Abstract
Background
Rare autoimmune neurological events have been reported during the ongoing global drive for mass vaccination as a means of controlling the Covid-19 pandemic. Guillain-Barré syndrome, an acute inflammatory neuropathy well recognised as a rare complication of influenza vaccination, has been reported to follow administration of the ChAdOx1 nCoV-19 (AstraZeneca) vaccine.
Methods
We report four patients with inflammatory demyelinating polyneuropathy after vaccination in whom a relapsing or progressive course indicated the development of chronic inflammatory demyelinating polyneuropathy (CIDP).
Conclusions
Awareness of this complication and distinction from Guillain-Barré syndrome enables the timely institution of maintenance immunomodulatory treatment. Our report also highlights the likely relationship between vaccination and the subsequent development of CIDP, but definitive demonstration of a causal link needs larger studies.
Keywords: Covid-19, AstraZeneca vaccine, CIDP, Guillain-Barré syndrome, Inflammatory demyelinating neuropathy
1. Introduction
Inflammatory neuropathy following vaccination first came to attention in 1976, when the swine influenza vaccine was associated with an increased incidence of Guillain-Barré syndrome (GBS) [1]. Subsequent studies provided a more nuanced picture, with the cumulative GBS risk from influenza being reported to be significantly higher than that from influenza vaccines [2]. In a similar fashion, although infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) responsible for the ongoing coronavirus disease (Covid-19) pandemic has been commonly associated with a spectrum of inflammatory neuropathies including GBS [3] and exacerbation of pre-existing CIDP [4,5], there have been multiple reports of GBS-like acute neuropathy following vaccination against SARS-CoV-2 even in the absence of previous Covid-19 [[6], [7], [8], [9]]. In our earlier report [10] we described four patients with inflammatory demyelinating polyneuropathy following the ChAdOx1 nCoV-19 (AstraZeneca, AZ) vaccine. While three had an acute-onset neuropathy diagnosed as GBS, one had an acute worsening of pre-existing neuropathic symptoms. All patients initially responded to intravenous immunoglobulin (IVIg) therapy, but on longitudinal follow-up only two had the monophasic course typical of GBS. In this report, we describe a relapsing or progressive inflammatory demyelinating neuropathy, fulfilling criteria for a diagnosis of chronic inflammatory demyelinating polyneuropathy (CIDP) [11], in four patients who received the AZ vaccine.
While viruses and viral vaccines have been proposed as putative triggers in the pathogenesis of autoimmune diseases such as CIDP [12], the association of this condition to a triggering vaccination is much less well defined than with GBS, with a few as 1.5% to 11% of patients with CIDP reporting a preceding vaccination within 8 weeks from the onset of the first neuropathy symptoms [13,14]. Our report adds to the limited body of evidence that vaccination can play a role in the pathogenesis of CIDP, either as de novo symptoms or as a relapse, and provides one of the first detailed descriptions of chronic inflammatory neuropathies triggered by the AZ vaccine.
2. Case report
We present four patients with CIDP following administration of the AZ vaccine, either de novo (three patients) or a relapse of a mild pre-existing demyelinating neuropathy. The patients were all male aged between 51 and 72 years and had received the first dose of the AZ vaccine 2–3 weeks before onset of symptoms. No patient had prior SARS-CoV-2 exposure. Results of serial nerve conduction studies are presented in the Table 1 .
Table 1.
Results of nerve conduction studies on four patients with CIDP following administration of the AZ vaccine.
