Summary
Background
Before widespread coronavirus disease (COVID-19) vaccinations, Japan experienced three COVID-19 epidemic waves. This study aimed to evaluate the characteristics of hospitalised COVID-19 patients and reveal temporal changes.
Methods
This study included 33,554 hospitalised patients with COVID-19 from 553 healthcare facilities. Data were analysed by age group and epidemic wave (first wave, 01/01/2020–05/31/2020; second wave, 06/01/2020–10/31/2020; and third wave, 11/01/2020–03/31/2021).
Findings
By age group, 3% (under 18), 22% (young), 34% (middle-aged), and 41% (older patients) were aged 0-17, 18-39, 40-64, and >65 years; while 16%, 35%, and 49% were in the first, second, and third wave, respectively. The patients’ overall median age (58 years; interquartile range, 39–74) was lowest and highest during the second and third waves, respectively. The frequency of any comorbidity was lowest and highest during the second (44·5%) and third (63·6%) waves, respectively. The symptoms at admission and exposure history differed considerably with age. The overall case fatality rate (5%) was highest among older patients (11·4%). Case fatality rate was highest and lowest during the first (7·3%) and second (2·8%) waves, respectively. Medication use changed over time.
Interpretation
Although the overall case fatality rate remained relatively low, it was more than twice as high among older patients. After adjusting for age and comorbidities, the risk of death was highest in the first wave.
Funding
This work was supported by the Ministry of Health, Labour and Welfare “Research on Emerging and Re-emerging Infectious Diseases and Immunization” 19HA1003].
Keywords: SARS-CoV-2, Inpatients, Wave, Case fatality rate
Research in context.
Evidence before this study
The novel coronavirus infection (COVID-19) has become a public health threat worldwide; however, the epidemiological characteristics of patients differ significantly between Western countries and Asia. Although multiple epidemic waves of COVID-19 have occurred in Japan, there has been no study using nationwide registry that have clarified changes in the epidemiological characteristics of patients hospitalised for COVID-19 during the epidemic waves.
We conducted a PubMed search between 1 December 2019, and 30 November 2021, for articles published in English using the keywords: (“COVID-19” OR “SARS-CoV-2” OR “novel coronavirus” OR “2019-nCoV”) AND (“Japan”) AND (“registry”) AND (“wave”). Our search identified eight publications, of which five were related to occupational health (two were on healthcare workers), two were on response and preparedness for COVID-19, and one was on the epidemiology of subarachnoid haemorrhage during the COVID-19 pandemic. We found no report that used registry data to determine the epidemiological status of hospitalised patients during the epidemic waves in Japan. We also conducted a PubMed search using the keywords: ("COVID-19" OR "SARS-CoV-2" OR "novel coronavirus" OR "2019-nCoV") AND ("Japan") AND ("wave") AND (" comparison"). Our search identified 11 papers. Of these, four reported on prediction using mathematical models in Japan; the number of infected people and deaths using artificial intelligence in multiple countries; mortality statistics; and influence of population density, temperature, and absolute humidity on the spread and decay durations of COVID-19. Three studies focused on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) genome sequences, cross-neutralizing activity against SARS-CoV-2 variants, and SARS-CoV-2 PCR positivity. Two studies examined the effect of COVID-19 on swimming and the step counts. One was a single-centre comparison of the first-third waves with the fourth wave. Another was an international comparison of perception of and anxiety about COVID-19 infection and risk behaviours.
Added value of this study
To the best of our knowledge, this is the largest study to date describing the changes of clinical epidemiological characteristics of inpatients with COVID-19 observed during the three epidemic waves in Japan that occurred prior to mass vaccination. Although the overall case fatality rate remained low, the case fatality rate was approximately twice as high among older patients and varied with epidemic wave. Notably, case fatality rates reduced in the third wave compared to the first wave despite an increase in the median age and comorbidity. The medications and supportive care for COVID-19 changed over time in Japan. The epidemiological and clinical characteristics differed greatly among each age group.
Implications of all available evidence
We present important epidemiological information in an Asian population with lower comorbidities than other populations. As vaccination rates and virus variants increase, changes in the epidemiological characteristics of COVID-19 are inevitable. Our findings could provide baseline data to track these changes. After adjusting for the age and comorbidities, the risk of death was highest in the first wave. The case fatality rate among older adults remained high, suggesting the need to target this age group for prevention and treatment.
Alt-text: Unlabelled box
Introduction
Coronavirus disease (COVID-19) has been prevalent in Japan since January 2020 with substantial variation in the number of cases. As of August 2021, the fifth wave of the epidemic is underway.1 During this period, there have been various changes in approaches to COVID-19 prevention, diagnosis, and treatment strategies, including changes in the criteria for hospitalisation, and the start of COVID-19 vaccination.
Various drugs, including those with uncertain efficacy at the time, were initially used in Japan;2 however, emerging evidence continues to influence domestic guidelines and clinical practice.3 The criteria for hospitalisation have also changed. In the beginning of the pandemic, almost all patients with COVID-19 were hospitalised; however, as the spread of the infection progressed, the target population for hospitalisation was changed to patients with or at risk of severe disease.4
In Japan, COVID-19 vaccination began in February 2021, and healthcare workers and older adults were prioritised. As of 31 March 2021, the proportion of people fully vaccinated against COVID-19 was 0·1% (n=125,580) (partly vaccinated, 0·7% [n=877,157]) of the total population of Japan.5
The characteristics of hospitalised patients with COVID-19 in Japan during the early phase of the epidemic were previously reported using a nationwide registry. The epidemiological characteristics were different from those in Western countries, with lower case fatality rate and comorbidity frequency in our cohort.2 To the best of our knowledge, no comprehensive analysis examining whether case fatality rate and clinical epidemiological characteristics had changed during subsequent epidemic waves was conducted. A previous study compared patients by severity of illness,2 but not by age group. Little is known regarding the effect of repeated epidemic waves on the clinical epidemiology of COVID-19 inpatients in Asian countries, where individuals have fewer comorbidities and lower body mass index (BMI) measures than individuals in Western countries.6
This study aimed to determine the characteristics of hospitalised COVID-19 patients in each epidemic wave and different age groups prior to widespread vaccination.
