Key Points
Recipients of allo-HSCT show weaker immune responses to COVID-19 vaccines than healthy controls.
In allo-HSCT, deficiency of SARS-CoV-2–specific T-cell responses after vaccination translates into insufficient humoral immunity.
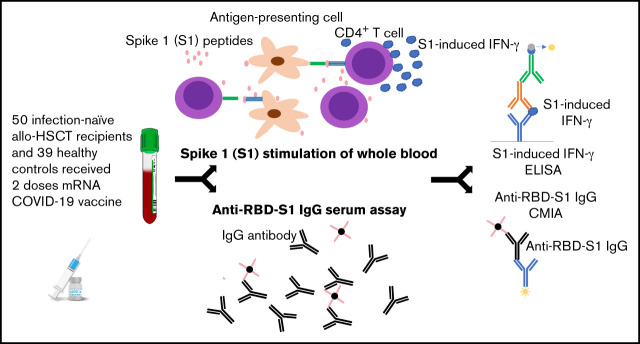
Visual Abstract
Abstract
Recipients of allogeneic hematopoietic stem cell transplantation (allo-HSCT) for hematological diseases are at risk of severe disease and death from COVID-19. To determine the safety and immunogenicity of BNT162b2 and mRNA-1273 COVID-19 vaccines, samples from 50 infection-naive allo-HSCT recipients (median, 92 months from transplantation, range, 7-340 months) and 39 healthy controls were analyzed for serum immunoglobulin G (IgG) against the receptor binding domain (RBD) within spike 1 (S1) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; anti–RBD-S1 IgG) and for SARS-CoV-2–specific T-cell immunity, reflected by induction of T-cell–derived interferon-γ in whole blood stimulated ex vivo with 15-mer SI-spanning peptides with 11 amino acid overlap S1-spanning peptides. The rate of seroconversion was not significantly lower in allo-transplanted patients than in controls with 24% (12/50) and 6% (3/50) of patients remaining seronegative after the first and second vaccination, respectively. However, 58% of transplanted patients lacked T-cell responses against S1 peptides after 1 vaccination compared with 19% of controls (odds ratio [OR] 0.17; P = .009, Fisher’s exact test) with a similar trend after the second vaccination where 28% of patients were devoid of detectable specific T-cell immunity, compared with 6% of controls (OR 0.18; P = .02, Fisher’s exact test). Importantly, lack of T-cell reactivity to S1 peptides after vaccination heralded substandard levels (<100 BAU/mL) of anti–RBD-S1 IgG 5 to 6 months after the second vaccine dose (OR 8.2; P = .007, Fisher’s exact test). We conclude that although allo-HSCT recipients achieve serum anti–RBD-S1 IgG against SARS-CoV-2 after 2 vaccinations, a deficiency of SARS-CoV-2–specific T-cell immunity may subsequently translate into insufficient humoral responses.
Introduction
Long-term survivors after allogeneic hematopoietic stem cell transplantation (allo-HSCT) for hematological diseases frequently remain in a state of depressed T-cell–mediated immunity because of incomplete immune reconstitution and immunosuppressive therapy to mitigate graft-versus-host disease (GVHD).1 Large studies report mortality rates of COVID-19 that may exceed 20% among recipients of allo-HSCT.2,3 Hence, the risk of severe COVID-19 in this patient group is considerably higher than that of the general population. In addition, immunosuppressed patients have been documented to drive the emergence and evolution of new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants,4 further supporting the need to mitigate morbidity rates.
In healthy subjects, 2 doses of messenger RNA (mRNA)-based COVID-19 vaccines result in 94% to 95% protection against COVID-19 lasting for at least 6 months with similar rates of seropositivity against the S1 portion of SARS-CoV-2.5,6 In contrast, lower seropositivity rates of 68% to 86% were achieved among allo-transplanted patients following 2 doses of the BNT162b2 mRNA vaccine,7-10 with reduced vaccine responsiveness being most prominent during the first-year posttransplant.8 In addition, concerns exist that COVID-19 vaccination may elicit or worsen GVHD.7,10,11
The induction of interferon-γ (IFN-γ) in whole-blood samples exposed to an array of SARS-CoV-2 peptides ex vivo reflects virus-specific T-cell immunity contributed by CD4+ and CD8+ T-cell subsets.12-14 This study thus aimed at determining the humoral and cellular immunogenicity of mRNA COVID-19 vaccines in long-term survivors after allo-HSCT for hematological disease.
