-3ζ is potentially transferred from HCC cells to T cells partially through exosomes (46). Exosomes with increased C-X-C motif chemokine receptor 4 (CXCR4) levels secreted by high metastatic mouse hepatocarcinoma Hca-F cells were confirmed to increase the migration and invasion of paired syngeneic Hca-P cells with low metastatic capacities. The increase in cell migratory and invasive capacities of Hca-P cells was facilitated by the internalization of exosomes isolated from Hca-F cells and subsequent transferring of CXCR4 via exosomes. Hca-F cell migration and invasion were increased by lymphatic endothelial cells via upregulation of stromal cell-derived factor-1α (SDF-1α) binding to C-X-C motif chemokine receptor 4 (CXCR4) in Hca-F cells and subsequently upregulating the secretion of vascular endothelial growth factor (VEGF)-C, MMP-9 and MMP-2. In addition, CXCR4 derived from Hca-F exosomes significantly increased the abilities of lymphatic endothelial cell proliferation and lymphatic tube formation (47). C-type lectin domain family 3 member B (CLEC3B) downregulation in HCC was found to suggest a poor prognosis. Exosomes secreted by HCC with downregulated CLEC3B significantly increased the epithelial-mesenchymal transition (EMT), migration and invasion of tumor cells. CLEC3B downregulation in HCC exosomes decreased VEGF secretion in HCC cells and inhibited angiogenesis. Mechanistically, VEGF expression mediated by CLEC3B in tumor cells relied on the activation of the AMP-activated protein kinase signaling pathway (48). Both in vivo and in vitro, overexpression of chitinase 3 like 1 (CHI3L1), a cancer poor prognosis-associated secreted glycoprotein, significantly facilitated the growth, migration and invasion of liver cancer cells. Overexpression of CHI3L1 influenced cell-cell adhesion, extracellular exosomes and adherent junction-related genes via TGF-β signaling pathway activation (49). The expression of golgi membrane protein 1 (GOLM1) was inhibited by miR-145 via targeting a coding sequence of the GOLM1 gene. The expression of GOLM1 and miR-145 showed an inverse correlation in human HCC tissues. Mechanistically, exosomes with GOLM1 enrichment activated the GSK-3β/MMP signaling axis of recipient cells, and accelerated HCC migration and proliferation (50).
4. Lipid transduction by HCC-derived exosomes
Exosome structures and contents consist of proteins, nucleic acids and lipids, which are mostly derived from contributor cells. Similar to structures such as cell membranes, exosomes contain a lipid bilayer membrane that protects the encapsulated material (nucleic acids, proteins, metabolites and lipids) from the extracellular environment (33,51). Lipids not only act as essential components of exosomal membranes, which protect exosome contents from various stimuli in the circulating body fluids, but also are necessary players in exosome formation and release to the extracellular environment (33). Increasing evidence suggests that specific lipid components are enriched in exosome particles compared with their parental cells.
In contrast to those of proteins and nucleic acids, the functions of exosomal lipids in HCC development, progression, radioresistance and chemoresistance are less established. Increasing data suggest that lipid molecules in exosomes may serve as potential cancer diagnostic biomarkers, as confirmed by data obtained in urine (52) and cancer cell lines (53). Exosomes derived from the oligodendroglial precursor cell line Oli-neu were enriched in cholesterol, and contained higher levels of sphingolipids (sphingomyelin and hexosylceramide) and lower levels of phosphatidylcholine than those found in the cell membrane (54). In previous studies on hepatitis and HCC, exosomes were found to play key roles in hepatitis E virus (HEV) egress; however, HEV infection did not modify the characteristics of exosomes produced by infected cells. Enrichment in phosphatidylserines and cholesterol in these exosomes could be critical for HEV entry into cells. Meanwhile, HEV particles in exosomes are protected from the attack of the immune system, which leads to wide spreading of HEV in the circulatory system of the host (55). A previous high-resolution lipidomic and proteomic study reported the analysis of exosomes and extracellular vesicles obtained by differential ultracentrifugation from the glioblastoma cell line U87, the HCC cell line Huh7 and human bone marrow-derived mesenchymal stem cells (MSCs). Exosomes enriched in glycolipids and characterized by the presence of free fatty acids, as well as exosomes from Huh7 cells and MSCs were specifically enriched in cardiolipins (56). Unlike circulating tumor cells, exosomes are highly abundant in biofluids and cells, and are diverse in vesicle type and lipid composition. Different from other single-molecule circulating biomarkers, exosomes protect their molecular cargo from degradation and may carry molecular signatures involved in specific phenotypes (57). Increased understanding of the role of exosomal lipids in HCC progression and radioresistance suggests that exosome-derived lipids have a considerable potential as HCC biomarkers and a tool to monitor radiotherapy in the future (26).
5. Nucleic acids derived from HCC exosomes
Exosome-derived nucleic acids are diverse, but the majority of the currently identified exosome-derived nucleic acids are RNAs, and few studies have focused on exosomal DNA in HCC.
DNA
Analyses of circulating cell-free tumor DNA and circulating tumor cells have paved the way for novel diagnostic approaches, and are the basis of diagnostic techniques based on liquid biopsies. The application of circulating cell-free tumor DNA on early cancer detection is of high public interest (58). Exosomal DNA has attracted broad interest in cancer research; however, few studies on HCC have been conducted to date (36,59). The majority of DNA associated with tumor exosomes has been confirmed to be double-stranded in three different cancer models: human chronic myeloid leukemia cell model K-562; human colorectal carcinoma cell model HCT116; and murine melanoma cell model B16-F10. This double-stranded DNA in exosomes represents the whole genomic DNA. Exosomal DNA has been confirmed to be used to identify mutations in parental tumor cells, which suggests its translational potential as a circulating biomarker of cancer in the clinic (36). A recent study on liquid biopsy samples of 194 patients, who were undergoing treatment for localized or metastatic pancreatic adenocarcinoma, showed a marked increase in exosomal DNA levels after neoadjuvant therapy, which suggested a close association with disease progression. Significantly shorter times of progression-free and overall survival were found in patients with metastases and detectable circulating tumor DNA (ctDNA) at baseline status compared with those of patients without detectable ctDNA (60). Plasma cell-free DNA (cfDNA) levels in patients with HCC and hepatitis B virus-related liver fibrosis were closely associated with the degree of liver inflammation, body mass index and α-fetoprotein level, but showed no association with liver fibrosis stage. Significantly elevated plasma cfDNA levels were detected in patients with HCC compared with those found in patients without HCC. The HCC index, which is generated by a combination model including age, and cfDNA and α-fetoprotein levels, had an area of 0.98 under the receiver operating characteristics curve for the diagnosis of HCC, suggesting that the diagnostic power of the HCC index was more promising than that of cfDNA or α-fetoprotein levels alone (61).
miRNA
Exosomal RNAs include miRNAs, lncRNAs, mRNAs, circRNAs and other non-coding ncRNAs (37). ncRNA families, including miRNAs, lncRNAs, circRNAs and other ncRNAs, are not functional to encode proteins, but function as specific RNA inhibitors by degradation or transformation to regulate post-transcriptional gene expression, which is associated with the control of nuclear architecture, transcription in the nucleus, and modulation of mRNA stability, translation and post-translational modifications in the cell cytoplasm (62,63).