Methods
Study design and patients
This was an observational study. Healthcare facilities that voluntarily participated in the COVID-19 Registry Japan (COVIREGI-JP) enrolled the patients. Research collaborators in each facility manually input the data into the registry. The inclusion criteria for enrolment were (i) a positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) test result7 and (ii) inpatient treatment at a healthcare facility. COVIREGI-JP details are described elsewhere.2,8 In this analysis, we also included the hospitalisation episodes of patients who were transferred from other facilities and who met the aforementioned inclusion criteria.
Data collection and case report form
We modified the case report form of the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) to enable the collection of clinical epidemiological information and treatment data in Japan.9 The study data were collected and managed using Research Electronic Data Capture (REDCap), a secure web-based data capture application hosted at the Japan Clinical Research Assist Center (JCRAC) of the National Center for Global Health and Medicine (NCGM).10
Data set
The COVIREGI-JP was started on 2 March 2020; however, data could be entered retrospectively. We used data for patients who were admitted on or before 31 March 2021, and whose data, including all of the following major items, had been entered and fixed (i.e., the data of these major items were fixed and the facility was unable to make any more modification) as of 30 April 2021: demographics and epidemiological characteristics, comorbidities, signs and symptoms at admission, outcome at discharge, supportive care, history of drug administration, and complications during hospitalisation. Available number of cases for each parameter differed due to missing data. If an “Unknown” option was selected instead of none of the options selected, “Unknown” was not treated as missing data; it was counted separately. Only the following parameters included “Unknown” as an option: exposure within 14 days, smoking/drinking history, symptoms, and complications during admission. The number of patients for parameters with “Unknown” information are listed in Table 1 or in Supplementary Table 1.
Table 1.
Patient demographics and clinical characteristics on admission
| Age category |
Wave category |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of cases | Subcategories | Total | Under 18 (0–17 years) | Young (18–39 years) | Middle age (40–64 years) | Older (≥65 years) | Wave 1 | Wave 2 | Wave 3 | |
| Demographics | ||||||||||
| Male sex | 33,551 | 18,985 (56·6) | 608 (55·1) | 4030 (55·2) | 7424 (64·9) | 6917 (50·5) | 3139 (58·5) | 6784 (58) | 9062 (55) | |
| Female sex | 14,566 (43.4) | 496 (44.9) | 3277 (44.8) | 4020 (35.1) | 6770 (49.5) | 2233 (41.5) | 4908 (42) | 7428 (45) | ||
| Age (median) [IQR], years | 33,542 | 58 [39, 74] | 9 [3, 14] | 28 [23, 34] | 53 [47, 58] | 77 [71, 85] | 56 [40, 71] | 50 [30, 69] | 64 [47, 78] | |
| Wave | 33,554 | First | 5372 (16) | 109 (9·9) | 1175 (16·1) | 2138 (18·7) | 1949 (14·2) | |||
| Second | 11,692 (34·8) | 459 (41·6) | 3772 (51·6) | 3924 (34·3) | 3532 (25·8) | |||||
| Third | 16,490 (49·1) | 536 (48·6) | 2360 (32·3) | 5382 (47) | 8206 (60) | |||||
| Japanese ethnicity | 33,190 | 31,680 (95·5) | 955 (87·5) | 6561 (90·7) | 10,747 (95) | 13,407 (99) | 5125 (95·7) | 10,950 (94·1) | 15,605 (96·3) | |
| Former or current smoker | 33,460 | 11,721 (35) | 12 (1.1) | 2399 (32.9) | 4977 (43·6) | 4332 (31·7) | 1882 (35·2) | 4420 (38) | 5419 (32·9) | |
| Alcohol consumption a | 32,866 | Daily | 2297 (7) | 2 (0·2) | 589 (8·2) | 1130 (10·1) | 576 (4·3) | 347 (7·3) | 1049 (9) | 901 (5·5) |
| Occasional | 10,525 (32) | 7 (0·6) | 2941 (41·2) | 4553 (40·9) | 3021 (22·4) | 1436 (30·1) | 4157 (35·7) | 4932 (30) | ||
| BMI (median) [IQR], kg/m2 | 27,180 | 23·3 [20·7, 26·3] | 18·2 [16, 20·7] | 22·4 [20·1, 25·7] | 24·7 [22·1, 27·8] | 22·8 [20·3, 25·3] | 23·1 [20·5, 26] | 23·1 [20·5, 26·3] | 23·4 [20·8, 26·4] | |
| Immunosuppressionb | 32,840 | 708 (2·2) | 8 (0·7) | 57 (0·8) | 212 (1·9) | 431 (3·2) | 145 (2·8) | 199 (1·7) | 364 (2·3) | |
| Exposure within 14 days | ||||||||||
| Travel to COVID-19 epidemic countries | 33,329 | 564 (1·7) | 36 (3·3) | 189 (2·6) | 196 (1·7) | 143 (1·1) | 345 (6·4) | 132 (1·1) | 87 (0·5) | |
| Close contact with COVID-19 casesc | 33,358 | 18,269 (54·8) | 914 (83) | 3671 (50·5) | 5475 (48·2) | 8203 (60·3) | 3035 (56·5) | 6039 (51·8) | 9195 (56·3) | |