Methods
Study population and design
This study was conducted between March and November 2021 at the Sahlgrenska University Hospital in Gothenburg, Sweden. Allo-HSCT recipients (n = 58), identified in local transplant registries in the Region Västra Götaland (population of ∼1.6 million), and healthy controls (n = 39) were recruited to participate in this substudy within the DurIRVac study. Enrolled transplant recipients fulfilled the following predetermined criteria: (i) to be at least 3 months post–allo-HSCT, (ii) to not have received rituximab during the last 6 months, and (iii) to be without uncontrolled acute GVHD (grade 3 to 4), all according to The European Society for Blood and Marrow Transplantation (EBMT) guidelines for COVID-19 vaccination (Version 2.0, December 21, 2020).
Five patients were excluded due to previous polymerase chain reaction–confirmed COVID-19 infection. Prior to vaccination, the allo-HSCT recipients were screened for previous COVID-19 infection by serology. Three additional patients were excluded because they exhibited anti–receptor binding domain (RBD)–immunoglobulin G (IgG). Five patients showed weak T-cell reactivity prior to vaccination that may indicate prior COVID-19. However, none of these patients were reactive against the RBD within spike 1 (S1) of SARS-CoV-2 (anti-RBD-S1 IgG) or antinucleocapsid IgG tests and were thus regarded as infection-naive. The 50 allo-HSCT recipients included in the study were intramuscularly vaccinated at a median of 41 days (range, 40 to 50) between doses with the mRNA-based SARS-CoV-2 vaccines BNT162b2 (Pfizer-BioBTech Comirnaty; n = 32) or mRNA-1273 (Moderna Spikevax; n = 18) according to the standard protocol at Sahlgrenska University Hospital. Peripheral blood was collected from transplant recipients at baseline, before the first immunization, 4 weeks after the first and second vaccine doses, and 4 to 5 months after the second dose. Sampling from all healthy controls was not possible prior to vaccination. However, no controls had had symptomatic COVID-19 nor had any serological signs of prior infection with SARS-CoV-2. Similarly, sampling from healthy controls was not available 4 to 5 months after the second dose. Baseline characteristics of allo-HSCT recipients and healthy controls are detailed in Table 1. Serum levels of anti–RBD-S1 IgG and the magnitude of S1-specific IFN-γ responses after the first and second vaccine doses, as well as their interplay were predefined primary study endpoints.
Table 1.
Baseline characteristics of allo-HSCT recipients and healthy controls
| Allo-HSCT* (n = 50) | Healthy controls (n = 39) | |
|---|---|---|
| Age at vaccination median, y (range) | 54 (29-78) | 60 (25-86) |
| Sex, female/male | 24/26 | 24/15 |
| Vaccine, Moderna/Pfizer-BioBTech | 18/32 | 2/37 |
| Days from dose 2 to sampling, median (range) | 27 (16-33) | 28 (14-147) |
| Months from transplantation, median (range) | 92 (7-340) | NA |
| Chronic GVHD (yes/no) | 18/32 | NA |
| Diagnosis (n) | ||
| Acute myeloid leukemia | 20 | NA |
| Acute lymphatic leukemia | 5 | NA |
| Chronic myeloid leukemia | 7 | NA |
| Chronic lymphatic leukemia | 3 | NA |
| Lymphoma | 2 | NA |
| Myelodysplastic syndrome | 7 | NA |
| Myelofibrosis | 2 | NA |
| Aplastic anemia | 2 | NA |
| Multiple myeloma | 1 | NA |
| Thalassemia | 1 | NA |
| Donor RD/URDhaplo | 15/34/1 | NA |
| Conditioning RIC/MAC | 25/25 | NA |
| Immunosuppressive therapy at time of first vaccination (n) | 9 | 0 |
| Corticosteroids | 7 | 0 |
| Ibrutinib | 2 | 0 |
| Ciclosporin | 1 | 0 |
| Imatinib | 2 | 0 |
MAC, myeloablative conditioning; NA, not applicable; RD, related donor; RIC, reduced intensity conditioning; URD, unrelated donor.
Allogeneic hematopoietic stem cell transplantation.
Adverse events
All allo-HSCT recipients completed a questionnaire following each vaccine dose. Side effects were categorized per the Common Terminology Criteria for Adverse Events standards. Information about GVHD using National Institutes of Health global severity score was assessed through the medical notes as well as patients’ self-reporting by means of questionnaire.