miRNAs are small ncRNAs of 17-24 nt, which mediate post-transcriptional gene silencing by binding to the 3′untranslated or open reading frame region of target mRNAs (64). miRNAs have been detected in exosomes, which have been found to be taken up by neighbor or distant cells, and then modulate the activities of recipient cells. Accumulating evidence has shown that miRNAs can be stable in body fluids, including saliva, urine, breast milk and blood. Besides being packed into exosomes or other microvesicles, extracellular miRNAs can also be loaded into high-density lipoprotein (65) or be bound by argonaute-2 protein outside these microvesicles (66). All these three modes of action protect miRNAs from degradation and ensure their stability (67). Changes in the expression levels of miRNAs contribute to the pathogenesis of numerous human malignancies. These changes are potentially caused by multiple different mechanisms, such as gene mutations, amplifications or deletions involving miRNA loci, epigenetic silencing or dysregulation of transcription factors targeting specific miRNAs. Malignant cells depend on the dysregulated expression of multiple miRNAs, and in turn control or are controlled by dysregulation of multiple protein-coding oncogenes or tumor-suppressor genes, which suggests that these small RNAs may provide opportunities for the development of novel miRNA-based therapies (68). Circulating exosomal miRNA-21 has been associated with TNM stages and other prognostic factors, including T stage and portal vein thrombosis, in HCC. High miRNA-21 level was reported to be a promising predictor of increased mortality and disease progression, as well as larger tumor size and higher C-reactive protein levels. Patients with higher circulating levels of exosomal miRNA-21 exhibited significantly lower overall and progression-free survival (69). Another study found that the migration, invasion and proliferation of recipient HCC cells were significantly increased after treatment with exosomes secreted by HCC cells cultured in acidic medium. miR-21 and miR-10b were the most critically functional miRNAs in acidic HCC cell-derived exosomes. Activation of hypoxia-inducible factor (HIF)-1α and HIF-2α was facilitated by the acidic microenvironment, which also stimulated the expression and secretion of exosomal miR-21 and miR-10b, substantially promoting HCC cell proliferation, invasion, migration and proliferation both in vivo and in vitro. Advanced tumor stage, and HIF-1α and HIF-2α expression in patients with early HCC were closely associated with serum exosomal miR-21 and miR-10b levels, suggesting that exosomal miR-21 and miR-10b were independent prognostic factors for disease-free survival of patients with early HCC (70). A previous study demonstrated that the levels of 19 miRNAs were markedly upregulated in the sera of patients with HCC (71). A following study demonstrated that HCC cells secreted miR-210 via exosomes. The in vitro tubulogenesis of endothelial cells and the in vivo angiogenesis of HCC were significantly promoted by exosomal miR-210 by inhibiting the expression levels of SMAD4 and STAT6 in the recipient endothelial cells (72). Another study focused on the functions of exosomal miR-224 in the development of HCC, and assessed its diagnostic and prognostic value in HCC. Patients with HCC who had high expression levels of serum exosomal miR-224 exhibited a lower overall survival, suggesting that exosomal miR-224 is potentially a tumor-promoting gene, and may serve as a biomarker for diagnosis and prognosis in patients with HCC (73). In addition to these studies, a number of other exosome-derived miRNAs have been found to be closely associated with the pathogenesis and progression of HCC. Their potential roles include being biomarkers for HCC diagnosis and serve as therapeutic targets for HCC treatment.
lncRNAs
lncRNAs, another type of ncRNAs with a size >200 nt that lack protein-coding functions, have been reported to be critical modulators of intercellular communications (74). Cancer cell-derived exosomes have been confirmed to contain various lncRNAs, usually defined exosomal lncRNAs, which regulate the cancer microenvironment by modulating multiple cellular functions, including regulation of the transcription of certain critical genes that determine cancer growth and progression, thus defining the biological functions of cancer exosomes (75,76). Furthermore, deregulation of lncRNA expression has been identified in multiple human tumors, and lncRNAs may serve as attractive diagnostic biomarkers to predict different cancer types (77).
Similar to miRNAs, exosomal lncRNAs are also important contributors to the occurrence, development and transferring of HCC. A stress-responsive lncRNA, long intergenic non-protein coding RNA, regulator of reprogramming (linc-ROR), was significantly upregulated in HCC cells and was also found to be enriched in tumor cell-derived extracellular vesicles. Incubation with HCC-derived extracellular vesicles significantly increased the expression of linc-ROR and blunt chemotherapy-induced cell death in recipient cells (78). Incubation with linc-very low density lipoprotein receptor (VLDLR)-enriched exosomes reduced chemotherapy-induced cell death in HCC cells, and increased the expression of linc-VLDLR in recipient cells (79). The expression levels of lncRNA-focally amplified lncRNA on chromosome 1 (lnc-FAL1) were significantly upregulated in HCC tissues, and lnc-FAL1 was found to function as an oncogene in HCC (80). In serum exosomes of patients with HCC, the levels of lnc-FAL1 were upregulated, and transferring lnc-FAL1 to HCC cells increased their cell migration and proliferation abilities (81).
Numerous studies have reported HCC-derived exosomes as cargo of novel lncRNA biomarkers. Exosomes derived from non-HCC serum contained a large number of lncRNAs with a high level of alternative splicing compared with those found in patients with other hepatic diseases. Exosomal lncRNAs, including lnc-G protein-coupled receptor 89B-15 (lnc-GPR89B-15), lnc-family with sequence similarity 72 member D-3 (lnc-FAM72D-3), lnc-enhancer of polycomb homolog1-4 (lnc-EPC1-4) and lnc-zinc finger E-box binding homeobox 2-19 (lncZEB2-19), showed differential expression in the serum of patients with HCC (82). Another study revealed that five lncRNAs, namely CTD-2116N20.1, AC012074.2, RP11-538D16.2, long intergenic non-protein coding RNA (LINC) 00501 and RP11-136I14.5, showed significantly different expression. Co-expression and competing endogenous (ce)RNA network analyses revealed possible mechanisms of CTD-2116N20.1 and RP11-538D16.2 as exosome-related lncRNAs, and CTD-2116N20.1 and RP11-538D16.2 were correlated with poor prognosis in patients with HCC (83). The expression of lncRNA-HEIH (lnc-HEIH) was increased in the serum and exosomes of patients with hepatitis C virus-related HCC compared with the findings in patients with chronic hepatitis C (84). High levels of ENSG00000258332.1 in HCC were associated with overall survival, portal vein tumor emboli, TNM stage and lymph node metastasis, and high levels of LINC00635 were closely associated with overall survival, TNM stage and lymph node metastasis (85). Another study evaluated the relative expression levels of 8 selected serum lncRNAs in the training and validation sets of patients with HCC and matched healthy controls. The expression of LINC00161 was markedly upregulated, and exhibited high stability and specificity in serum samples of patients with HCC, indicating the biomarker potential of LINC00161 in HCC diagnosis and prognosis (86). The expression level of X-inactive-specific transcript (Xist), another lncRNA, was significantly increased in peripheral blood mononuclear cells and granulocytes of patients with HCC. Another lncRNA, Jpx, also showed increased upregulation in mononuclear cells, granulocytes and exosomes of female patients with HCC. Delivery of Jpx from HCC cells to blood cells via exosomes activated Xist expression in blood cells, possibly by inhibiting the trans-regulatory effects of CCCTC-binding factor (87). A previous study also identified Xist as a mediator of miRNA-92b, which promoted HCC progression by targeting SMAD7 (88). Another commonly reported HCC-associated lncRNA is TUC339. The most highly significantly expressed lncRNA in extracellular vesicles derived from HCC cells was identified as TUC339 in a previous study. Functionally, TUC339 was found to be involved in regulating tumor cell adhesion and growth (89). Compared with those from healthy controls, exosomes derived from HCC cells contained upregulated levels of TUC339, and HCC-secreted exosomes could be taken up by THP-1 cells. These THP-1 cells also showed a significant increase in pro-inflammatory cytokine production, increased co-stimulatory molecule expression and enhanced phagocytosis upon inhibition of TUC339 (90). LncRNA acetylserotonin O-methyltransferase like antisense RNA 1 (ASMTL-AS1) was found to be upregulated in HCC tissues, and exhibited increased expression in tumors after radiofrequency ablation (RFA) insufficiency. The expression levels of ASMTL-AS1 were found to be closely associated with disease stage, metastasis and prognosis of HCC, and were found to contribute to the malignancy of HCC cells. ASMTL-AS1 could be packaged by exosomes and then contribute to the malignancy of HCC cells via the nemo-like kinase (NLK)/yes-associated protein (YAP) regulatory axis between cells even in residual HCC after RFA insufficiency (91). High expression of exosomal H19, an exosomal lncRNA, could enhance the motility and proliferation of HCC cells while reducing their apoptosis through binding to and sponging miR-520a-3p (92).