| Unknown | 3915 (11·7) | 40 (3·6) | 793 (10·9) | 1468 (12·9) | 1614 (11·9) | 654 (12·2) | 1212 (10·4) | 2049 (12·6) | ||
| Contact details | 33,554 | Family | 6968 (20·8) | 713 (64·6) | 948 (13) | 2178 (19) | 3128 (22·9) | 912 (17) | 2273 (19·4) | 3783 (22·9) |
| Workplace | 3354 (10) | 2 (0·2) | 1288 (17·6) | 1656 (14·5) | 407 (3) | 601 (11·2) | 1265 (10·8) | 1488 (9) | ||
| Healthcare facility | 4785 (14·3) | 2 (0·2) | 346 (4·7) | 675 (5·9) | 3760 (27·5) | 913 (17) | 1049 (9) | 2823 (17·1) | ||
| Educational facility | 253 (0·8) | 143 (13) | 75 (1) | 24 (0·2) | 10 (0·1) | 15 (0·3) | 108 (0·9) | 130 (0·8) | ||
| Meals with more than 3 persons (excl. family members) | 32,783 | 4643 (14·2) | 95 (8·7) | 1605 (22·5) | 1595 (14·4) | 1346 (10) | 539 (11·2) | 2030 (17·4) | 2074 (12·7) | |
| Unknown | 9554 (29·1) | 204 (18·7) | 2050 (28·7) | 3537 (31·9) | 3763 (28) | 1951 (40·4) | 3218 (27·6) | 4385 (26·9) | ||
| Stay in a closed and crowded spaced | 32,761 | 4533 (13·8) | 74 (6·8) | 1645 (23·1) | 1561 (14·1) | 1250 (9·3) | 658 (13·6) | 2177 (18·7) | 1698 (10·4) | |
| Unknown | 8745 (26·7) | 181 (16·6) | 1960 (27·5) | 3389 (30·6) | 3215 (23·9) | 1683 (34·9) | 2875 (24·7) | 4187 (25·7) | ||
| Occupation | ||||||||||
| Healthcare worker | 32,811 | 1753 (5·3) | 3 (0·3) | 667 (9·3) | 894 (8) | 188 (1·4) | 455 (9·4) | 507 (4·3) | 791 (4·9) | |
| Restaurant worker | 32,753 | 1128 (3·4) | 1 (0·1) | 423 (5·9) | 427 (3·8) | 277 (2·1) | 164 (3·4) | 555 (4·8) | 409 (2·5) | |
| Specific service workere | 32,744 | 775 (2·4) | 2 (0·2) | 503 (7·1) | 200 (1·8) | 70 (0·5) | 126 (2·6) | 446 (3·8) | 203 (1·2) | |
| Conditions at admissionf | ||||||||||
| Days from symptom onset to hospitalisation (Median) [IQR] | 30,244 | 5 [2, 7] | 3 [1, 5] | 4 [2, 7] | 5 [3, 8] | 4 [2, 7] | 7 [4, 10] | 4 [2, 7] | 4 [2, 7] | |
| Severe condition at admission | 33,554 | 9587 (28·6) | 214 (19·4) | 567 (7·8) | 2906 (25·4) | 5898 (43·1) | 1767 (32·9) | 2544 (21·8) | 5276 (32) | |
| O2 administration method | 33,287 | Room air | 28,537 (85·7) | 1045 (99·4) | 7115 (98·1) | 10,031 (88·2) | 10,335 (76) | 4417 (82·7) | 10,451 (90·1) | 13,669 (83·6) |
| O2 administration | 4312 (13) | 6 (0·6) | 129 (1·8) | 1174 (10·3) | 3002 (22·1) | 779 (14·6) | 1025 (8·8) | 2508 (15·3) | ||
| BiPAP/CPAP, IMV, ECMO | 438 (1·3) | 0 (0) | 7 (0·1) | 173 (1·5) | 258 (1·9) | 143 (2·7) | 121 (1) | 174 (1·1) | ||
| O2 administered through | 4306 | Cannula | 2941 (68·3) | 4 (66·7) | 102 (79·1) | 836 (71·4) | 1998 (66·6) | 490 (63) | 734 (71·8) | 1717 (68·5) |
| Mask | 812 (18·9) | 2 (33·3) | 22 (17·1) | 201 (17·2) | 587 (19·6) | 152 (19·5) | 167 (16·3) | 493 (19·7) | ||
| Reservoir mask | 484 (11·2) | 0 (0) | 5 (3·9) | 117 (10) | 362 (12·1) | 126 (16·2) | 106 (10·4) | 252 (10·1) | ||
| High-flow therapy | 69 (1·6) | 0 (0) | 0 (0) | 17 (1·5) | 52 (1·7) | 10 (1·3) | 15 (1·5) | 44 (1·8) | ||
| Signs and symptoms on admissiong | ||||||||||
| Asymptomatic on admission | 33,084 | 3029 (9·2) | 320 (29·2) | 582 (8·1) | 621 (5·5) | 1505 (11·1) | 321 (6·5) | 1183 (10·1) | 1525 (9·3) | |
| Fever (≥37·5°C) | 33,536 | 16,208 (48·3) | 319 (28·9) | 2911 (39·9) | 6222 (54·4) | 6751 (49·4) | 2807 (52·3) | 5198 (44·5) | 8203 (49·8) | |
| Cough | 33,536 | 16,295 (48·6) | 306 (27·7) | 3464 (47·4) | 6493 (56·8) | 6025 (44) | 2816 (52·5) | 5228 (44·7) | 8251 (50·1) | |
| Bloody sputum | 16,252 | 242 (1·5) | 2 (0·7) | 41 (1·2) | 95 (1·5) | 103 (1·7) | 43 (1·5) | 69 (1·3) | 130 (1·6) | |
| Sore throat | 33,539 | 5415 (16·1) | 133 (12·1) | 1763 (24·1) | 2247 (19·6) | 1270 (9·3) | 722 (13·4) | 2050 (17·5) | 2643 (16) | |
| Rhinorrhoea | 33,539 | 3084 (9·2) | 246 (22·3) | 1060 (14·5) | 1090 (9·5) | 687 (5) | 441 (8·2) | 898 (7·7) | 1745 (10·6) | |
| Dyspnoea | 33,542 | 6941 (20·7) | 18 (1·6) | 988 (13·5) | 2857 (25) | 3078 (22·5) | 1350 (25·1) | 2005 (17·2) | 3586 (21·8) | |
| Chest pain | 33,541 | 932 (2·8) | 4 (0·4) | 240 (3·3) | 448 (3·9) | 240 (1·8) | 182 (3·4) | 332 (2·8) | 418 (2·5) | |
| Myalgia | 33,542 | 2876 (8·6) | 21 (1·9) | 777 (10·6) | 1426 (12·5) | 651 (4·8) | 372 (6·9) | 1005 (8·6) | 1499 (9·1) | |
| Headache | 33,535 | 5119 (15·3) | 115 (10·4) | 1721 (23·6) | 2429 (21·2) | 852 (6·2) | 717 (13·4) | 1994 (17·1) | 2408 (14·6) | |
| Fatigue | 33,535 | 11,700 (34·9) | 101 (9·1) | 2472 (33·8) | 4795 (41·9) | 4328 (31·6) | 2076 (38·7) | 3948 (33·8) | 5676 (34·4) | |