Analysis of IgG against the RBD within S1 of SARS-CoV-2 (anti–RBD-S1 IgG)
Chemiluminescent microparticle immunoassays were performed on serum using the automated Alinity system for quantitative measurement of IgG antibodies against the RBD of the spike protein of SARS-CoV-2 (SARS-CoV-2 IgG II Quant; Abbott, Abbott Park, IL) with levels reported in the World Health Organization International Standard Binding Antibody Units (BAU)/mL (quantitative detection range of 14 to 5680 BAU/mL). Samples reaching 5680 BAU/mL were diluted with seronegative serum and reanalyzed allowing for an upper detection limit of >5680 BAU/mL.15
Assay of virus-specific T-cell–derived IFN-γ in whole blood
Peripheral blood was collected in vacutainer lithium-heparin tubes (BD, Plymouth, UK). Within 24 hours of collection, 1 mL of whole blood was transferred to 10-mL tubes (Sarstedt) and stimulated or not with 1 µg/mL per peptide of 15-mer peptides with 11-amino-acid overlap spanning the N-terminal S1 domain of the SARS-CoV-2 surface glycoprotein S1 (product number: 130-127-041; Miltenyi Biotec). The samples were incubated for 2 days at 37°C with 5% CO2 to allow release of cytokines from peptide-responsive T cells. Thereafter, tubes were centrifuged for 5 minutes at 1500 rpm followed by recovery of plasma and storage at −80°C until analysis of IFN-γ.
IFN-γ enzyme-linked immunosorbent assay
Plasma recovered from S1 peptide-stimulated and unstimulated whole-blood samples was assessed for levels of IFN-γ by enzyme-linked immunosorbent assay (DY285B; R&D Systems) according to the manufacturer’s instructions. Plasma was diluted (1:2) in phosphate-buffered saline containing 1% bovine serum albumin and 10% mouse serum (Invitrogen) to minimize unspecific reactivity. Optical density was measured at 450 nm and 570 nm using a FLUOstar Omega plate reader (BMG, Ortenberg, Germany). The lower detection limit of the assay was 10 pg/mL. This assay was previously reported to discriminate individuals with prior SARS-CoV-2 infection from uninfected individuals with high accuracy.12
Ethical considerations and trial registration
All research participants gave written informed consent before enrollment. The DurIRVac study was approved by the Swedish Ethical Review Authority (permit nos. 2020-03276, 2021-00374, and 2021-00539) and by the Swedish Medical Products Agency (Dnr: 5.1-2021-11118) and has been registered at the European Union Drug Regulating Authorities Clinical Trials Database (EudraCT no. 2021-000349-42).
Statistical analysis
Continuous variables were described as median and range, that is, min-max values, as applicable. Categorical data were described with contingency tables including frequency and percent. Serological and cellular responses following vaccination, as well as differences in vaccine response between allo-HSCT recipients and controls, were analyzed based on the empirical distribution of 100 000 permutations of the average differences. Fisher’s exact test was used to calculate differences between observed frequencies in a contingency table. The association between continuous parameters was determined using Spearman’s correlation. The association between various parameters was further determined using logistic regression. Parameters with a univariate P value <.1 were included in multivariate analysis. For some figures, results were log-transformed as indicated in the figure text. Statistical analyses were performed using SPSS statistical software package (version 28) or GraphPad Prism software (version 9). P values are designated as follows: *P < .05, **P < .01, ***P < .001, and ****P < .0001. All indicated P values are 2-sided.
Results
Serological response to COVID-19 mRNA vaccination in allo-HSCT recipients
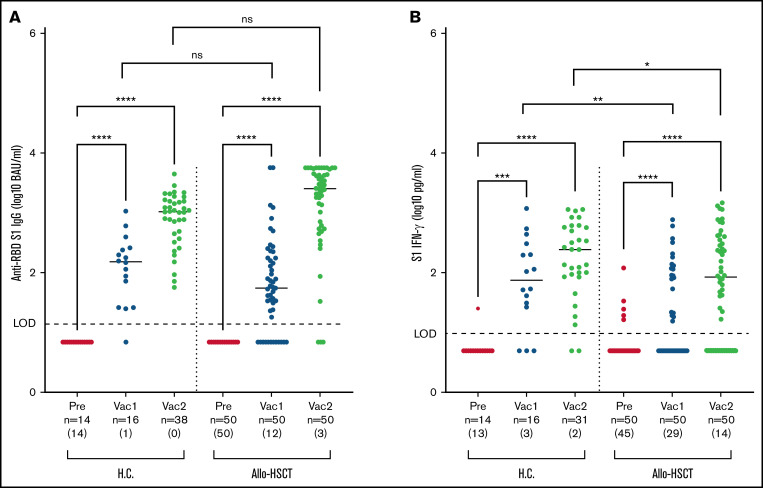
The majority of allo-HSCT recipients developed anti–RBD-S1 IgG after COVID-19 vaccination (Figure 1A). However, 12 of 50 (24%) of the transplanted patients remained seronegative (ie, below the level of detection of 14 BAU/mL) after the first vaccine dose and 3/50 (6%) remained seronegative after the second dose. Despite 2 vaccine doses, 5/50 (10%) of the allo-transplanted patients had 1 month after vaccination anti–RBD-S1 IgG <100 BAU/mL, a level above which has been proposed to provide protection against COVID-19.16 Characteristics of transplant recipients achieving anti–RBD-S1 IgG below or above 100 BAU/mL following 2 immunizations are detailed in Table 2, where transplantation within 24 months was significantly associated with a diminished antibody response (P = .009).
