Abstract
Trovafloxacin, a new fluoroquinolone, produced bactericidal activity (−0.33 ± 0.13 Δlog10 CFU/ml · h; intravenously [i.v.] administered dose, 15 mg/kg) comparable to that of vancomycin (−0.39 ± 0.18 Δlog10 CFU/ml · h; i.v. administered dose, 20 mg/kg) in the treatment of experimental meningitis in rabbits due to a pneumococcal strain highly resistant to penicillin (MIC of penicillin G, 4 μg/ml). The combination of both drugs significantly increased (P < 0.05) the killing rate (−0.60 ± 0.23 Δlog10 CFU/ml · h) compared to that produced by either monotherapy. These results were also confirmed in vitro.
The worldwide spread of penicillin-resistant pneumococci presents a major challenge for clinicians. The new quinolone trovafloxacin {7-(3-azabicyclo[3.1.0]hexyl) quinolone} has excellent antimicrobial activity against penicillin-sensitive and penicillin-resistant pneumococci in vitro and good penetration into the cerebrospinal fluid (CSF) due to its lipophilic properties (2, 7, 15). In previous studies, it has been demonstrated that trovafloxacin is very active against penicillin-resistant pneumococci in the rabbit meningitis model, provided that the antibiotic level in the CSF remains above the MIC (12, 17). In the present study, we tested trovafloxacin alone and in combination with vancomycin against a pneumococcus highly resistant to penicillin in the rabbit meningitis model and in vitro.
Rabbit meningitis model.
The meningitis model originally described by Dacey and Sande (4) was used in a slightly modified way. In brief, young New Zealand White rabbits weighing 2 to 2.5 kg were anesthetized by intramuscular injections of ketamine (30 mg/kg) and xylazine (15 mg/kg) and were immobilized in stereotactic frames for induction of meningitis and CSF samplings. An inoculum containing 105 CFU of pneumococcus serotype 6 was directly injected into the cisterna magna. The organism had originally been isolated from a patient with pneumonia at the University Hospital of Berne, Switzerland, and was highly resistant to penicillin (the MICs were as follows: penicillin G, 4 μg/ml; ceftriaxone, 0.5 μg/ml; vancomycin, 0.12 to 0.25 μg/ml; and trovafloxacin, 0.12 μg/ml). A long-acting anesthetic (ethyl carbamate, or urethane; 3.5 g/rabbit) was injected subcutaneously. The animals were then returned to their cages. Fourteen hours later the cisterna magna was punctured again for periodic CSF sampling before and 0.75, 2.5, 4, 6, and 8 h after initiation of therapy. Antibiotics were administered through a peripheral ear vein as bolus injections at the following concentrations: alatrofloxacin (a prodrug of trovafloxacin with activity corresponding to 80.2% of the activity of trovafloxacin), 15 mg/kg; and vancomycin, 20 mg/kg. Alatrofloxacin was injected at hour 0, and vancomycin was injected at hours 0 and 4. During injection, alatrofloxacin was protected from light. Untreated controls received the same volume of saline.
Bacterial concentrations were determined as described by Kim et al. (12). In order to determine a possible carryover effect, samples were plated undiluted and at dilutions of 1:10 and 1:100 and titers were compared. No obvious carryover effect was noted. The antimicrobial activities of the regimens during the 8-h treatment were calculated by linear regression analysis and expressed as the decrease of log10 CFU per milliliter per hour (Δlog10 CFU/ml · h), as previously described (12). The results were expressed as means ± standard deviations (SDs). Statistical significance was determined by the Newman-Keuls test.
Measurement of antibiotic concentrations in the CSF.
Trovafloxacin and vancomycin concentrations in the CSF of every treated rabbit were determined by an agar diffusion method with antibiotic medium 11 for trovafloxacin and antibiotic medium 1 for vancomycin (Difco Laboratories, Detroit, Mich.). Standard curves were generated by measuring levels in saline with 5% rabbit serum, approximating the CSF protein concentration during meningitis (12, 15). Bacillus subtilis (ATCC 6633) was used as a test strain (18). Inter- and intraday assay variabilities were both less than 10%. The limits of detection were 0.16 to 0.20 μg/ml for trovafloxacin and 0.5 μg/ml for vancomycin and thus below the lowest concentration measured in the CSF.
In vitro assays.