| Abdominal pain | 33,528 | 608 (1·8) | 21 (1·9) | 176 (2·4) | 227 (2) | 184 (1·3) | 124 (2·3) | 216 (1·8) | 268 (1·6) | |
| Vomit | 33,528 | 1230 (3·7) | 25 (2·3) | 302 (4·1) | 452 (4) | 451 (3·3) | 207 (3·9) | 444 (3·8) | 579 (3·5) | |
| Diarrhoea | 33,530 | 3156 (9·4) | 69 (6·3) | 818 (11·2) | 1393 (12·2) | 875 (6·4) | 604 (11·3) | 1232 (10·5) | 1320 (8) | |
| Dysgeusia | 33,535 | 5415 (16·1) | 101 (9·1) | 2238 (30·6) | 2145 (18·8) | 929 (6·8) | 894 (16·6) | 2397 (20·5) | 2124 (12·9) | |
| Olfactory abnormality | 33,532 | 4878 (14·5) | 87 (7·9) | 2219 (30·4) | 1881 (16·4) | 689 (5) | 734 (13·7) | 2239 (19·2) | 1905 (11·6) | |
| Rash | 32,897 | 224 (0·7) | 6 (0·5) | 58 (0·8) | 101 (0·9) | 59 (0·4) | 38 (0·8) | 93 (0·8) | 93 (0·6) | |
Data are presented as n (%) unless otherwise indicated.
As the number of missing values differed for each parameter, the number of cases in each parameter's age/wave category was used as the denominator for calculating the percentage.
Abbreviations: BiPAP/CPAP, bilevel positive airway pressure/continuous positive airway pressure; BMI, body mass index; IMV, invasive mechanical ventilation; ECMO, extracorporeal membrane oxygenation; IQR, interquartile range.
Daily, ≥3 cans of beer per day; occasional includes daily alcohol intake of <3 cans beer per day.
Immunosuppression includes neutropenia (<500 neutrophils/μL); glucocorticoid/steroid use within 1 month (doses greater or equal to an equivalent of 20 mg of prednisone per day for at least 1 month); chemotherapy, radiation therapy, or immunosuppressant use (such as antitumor necrosis factor α therapy, anti-IL-6 receptor/anti-CD20 monoclonal antibodies, selective T-cell co-stimulation blocker, methotrexate, tacrolimus) in the past 3 months; and/or post-transplantation, asplenia, or primary immunodeficiency syndrome. Multiple immunosuppressants were used in some cases.
“Close contact” was defined as in the International Severe Acute Respiratory and Emerging Infection Consortium case record form.9
Close contact in closed and crowded spaces, include sports gyms, live music clubs, karaokes, game centers, buffets, indoor parties, conferences, or nightclubs/bars.
Included nightlife business workers at bars and pubs, host and hostess clubs, nightclubs, and other similar businesses.28
First available data at presentation/admission within 24 hours.
Signs and symptoms observed/reported at admission and associated with the presented episode of acute illness.
Age groups
Age was categorized and analysed in four groups as follows: (i) under 18 (0–17 years), (ii) young (18–39 years), (iii) middle age (40–64 years), and (iv) older patients (≥65 years).
COVID-19 epidemic waves
COVID-19 epidemic waves were defined as follows using the admission date: (i) first wave (Wave 1), 01/01/2020–05/31/2020; (ii) second wave (Wave 2), 06/01/2020–10/31/2020; and (iii) third wave (Wave 3), 11/01/2020–03/31/2021.1,11 For cases where the SARS-CoV-2 test date was after the admission date (e.g., hospital-acquired COVID-19 cases), the test date was used instead of the admission date.
Definitions
“Severe disease at admission” was defined as requiring invasive or non-invasive mechanical ventilation, supplemental oxygen, SpO2 ≤ 94% on room air, and/or tachypnoea (respiratory rate ≥24 breaths per minute).12
“Obesity” was defined as a body mass index (BMI) ≥ 25 due to the small number of patients with BMIs ≥ 30.
Oxygen support
If the patient received more than one type of oxygen support during the hospitalisation, only one higher level of oxygen support was counted (i.e. extracorporeal membrane oxygenation (ECMO)> invasive mechanical ventilation (IMV)> bilevel positive airway pressure/continuous positive airway pressure (BIPAP/CPAP)>high-flow nasal cannula>other oxygen support).
History of drug administration during hospitalisation
Information on SARS-CoV-2 antiviral drugs, medications with immunomodulatory and/or immunosuppressive effects against COVID-19, antibiotics, antifungal drugs, and anticoagulants was collected. These medications were included in the analysis as described in the statistical analysis section, if they were administered at least once during the hospitalisation period. If more than one drug was used in one patient, each drug was counted separately, regardless of whether they were administered concurrently. If one drug was administered during the different periods of hospitalisation, it was counted as one.