Figure 1.
Humoral and T-cell–mediated immune responses to vaccination in healthy controls and allo-HSCT recipients. S1-specific immune responses were determined in healthy controls (H.C.) and allo-HSCT recipients before vaccination (pre), after the first (Vac1), or second vaccine dose (Vac2). IgG antibody levels in serum against the RBD in S1 (A), and IFN-γ production in supernatant plasma following stimulation of whole blood with S1 peptides (B). The number of samples below the level of detection (LOD) for each assay (anti-RBD S1 IgG < 14 BAU/AU/mL and S1 IFN-γ < 10 pg/mL) is shown in brackets under each bar. Statistical comparisons were calculated by empirical distribution of 100 000 permutations of the average differences. ns, not significant.
Table 2.
Characteristics of allo-HSCT recipients with sufficient compared with suboptimal anti–RBD-S1 IgG (<100 BAU/mL) or nondetectable S1–IFN-γ after 2 doses of vaccinations
| Anti–RBD-S1 IgG<100 BAU/mL (n = 5) |
Anti–RBD-S1 IgG ≥100 BAU/mL (n = 45) |
OR* (95% CI) | P † | IFN-γ production <10 pg/mL (n = 14) |
IFN-γ production ≥10 pg/mL (n = 36) |
OR* (95% CI) | P † | |
|---|---|---|---|---|---|---|---|---|
| Median age at vaccination, y (range) | 62 (31-71) | 53 (29-78) | 1.0 (0.9-1.0) |
.63 | 61 (42-78) | 52.5 (29-75) | 0.96 (0.9-1.0) |
.09 |
| Time after allo-HSCT <24 mo, n (%) | 3 (60) | 3 (7) | 21.0 (2.5-178.2) |
.009 | 3 (21) | 3 (8) | 3.0 (0.5-17.3) |
.22 |
| Sex female/male, n (%) | 1 (20)/4 (80) | 23 (51)/22 (49) | 4.2 (0.4-40.4) |
.35 | 6 (43)/8 (57) | 18 (50)/18 (50) | 1.6 (0.4-4.6) |
.65 |
| Vaccine (Pfizer-BionTech/Moderna), n (%) | 3 (60)/2 (40) | 29 (64)/16 (36) | 1.2 (0.2-8.0) |
1.00 | 8 (53)/7 (47) | 24 (69)/11 (31) | 1.5 (0.4-5.3) |
.53 |
| Chronic GVHD, n (%) | 3 (60) | 15 (33) | 3.0 (0.5-6.8) |
.34 | 6 (43) | 12 (33) | 1.5 (0.4-5.3) |
.53 |
| Ongoing immunosuppressive treatment, n (%) | 2 (40) | 7 (16) | 3.6 (0.5-25.8) |
.22 | 2 (14) | 7 (19) | 0.7 (0.1-3.8) |
.67 |
Odds ratio with confidence intervals (CI).
P values by logistic regression; all P values shown are univariate.
Although there were no significant differences regarding antibody levels between patients and healthy controls after 2 vaccinations, the allo-HSCT recipients demonstrated a trend toward delayed serological response with a larger portion below the cutoff level of detection (<14 BAU/mL) of the assay (Figure 1A). Interestingly, after 2 vaccine doses, there was a considerable spread in antibody levels among allo-HSCT recipients (Figure 1A). A subset of the transplanted patients 10/50 (20%) thus produced very high levels of anti–RBD-S1 IgG (>5680 BAU/mL) following 2 vaccinations. None of the controls achieved similarly high anti–RBD-S1 IgG levels (P = .004, comparing the fraction of high responders among allo-HSCT recipients and heathy controls, Fisher’s exact test). Five of these 10 high-responding patients (50%) had ongoing low-grade chronic GVHD. Among the 5 high responders without previous chronic GVHD, 2 developed de novo onset mild chronic GVHD after the first vaccine dose. Two study patients had received rituximab because of Epstein-Barr virus reactivation <12 months (but >6 months) prior to inclusion in the present study. Both patients were seronegative after 2 vaccine doses. One of the patients, however, developed T-cell reactivity following the second vaccine dose.