| Patient | Days since symptom onset | UL sensory responses |
LL sensory responses |
UL motor responses |
LL motor responses |
F-waves |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amplitude | NCV | Amplitude | NCV | Distal latencies | Amplitude | NCV | Distal latencies | Amplitude | NCV | UL | LL | ||
| 1 | 9 | ↓ | ↓ | NR | NR | ↑ | N | ↓ | ↑ | N | ↓ | NR | NR |
| 50 | NR | NR | NR | NR | ↑ | ↓, TD | ↓ | ↑ | ↓, TD, CB | ↓ | NR | NR | |
| 2 | 110 | ↓ | N | NR | NR | ↑ | ↓, TD | ↓ | N | ↓ | ↓ | ↑ | ↑ |
| 3 | 25 | NR | NR | ↓ | N | ↑ | ↓, CB | ↓ | ↑ | ↓, CB | ↓ | NR | NR |
| 4 | (2017) | NR | NR | NR | NR | N | ↓ | ↓ | ↑ | ↓, TD, CB | ↓ | – | NR |
| 21 | NR | NR | NR | NR | ↑ | ↓ | ↓ | ↑ | ↓, TD | ↓ | ↑ | NR | |
| 70 | – | – | NR | NR | – | – | – | ↑ | ↓, TD | ↓, focal slowing in the leg | – | NR | |
↓: reduced; ↑: prolonged; CB: conduction block; LL: lower limbs; N: normal; NCV: nerve conduction velocity; NR: not recordable; TD: temporal dispersion; UL: upper limbs.
2.1. Patient 1
A 51-year-old man with congenital deafness and coronary artery disease presented two weeks after the first dose of the AZ vaccine with six days of low back pain followed by lower limb and bifacial weakness evolving to a severe areflexic quadriparesis with mild sensory loss in the feet. Within the next 48 h he developed neck weakness, dysphagia, dysphonia and respiratory weakness necessitating endotracheal intubation and mechanical ventilation. CSF showed albumino-cytologic dissociation with protein 0.70 g/L with one lymphocyte. He responded to IVIg 2 g/kg and was extubated in one week. His clinical course was marked by worsening of limb and respiratory function at 3, 5, and 9 weeks after hospital presentation leading to further mechanical ventilation and tracheostomy. These were managed with therapeutic plasma exchange which produced only ill-sustained and partial improvement, followed by two further cycles of IVIg at 2 g/kg. After the third course of IVIg he was commenced on maintenance treatment at 0.5 g/kg every four weeks, with sustained clinical improvement. He underwent extensive rehabilitation and at last follow-up eight months after initial presentation had completed seven cycles of IVIg and was ambulant with a single-point cane. Mild hand weakness persists.
2.2. Patient 2
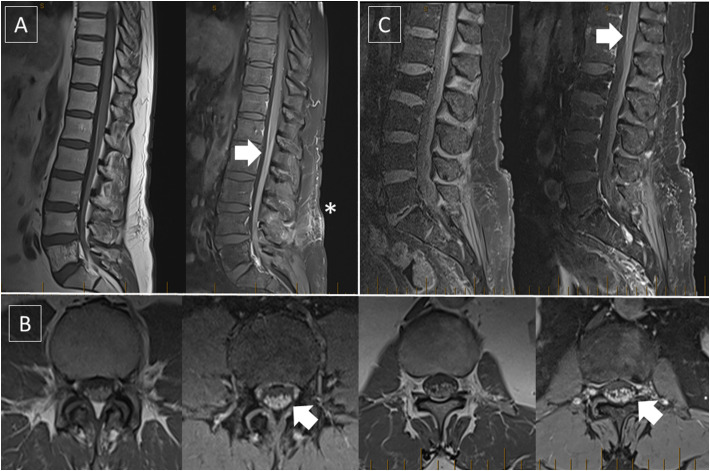
A 72-year-old man with localised prostate cancer, type 2 diabetes, hypertension, and hypothyroidism presented with two weeks of bilateral progressive paraesthesia of the lower limbs, dysarthria and gait dysfunction beginning three weeks after the first dose of the AZ vaccine. He was initially admitted for workup of haematological abnormalities that were diagnosed as a vaccine-induced immune thrombocytopenia and pancytopenia (normal bone marrow biopsy, cytogenetics, flow cytometry and myeloid gene panel), and managed with low-dose prednisolone, but suffered a subsequent left thalamic and posterior cerebral territory stroke. On referral to our service, he was found to have right homonymous hemianopia, bilateral proximal lower limb weakness with hyporeflexia, sensory ataxia, and loss of proprioception and vibration and pain perception in the lower extremities. A mixed axonal and demyelinating polyradiculo-neuropathy was detected on nerve conduction studies (Table 1) and CSF showed elevated protein levels (2.02 g/L) without pleocytosis. Imaging of the spine demonstrated surface enhancement of the lower dorsal cord (Fig. 1 ). In view of CSF albumino-cytologic dissociation and unequivocal demyelinating features on electrophysiology IVIg was commenced at 2 g/kg followed by 0.6 g/kg monthly, with improvement in his sensory deficits and balance at eight months follow-up.