The pneumococcal strain was grown in C+Y medium (13) to an optical density at 590 nm of 0.3 and then diluted 40-fold to 106 CFU/ml, corresponding to the CSF bacterial titer in rabbits before initiation of therapy. Antibiotics were added at concentrations approximating levels achievable in the CSF, as follows: vancomycin, 0.06 to 2.5 μg/ml (MIC, 0.25 μg/ml); and trovafloxacin mesylate, 0.06 to 0.6 μg/ml (MIC, 0.12 μg/ml). Combinations of trovafloxacin (0.12 μg/ml) with vancomycin (0.12 μg/ml) were also tested. Bacterial titers were determined at 0, 2, 4, and 6 h by serial dilution of samples, and the samples were plated on agar plates containing 5% sheep blood and incubated at 37°C for 24 hours. Experiments were performed in triplicate, and results were expressed in means ± SDs.
Results and discussion.
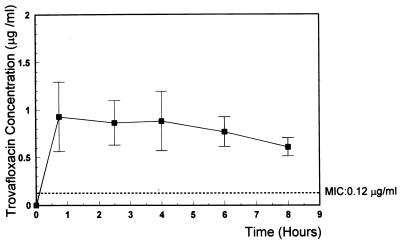
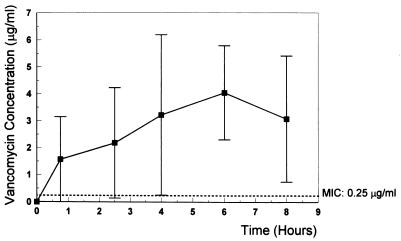
The results summarized in Table 1 confirm previous studies that demonstrated good activity of trovafloxacin against penicillin-sensitive and -resistant pneumococci and good CSF penetration in the rabbit meningitis model (17). To date, the optimal dosage for treatment of bacterial meningitis in humans has not been established. On account of this fact, we used a dose (15 mg/kg) that led to concentrations in CSF closely comparable to those found in healthy human volunteers following intravenous infusion of 300 mg of alatrofloxacin (5) (Fig. 1). Due to its long half-life, a single 300-mg dose of alatrofloxacin is usually recommended for severe infections (10). The CSF vancomycin concentration (Fig. 2) corresponded to the level measured in the CSF in humans (1, 11). Although the trovafloxacin level after a single dose (15 mg/kg) of alatrofloxacin remained far above the MIC during the treatment period (the lowest level in the CSF after 8 h was 0.6 μg/ml [MIC, 0.12 μg/ml]), trovafloxacin had only moderate bactericidal activity (Δlog10 CFU/ml · h, −0.33 ± 0.13 [Table 1]). The minimal CSF trovafloxacin concentration/MIC ratio was 5. The bactericidal activity of vancomycin (Δlog10 CFU/ml · h, −0.39 ± 0.18) was similar to that of trovafloxacin (it showed no statistical difference compared to trovafloxacin), confirming the findings of previous studies (9). Combination of trovafloxacin with vancomycin improved the killing rate significantly (Δlog10 CFU/ml · h, −0.60 ± 0.23) compared to that of either compound alone (P < 0.05). The regimens tested were bactericidal during therapy, sterilizing the CSF in four of nine animals treated with vancomycin, none of seven animals in the alatrofloxacin group, and four of seven animals treated with the combination.
TABLE 1.
Bactericidal activities of alatrofloxacin (prodrug of trovafloxacin) and vancomycin alone and in combination in experimental meningitis due to Streptococcus pneumoniae highly resistant to penicillin
| Antibiotic(s) | No. of rabbits | Killing rate (Δlog10 CFU/ml · h [mean ± SD]) |
|---|---|---|
| Controls | 5 | 0.06 ± 0.10 |
| Vancomycin | 9 | −0.39 ± 0.18 |
| Alatrofloxacin | 7 | −0.33 ± 0.13 |
| Alatrofloxacin + vancomycin | 7 | −0.60 ± 0.23a |
P < 0.05 versus all other treatments, by Newman-Keuls multiple comparison test.
FIG. 1.
Mean trovafloxacin concentrations in CSF at 0.75, 2.5, 4, 6, and 8 h after intravenous injection of 15 mg of alatrofloxacin per kg. The concentration of trovafloxacin remained above the MIC (0.12 μg/ml) during the entire treatment period. Vertical bars show ± SD.
FIG. 2.
Mean vancomycin concentration in the CSF at 0.75, 2.5, 4, 6, and 8 h after intravenous injection of vancomycin (20 mg/kg). Vancomycin was injected intravenously at 4 h at the same dose. The MIC for vancomycin was 0.25 μg/ml, and the concentrations in CSF remained above the MIC for the entire treatment period. Vertical bars show ± SD.