S1-specific T-cell responses to COVID-19 mRNA vaccination
T-cell responses to vaccination were assessed by measuring released IFN-γ in plasma following stimulation of whole-blood samples with peptides spanning the S1 region of spike.12 Among allo-HSCT recipients, 29/50 (58%) did not achieve a detectable S1–IFN-γ response after 1 vaccine dose, and 14/50 (28%) failed to do so also after 2 doses (Figure 1B). As compared with healthy controls, patients had significantly lower S1–IFN-γ levels after both the first (median, 0 vs 105 pg/mL; P = .004, permutation test) and the second (median, 88 vs 243 pg/mL; P = .036, permutation test) vaccination (Figure 1B). The characteristics of allo-HSCT recipients with undetectable virus-specific T-cell responses after 2 vaccine doses are detailed in Table 2. No significant differences were noted for the T-cell or humoral responses between the 2 mRNA vaccines.
Humoral response in relation to T-cell responses after COVID-19 mRNA vaccination
Among allo-HSCT recipients, failure to mount anti–RBD-S1 IgG after 1 vaccination predicted failure to produce T-cell–derived S1–IFN-γ after 2 vaccinations (P < .0001; Fisher’s exact test). In addition, anti–RBD-S1 IgG and S1–IFN-γ levels after 2 immunizations were significantly correlated in both allo-HSCT recipients (r = 0.52, P = .001) and healthy controls (r = 0.41, P = .02). It is, however, noteworthy that cellular and humoral responses were not associated in all participants. For example, after 2 vaccine doses, 2/3 of the seronegative allo-HSCT recipients showed detectable virus-specific IFN-γ induction, and 11/14 (79%) patients with undetectable IFN-γ induction had anti–RBD-S1 IgG ≥ 100 BAU/mL.
S1-specific T-cell responses in dual vaccinated individuals predict durability of serological responses
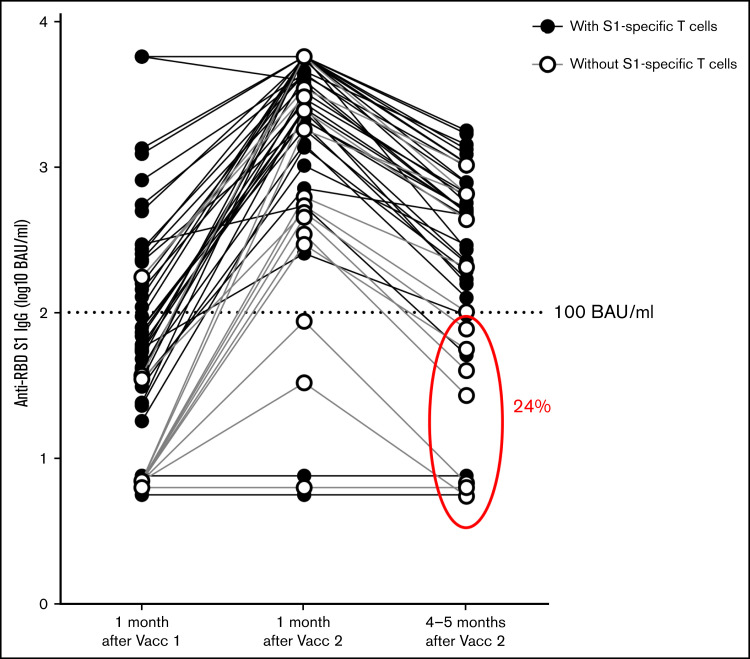
To further elucidate the durability of the COVID-19–specific T-cell and serological responses, 45 of the allo-HSCT recipients underwent an additional blood sampling 4 to 5 months after the second vaccine dose (median, 165; range, 114 to 169 days). Of note, the anti–RBD-S1 IgG levels in 11/45 patients (24%) were below the proposed protective level of 100 BAU/mL 4 to 5 months after the second vaccine dose (Figure 2). Among these 11 patients, 4/11 (36%) did not fulfill the criteria for receiving a third dose prior to the general population. Furthermore, of the 13 patients with follow-up samples who were devoid of a S1-specific IFN-γ response 1 month after the second dose of vaccination, the anti–RBD-S1 IgG levels were <100 BAU/mL 4 to 5 months later in 7 individuals (54%). In contrast, among patients with specific T-cell responses 3 to 4 weeks after receiving the second vaccine dose, only 4/32 (12%) had antibody levels <100 BAU/mL (P = .007, Fisher’s exact test) 4 to 5 months after the second immunization. Thus, the lack of a S1-specific IFN-γ response after 2 vaccine doses was predictive of a suboptimal humoral response 4 to 5 months later, while the lack of early serological response predicted lack of T-cell responsiveness. Interestingly, 2 patients had a detectable IFN-γ response after the second vaccine dose but lacked detectable anti–RBD-S1 IgG levels at any sampling throughout the study.
Figure 2.