Fig. 1.
Pre- and post-contrast T1-weighted MR images of the lumbosacral spine of patient 3 (A,B) and patient 2 (C) showing contrast enhancement over the cauda equina and surface of the lower dorsal spinal cord (arrows). The asterisk denotes enhancement at the site of recent lumbar puncture.
2.3. Patient 3
A 72-year-old man with hypertension and vertigo presented to hospital with one week of paraesthesia and numbness in the hands and feet followed the next day by lower limb weakness, areflexia, difficulty ambulating, bifacial weakness, right abducent nerve palsy, dysarthria, and dysphagia. He had received the first dose of the AZ vaccine two weeks before symptoms onset. CSF protein was elevated (1.964 g/L) with nine lymphocytes. MRI of the spine demonstrated thickening and enhancement of lumbosacral nerve roots (Fig. 1). IVIg at 2 g/kg produced rapid improvement in facial, bulbar and limb weakness, but further deterioration of limb power two weeks later prompted repeat treatment. He had slow recovery of limb power, with his course complicated by autonomic dysfunction and aspiration pneumonia. Repeat CSF evaluation showed elevated protein at 1.26 g/L and five lymphocytes.
While awaiting transfer to the rehabilitation service, twelve weeks after symptom onset, he developed severe weakness in both upper and lower limbs. He remained areflexic, with absent proprioception and vibration perception in the lower limbs and in the hands. Further treatment with IVIg at 1 g/kg every 4 weeks led to improvement, and at five months follow-up he was ambulant with a four-wheeled walker. Recurrent urinary tract infections with bacteraemia complicated rehabilitation efforts.
2.4. Patient 4
A 72-year-old man had a previous diagnosis of a demyelinating neuropathy on EMG in 2017 which had remained stable without treatment for three years, with mild and non-disabling paraesthesia and numbness in the distal lower limbs. He presented with two weeks of thigh and buttock muscle pain, back pain, unsteadiness, lower limb weakness and worsening of pre-existing paraesthesia beginning three weeks after receiving the first dose of the AZ vaccine. He had proximal weakness of all limbs, worse in the lower limbs, with areflexia and hypoalgesia in the hands, legs, and feet. Proprioception was impaired distally in all limbs with sensory ataxia. CSF was acellular with elevated protein levels at 0.55 g/L. He received IVIg at 2 g/kg with good recovery within four weeks, almost to baseline apart from mild difficulty walking at speed. Ten weeks after his initial event he reported worsening leg paraesthesia and poor balance, and repeat electrophysiology confirmed ongoing demyelination (Table 1). He had a repeat course of IVIg with ongoing maintenance at 0.4 g/kg monthly with an excellent response over seven months of follow-up.
3. Discussion
Following strict border closures, Tasmania experienced a lull in the spread of Covid-19 with no community transmission noted for 18 months prior to border re-opening in December 2021. This reprieve was put to good use, with >99% of adults among the 261,778 people in the northern half of the state having received one dose of a Covid-19 vaccine and 97.48% having received two. We report four patients with inflammatory demyelinating polyneuropathy following administration of the AZ vaccine, two with slowly progressive symptoms (one beginning de novo after the first vaccine dose in association with immune thrombocytopenia, and another with recurrent worsening of a pre-existing neuropathy after vaccination) and two with severe deficits due to acute-onset CIDP (aCIDP) who had initial diagnoses of GBS, experiencing significant improvement following IVIg only to suffer multiple relapses. Low back pain was an initial feature in two patients. All but one had evidence of cranial neuropathy including bifacial weakness (two patients), dysphagia, dysarthria, or diplopia. A good response to maintenance IVIg treatment was seen in all patients.