Statistical analysis
Continuous variables are described as medians and interquartile ranges (IQRs). As the number of missing values differed for each parameter, the number of cases in each parameter's age/wavecategory was used as the denominator for calculating the percentage.
To compare the major characteristics and drug administration during hospitalisation among the waves, we conducted Kruskal-Wallis Rank Sum test for age, and chi-square test for other variables. Bonferroni correction was used for pairwise comparisons among waves (threshold of significant difference was 0.0167), and corresponding 98.33% confidence intervals were provided. To evaluate the effect of each wave on death, we conducted a multivariable analysis using a relative risk regression model, with death during hospitalisation as the outcome. The parameters in the model were selected by referring to previous evaluations.3,13 All statistical analyses were conducted using R, version 3.5.1 (R Core Team, Vienna, Austria).
Ethics
This study was approved by the NCGM ethics review board (NCGM-G-004232-00). Information regarding opting out of our study is available on the registry website.
Role of the funding source
The funding source was not involved in the study.
Results
Data of 33,554 patients from 553 healthcare facilities were included in the analysis (Figure 1). The available number of cases differed depending on each parameter due to missing data. The numbers of patients in each age category were as follows: under 18, 1104 (3%); young, 7307 (22%); middle age, 11,444 (34%); and older, 13,687 (41%). The numbers of patients in each COVID-19 wave category were as follows: Wave 1, 5372 (16%); Wave 2, 11,692 (35%); and Wave 3, 16,490 (49%).
Figure 1.
The flowchart of the number of cases included in the study.
Twelve patients whose age was unknown were excluded in this analysis.
Details of “Non-fixed” cases (n=1,943: 5.5%) were as follows:
Inclusion criteria, 446 (1.3%); demographics and epidemiological characteristics,740 (2.1%); comorbidities, 710 (2.0%); signs and symptoms, 1,049 (3.0%); outcome at discharge,1,416 (4.0%); supportive care, 1,368 (3.9%); history of drug administration, 1,315 (3.7%); complication during hospitalisation, 1,390 (3.9%)
Patient demographics and characteristics on admission
More than half of the patients were male (18,985 [56·6%]), and 65% of middle-aged patients were males (Table 1). There were fewer male patients in Wave 3 than in Wave 1 or 2 (estimated difference [ED]of Wave 1 vs Wave 3: 3.5% [1.6, 5.4], Wave 2 vs Wave 3: 3.1% [1.6, 4.5]) (Table 3). Most patients (31,680 [95·5%]) were Japanese. The median age of the entire cohort was 58 (IQR, 39–74) years. The median age was highest in Wave 3 (64 [47–78]), and lowest in Wave 2 (50 [30-69]); ED of Wave 1 vs Wave 3: -6.0 [-7.0, 5.0], Wave 2 vs Wave 3: -12.0 [-12.0, -11.0]) (Table 3). Waves 1, 2, and 3 comprised 36·3% (1949/5372), 30·2% (3532/11692), and 49·8% (8206/16490) of older patients, respectively.
Table 3.
Comparison of the major characteristics, drug administration, and oxygen support during hospitalisation among each wave.
| Variable | Wave 1 | Wave 2 | Wave 3 | Wave 1 vs Wave 2 | Wave 1 vs Wave 3 | Wave 2 vs Wave 3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number (%) | Estimated difference* [confidence interval†] | P value‡ | Estimated difference* [confidence interval†] | P value‡ | Estimated difference* [confidence interval†] | P value‡ | |||||
| Patient demographics and characteristics on admission | |||||||||||
| Age, median [IQR], years | 56 [40, 71] |
50 [30, 69] |
64 [47, 78] |
6.0 [5.0, 7.0] |
<0.001 | -6.0 [-7.0, -5.0] |
<0.001 | -12.0 [-12.0, -11.0] |
<0.001 | ||
| Male sex | 3139 (58.5) | 6784 (58) |
9062 (55) | 0.004 [-0.015, 0.024] |
0.598 | 0.035 [0.016, 0.054] |
<0.001 | 0.031 [0.016, 0.045] |
<0.001 | ||
| Severe disease at admission | 1767 (32.9) | 2544 (21.8) | 5276 (32) | 0.111 [0.093, 0.129] |
<0.001 | 0.009 [-0.009, 0.027] |
0.228 | -0.102 [-0.115, -0.090] |
<0.001 | ||
| ComorbiditiesA | |||||||||||
| Cardiovascular disease | 268 (5) |
408 (3.5) |
973 (5.9) | 0.015 [0.007, 0.023] |
<0.001 | -0.009 [-0.018, -0.001] |
0.001 | -0.024 [-0.030, -0.018] |
<0.001 | ||
| Chronic respiratory disease | 109 (2) |
133 (1.1) |
263 (1.6) | 0.010 [0.002, 0.018] |
0.001 | -0.003 [-0.010, 0.005] |
0.381 | -0.013 [-0.018, -0.007] |
<0.001 | ||
| Liver disease | 130 (2.4) | 235 (2) |
422 (2.6) | 0.004 [-0.002, 0.010] |
0.096 | -0.001 [-0.007, 0.005] |
0.607 | -0.005 [-0.010, -0.001] |
0.003 | ||
| Diabetes Mellitus | 856 (15.9) | 1509 (12.9) | 3191 (19.4) | 0.030 [0.016, 0.044] |
<0.001 | -0.034 [-0.048, -0.020] |
<0.001 | -0.064 [-0.075, -0.054] |
<0.001 | ||
| Cerebrovascular disease | 337 (6.3) | 552 (4.7) |
1342 (8.1) | 0.016 [0.006, 0.025] |
<0.001 | -0.019 [-0.028, -0.009] |
<0.001 | -0.034 [-0.041, -0.027] |
<0.001 | ||
| Hypertension | 1050 (19.5) | 2831 (24.2) | 6068 (36.8) | -0.047 [-0.063, -0.030] |
<0.001 | -0.173 [-0.188, -0.157] |
<0.001 | -0.126 [-0.139, -0.113] |