Waning COVID-19–specific antibodies in allo-HSCT recipients following the second vaccine (Vacc) dose. Levels of IgG antibodies in serum against the RBD in S1 were determined 1 month after the first dose of vaccination (n = 50) and 1 (n = 50) and 4 to 5 months (n = 45) after the second COVID-19 vaccine dose. Patients responsive to S1-specific peptides, reflecting virus-specific T-cell reactivity, at 1 month after the second vaccine dose are shown with filled rings. Patients without a detectable S1 IFN-γ Τ-cell response 1 month after second vaccine dose are shown with open rings. Encircled are the 11/45 (24%) samples with below 100 BAU/ml anti-RBD S1 IgG levels 4-5 months after the second immunization.
Multivariate analyses
We performed logistic regression analyses to define the potential influence of confounders on the observed differences in vaccine responses among allo-HSCT recipients and controls. In these analyses, S1–IFN-γ levels were dichotomized based on below or >10 pg/mL IFN-γ in plasma supernatants or anti–RBD-S1 IgG below or above/equal to 100 BAU/mL in serum. Having undergone allo-HSCT remained significantly associated with S1–IFN-γ levels <10 pg/mL in plasma supernatants, after both the first and the second vaccine dose, when taking sex, age, type of vaccine, and time from second vaccination to sampling into account (adjusted P value, P = .01 after the first vaccine dose and P = .03 after the second dose). In contrast, no significant variables were found with regards to the antibody response when analyzing the entire cohort, including healthy controls.
Tolerability and safety
Adverse events were reported in 34/45 (76%) allo-HSCT recipients after the first vaccine dose; 31 of these were categorized as mild and were predominantly local injection-related reactions. Three reactions were categorized as moderate, marked by local swelling and pain lasting 7 to 14 days after immunization. Following the second vaccine dose, 26/47 (55%) reported adverse events, all of which were categorized as mild reactions. Similarly, only mild adverse events were reported among healthy controls.
GVHD
Eighteen patients had ongoing chronic GVHD at baseline prior to the first vaccination (10 mild, 7 moderate, and 1 severe). Two patients reported occurrence of oral blisters after the first immunization, assessed as de novo onset of mild chronic GVHD, which rapidly spontaneously resolved before the second vaccine dose. One patient with ongoing low-grade chronic GVHD reported a skin reaction following the first vaccine dose that resolved with topical therapy. Two patients reported worsening of chronic GVHD, of whom one required modest augmentation of prednisone dosing. No patient developed exacerbation or onset of GVHD after the second immunization.
There were no significant differences in humoral or cellular vaccine responses among patients with or without chronic GVHD. None of the patients with ongoing prednisone treatment (n = 7) (median dose, 5 mg per day; range, 1.25 to 11 mg per day) achieved detectable S1–IFN-γ levels after the first vaccine dose compared with 21/43 of the patients not receiving prednisone (P = .01; Fisher’s exact test). This difference was not significant following the second vaccination.
Documented COVID-19 during the study
One allo-HSCT recipient developed verified COVID-19 after the first vaccine dose with mild symptoms not requiring treatment or hospitalization. Of note, this patient had undetectable levels of anti–RBD-S1 IgG as well as S1–IFN-γ after the first immunization.
Discussion
We evaluated the safety and immunogenicity of mRNA-based COVID-19 vaccines in allo-HSCT survivors and noted that these vaccines were safe in these patients, although mild and infrequent exacerbations of chronic GVHD were observed in a similar frequency as reported by others.7,10,17 Our main finding was that although the majority of allo-transplanted patients achieved serum anti–RBD-S1 IgG after 2 vaccinations, a deficiency of SARS-CoV-2–specific T-cell immunity rapidly translated into lower antibody levels, below the proposed protective anti–RBD-S1 IgG concentration of 100 BAU/mL.
Vaccination elicited seroconversion, reflected by detectable serum anti–RBD-S1 IgG levels, in most patients. However, in one-third of recipients, in particular, those having undergone allo-HSCT within 2 years, humoral and/or cellular responses remained suboptimal (defined as anti–RBD-S1 IgG < 100 BAU/mL and/or S1–IFN-γ < 10 pg/mL) despite 2 vaccine doses. A deficiency in mounting a T-cell response was overrepresented among patients with ongoing immunosuppressive therapy. These observations confirm and extend results from previous studies in which shorter time after allo-HSCT and, in some studies, ongoing immunosuppressive therapy were associated with less pronounced vaccine responses.8-10,18 It should be noted that in the present study, most patients were vaccinated several years after allo-HSCT, which might have influenced the results regarding both vaccine efficacy and safety.
It was recently recognized that immune responses to SARS-CoV-2, both infection- and vaccine-induced, decline over time,19 and that additional booster vaccine doses are likely required to maintain protective immunity, in particular, in immunosuppressed subjects and elderly people.20 In addition, the recent identification of the Οmicron variant challenges the protective effect of vaccination against infection, although it is possible that the protection against severe and possibly fatal disease might be better. Of interest, we observed that patients lacking detectable T-cell responses to S1 peptide 4 weeks after the second vaccine dose were at marked risk of diminishing S1 RBD-specific antibody levels.