3.1. The distinction between GBS and CIDP
Strict separation of GBS and CIDP, although desirable and straightforward in most cases, is not always easy in clinical practice [15]. Unlike the more usual slowly progressive and/or relapsing course over >8 weeks typical of most patient with CIDP, 16% of patients may present acutely simulating GBS, with temporal evolution of symptoms in less than 8 weeks. While 8–16% of patients with GBS may suffer worsening of weakness after commencement of treatment – referred to as treatment-related fluctuations (TRFs) - aCIDP needs to be considered when this occurs three times or more or when deterioration takes place after eight weeks from onset of disease [[16], [17], [18], [19], [20]]. The mean time to a nadir in symptoms is significantly shorter in patients with GBS-TRF than in patients with aCIDP (<4 weeks compared to 4–8 weeks), but this nadir may be less severe in aCIDP [16,21] . Our patients presented between 6 and 15 days after symptom onset. Symptoms due to aCIDP often lead to later hospital presentation than in patients with GBS [21]. A preceding vaccination was noted in one patient with aCIDP and no patients with GBS-TRF in a prospective Dutch study. Unlike our patients with aCIDP after the AZ vaccine and previously reported patients with GBS-TRF who had severe limb and respiratory muscle weakness, over half of the patients in this study had remained able to walk independently, with none needing artificial ventilation. This suggests aCIDP following the AZ vaccine may be more severe at initial presentation and may mimic GBS. Cranial nerve involvement including bifacial paresis was noted in our patients, in distinction to other patients with aCIDP who do not often develop cranial neuropathy [16,21].
3.2. CIDP and vaccination
Autoimmune neuropathies may be triggered by viruses and vaccines, through mechanisms that include antigen mimicry, triggering self-reactive T-cell clones, and cytokine upregulation that may induce aberrant MHC class II expression [22]. About 70% of cases of GBS are preceded by an infectious respiratory or gastrointestinal illness [23]. Twenty-two percent of the GBS patients included in the prospective International GBS Outcome Study during the first four months of the Covid-19 pandemic had a preceding SARS-CoV-2 infection, and data indicate this to be due to a post-infectious disease mechanism rather than direct viral invasion [24]. In CIDP, however, there is paucity as well as heterogeneity of data on preceding infections, with these implicated in approximately 10–20% [15,25,26]. In contrast to the occurrence of GBS after Covid-19, CIDP is not thought to be triggered or exacerbated by infection with SARS-CoV-2. While a small excess risk of GBS after influenza vaccines exists (around one case of GBS per million persons vaccinated) [13,[27], [28], [29], [30]], the risk is believed to be much lower for CIDP and other immune neuropathies [13]. Five of 65 British patients with CIDP who had been immunised after disease onset against tetanus, influenza or pneumococcus reported worsening of neurological symptoms in one retrospective questionnaire-based study, but only three needed treatment for this flare, suggesting that the risk of relapse following immunisation is low [14]. An Australian man homozygous for HLA-B8 experienced relapses of an inflammatory demyelinating polyneuropathy after each of three doses of tetanus toxoid and histological features of CIDP were demonstrated by sural nerve biopsy [31]. Kelkar reported three patients with relapsing or progressive-relapsing inflammatory demyelinating polyneuropathy beginning 2–21 days after influenza vaccination, responding to corticosteroids or IVIg combined with mycophenolate [32]. Studies, including a recent database review from Italy, have indicated that onset of CIDP could be temporally linked to an infection (most often a flu-like illness) in about 10% and vaccination against influenza in 1.2–1.5% of cases and occurred earlier by about a decade compared to those CIDP patients without antecedent events. Similar to our patients, aCIDP and cranial nerve involvement was reportedly more common in this setting, with symptom onset at a mean of 16.5 days after infection or vaccination [15,33].