lncRNAs may play a role in HCC recurrence. A set of deregulated lncRNAs showed potential to differentiate HCC from cirrhotic tissue. Among these lncRNAs, cancer susceptibility 9 (CASC9) and lung cancer associated transcript 1 (LUCAT1) showed upregulation in a subset of HCC-derived cell lines and in certain HCC tissues, whose donors exhibited decreased recurrence after surgery. LUCAT1 was found to directly sponge the onco-miR-181d-5p, a novel regulatory element of liver cancer stem/progenitor cells. Both lncRNAs, LUCAT1 and CASC9, were detected in secreted exosomes, and upregulated circulating CASC9 levels were closely associated with tumor size and HCC recurrence after surgery in human patients (93).
circRNAs
circRNAs, first identified in RNA viruses in 1976 by electron microscopy (94), are another type of ncRNAs that form a covalently circled continuous loop via the process of back-splicing, during which the downstream splice donor site is joined with the upstream splice acceptor site (95). Different to common linear RNAs, circRNAs are characterized by featuring a covalently circled continuous loops without 5′ or 3′polarities (96). Previous studies have demonstrated that circRNAs are capable of absorbing miRNAs by stably competitively binding and play a role as sponges of miRNAs to regulate gene expression (97). Numerous studies have demonstrated that circRNAs are abundant, stable and one of the major components of exosomes (98). Increasing evidence has indicated that dysregulation of exosomal circRNAs contributes to tumorigenesis and progression in human liver cancer (99).
circRNAs secreted by adipose tissue were found to regulate deubiquitination in HCC cells to facilitate cell growth. Exosome circ-de-ubiquitination (circ-DB) levels were significantly upregulated in patients with HCC who had higher body fat ratios. circ-DB has been demonstrated to promote HCC cell growth and inhibit DNA damage by suppressing miR-34a expression and upregulating the de-ubiquitination of ubiquitin specific protease 7 (USP7). The effects of adipose-secreted exosomes on HCC cells could be reversed by downregulation of circ-DB. These findings indicated that exosome circRNAs secreted from adipocytes promoted tumor growth and reduced DNA damage via miR-34a suppression and activation of the USP7/cyclin A2 signaling pathway axis in HCC cells (100). CircRNA circ-0051443 was significantly downregulated in the tissues and plasma exosomes of patients with HCC compared with the levels found in those of healthy controls, and was mainly packaged into exosomes in HCC cells. HCC cells could be distinguished from healthy control cells by the presence of circ-0051443 in exosomes. Transmitting circ-0051443 from healthy normal cells to HCC cells via exosomes significantly suppressed the malignancy of HCC cells via cell apoptosis increase and cell cycle arrest (101).
Numerous studies have reported critical roles of exosomal circRNAs in HCC progression. The level of another circRNA, circ-tripartite motif containing 33-12 (TRIM33-12), was also decreased significantly in HCC tissues and cell lines. circ-TRIM33-12 downregulation in HCC was closely associated with HCC malignant behavior, and served as a promising risk factor of recurrence-free and overall survival in patients with HCC after surgery (102). A recent study characterized the facilitation of MET expression by exosome circ-prostaglandin reductase 1 (PTGR1) competing with the seed sequence of miR-449a, a miRNA with roles in the inhibition of tumor growth and metastasis in HCC (103). Further mechanistic analyses confirmed that three different isoforms of exosomal circPTGR1 promoted HCC metastasis through the miR-449a/MET pathway (104). The level of circTMEM45A was markedly reduced in HCC. circTMEM45A levels were closely associated with clinicopathological features as well as decreased prognosis of patients with HCC. Clinically, circTMEM45A expression levels were markedly increased in exosomes from patients with HCC (105). Several studies have demonstrated that exosomal circRNAs, particularly those derived from pathogenic exosomes, may have an important influence on the pathophysiological stage and processes of HCC; however, only a limited number of circRNAs have been reported to establish critical functions or clinical applications (26). Exosomal circRNAs are likely to attract more interest in HCC research.
mRNAs
mRNAs are mediators of genetic information transferred from the cell nucleus to ribosomes in the cytoplasm, in which mRNAs serve as templates for protein coding and synthesis (106). Although relatively few studies have focused on exosomal mRNAs, recent reports have been published on the promising and increasing functions of exosomal mRNAs in cancer development and prognosis (18,107), particularly in liver disease and cancer (108). An increasing number of exosomal mRNAs have been reported to be potential biomarkers in HCC diagnosis. By using bioinformatics, a recent study identified an HCC-exosomal RNA-based biomarker selection panel, which was mostly associated with RAB11A gene expression and its competing endogenous network, including lncRNA-RP11-513I15.6 and miR-1262. The expression levels of this network were validated in the sera of 60 patients with HCC, and were compared with those found in 42 patients with chronic hepatitis C virus infection and 18 healthy donors. Panels of three novel exosomal RNA-based genes, including RAB11A, miR-1262 and lncRNA-RP11-513I15.6, displayed good sensitivity and specificity in predicting and differentiating patients with HCC from patients with chronic hepatitis C virus infection and healthy donors. Of these three RNAs, the RAB11A mRNA levels in serum were the most independent and promising prognosis factor (109). Another cohort study on patients with HCC, liver cirrhosis, chronic hepatitis B and healthy controls quantified serum exosomal heterogeneous nuclear ribonucleoprotein H1 (hnRNPH1) mRNA, and found that the hnRNPH1 mRNA levels in serum exosomes from patients with HCC were markedly upregulated compared with those in the other groups. The mRNA levels of hnRNPH1 discriminated patients with HCC from patients with chronic hepatitis B. Furthermore, the hnRNPH1 mRNA levels in serum exosomes from patients with HCC were closely associated with portal vein tumor emboli, lymph node metastasis, Child-Pugh classification, overall survival and TNM stage. These data indicated that hnRNPH1 mRNA levels in serum exosomes were potentially a promising biomarker for HCC diagnosis in areas with a high prevalence of patients with hepatitis B virus infection (110). A recent study enrolled a cohort consisting of healthy donors (n=159), as well as patients with benign hepatic tumors (n=24), hepatitis (n=11), hepatic cirrhosis (n=8), HCC (n=104), and breast (n=10), gastric (n=9), kidney (n=15) and colorectal cancer (n=12). The authors detected >10,000 extracellular vesicle-derived long RNAs, including mRNAs, circRNAs and lncRNAs, in plasma. Among these RNAs, a majority of the total mapped reads were mRNAs. Blood extracellular vesicles contained a substantial fraction of intact mRNAs, and 8 extracellular vesicle-derived long RNAs were suggested to serve as potential biomarkers for HCC diagnosis in areas with high diagnostic efficiency (108).