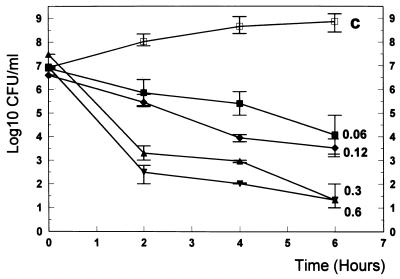
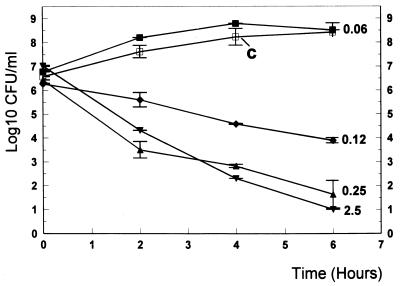
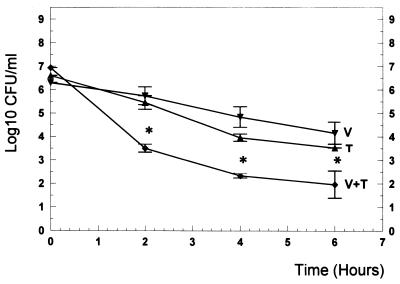
In vitro, trovafloxacin showed good bactericidal activity provided that the antibiotic concentrations were above the MIC (Fig. 3). Vancomycin at concentrations ranging from 0.06 to 2.5 μg/ml produced bactericidal rates comparable to those of trovafloxacin (Fig. 4). The combination enhanced the killing rates by nearly 2 log10 CFU/ml after 2, 4, and 6 h of incubation (P < 0.05) (Fig. 5).
FIG. 3.
Killing rates of trovafloxacin (■) in vitro at concentrations ranging from 0.06 μg/ml (1/2 of the MIC) to 0.6 μg/ml (5× the MIC). □, data for untreated controls (C). Experiments were performed in triplicate, and killing rates are expressed as means ± SDs.
FIG. 4.
Bactericidal activity of vancomycin in vitro. Concentrations of vancomycin ranging from 0.06 μg/ml (1/4 of the MIC) to 2.5 μg/ml (10× the MIC) were tested. □, data for untreated controls (C). Experiments were performed in triplicate, and results are expressed as means ± SDs.
FIG. 5.
Killing rates of trovafloxacin (T) (0.12 μg/ml; 1× the MIC), vancomycin (V) (0.12 μg/ml; 1/2 of the MIC), and the combination of the two substances (V+T) in vitro. The experiments were performed in triplicate, and results are expressed as means ± SDs. ∗, P < 0.05 versus either single-drug therapy.
In summary, addition of vancomycin improved the bactericidal activity of trovafloxacin in vitro and in vivo. Synergistic effects of quinolones with other antibiotics, e.g., rifampin, have been documented, and their therapeutic benefit in certain clinical situations is suggested (6, 13). To our knowledge, an increased bactericidal efficacy against pneumococci of the combination of trovafloxacin and vancomycin compared to single drugs in vivo has not been described. Recently, Nicolau et al. (16) demonstrated a synergy between trovafloxacin and vancomycin in vitro by calculating fractional inhibitory concentration (FIC) indices, as described by Eliopoulos and Moellering (8). However, synergy was observed in only 6% of all resistant isolates tested (16). The FIC indices of the combination against the resistant pneumococcal isolate we studied showed a neutral effect (FIC indices were 1.0). Time-killing curves constructed by using data measured in vitro, as used in our study, may correlate more closely with the situation in the CSF in vivo. The findings of the present study might be of relevance to pneumococcal diseases like meningitis, where the penetration of antibiotics is limited and bactericidal activity of therapeutic regimens is an imperative requirement.
Acknowledgments
This work was supported by grants from Pfizer Corporation and the Wander Foundation, Berne, Switzerland.