3.3. GBS and CIDP following vaccination against Covid-19
Over a six-month period from January 2021 a 2.7-fold increase in admissions for acute-onset polyradiculoneuropathy to two hospitals in the Midlands was recorded compared to the same period in 2020, with 16 (66.7%) having received the first dose of any SARS-CoV2 vaccine in the preceding 4 weeks before symptom onset. Fourteen of these had received the AZ vaccine, at a mean of 14.4 days prior to onset of symptoms. Four (25%) had a subsequent clinical course and electrophysiology meeting criteria for aCIDP, re-treated with IVIg, plasma exchange or corticosteroids [34]. These findings were supported by a recent review of the English National Immunisation Database of COVID-19 vaccination, which found an increased risk of hospital admission or death due to GBS 15–28 days following the first dose of the AZ vaccine, as well an increased risk of facial palsy in the same period. 38 excess GBS events were observed per 10 million people vaccinated in the 1–28 days period after vaccination [35]. This is lower than the incidence of inflammatory neuropathy in our population, in which four patients were diagnosed over six months with CIDP following the AZ vaccine in addition to two previously reported patients who developed GBS after receiving the AZ vaccine [10]. We are unable to explain the high incidence of post-vaccinal neuropathy in northern Tasmania relative to other studies but note that our report consists only of clinical observations from a single centre. Temporal association does not necessarily imply causality and further epidemiological investigation on a larger population is needed to resolve this issue.
There have been single case reports of CIDP beginning after vaccination against Covid-19. A 49-year-old Italian man developed bifacial palsy with lower limb areflexia 16 days after receiving the AZ vaccine, with contrast enhancement of the cauda equina on MRI and CSF albumino-cytologic dissociation. Two months later he developed lower limb paraesthesia and weakness, sensory ataxia, and electrophysiological evidence of worsening neuropathy, treated with maintenance IVIg to good effect [36]. A 47-year-old Indian man who had had Covid-19 seven months before the first dose of the AZ vaccine developed rapidly progressive severe flaccid quadriparesis with bifacial weakness 17 days after vaccination. He improved significantly with IVIg therapy within ten days, only to experience two recurrences three and six weeks later with limb and bifacial weakness and right abducent nerve palsy. Albumino-cytologic dissociation was noted in CSF, with a demyelinating polyradiculo-neuropathy on EMG and thickened enhancing lumbosacral nerve roots on MRI. Repeat IVIg in combination with maintenance prednisolone and azathioprine led to recovery with no further relapses. A marked elevation of IgG antibody against SARS-CoV-2 was attributed by the authors to the combined effect of COVID-19 infection and AZ vaccination and was thought to play a role in the genesis of the autoimmune neuropathy [17]. A 49-year-old American woman developed ascending limb weakness, ataxia and areflexia evolving over 10–12 weeks, beginning 10 days after receiving the first dose of the mRNA-1273 (Moderna) vaccine and culminating in respiratory compromise necessitating mechanical ventilation. CSF albumino-cytological dissociation and EMG confirmed an inflammatory demyelinating polyneuropathy that responded to plasma exchange. The temporal course with evolution over >8 weeks suggests aCIDP, with a relatively severe course similar to our patients [37].
This last report notwithstanding, inflammatory neuropathies have been associated predominantly with the AZ vaccine, analogous to vaccine-induced immune thrombocytopenia which was also seen in one of our patients with CIDP. It is conceivable that antibodies produced after vaccination may react with neural tissue. This may involve the SARS-CoV-2 spike protein, or possibly the adenovirus vector. The latter hypothesis is an attractive explanation for the rarity of cases of GBS after administration of the mRNA vaccines against SARS-CoV-2 [32,38].
4. Conclusions
Inflammatory demyelinating neuropathy observed after administration of the AZ vaccine may follow a chronic course indistinguishable clinically or electrophysiologically from CIDP. In contrast to previous descriptions of aCIDP, the acute illness may be severe and associated with cranial nerve dysfunction, particularly bifacial weakness. Once differentiated from GBS-TRF, maintenance treatment based on the recommendations for CIDP should be considered. Our report also highlights the likely relationship between vaccination and the subsequent development of CIDP, but definitive demonstration of a causal link needs larger studies. It must be emphasized however that vaccination - ranked among the most impressive achievements of modern scientific medicine - is an essential and urgent component of the global response to the pandemic, and the relatively rare occurrence of inflammatory demyelinating polyneuropathy after the AZ vaccine needs to be balanced against the 5.25-fold increased risk of GBS in the 28 days following Covid-19 [35]. The rare occurrence of restricted patterns of autoimmune neurological damage does not detract from the vital need for mass vaccination and compliance with immunisation campaigns.