6. Exosomes in the tumor microenvironment of HCC
Tumor progression depends on the complexity and heterogeneity of the tumor microenvironment, which consists of a network of cellular and acellular constituents. Increasing evidence reveals that this process is also impacted by exosome-mediated cross-talks or communications within the tumor microenvironment (111). Communications between HCC cells and their microenvironmental cell components are necessary mechanisms that modulate tumor development and progression. As one of the major components of the ncRNA exosomal cargo, miRNAs play multiple critical roles in modulating cellular pathways in targeted cells and microenvironments, and in regulating multiple processes associated with tumor progression, invasion and metastasis, including EMT, invasion, angiogenesis, multi-drug resistance and immune escape. Increasing evidence suggests that exosomal miRNAs function as important players in the dynamic crosstalk among cancerous, stromal and immune cells to establish the microenvironment of tumorigenesis (112). In the HCC microenvironment, exosomes participate in multiple steps during tumor progression and radioresistance, and numerous cell types (particularly stromal and inflammatory cells) within the microenvironment are involved in these processes (113). Exosomes have been reported to be critical in mediating the regulation of the inflammatory microenvironment to promote cancer progression and metastasis (114). HCC cells exposed to arsenite secreted miR-155-rich exosomes that enhanced inflammation and were positively correlated with IL-6 or IL-8 levels (115). The bidirectional communication between HCC and its exosome-mediated inflammatory microenvironment was also confirmed by a previous study, which reported that the inflammatory microenvironment promoted tumorigenesis while the tumor also created an inflammatory environment that promoted its own development. The β1-integrin/NF-κB signaling pathway in cancer-associated fibroblasts (CAFs) was activated by exosomes with miR-1247-3p secreted by high-metastatic HCC cells via directly targeting β-1,4-galactosyltransferase 3. These activated CAFs further promoted pleiotropic actions, including the secretion of proinflammatory cytokines, such as IL-6 and IL-8, that governed tumor progression (116). Exosomes with miR-320a overexpression derived from CAFs showed potential to inhibit tumorigenesis, thus suggesting a possible cause of HCC progression mediated by CAFs by deficiency in antitumor miR-320a in CAF-derived exosomes (117). CAFs could transfer miR-320a to HCC cells via exosomes and then inhibit EMT, while loss of antitumor miR-320a in CAF-derived exosomes could induce EMT and promote tumor progression. In solid HCC tumors, a number of the main components of the extracellular matrix, such as collagens, laminins, fibronectin, glycosaminoglycans and proteoglycans, play crucial roles in changing the phenotypic and functional characteristics of HCC and stroma cells via various mechanisms, including exosome-involved interaction (118). Beside these studies, additional evidence in recent years has implied the roles of exosomes in establishing and modifying the HCC microenvironment in a manner that enhances tumor progression, metastasis and tumor resistance.
7. Other extracellular vesicle subtypes in HCC progression and radioresistance
Besides exosomes, which are well studied, the other two major extracellular vesicle subtypes, microvesicles and apoptotic bodies (which are differentiated based upon their biogenesis, release pathways, size, content and function) (119), are less studied in the context of the development, progression and radioresistance of HCC. Microvesicles derived from human liver stem cells induced the in vitro proliferation and apoptosis resistance of hepatocytes by transporting mRNA into hepatocytes (120). Microvesicles derived from human adult liver stem cells may reprogram in vitro HepG2 liver cancer and primary HCC cells by inhibiting their growth and survival. In vivo intratumor administration of microvesicles induced the regression of ectopic tumors developed in SCID mice by a mechanism involving the delivery of miRNAs from human adult liver stem cell-derived microvesicles to tumor cells according to various studies. The antitumor effect of adult liver stem cell-derived microvesicles was also observed in tumors other than liver, such as lymphoblastoma and glioblastoma (121). These studies suggest that the delivery of selected miRNAs by microvesicles derived from stem cells may inhibit tumor growth and stimulate apoptosis. Apoptotic bodies, another major class of extracellular vesicles released as a product of apoptotic cell disassembly as well as insufficient apoptosis, have been associated with the development and progression of tumors of the liver and biliary tree (122). Accumulating evidence suggests a defective apoptotic process in human HCC, and numerous potential therapeutic strategies that induce the apoptotic process in various ways have the potential to aid the management of HCC (123).
8. Conclusions and perspectives
Exosomes are emerging as novel modes of intercellular communications in human homeostasis and diseases. Exosomes act as bioactive components that transfer proteins, lipids, DNA, mRNAs, ncRNAs and recently identified molecules from contributor to recipient cells, which leads to the exchange of genetic information and the transcriptional re-programming of recipient cells (22,124). Circulating exosomes, as well as their main components, may potentially be used as liquid biopsies, and are promising biomarkers for early detection, diagnosis and multi-therapy in patients with cancer (125), such as HCC (59,126). Clinically, HCC is considered a radioresistant tumor, and increasing evidence suggests a critical role of HCC-derived exosomes in radioresistance (13,127), due to their influence on tumor initiation, growth, progression, metastasis, drug radioresistance and recurrence. Despite these adverse conditions in HCC therapy, as increasing novel technologies are developed and to monitor and modify exosomes in cancer progression and radiotherapy, exosome-associated HCC diagnosis, therapies and post-surgery prediction are becoming more promising, and exosome-containing molecules are valuable in HCC research as novel biomarkers.
Acknowledgments
Not applicable.
Abbreviations
- HCC
hepatocellular carcinoma
- mRNA
messenger RNA
- miRNA
micro-RNA
- lncRNA
long non-coding RNA
- circRNA
circular RNA
- ncRNA
non-coding RNA
- HEV
hepatitis E virus
- ctDNA
circulating tumor DNA
- EMT
epithelial-mesenchymal transition
- TNM
tumor, node, metastasis
- ceRNA
competitive endogenous RNA
Funding Statement
The current study was funded by the Natural Science Foundation of Liaoning Province (2021-MS-181 to CW) and the Young and Middle-Aged Scientific and Technological Talents Support Program of Shenyang City (RC200554 to CW).
Availability of data and materials
Not applicable.