A seroconversion rate of 75% was previously reported in the prospective study by Ram et al among patients having undergone allogeneic SCT or CD19-based chimeric antigen receptor–T-cell therapy.10 In this latter study, however, a subset of patients was evaluated for virus-specific T-cell reactivity by ELIspot, with 36% having detectable cellular immune responses. Aside from using a different method for evaluating T-cell responses, it should be noted that the median time from transplant was 32 months,10 substantially shorter than in the present study.
In most other trials evaluating vaccine responses among recipients of allo-HSCT or solid organ transplantation,10,21 BNT162b2 vaccinations were given with a median of 21 days between immunizations, per label. In our study, however, this interval was longer (median, 42 days) secondary to limitations in vaccine supplies as well as regional allocation plans in the early phases of COVID-19 vaccine rollout. As longer intervals between doses often tend to improve immune responses,22 this may, together with the fact that vaccinations were performed late (≥2 years) after allo-HSCT in most (44/50) patients, have contributed to the favorable antibody responses noted in the majority of participants in the present study.
Interestingly, vaccine-associated adverse events among allo-HSCT recipients in our study did not differ from those observed in healthy controls, and no severe exacerbations of chronic GVHD were observed. In the only prospective clinical trial reported in allo-transplanted patients,7 the same type and frequency of side effects were seen in patients as in healthy controls, although there was a risk for significant GVHD. However, it should be noted that patients in our study were at low risk for chronic GVHD declension, as most enrolled patients were long-time survivors after transplantation.
This study has strengths and limitations. The strengths include the parallel monitoring of aspects of virus-specific B- and T-cell–mediated immunity as well as the complete sampling performed in all enrolled patients. Although our major findings were based on observations within the group of transplanted patents, a limitation of the study is the suboptimal control group in which complete sampling was only available in 14 subjects. Therefore, we collected further control samples from 22 staff members and their relatives at the Hematology Department with samples available uniquely after 2 vaccinations. We thus cannot exclude that some control subjects were seropositive before the first vaccination. In the present study, the healthy controls were older than allo-HSCT recipients, and some were sampled later after immunization, which may have resulted in a greater decline of immune responses among controls, thus possibly overestimating the efficacy of vaccination in allo-HSCT recipients. Also, importantly, only 2 patients in the present study were vaccinated within 1 year of allo-transplantation, and the responses in these 2 patients were poor, consistent with the findings in Bergman et al,7 where patients transplanted within 12 months showed a lower proportion of seroconversion. Thus, our findings might be most applicable to long-term allo-HSCT survivors. In addition, to define an insufficient antibody response, we used an arbitrarily chosen discriminatory anti–RBD-S1 IgG level of 100 BAU/mL. This cutoff value was previously used as a prespecified marker of poor response in a human therapeutic SARS-CoV-2 vaccine trial,23 where antibody levels were reported in U/mL, which are equivalent to BAU/mL with no conversion of units required.24 However, this threshold remains to be prospectively validated as a correlate of protection against mild or severe COVID-19 and against variants of SARS-CoV-2.
In conclusion, COVID-19 mRNA vaccines are safe in long-time allo-HSCT survivors and resulted in robust immune responses among the majority of patients, especially in those having undergone transplantation >2 years previously. However, a subset of transplanted patients showed deficient T-cell responses to SARS-CoV-2 peptides that heralded nonprotective humoral immunity, highlighting the need for further preventive and preemptive measures along with continued vigilance in these patients.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by the Swedish Medical Research Council Vetenskapsrådet, Diarienr 2021-04779 and 2020-01437 and ALF Funds at Sahlgrenska University Hospital Diarienr ALFGBG-438371.
Authorship
Contribution: S.E. and M. Lagging were responsible for designing and writing the protocol, conducting the study, extracting and analyzing data, interpreting results, writing the manuscript, updating reference lists, and creating tables and figures; A.M., K.H., and M. Lagging were responsible for designing and writing the protocol, extracting and analyzing data, interpreting results, writing the manuscript, updating reference lists, and creating tables and figures; J.W. and M. Nicklasson participated in interpreting results, writing the manuscript, and creating the figures; J.R., M.A., A.T., H.G.W., and R.B. were responsible for T-cell analyses and participated in extracting and analyzing data and interpreting results; J.W. and S.N. performed statistical analyses; M. Nilsson collected data for healthy controls and participated in interpretation of results; M. Lisak, M.V., V.F., and S.A.-D. participated in extracting and analyzing data and interpreting results; and T.B., P.L., and M.B. participated in designing and writing the protocol, interpreting results, writing the manuscript, and updating reference lists. All authors approved the final version of the manuscript.