Author statement
There is no objection to the above details being published. No person who had contributed substantially to the production of this manuscript had been excluded from authorship. The author affirms that there is no conflict of interest to declare. No funding was received from any source. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Schonberger L.B., Bregman D.J., Sullivan-Bolyai J.Z., et al. Guillain-Barré syndrome following vaccination in the National Influenza Immunization Program, United States, 1976–1977. Am. J. Epidemiol. 1979;110(2):105–123. doi: 10.1093/oxfordjournals.aje.a112795. [DOI] [PubMed] [Google Scholar]
- 2.Vellozzi C., Iqbal S., Stewart B., Tokars J., DeStefano F. Cumulative risk of Guillain-Barré syndrome among vaccinated and unvaccinated populations during the 2009 H1N1 influenza pandemic. Am. J. Public Health. 2014;104(4):696–701. doi: 10.2105/AJPH.2013.301651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abu-Rumeileh S., Abdelhak A., Foschi M., et al. Guillain-Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J. Neurol. 2021;268:1133–1170. doi: 10.1007/s00415-020-10124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Looy E., Veenker L., Steyaert A., et al. COVID-19- induced exacerbation of chronic inflammatory demyelinating polyneuropathy. J. Neurol. 2021;268:3129–3131. doi: 10.1007/s00415-021-10417-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abu-Rumeileh S., Garibashvili T., Ruf W., et al. Exacerbation of chronic inflammatory demyelinating polyneuropathy in concomitance with COVID-19. J. Neurol. Sci. 2020;418 doi: 10.1016/j.jns.2020.117106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen C.M., Ramsamy S., Tarr A.W., Tighe P.J., Irving W.L., Tanasescu R., Evans J.R. Guillain-Barré syndrome variant occurring after SARS-CoV-2 vaccination. Ann. Neurol. 2021;90:315–318. doi: 10.1002/ana.26144. [DOI] [PubMed] [Google Scholar]
- 7.Maramattom B.V., Krishnan P., Paul R., Padmanabhan S., Cherukudal Vishnu Nampoothiri S., Syed A.A., Mangat H.S. Guillain-Barré syndrome following ChAdOx1-S/nCoV-19 vaccine. Ann. Neurol. 2021;90:312–314. doi: 10.1002/ana.26143. [DOI] [PubMed] [Google Scholar]
- 8.Azam S., Khalil A., Taha A. Guillain-Barré syndrome in a 67-year-old male post COVID-19 vaccination (Astra Zeneca) Am. J. Med. Case Rep. 2021;9:424–427. [Google Scholar]
- 9.Patel S.U., Khurram R., Lakhani A., Quirk B. Guillain-Barre syndrome following the first dose of the chimpanzee adenovirus-vectored COVID-19 vaccine, ChAdOx1. BMJ Case. Rep. 2021;14 doi: 10.1136/bcr-2021-242956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oo W.M., Giri P., de Souza A. AstraZeneca COVID-19 vaccine and Guillain- Barré syndrome in Tasmania: a causal link? J. Neuroimmunol. 2021 Nov 15;360:577719. doi: 10.1016/j.jneuroim.2021.577719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Den Bergh P.Y.K., Hadden R.D.M., Bouche P., Cornblath D.R., Hahn A., Illa I., Koski C.L., Leger J.-M., Nobile-Orazio E., Pollard J., Sommer C., van Doorn P.A., van Schaik I.N. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society — First Revision. Eur. J. Neurol. 2010;17:356–363. doi: 10.1111/j.1468-1331.2009.02930.x. [DOI] [PubMed] [Google Scholar]