Authors' contributions
QF, YY and CW designed and wrote the manuscript. YZ and SZ collected and summarized the related papers and references. CW revised and conceived the final approval of the version to be submitted and obtaining of the funding.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- 2.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380:1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 3.Nair H, Simoes EA, Rudan I, Gessner BD, Azziz-Baumgartner E, Zhang JSF, Feikin DR, Mackenzie GA, Moiïsi JC, Roca A, et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: A systematic analysis. Lancet. 2013;381:1380–1390. doi: 10.1016/S0140-6736(12)61901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Burden of Disease Liver Cancer Collaboration. Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: Results from the global burden of disease study 2015. JAMA Oncol. 2017;3:1683–1691. doi: 10.1001/jamaoncol.2017.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Fan D. Hepatitis B in China. Lancet. 2007;369:1582–1583. doi: 10.1016/S0140-6736(07)60723-5. [DOI] [PubMed] [Google Scholar]
- 7.Laursen L. A preventable cancer. Nature. 2014;516:S2–S3. doi: 10.1038/516S2a. [DOI] [PubMed] [Google Scholar]
- 8.European Association for the study of the Liver and European Organisation for research and treatment of Cancer EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, Imamura H, Sugawara Y, Kokudo N, Makuuchi M. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908–1916. doi: 10.1053/j.gastro.2008.02.091. [DOI] [PubMed] [Google Scholar]
- 10.Yu Y, Feng M. Radiotherapy for hepatocellular carcinoma. Semin Radiat Oncol. 2018;28:277–287. doi: 10.1016/j.semradonc.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Bang A, Dawson LA. Radiotherapy for HCC: Ready for prime time? JHEP Rep. 2019;1:131–137. doi: 10.1016/j.jhepr.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohri N, Dawson LA, Krishnan S, Seong J, Cheng JC, Sarin SK, Kinkhabwala M, Ahmed MM, Vikram B, Coleman CN, Guha C. Radiotherapy for hepatocellular carcinoma: New indications and directions for future study. J Natl Cancer Inst. 2016;108:djw133. doi: 10.1093/jnci/djw133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ni J, Bucci J, Malouf D, Knox M, Graham P, Li Y. Exosomes in cancer radioresistance. Front Oncol. 2019;9:869. doi: 10.3389/fonc.2019.00869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15:409–425. doi: 10.1038/nrc3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan R, Sethi P, Jyoti A, McGarry R, Upreti M. Investigating the radioresistant properties of lung cancer stem cells in the context of the tumor microenvironment. Radiat Res. 2016;185:169–181. doi: 10.1667/RR14285.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swartz MA, Iida N, Roberts EW, Sangaletti S, Wong MH, Yull FE, Coussens LM, DeClerck YA. Tumor microenvironment complexity: Emerging roles in cancer therapy. Cancer Res. 2012;72:2473–2480. doi: 10.1158/0008-5472.CAN-12-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Son B, Lee S, Youn H, Kim E, Kim W, Youn B. The role of tumor microenvironment in therapeutic resistance. Oncotarget. 2017;8:3933–3945. doi: 10.18632/oncotarget.13907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q, Evans R, et al. Reassessment of exosome composition. Cell. 2019;177:428–445.e18. doi: 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tai YL, Chen KC, Hsieh JT, Shen TL. Exosomes in cancer development and clinical applications. Cancer Sci. 2018;109:2364–2374. doi: 10.1111/cas.13697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai J, Su Y, Zhong S, Cong L, Liu B, Yang J, Tao Y, He Z, Chen C, Jiang Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther. 2020;5:145. doi: 10.1038/s41392-020-00261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 23.Sato S, Zhu XL, Sly WS. Carbonic anhydrase Isozymes IV and II in urinary membranes from carbonic anhydrase II-deficient patients. Proc Natl Acad Sci USA. 1990;87:6073–6076. doi: 10.1073/pnas.87.16.6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Yu D. Exosomes in cancer development, metastasis, and immunity. Biochim Biophys Acta Rev Cancer. 2019;1871:455–468. doi: 10.1016/j.bbcan.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487–514. doi: 10.1146/annurev-biochem-013118-111902. [DOI] [PubMed] [Google Scholar]
- 26.Chen W, Mao Y, Liu C, Wu H, Chen S. Exosome in hepatocellular carcinoma: An update. J Cancer. 2021;12:2526–2536. doi: 10.7150/jca.54566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang HB, Lu ZM, Zhao XX. Tumorigenesis, diagnosis, and therapeutic potential of exosomes in liver cancer. J Hematol Oncol. 2019;12:133. doi: 10.1186/s13045-019-0806-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, Samuel M, Pathan M, Jois M, Chilamkurti N, et al. ExoCarta: A Web-Based compendium of exosomal cargo. J Mol Biol. 2016;428:688–692. doi: 10.1016/j.jmb.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nabet BY, Qiu Y, Shabason JE, Wu TJ, Yoon T, Kim BC, Benci JL, DeMichele AM, Tchou J, Marcotrigiano J, Minn AJ. Exosome RNA unshielding couples stromal activation to pattern recognition receptor signaling in cancer. Cell. 2017;170:352–366.e13. doi: 10.1016/j.cell.2017.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li I, Nabet BY. Exosomes in the tumor microenvironment as mediators of cancer therapy resistance. Mol Cancer. 2019;18:32. doi: 10.1186/s12943-019-0975-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conigliaro A, Costa V, Lo Dico A, Saieva L, Buccheri S, Dieli F, Manno M, Raccosta S, Mancone C, Tripodi M, et al. CD90+liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol Cancer. 2015;14:155. doi: 10.1186/s12943-015-0426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ridder K, Keller S, Dams M, Rupp AK, Schlaudraff J, Del Turco D, Starmann J, Macas J, Karpova D, Devraj K, et al. Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol. 2014;12 doi: 10.1371/journal.pbio.1001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skotland T, Sandvig K, Llorente A. Lipids in exosomes: Current knowledge and the way forward. Prog Lipid Res. 2017;66:30–41. doi: 10.1016/j.plipres.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Li WH, Li CY, Zhou T, Liu X, Liu X, Li X, Chen D. Role of exosomal proteins in cancer diagnosis. Mol Cancer. 2017;16:145. doi: 10.1186/s12943-017-0706-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai J, Han Y, Ren HM, Chen C, He D, Zhou L, Eisner GM, Asico LD, Jose PA, Zeng C. Extracellular vesicle-mediated transfer of donor genomic DNA to recipient cells is a novel mechanism for genetic influence between cells. J Mol Cell Biol. 2013;5:227–238. doi: 10.1093/jmcb/mjt011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thakur BK, Zhang H, Becker A, Matei I, Huang Y, Costa-Silva B, Zheng Y, Hoshino A, Brazier H, Xiang J, et al. Double-stranded DNA in exosomes: A novel biomarker in cancer detection. Cell Res. 2014;24:766–769. doi: 10.1038/cr.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong CM, Tsang FHC, Ng IOL. Non-coding RNAs in hepatocellular carcinoma: Molecular functions and pathological implications. Nat Rev Gastro Hepat. 2018;15:137–151. doi: 10.1038/nrgastro.2017.169. [DOI] [PubMed] [Google Scholar]