<0.001 | ||
| BMI>=25 | 1366 (32.8) | 3171 (33.2) | 4762 (35.4) | -0.004 [-0.025, 0.017] |
0.688 | -0.025 [-0.045, -0.005] |
<0.003 | -0.021 [-0.037, -0.006] |
0.001 | ||
| Drug administration | |||||||||||
| Steroid (excl. ciclesonide) | 497 (9.4) | 2384 (20.4) | 6569 (39.9) | -0.110 [-0.124, -0.097] |
<0.001 | -0.305 [-0.318, -0.292] |
<0.001 | -0.195 [-0.207, -0.182] |
<0.001 | ||
| Tocilizumab | 66 (2.6) |
259 (5.6) |
393 (4.7) | -0.030 [-0.041, -0.019] |
<0.001 | -0.022 [-0.032, -0.012] |
<0.001 | 0.008 [-0.002, 0.018] |
0.051 | ||
| Remdesivir | 2 (0.9) |
1074 (23.2) | 2682 (32.4) | -0.223 [-0.239, -0.208] |
<0.001 | -0.315 [-0.329, -0.302] |
<0.001 | -0.092 [-0.112, -0.073] |
<0.001 | ||
| Favipiravir | 2037 (69.5) | 2664 (57.4) | 4415 (53.3) | 0.122 [0.095, 0.149] |
<0.001 | 0.164 [0.139, 0.188] |
<0.001 | 0.042 [0.020, 0.064 |
<0.001 | ||
| Anticoagulant | 538 (10.0) | 1416 (12.1) | 3674 (22.3) | -0.021 [-0.034, -0.009] |
<0.001 | -0.123 [-0.136, -0.111] |
<0.001 | -0.102 [-0.113, -0.091] |
<0.001 | ||
| Oxygen support | |||||||||||
| Any formB | 2063 (38.4) | 3069 (26.3) | 6554 (39.7) | 0.070 [0.055, 0.084] |
<0.001 | -0.007 [-0.021, 0.007] |
0.254 | -0.076 [-0.087, -0.066] |
<0.001 | ||
| High-flow nasal canula | 110 (2.0) |
181 (1.5) |
415 (2.5) | 0.005 [-0.001, 0.010] |
0.025 | -0.004 [-0.010, 0.001] |
0.064 | -0.009 [-0.013, -0.005] |
<0.001 | ||
| BiPAP/CPAP | 32 (0.6) |
103 (0.9) |
101 (0.6) | -0.003 [-0.006, 0.001] |
0.065 | -0.0002 [-0.003, 0.003] |
0.972 | 0.003 [-0.0001, 0.005] |
0.011 | ||
| IMV | 441 (8.2) | 371 (3.2) |
629 (3.8) | 0.045 [0.036, 0.054] |
<0.001 | 0.039 [0.030, 0.048] |
<0.001 | -0.006 [-0.011, -0.001] |
0.006 | ||
| ECMO | 83 (1.5) |
29 (0.2) |
35 (0.2) |
0.013 [0.008, 0.017] |
<0.001 | 0.013 [0.009, 0.017] |
<0.001 | 0.0004 [-0.001, 0.002] |
0.622 | ||
Earlier wave was used as reference.
†98.33% confidence intervals were calculated because significance threshold was corrected by Bonferroni method.
Bonferroni correction was used for pairwise comparisons among waves (threshold of significant difference was 0.0167). Statistically significant values are shown in bold.
Abbreviations. BiPAP/CPAP, bilevel positive airway pressure/continuous positive airway pressure; BMI, body mass index; ECMO, extracorporeal membrane oxygenation; IMV, invasive mechanical ventilation; IQR, interquartile range.
A Cardiovascular disease includes myocardial infarction/Congestive heart failure. Chronic respiratory disease was defined as pulmonary disease in patients who were dyspneic with slight activity and included chronic obstructive pulmonary disease.
B Any form of oxygen support includes high-flow nasal canula, non-IMV, IMV, ECMO, and other oxygen support (e.g. nasal cannula, mask).
Regarding exposure within 14 days of admission, contact with positive or probable COVID-19 patient accounted for 54·8% (n=18,269) of cases. This was most prevalent in the under 18 group (914 [83%]). The frequent categories of exposure were different between age groups and COVID-19 waves. Family, workplace, and healthcare facility contact were more common among patients aged <18 years, young and middle-aged patients, and older patients, respectively. The proportion of infections from family contact increased gradually in the later waves. Young patients had the highest frequency of having meals with more than three persons or staying in crowded places. Healthcare workers constituted 5·3% (n=1,753) of the cases. This proportion was highest during Wave 1 (455 [9·4%]). The proportion of restaurant and nightlife business workers was highest during Wave 2.
Regarding conditions at admission, the median duration from symptom onset to hospitalisation in days was longest in the middle-aged group (5 [3, 8]) and during Wave 1 (7 [4, 10]). Young patients had least severe disease at admission (567 [7·8%]). The incidence of severe disease at admission was lowest during Wave 2 (2,544 [21·8%]) (ED of Wave 1 vs Wave 2: 11.1 % [9.3, 12.9], Wave 2 vs Wave 3: -10.2% [-11.5, -9.0]) (Table 3). Oxygen was administered to 13% (n=4,312) of all patients on admission. Oxygen administration was highest among older patients (3,002 [22·1%]).
Regarding the signs and symptoms at hospital admission, overall, 9·2% (n=3,029) reported no symptoms. In the under 18 group, 29·2% (n=320) were asymptomatic at hospital admission. Approximately half of the patients had a fever and cough. The frequency of some symptoms varied considerably with age. Sore throat was more common among young (1,763 [24·1%]) and middle-aged (2,247 [19·6%]) patients, and rhinorrhoea was more common among under 18 (246 [22·3%]) and young (1,060 [14·5%]) patients. Dyspnoea was most common among middle-aged patients (2,857 [25%]). Muscle pain, headache, and diarrhoea were more common among young and middle-aged patients. Fever, cough, and fatigue were most frequent among middle-aged patients. Taste (2,238 [30·6%]) and smell disorders (2,219 [30·4%]) were most common among young patients.