- 12.Schattner A. Consequence or coincidence? The occurrence, pathogenesis and significance of autoimmune manifestations after viral vaccines. Vaccine. 2005;23:3876–3886. doi: 10.1016/j.vaccine.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Doneddu P.E., Spina E., Briani C., Fabrizi G.M., Manganelli F., Nobile-Orazio E. Acute and chronic inflammatory neuropathies and COVID-19 vaccines: practical recommendations from the task force of the Italian Peripheral Nervous System Association (ASNP) J. Peripher. Nerv. Syst. 2021;26:148–154. doi: 10.1111/jns.12435. [DOI] [PubMed] [Google Scholar]
- 14.Pritchard J., Mukherjee R., Hughes R.A.C. Risk of relapse of Guillain-Barré syndrome or chronic inflammatory demyelinating polyradiculoneuropathy following immunisation. J. Neurol. Neurosurg. Psychiatry. 2002;73:348–349. doi: 10.1136/jnnp.73.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajabally Y.A., Peric S., Bozovic I., Loo L.K., Kalac A., Palibrk A., Basta I. Antecedent infections and vaccinations in chronic inflammatory demyelinating polyneuropathy: a European collaborative study. Muscle Nerve. 2021;1–5 doi: 10.1002/mus.27374. [DOI] [PubMed] [Google Scholar]
- 16.Ruts L., van Koningsveld R., van Doorn P.A. Distinguishing acute-onset CIDP from Guillain-Barré syndrome with treatment related fluctuations. Neurology. 2005;65:138–140. doi: 10.1212/01.wnl.0000167549.09664.b8. [DOI] [PubMed] [Google Scholar]
- 17.Suri V., Pandey S., Singh J., Jena A. Acute-onset chronic inflammatory demyelinating polyneuropathy after COVID-19 infection and subsequent ChAdOx1 nCoV-19 vaccination. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-245816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleyweg R.P., van der Meché F.G. Treatment related fluctuations in Guillain–Barré syndrome after high-dose immunoglobulins or plasma exchange. J. Neurol. Neurosurg. Psychiatry. 1991;54:957–960. doi: 10.1136/jnnp.54.11.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Visser L.H., van der Meché F.G., Meulstee J., van Doorn P.A. Risk factors for treatment related clinical fluctuations in Guillain-Barré syndrome. Dutch Guillain-Barré study group. J. Neurol. Neurosurg. Psychiatry. 1998;64:242–244. doi: 10.1136/jnnp.64.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCombe P.A., Pollard J.D., McLeod J.G. Chronic inflammatory demyelinating polyradiculoneuropathy. A clinical and electrophysiological study of 92 cases. Brain. 1987;110:1617–1630. doi: 10.1093/brain/110.6.1617. [DOI] [PubMed] [Google Scholar]
- 21.Ruts L., Drenthen J., Jacobs B.C., van Doorn P.A. Distinguishing acute-onset CIDP from fluctuating Guillain-Barré syndrome: a prospective study. Neurology. 2010;74:1680–1686. doi: 10.1212/WNL.0b013e3181e07d14. [DOI] [PubMed] [Google Scholar]
- 22.Albert L.J., Inman R.D. Molecular mimicry and autoimmunity. N. Engl. J. Med. 1999;341:2068–2074. doi: 10.1056/NEJM199912303412707. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs B.C., Rothbarth P.H., van der Meché F.G., et al. The spectrum of antecedent infections in Guillain-Barré syndrome: a case-control study. Neurology. 1998;51:1110–1115. doi: 10.1212/wnl.51.4.1110. [DOI] [PubMed] [Google Scholar]
- 24.Luijten L.W.G., Leonhard S.E., van der Eijk A.A., Doets A.Y., Appeltshauser L., Arends S., et al. Guillain-Barre´ syndrome after SARS-CoV-2 infection in an international prospective cohort study. Brain. 2021;144:3392–3404. doi: 10.1093/brain/awab279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiò A., Cocito D., Bottacchi E., et al. Idiopathic chronic inflammatory demyelinating polyneuropathy: an epidemiological study in Italy. J. Neurol. Neurosurg. Psychiatry. 2007;78:1349–1353. doi: 10.1136/jnnp.2007.114868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuitwaard K., Bos-Eyssen M.E., Blomkwist-Markens P.H., van Doorn P.A. Recurrences, vaccinations and long-term symptoms in GBS and CIDP. J. Peripher. Nerv. Syst. 2009;14:310–315. doi: 10.1111/j.1529-8027.2009.00243.x. [DOI] [PubMed] [Google Scholar]