- 38.He M, Qin H, Poon TC, Sze SC, Ding X, Co NN, Ngai SM, Chan TF, Wong N. Hepatocellular carcinoma-derived exosomes promote motility of immortalized hepatocyte through transfer of oncogenic proteins and RNAs. Carcinogenesis. 2015;36:1008–1018. doi: 10.1093/carcin/bgv081. [DOI] [PubMed] [Google Scholar]
- 39.Wang S, Xu M, Li X, Su X, Xiao X, Keating A, Zhao RC. Exosomes released by hepatocarcinoma cells endow adipocytes with tumor-promoting properties. J Hematol Oncol. 2018;11:82. doi: 10.1186/s13045-018-0625-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee HY, Chen CK, Ho CM, Lee SS, Chang CY, Chen KJ, Jou YS. EIF3C-enhanced exosome secretion promotes angiogenesis and tumorigenesis of human hepatocellular carcinoma. Oncotarget. 2018;9:13193–13205. doi: 10.18632/oncotarget.24149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang S, Chen G, Lin X, Xing X, Cai Z, Liu X, Liu J. Role of exosomes in hepatocellular carcinoma cell mobility alteration. Oncol Lett. 2017;14:8122–8131. doi: 10.3892/ol.2017.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J, Lu S, Zhou Y, Meng K, Chen Z, Cui Y, Shi Y, Wang T, He QY. Motile hepatocellular carcinoma cells preferentially secret sugar metabolism regulatory proteins via exosomes. Proteomics. 2017;17:1700103. doi: 10.1002/pmic.201700103. [DOI] [PubMed] [Google Scholar]
- 43.Conde-Vancells J, Rodriguez-Suarez E, Gonzalez E, Berisa A, Gil D, Embade N, Valle M, Luka Z, Elortza F, Wagner C, et al. Candidate biomarkers in exosome-like vesicles purified from rat and mouse urine samples. Proteom Clin Appl. 2010;4:416–425. doi: 10.1002/prca.200900103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fu Q, Zhang Q, Lou Y, Yang J, Nie G, Chen Q, Chen Y, Zhang J, Wang J, Wei T, et al. Primary tumor-derived exosomes facilitate metastasis by regulating adhesion of circulating tumor cells via SMAD3 in liver cancer. Oncogene. 2018;37:6105–6118. doi: 10.1038/s41388-018-0391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye L, Zhang Q, Cheng Y, Chen X, Wang G, Shi M, Zhang T, Cao Y, Pan H, Zhang L, et al. Tumor-derived exosomal HMGB1 fosters hepatocellular carcinoma immune evasion by promoting TIM-1 + regulatory B cell expansion. J Immunother Cancer. 2018;6:145. doi: 10.1186/s40425-018-0451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, Shen H, Zhangyuan G, Huang R, Zhang W, He Q, Jin K, Zhuo H, Zhang Z, Wang J, et al. 14-3-3ζ delivered by hepatocellular carcinoma-derived exosomes impaired anti-tumor function of tumor-infiltrating T lymphocytes. Cell Death Dis. 2018;9:159. doi: 10.1038/s41419-017-0180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li M, Lu Y, Xu Y, Wang J, Zhang C, Du Y, Wang L, Li L, Wang B, Shen J, et al. Horizontal transfer of exosomal CXCR4 promotes murine hepatocarcinoma cell migration, invasion and lymphangiogenesis. Gene. 2018;676:101–109. doi: 10.1016/j.gene.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 48.Dai WJ, Wang YL, Yang TX, Wang J, Wu WC, Gu JX. Downregulation of exosomal CLEC3B in hepatocellular carcinoma promotes metastasis and angiogenesis via AMPK and VEGF signals. Cell Commun Signal. 2019;17:113. doi: 10.1186/s12964-019-0423-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qiu QC, Wang L, Jin SS, Liu GF, Liu J, Ma L, Mao RF, Ma YY, Zhao N, Chen M, Lin BY. CHI3L1 promotes tumor progression by activating TGF-β signaling pathway in hepatocellular carcinoma. Sci Rep. 2018;8:15029. doi: 10.1038/s41598-018-33239-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gai X, Tang B, Liu F, Wu Y, Wang F, Jing Y, Huang F, Jin D, Wang L, Zhang H. mTOR/miR-145-regulated exosomal GOLM1 promotes hepatocellular carcinoma through augmented GSK-3 beta/MMPs. J Genet Genomics. 2019;46:235–245. doi: 10.1016/j.jgg.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 51.Skotland T, Sagini K, Sandvig K, Llorente A. An emerging focus on lipids in extracellular vesicles. Adv Drug Deliv Rev. 2020;159:308–321. doi: 10.1016/j.addr.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 52.Skotland T, Ekroos K, Kauhanen D, Simolin H, Seierstad T, Berge V, Sandvig K, Llorente A. Molecular lipid species in urinary exosomes as potential prostate cancer biomarkers. Eur J Cancer. 2017;70:122–132. doi: 10.1016/j.ejca.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 53.Lydic TA, Townsend S, Adda CG, Collins C, Mathivanan S, Reid GE. Rapid and comprehensive 'Shotgun' lipidome profiling of colorectal cancer cell derived exosomes. Methods. 2015;87:83–95. doi: 10.1016/j.ymeth.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular Endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 55.Chapuy-Regaud S, Dubois M, Plisson-Chastang C, Bonnefois T, Lhomme S, Bertrand-Michel J, You B, Simoneau S, Gleizes PE, Flan B, et al. Characterization of the lipid envelope of exosome encapsulated HEV particles protected from the immune response. Biochimie. 2017;141:70–79. doi: 10.1016/j.biochi.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 56.Haraszti RA, Didiot MC, Sapp E, Leszyk J, Shaffer SA, Rockwell HE, Gao F, Narain NR, DiFiglia M, Kiebish MA, et al. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J Extracell Vesicles. 2016;5:32570. doi: 10.3402/jev.v5.32570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sadovska L, Eglitis J, Line A. Extracellular vesicles as biomarkers and therapeutic targets in breast cancer. Anticancer Res. 2015;35:6379–6390. [PubMed] [Google Scholar]
- 58.Alix-Panabieres C, Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016;6:479–491. doi: 10.1158/2159-8290.CD-15-1483. [DOI] [PubMed] [Google Scholar]
- 59.Li X, Li C, Zhang L, Wu M, Cao K, Jiang F, Chen D, Li N, Li W. The significance of exosomes in the development and treatment of hepatocellular carcinoma. Mol Cancer. 2020;19:1. doi: 10.1186/s12943-019-1085-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bernard V, Kim DU, San Lucas FA, Castillo J, Allenson K, Mulu FC, Stephens BM, Huang J, Semaan A, Guerrero PA, et al. Circulating nucleic acids are associated with outcomes of patients with pancreatic cancer. Gastroenterology. 2019;156:108–118.e4. doi: 10.1053/j.gastro.2018.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yan LL, Chen YH, Zhou JY, Zhao H, Zhang HH, Wang GQ. Diagnostic value of circulating cell-free DNA levels for hepatocellular carcinoma. Int J Infect Dis. 2018;67:92–97. doi: 10.1016/j.ijid.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 62.Slack FJ, Chinnaiyan AM. The role of Non-coding RNAs in oncology. Cell. 2019;179:1033–1055. doi: 10.1016/j.cell.2019.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 64.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 65.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang J, Li S, Li L, Li M, Guo C, Yao J, Mi S. Exosome and exosomal MicroRNA: Trafficking, sorting, and function. Genom Proteom Bioinf. 2015;13:17–24. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee YR, Kim G, Tak WY, Jang SY, Kweon YO, Park JG, Lee HW, Han YS, Chun JM, Park SY, Hur K. Circulating exosomal noncoding RNAs as prognostic biomarkers in human hepatocellular carcinoma. Int J Cancer. 2019;144:1444–1452. doi: 10.1002/ijc.31931. [DOI] [PubMed] [Google Scholar]