Comorbidities
The complication rate of any comorbidity increased with age (Figure 2). Obesity was the most common comorbidity among young and middle-aged patients. Hypertension, obesity, and diabetes were the most common comorbidities among older patients. The frequency of any comorbidity was lowest and highest during Waves 2 and 3, respectively. In comparison of the major comorbidities between the waves, the frequency of cardiovascular disease and diabetes mellitus was highest in Wave 3, followed by Waves 1 and 2 (ED of Wave 1 vs Wave 3: -0.9% [-1.8, -0.1] for cardiovascular disease, -3.4% [-4.8, -2.0] for diabetes; Wave 2 vs Wave 3: -2.4% [-3.0, -1.8] for cardiovascular disease, -6.4% [-7.5, -5.4] for diabetes) (Table 3). Hypertension was most frequent in Wave 3, followed by Waves 2 and 1 (ED of Wave 2 vs Wave 3: -12.6% [-13.9, -11.3]; Wave 1 vs Wave 3: -17.3% [-18.8, -15.7]). Chronic respiratory disease was less frequent in Wave 2 than in the other waves (ED of Wave 1 vs Wave 2: 1.0% [0.2, 1.8]; Wave 2 vs Wave 3:-1.3% [-1.8, -0.7]). Liver disease was more frequent in Wave 3 than Wave 2 (ED of Wave 2 vs Wave 3: -1.3% [-1.8, -0.7]. For obesity, the frequency was highest in Wave 3 and lowest in Wave 1. (ED of Wave 1 vs Wave 3: -2.5% [-4.5, -0.5]; Wave 2 vs Wave 3: -2.1% [-3.7, -0.6])
Figure 2.
Comorbidities existing prior to COVID-19 hospitalisation.
Comorbidities that existed prior to the hospitalisation for coronavirus disease (COVID-19) were included. New-onset diseases associated with COVID-19 were excluded. The number of cases with comorbidities data were 33,554 (obesity n=27,180). Definitions were based on Charlson scores, unless otherwise specified 27. Cardiovascular disease includes myocardial infarction and congestive heart failure. Chronic respiratory disease was defined as pulmonary disease in patients who were dyspnoeic with slight activity and included chronic obstructive pulmonary disease. Bronchial asthma was diagnosed based on the physician's diagnosis. Obesity was defined as a body mass index ≥ 25.
Supportive care, outcomes, and complications during hospitalisation
In total, 7·2% (n=2,409) of all patients and 10·9% (n=1,484) of the older patients were admitted to the intensive care unit during their stays. This proportion was highest during Wave 1 (Table 2). Overall, oxygen was not administered in 65·2% (n=21,589) of cases; oxygen, such as nasal cannula, mask, or reservoir mask, was administered in 27·3% (n=9,156) of cases; and high-flow oxygen therapy was used in 2·1% (n=706) of cases. Furthermore, 4·3% (n=1,441) and 0·4% (n=147) of the patients were treated with IMV and ECMO, respectively. A high-flow oxygen therapy was most frequently used among older patients (3·7% [509]) and during Wave 3 (2·5% [415]). IMV and BIPAP/CPAP use were highest among older patients (IMV: 6·8% [926], BIPAP/CPAP: 1% [134]). IMV was most frequently used during Wave 1 (8.2% [441]), and BIPAP/CPAP was most frequently used during Wave 2 (0.9% [103]). ECMO was most frequently used among middle-aged patients (0·8% [86]) and during Wave 1 (1·5% [83]). Any form oxygen use was lower in the Wave 2 than in Waves 1 and 3 (Table 3). (ED of Wave 1 vs Wave 2: 7.0% [5.5, 8.4]; Wave 2 vs Wave 3: -7.6% [-8.7, -6.6]). The use of high-flow nasal cannula was most common in Wave 3 and least common in Wave 2 (ED of Wave 2 vs Wave 3: -0.9% [-1.3, -0.5]). BIPAP/CPAP use was more common in Wave 2 than in the other two waves (ED of Wave 1 vs Wave 2: -0.3% [-0.6, 0.1], Wave 2 vs Wave 3: 0.3% [-0.01, 0.5]). IMV use was more common in Wave 1, followed by Waves 3 and 2 (ED of Wave 1 vs Wave 3: 3.9% [3.0, 4.8], Wave 1 vs Wave 2: 4.5% [3.6, 5.4]). ECMO use was highest in Wave 1, with similar frequency in Waves 2 and 3 (ED of Wave 1 vs Wave 3: 1.3% [0.9, 1.7], Wave 1 vs Wave 2: 1.3% [0.8, 1.7].
Table 2.
Supportive care, outcomes, and complications during hospitalisation.