REFERENCES
- 1.Ahmed A. A critical evaluation of vancomycin for treatment of bacterial meningitis. Pediatr Infect Dis J. 1997;16:895–903. doi: 10.1097/00006454-199709000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Briggs Gooding B, Jones R N. In vitro antimicrobial activity of CP-99,219, a novel azabicyclo-naphthyridone. Antimicrob Agents Chemother. 1993;37:349–353. doi: 10.1128/aac.37.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cutler N R, Vincent J, Jhee S S, Teng R, Wardle T, Lucas G, Dogolo L C, Stramek J J. Penetration of trovafloxacin into cerebrospinal fluid in humans following intravenous infusion of alatrofloxacin. Antimicrob Agents Chemother. 1997;41:1298–1300. doi: 10.1128/aac.41.6.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dacey R G, Sande M A. Effect of probenecid on cerebrospinal fluid concentrations of penicillin and cephalosporin derivatives. Antimicrob Agents Chemother. 1974;6:437–441. doi: 10.1128/aac.6.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doit C, Barre J, Cohen R, Bonacorsi S, Bourillon A, Bingen E H. Bactericidal activity against intermediately cephalosporin-resistant Streptococcus pneumoniae in cerebrospinal fluid of children with bacterial meningitis treated with high doses of cefotaxime and vancomycin. Antimicrob Agents Chemother. 1997;41:2050–2052. doi: 10.1128/aac.41.9.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drancourt M, Stein A, Argenson J N, Zannier A, Curvale G, Raoult D. Oral rifampin plus ofloxacin for treatment of staphylococcus-infected orthopedic implants. Antimicrob Agents Chemother. 1993;37:1214–1218. doi: 10.1128/aac.37.6.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eliopoulos G M, Klimm K, Eliopoulos C T, Ferraro M J, Moellering R C., Jr In vitro activity of CP-99,219, a new fluoroquinolone, against clinical isolates of gram-positive bacteria. Antimicrob Agents Chemother. 1993;37:366–370. doi: 10.1128/aac.37.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eliopoulos G M, Moellering R C. Antimicrobial combinations. In: Lorian V, editor. Antibiotics in laboratory medicine. 4th ed. Baltimore, Md: The Williams & Wilkins Co.; 1991. pp. 330–396. [Google Scholar]
- 9.Friedland I R, Paris M, Ehret S, Hickey S, Olsen K, McCracken G H., Jr Evaluation of antimicrobial regimens for treatment of experimental penicillin- and cephalosporin-resistant pneumococcal meningitis. Antimicrob Agents Chemother. 1993;37:1630–1636. doi: 10.1128/aac.37.8.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haria M, Lamb H M. Trovafloxacin. Drugs. 1997;54:435–455. doi: 10.2165/00003495-199754030-00006. [DOI] [PubMed] [Google Scholar]
- 11.Hawley H B, Gump D W. Vancomycin therapy in bacterial meningitis. Am J Dis Child. 1973;126:261–264. doi: 10.1001/archpedi.1973.02110190231025. [DOI] [PubMed] [Google Scholar]
- 12.Kim Y S, Liu Q, Chow L L, Täuber M G. Trovafloxacin in treatment of rabbits with experimental meningitis caused by high-level penicillin-resistant pneumococci. Antimicrob Agents Chemother. 1997;41:1186–1189. doi: 10.1128/aac.41.5.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lack S, Hotchkiss R D. A study of the genetic material determining an enzyme activity in pneumococcus. Biochim Biophys Acta. 1960;39:508–518. doi: 10.1016/0006-3002(60)90205-5. [DOI] [PubMed] [Google Scholar]
- 14.Lucet J-C, Herrmann M, Rohner P, Auckenthaler R, Waldvogel F A, Lew D P. Treatment of experimental foreign body infection caused by methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1990;34:2312–2317. doi: 10.1128/aac.34.12.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nau R, Schmidt T, Kaye K, Froula J L, Täuber M G. Quinolone antibiotics in therapy of experimental pneumococcal meningitis in rabbits. Antimicrob Agents Chemother. 1995;39:593–597. doi: 10.1128/AAC.39.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicolau D P, Tessier P R, Quintiliani R, Nightingale C H. Synergistic activity of trovafloxacin and ceftriaxone or vancomycin against Streptococcus pneumoniae with various penicillin susceptibilities. Antimicrob Agents Chemother. 1998;42:991–992. doi: 10.1128/aac.42.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paris M M, Hickey S M, Trujillo M, Shelton S, McCracken G H., Jr Evaluation of CP-99,219, a new fluoroquinolone, for treatment of experimental penicillin- and cephalosporin-resistant pneumococcal meningitis. Antimicrob Agents Chemother. 1995;39:1243–1246. doi: 10.1128/aac.39.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simon H J, Yin E J. Microbioassay of antimicrobial agents. Appl Microbiol. 1970;19:573–579. doi: 10.1128/am.19.4.573-579.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]