- 70.Tian XP, Wang CY, Jin XH, Li M, Wang FW, Huang WJ, Yun JP, Xu RH, Cai QQ, Xie D. Acidic microenvironment Up-Regulates exosomal miR-21 and miR-10b in early-stage hepatocellular carcinoma to promote cancer cell proliferation and metastasis. Theranostics. 2019;9:1965–1979. doi: 10.7150/thno.30958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin XJ, Chong YT, Guo ZW, Xie C, Yang XJ, Zhang Q, Li SP, Xiong Y, Yuan Y, Min J, et al. A serum microRNA classifier for early detection of hepatocellular carcinoma: A multicentre, retrospective, longitudinal biomarker identification study with a nested case-control study. Lancet Oncol. 2015;16:804–815. doi: 10.1016/S1470-2045(15)00048-0. [DOI] [PubMed] [Google Scholar]
- 72.Lin XJ, Fang JH, Yang XJ, Zhang C, Yuan Y, Zheng L, Zhuang SM. Hepatocellular carcinoma cell-secreted exosomal MicroRNA-210 promotes angiogenesis in vitro and in vivo. Mol Ther Nucleic Acids. 2018;11:243–252. doi: 10.1016/j.omtn.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cui Y, Xu HF, Liu MY, Xu YJ, He JC, Zhou Y, Cang SD. Mechanism of exosomal microRNA-224 in development of hepatocellular carcinoma and its diagnostic and prognostic value. World J Gastroentero. 2019;25:1890–1898. doi: 10.3748/wjg.v25.i15.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Statello L, Guo CJ, Chen LL, Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 2021;22:96–118. doi: 10.1038/s41580-020-00315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pathania AS, Challagundla KB. Exosomal long Non-coding RNAs: Emerging players in the tumor microenvironment. Mol Ther Nucleic Acids. 2021;23:1371–1383. doi: 10.1016/j.omtn.2020.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prensner JR, Chinnaiyan AM. The Emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1:391–407. doi: 10.1158/2159-8290.CD-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bolha L, Ravnik-Glavac M, Glavac D. Long noncoding RNAs as biomarkers in cancer. Dis Markers. 2017;2017 doi: 10.1155/2017/7243968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takahashi K, Yan IK, Kogure T, Haga H, Patel T. Extracellular vesicle-mediated transfer of long non-coding RNA ROR modulates chemosensitivity in human hepatocellular cancer. FEBS Open Bio. 2014;4:458–467. doi: 10.1016/j.fob.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takahashi K, Yan IK, Wood J, Haga H, Patel T. Involvement of extracellular vesicle long noncoding RNA (linc-VLDLR) in tumor cell responses to chemotherapy. Mol Cancer Res. 2014;12:1377–1387. doi: 10.1158/1541-7786.MCR-13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen SY, Teng SC, Cheng TH, Wu KJ. miR-1236 regulates hypoxia-induced epithelial-mesenchymal transition and cell migration/invasion through repressing SENP1 and HDAC3. Cancer Lett. 2016;378:59–67. doi: 10.1016/j.canlet.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 81.Li BG, Mao R, Liu CF, Zhang WH, Tang Y, Guo Z. LncRNA FAL1 promotes cell proliferation and migration by acting as a CeRNA of miR-1236 in hepatocellular carcinoma cells. Life Sci. 2018;197:122–129. doi: 10.1016/j.lfs.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 82.Yao ZC, Jia CC, Tai Y, Liang H, Zhong Z, Xiong Z, Deng M, Zhang Q. Serum exosomal long noncoding RNAs lnc-FAM72D3 and lnc-EPC14 as diagnostic biomarkers for hepatocellular carcinoma. Aging (Albany NY) 2020;12:11843–11863. doi: 10.18632/aging.103355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hou YC, Yu Z, Tam NL, Huang S, Sun C, Wang R, Zhang X, Wang Z, Ma Y, He X, Wu L. Exosome-related lncRNAs as predictors of HCC patient survival: A prognostic model. Am J Transl Res. 2018;10:1648–1662. [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang C, Yang X, Qi Q, Gao YH, Wei Q, Han SW. lncRNA-HEIH in serum and exosomes as a potential biomarker in the HCV-related hepatocellular carcinoma. Cancer Biomark. 2018;21:651–659. doi: 10.3233/CBM-170727. [DOI] [PubMed] [Google Scholar]
- 85.Xu H, Chen YM, Dong XY, Wang XJ. Serum exosomal long noncoding RNAs ENSG00000258332.1 and LINC00635 for the diagnosis and prognosis of hepatocellular carcinoma. Cancer Epidem Biomar. 2018;27:710–716. doi: 10.1158/1055-9965.EPI-17-0770. [DOI] [PubMed] [Google Scholar]
- 86.Sun L, Su Y, Liu X, Xu M, Chen X, Zhu Y, Guo Z, Bai T, Dong L, Wei C, et al. Serum and exosome long non coding RNAs as potential biomarkers for hepatocellular carcinoma. J Cancer. 2018;9:2631–2639. doi: 10.7150/jca.24978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ma X, Yuan T, Yang C, Wang Z, Zang Y, Wu L, Zhuang L. X-inactive-specific transcript of peripheral blood cells is regulated by exosomal Jpx and acts as a biomarker for female patients with hepatocellular carcinoma. Ther Adv Med Oncol. 2017;9:665–677. doi: 10.1177/1758834017731052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhuang LK, Yang YT, Ma X, Han B, Wang ZS, Zhao QY, Wu LQ, Qu ZQ. MicroRNA-92b promotes hepatocellular carcinoma progression by targeting Smad7 and is mediated by long non-coding RNA XIST. Cell Death Dis. 2016;7:e2203. doi: 10.1038/cddis.2016.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kogure T, Yan IK, Lin WL, Patel T. Extracellular vesicle-mediated transfer of a novel long noncoding RNA TUC339: A mechanism of intercellular signaling in human hepatocellular cancer. Genes Cancer. 2013;4:261–272. doi: 10.1177/1947601913499020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li X, Lei Y, Wu M, Li N. Regulation of macrophage activation and polarization by HCC-Derived Exosomal lncRNA TUC339. Int J Mol Sci. 2018;19:2958. doi: 10.3390/ijms19102958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ma D, Gao X, Liu Z, Lu X, Ju H, Zhang N. Exosome-transferred long non-coding RNA ASMTL-AS1 contributes to malignant phenotypes in residual hepatocellular carcinoma after insufficient radiofrequency ablation. Cell Prolif. 2020;53:e12795. doi: 10.1111/cpr.12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang D, Xing N, Yang T, Liu J, Zhao H, He J, Ai Y, Yang J. Exosomal lncRNA H19 promotes the progression of hepatocellular carcinoma treated with Propofol via miR-520a-3p/LIMK1 axis. Cancer Med. 2020;9:7218–7230. doi: 10.1002/cam4.3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ji JF, Yamashita T, Budhu A, Forgues M, Jia HL, Li C, Deng C, Wauthier E, Reid LM, Ye QH, et al. Identification of MicroRNA-181 by genome-wide screening as a critical player in EpCAM-Positive hepatic cancer stem cells. Hepatology. 2009;50:472–480. doi: 10.1002/hep.22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sanger HL, Klotz G, Riesner D, Gross HJ, Kleinschmidt AK. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci USA. 1976;73:3852–3856. doi: 10.1073/pnas.73.11.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Seimiya T, Otsuka M, Iwata T, Shibata C, Tanaka E, Suzuki T, Koike K. Emerging roles of exosomal circular RNAs in cancer. Front Cell Dev Biol. 2020;8:568366. doi: 10.3389/fcell.2020.568366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang Y, Liu J, Ma J, Sun T, Zhou Q, Wang W, Wang G, Wu P, Wang H, Jiang L, et al. Exosomal circRNAs: Biogenesis, effect and application in human diseases. Mol Cancer. 2019;18:116. doi: 10.1186/s12943-019-1041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wilusz JE, Sharp PA. Molecular biology. A circuitous route to noncoding RNA. Science. 2013;340:440–441. doi: 10.1126/science.1238522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li SS, Yao JP, Xie MJ, Liu YN, Zheng M. Exosomal miRNAs in hepatocellular carcinoma development and clinical responses. J Hematol Oncol. 2018;11:54. doi: 10.1186/s13045-018-0579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Geng X, Lin X, Zhang Y, Li Q, Guo Y, Fang C, Wang H. Exosomal circular RNA sorting mechanisms and their function in promoting or inhibiting cancer. Oncol Lett. 2020;19:3369–3380. doi: 10.3892/ol.2020.11449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang H, Deng T, Ge S, Liu Y, Bai M, Zhu K, Fan Q, Li J, Ning T, Tian F, et al. Exosome circRNA secreted from adipocytes promotes the growth of hepatocellular carcinoma by targeting deubiquitination-related USP7. Oncogene. 2019;38:2844–2859. doi: 10.1038/s41388-018-0619-z. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 101.Chen W, Quan Y, Fan S, Wang H, Liang J, Huang L, Chen L, Liu Q, He P, Ye Y. Exosome-transmitted circular RNA hsa_ circ_0051443 suppresses hepatocellular carcinoma progression. Cancer Lett. 2020;475:119–128. doi: 10.1016/j.canlet.2020.01.022. [DOI] [PubMed] [Google Scholar]