- 27.Martín Arias L.H., Sanz R., Sáinz M., Treceño C., Carvajal A. Guillain-Barré syndrome and influenza vaccines: a meta-analysis. Vaccine. 2015;33(31):3773–3778. doi: 10.1016/j.vaccine.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Juurlink D.N., Stukel T.A., Kwong J., Kopp A., McGeer A., Upshur R.E., Manuel D.G., Moineddin R., Wilson K. Guillain-Barre syndrome after influenza vaccination in adults—a population-based study. Arch. Intern. Med. 2006;166(20):2217–2221. doi: 10.1001/archinte.166.20.2217. [DOI] [PubMed] [Google Scholar]
- 29.Wise M.E., Viray M., Sejvar J.J., et al. Guillain-Barre syndrome during the 2009–2010 H1N1 influenza vaccination campaign: population-based surveillance among 45 million Americans. Am. J. Epidemiol. 2012;175(11):1110–1119. doi: 10.1093/aje/kws196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sejvar J.J., Pfeifer D., Schonberger L.B. Guillain-Barre´ syndrome following influenza vaccination: causal or coincidental? Curr. Infect. Dis. Rep. 2011;13(4):387–398. doi: 10.1007/s11908-011-0194-8. [DOI] [PubMed] [Google Scholar]
- 31.Pollard J.D., Selby G. Relapsing neuropathy due to tetanus toxoid. J. Neurol. Sci. 1978;37:113–125. doi: 10.1016/0022-510x(78)90232-0. [DOI] [PubMed] [Google Scholar]
- 32.Kelkar P. Chronic inflammatory demyelinating polyneuropathy (CIDP) with rapid progression after influenza vaccination: a report of three cases. J. Clin. Neuromusc. Dis. 2006;8(1):20–25. [Google Scholar]
- 33.Doneddu P.E., Bianchi E., Cocito D., Managanelli F., Fazio R., Filosto M., et al. Risk factors for chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): antecedent events, lifestyle and dietary habits. Data from the Italian CIDP database. Eur. J. Neurol. 2020;27:136–143. doi: 10.1111/ene.14044. [DOI] [PubMed] [Google Scholar]
- 34.Loo L.K., Salim O., Liang D., Goel A., Sumangala S., Gowda A.S., Davies B., Rajabally Y.A. Acute-onset polyradiculoneuropathy after SARS-CoV2 vaccine in the West and North Midlands, United Kingdom. Muscle Nerve. 2022;65:233–237. doi: 10.1002/mus.27461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patone M., Handunnetthi L., Saatci D., Pan J., Katikireddi S.V., Hunt D., Mei X.W., et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat. Med. 2021 Dec;27(12):2144–2153. doi: 10.1038/s41591-021-01556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bagella C.F., Corda D.G., Zara P., Elia A.E., Ruiu E., Sechi E., Solla P. Chronic inflammatory demyelinating polyneuropathy after ChAdOx1 nCoV-19 vaccination. Vaccines. 2021;9:1502–1512. doi: 10.3390/vaccines9121502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagalli S., Kikkeri N.S. Sub-acute onset of Guillain-Barré syndrome post-mRNA-1273 vaccination: a case report. SN Comprehensive Clin. Med. 2022:4:41. doi: 10.1007/s42399-022-01124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGonagle D., De Marco G., Bridgewood C. Mechanisms of immunothrombosis in vaccine-induced thrombotic thrombocytopenia (VITT) compared to natural SARS-CoV-2 infection. Autoimmunity. 2021;121 doi: 10.1016/j.jaut.2021.102662. [DOI] [PMC free article] [PubMed] [Google Scholar]