Conflict-of-interest disclosure: P.L. has given lectures for Pfizer and will participate in a satellite symposium hosted by Moderna. The remaining authors have nothing to disclose.
Correspondence: Martin Lagging, Department of Infectious Diseases/Virology, Guldhedsgatan 10B, SE-413 46 Gothenburg, Sweden; e-mail: martin.lagging@medfak.gu.se; and Anna Martner, TIMM Laboratory, Sahlgrenska Center for Cancer Research, Medicinaregatan 1F, SE- 413 90 Gothenburg, Sweden; e-mail: anna.martner@microbio.gu.se.
References
- 1.Smith LA, Wright-Kanuth MS. Complications and risks in hematopoietic stem cell transplant patients. Clin Lab Sci. 2001;14(2):118-124. [PubMed] [Google Scholar]
- 2.Ljungman P, de la Camara R, Mikulska M, et al. COVID-19 and stem cell transplantation; results from an EBMT and GETH multicenter prospective survey. Leukemia. 2021;35(10):2885-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma A, Bhatt NS, St Martin A, et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol. 2021;8(3):e185-e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kemp SA, Collier DA, Datir RP, et al. ; COVID-19 Genomics UK (COG-UK) Consortium . SARS-CoV-2 evolution during treatment of chronic infection. Nature. 2021;592(7853):277-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergman P, Blennow O, Hansson L, et al. ; COVAXID-collaborator group (shown separately) . Safety and efficacy of the mRNA BNT162b2 vaccine against SARS-CoV-2 in five groups of immunocompromised patients and healthy controls in a prospective open-label clinical trial. EBioMedicine. 2021;74:103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhakal B, Abedin S, Fenske T, et al. Response to SARS-CoV-2 vaccination in patients after hematopoietic cell transplantation and CAR T-cell therapy. Blood. 2021;138(14):1278-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redjoul R, Le Bouter A, Beckerich F, Fourati S, Maury S. Antibody response after second BNT162b2 dose in allogeneic HSCT recipients. Lancet. 2021;398(10297):298-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ram R, Hagin D, Kikozashvilli N, et al. Safety and immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in patients after allogeneic HCT or CD19-based CART therapy—a single-center prospective cohort study. Transplant Cell Ther. 2021;27(9):788-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sahin U, Toprak SK, Atilla PA, Atilla E, Demirer T. An overview of infectious complications after allogeneic hematopoietic stem cell transplantation. J Infect Chemother. 2016;22(8):505-514. [DOI] [PubMed] [Google Scholar]
- 12.Törnell A, Wiktorin HG, Ringlander J, et al. Rapid cytokine release assays for analysis of severe acute respiratory syndrome coronavirus 2-specific T cells in whole blood [published online ahead of print 12 January 2022]. J Infectious Dis.. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan AT, Lim JM, Le Bert N, et al. Rapid measurement of SARS-CoV-2 spike T cells in whole blood from vaccinated and naturally infected individuals. J Clin Invest. 2021;131(17):e152379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heide J, Schulte S, Kohsar M, et al. Broadly directed SARS-CoV-2-specific CD4+ T cell response includes frequently detected peptide specificities within the membrane and nucleoprotein in patients with acute and resolved COVID-19 [published correction appears in PLoS Pathog. 2022;18(1):e1010220]. PLoS Pathog. 2021;17(9):e1009842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kristiansen PA, Page M, Bernasconi V, et al. WHO international standard for anti-SARS-CoV-2 immunoglobulin. Lancet. 2021;397(10282): 1347-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMahan K, Yu J, Mercado NB, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590(7847):630-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali H, Ngo D, Aribi A, et al. Safety and tolerability of SARS-CoV2 emergency-use authorized vaccines for allogeneic hematopoietic stem cell transplant recipients. Transplant Cell Ther. 2021;27(11):938.e931-938.e936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maneikis K, Šablauskas K, Ringelevičiūtė U, et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study. Lancet Haematol. 2021;8(8):e583-e592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Science Brief. SARS-CoV-2 infection-induced and vaccine-induced immunity. CDC COVID-19 Science Briefs. Atlanta (GA); 2020.
- 20.Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021; 385(24):e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments [published correction appears in Nat Rev Immunol. 2021;21(2):129]. Nat Rev Immunol. 2021;21(2):83-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385(13):1244-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jochum S, Kirste I, Hortsch S, et al. Clinical utility of Elecsys anti-SARS-CoV-2 S assay in COVID-19 vaccination: an exploratory analysis of the mRNA-1273 phase 1 trial. medRxiv. 2021;12:798117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.