- 102.Zhang PF, Wei CY, Huang XY, Peng R, Yang X, Lu JC, Zhang C, Gao C, Cai JB, Gao PT, et al. Circular RNA circTRIM33-12 acts as the sponge of MicroRNA-191 to suppress hepatocellular carcinoma progression. Mol Cancer. 2019;18:105. doi: 10.1186/s12943-019-1031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen SP, Liu BX, Xu J, Pei XF, Liao YJ, Yuan F, Zheng F. MiR-449a suppresses the epithelial-mesenchymal transition and metastasis of hepatocellular carcinoma by multiple targets. BMC Cancer. 2015;15:706. doi: 10.1186/s12885-015-1738-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang GY, Liu W, Zou Y, Wang G, Deng Y, Luo J, Zhang Y, Li H, Zhang Q, Yang Y, Chen G. Three isoforms of exosomal circPTGR1 promote hepatocellular carcinoma metastasis via the miR449a-MET pathway. EBioMedicine. 2019;40:432–445. doi: 10.1016/j.ebiom.2018.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang TT, Jing B, Bai YX, Zhang Y, Yu HY. Circular RNA circTMEM45A Acts as the Sponge of MicroRNA-665 to promote hepatocellular carcinoma progression. Mol Ther Nucleic Acids. 2020;22:285–297. doi: 10.1016/j.omtn.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shyu AB, Wilkinson MF, van Hoof A. Messenger RNA regulation: To translate or to degrade. Embo J. 2008;27:471–481. doi: 10.1038/sj.emboj.7601977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yu SL, Li YC, Liao Z, Wang Z, Wang Z, Li Y, Qian L, Zhao J, Zong H, Kang B, et al. Plasma extracellular vesicle long RNA profiling identifies a diagnostic signature for the detection of pancreatic ductal adenocarcinoma. Gut. 2020;69:540–550. doi: 10.1136/gutjnl-2019-318860. [DOI] [PubMed] [Google Scholar]
- 108.Li Y, Zhao J, Yu S, Wang Z, He X, Su Y, Guo T, Sheng H, Chen J, Zheng Q, et al. Extracellular vesicles long RNA sequencing reveals abundant mRNA, circRNA, and lncRNA in human blood as potential biomarkers for cancer diagnosis. Clin Chem. 2019;65:798–808. doi: 10.1373/clinchem.2018.301291. [DOI] [PubMed] [Google Scholar]
- 109.Abd El Gwad A, Matboli M, El-Tawdi A, Habib EK, Shehata H, Ibrahim D, Tash F. Role of exosomal competing endogenous RNA in patients with hepatocellular carcinoma. J Cell Biochem. 2018;119:8600–8610. doi: 10.1002/jcb.27109. [DOI] [PubMed] [Google Scholar]
- 110.Xu H, Dong XY, Chen YM, Wang XJ. Serum exosomal hnRNPH1 mRNA as a novel marker for hepatocellular carci-noma. Clin Chem Lab Med. 2018;56:479–484. doi: 10.1515/cclm-2017-0327. [DOI] [PubMed] [Google Scholar]
- 111.He R, Wang Z, Shi W, Yu L, Xia H, Huang Z, Liu S, Zhao X, Xu Y, Yam JWP, Cui Y. Exosomes in hepatocellular carcinoma microenvironment and their potential clinical application value. Biomed Pharmacother. 2021;138:111529. doi: 10.1016/j.biopha.2021.111529. [DOI] [PubMed] [Google Scholar]
- 112.Pascut D, Pratama MY, Vo NVT, Masadah R, Tiribelli C. The Crosstalk between tumor cells and the microenvironment in hepatocellular carcinoma: The role of exosomal microRNAs and their clinical implications. Cancers (Basel) 2020;12:823. doi: 10.3390/cancers12040823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu Q, Zhou L, Lv D, Zhu X, Tang H. Exosome-mediated communication in the tumor microenvironment contributes to hepatocellular carcinoma development and progression. J Hematol Oncol. 2019;12:53. doi: 10.1186/s13045-019-0739-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen X, Chi H, Zhao X, Pan R, Wei Y, Han Y. Role of exosomes in immune microenvironment of hepatocellular carcinoma. J Oncol. 2022;2022:2521025. doi: 10.1155/2022/2521025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen C, Luo F, Liu X, Lu L, Xu H, Yang Q, Xue J, Shi L, Li J, Zhang A, Liu Q. NF-kB-regulated exosomal miR-155 promotes the inflammation associated with arsenite carcinogenesis. Cancer Lett. 2017;388:21–33. doi: 10.1016/j.canlet.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 116.Fang T, Lv H, Lv G, Li T, Wang C, Han Q, Yu L, Su B, Guo L, Huang S, et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat Commun. 2018;9:191. doi: 10.1038/s41467-017-02583-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang Z, Li X, Sun W, Yue S, Yang J, Li J, Ma B, Wang J, Yang X, Pu M, et al. Loss of exosomal miR-320a from cancer-associated fibroblasts contributes to HCC proliferation and metastasis. Cancer Lett. 2017;397:33–42. doi: 10.1016/j.canlet.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 118.Goessler UR, Hormann K, Riedel F. Tissue engineering with chondrocytes and function of the extracellular matrix (Review) Int J Mol Med. 2004;13:505–513. [PubMed] [Google Scholar]
- 119.Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Herrera MB, Fonsato V, Gatti S, Deregibus MC, Sordi A, Cantarella D, Calogero R, Bussolati B, Tetta C, Camussi G. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J Cell Mol Med. 2010;14:1605–1618. doi: 10.1111/j.1582-4934.2009.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fonsato V, Collino F, Herrera MB, Cavallari C, Deregibus MC, Cisterna B, Bruno S, Romagnoli R, Salizzoni M, Tetta C, Camussi G. Human liver stem cell-derived microvesicles inhibit hepatoma growth in SCID mice by delivering antitumor microRNAs. Stem Cells. 2012;30:1985–1998. doi: 10.1002/stem.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fabregat I, Roncero C, Fernandez M. Survival and apoptosis: A dysregulated balance in liver cancer. Liver Int. 2007;27:155–162. doi: 10.1111/j.1478-3231.2006.01409.x. [DOI] [PubMed] [Google Scholar]
- 123.Kountouras J, Zavos C, Chatzopoulos D. Apoptosis in hepatocellular carcinoma. Hepatogastroenterology. 2003;50:242–249. [PubMed] [Google Scholar]
- 124.Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, et al. Minimal information for studies of extra-cellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McAndrews KM, Kalluri R. Mechanisms associated with biogenesis of exosomes in cancer. Mol Cancer. 2019;18:52. doi: 10.1186/s12943-019-0963-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sasaki R, Kanda T, Yokosuka O, Kato N, Matsuoka S, Moriyama M. Exosomes and hepatocellular carcinoma: From bench to bedside. Int J Mol Sci. 2019;20:1406. doi: 10.3390/ijms20061406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hennequin C, Quero L, Rivera S. Radiosensitivity of hepatocellular carcinoma. Cancer Radiother. 2011;15:39–42. doi: 10.1016/j.canrad.2010.11.004. In French. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.