| Age category |
Wave category |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of cases | Subcategories | Total | Under 18 (0–17 years) | Young (18–39 years) | Middle age (40–64 years) | Older (≥65 years) | Wave 1 | Wave 2 | Wave 3 | |
| Supportive therapy during admissiona | ||||||||||
| Stay in ICU | 33,539 | 2409 (7·2) | 1 (0·1) | 97 (1·3) | 826 (7·2) | 1484 (10·9) | 633 (11·8) | 709 (6·1) | 1067 (6·5) | |
| Oxygen supportb | 33,545 | No oxygen | 21859 (65.2) | 1086 (98.4) | 6866 (94) | 7778 (68) | 6121 (44.7) | 3304 (61.6) | 8620 (73.7) | 9935 (60.3) |
| Other oxygen support (e.g., nasal cannula, mask) | 9156 (27.3) | 18 (1.6) | 369 (5.1) | 2827 (24.7) | 5939 (43.4) | 1397 (26) | 2385 (20.4) | 5374 (32.6) | ||
| High-flow nasal cannula | 706 (2.1) | 0 (0) | 16 (0.2) | 181 (1.6) | 509 (3.7) | 110 (2) | 181 (1.5) | 415 (2.5) | ||
| BiPAP/CPAP | 236 (0.7) | 0 (0) | 17 (0.2) | 85 (0.7) | 134 (1) | 32 (0.6) | 103 (0.9) | 101 (0.6) | ||
| IMV | 1441 (4.3) | 0 (0) | 30 (0.4) | 484 (4.2) | 926 (6.8) | 441 (8.2) | 371 (3.2) | 629 (3.8) | ||
| ECMO | 147 (0.4) | 0 (0) | 8 (0.1) | 86 (0.8) | 53 (0.4) | 83 (1.5) | 29 (0.2) | 35 (0.2) | ||
| Prone therapy | 30,294 | 732 (2·4) | 0 (0) | 29 (0·4) | 276 (2·7) | 427 (3·4) | 141 (5·5) | 183 (1·6) | 408 (2·5) | |
| Nitric oxide inhalation therapy | 30,290 | 31 (0·1) | 0 (0) | 5 (0·1) | 9 (0·1) | 17 (0·1) | 9 (0·4) | 10 (0·1) | 12 (0·1) | |
| Muscle relaxant | 30,198 | 815 (2·7) | 0 (0) | 22 (0·3) | 309 (3·1) | 484 (3·9) | 240 (9·8) | 195 (1·7) | 380 (2·3) | |
| Inotropic | 33,525 | 837 (2·5) | 0 (0) | 15 (0·2) | 238 (2·1) | 584 (4·3) | 270 (5) | 206 (1·8) | 361 (2·2) | |
| RRT or dialysis | 33,504 | 459 (1·4) | 0 (0) | 6 (0·1) | 153 (1·3) | 300 (2·2) | 125 (2·3) | 112 (1) | 222 (1·3) | |
| Blood transfusion | 33,524 | 666 (2) | 0 (0) | 17 (0·2) | 167 (1·5) | 482 (3·5) | 261 (4·9) | 163 (1·4) | 242 (1·5) | |
| Immunoglobulin | 33,518 | 254 (0·8) | 2 (0·2) | 12 (0·2) | 79 (0·7) | 161 (1·2) | 110 (2·1) | 51 (0·4) | 93 (0·6) | |
| Outcome at discharge | ||||||||||
| Outcome | 33,531 | Discharge | 24,835 (74·1) | 1024 (92·8) | 6293 (86·1) | 9697 (84·7) | 7815 (57·2) | 3507 (65·3) | 9450 (80·9) | 11,878 (72·1) |
| Transfer to different hospital | 3983 (11·9) | 15 (1·4) | 200 (2·7) | 989 (8·6) | 2777 (20·3) | 852 (15·9) | 1070 (9·2) | 2061 (12·5) | ||
| Transfer to non-medical facilityc | 1656 (4·9) | 65 (5·9) | 801 (11) | 593 (5·2) | 194 (1·4) | 500 (9·3) | 545 (4·7) | 611 (3·7) | ||
| Transfer to long-term care facility | 1386 (4·1) | 0 (0) | 10 (0·1) | 55 (0·5) | 1321 (9·7) | 120 (2·2) | 290 (2·5) | 976 (5·9) | ||
| Dead | 1671 (5) | 0 (0) | 3 (0) | 108 (0·9) | 1559 (11·4) | 391 (7·3) | 324 (2·8) | 956 (5·8) | ||
| Number of deaths after oxygen supportb | 33545 | |||||||||
| 21,859 | No oxygen | 46 (0.2) | 0 (0) | 0 (0) | 6 (0.1) | 40 (0.7) | 9 (0.3) | 16 (0.2) | 21 (0.2) | |
| 9156 | Other oxygen support (e.g., nasal cannula, mask) | 994 (10.9) | 0 (0) | 1 (0.3) | 31 (1.1) | 962 (16.2) | 200 (14.3) | 165 (6.9) | 629 (11.7) | |
| 706 | High-flow nasal cannula | 168 (23.8) | 0 (0) | 0 (0) | 8 (4.4) | 160 (31.4) | 22 (20) | 38 (21) | 108 (26) | |
| 236 | BIPAP/CPAP | 51 (21.6) | 0 (0) | 0 (0) | 2 (2.4) | 49 (36.6) | 10 (31.2) | 16 (15.5) | 25 (24.8) | |
| 1441 | IMV | 369 (25.6) | 0 (0) | 2 (6.7) | 44 (9.1) | 322 (34.8) | 127 (28.8) | 82 (22.1) | 160 (25.4) | |
| 147 | ECMO | 43 (29·3) | 0 (0) | 0 (0) | 17 (19·8) | 26 (49·1) | 23 (27·7) | 7 (24·1) | 13 (37·1) | |
| Tracheotomyd | 31,808 | 241 (0·8) | 1 (0·1) | 18 (0·2) | 86 (0·8) | 136 (1·1) | 76 (1·5) | 70 (0·6) | 95 (0·6) | |
| Worsened self-care abilityd | 31,291 | 2667 (8·5) | 1 (0·1) | 49 (0·7) | 628 (5·6) | 1989 (16·9) | 514 (10·7) | 681 (6·1) | 1472 (9·6) | |
| Worsened walking abilityd | 30,562 | 2716 (8·9) | 1 (0·1) | 52 (0·7) | 637 (5·9) | 2026 (17·5) | 492 (11·9) | 723 (6·4) | 1501 (9·9) | |
| O2 administration at discharged | 31,822 | 2128 (6·7) | 3 (0·3) | 58 (0·8) | 673 (5·9) | 1394 (11·5) | 370 (7·5) | 572 (5) | 1186 (7·6) | |
| Complications during admission | ||||||||||
| Bacterial pneumoniae (incl. HAP/VAP) | 33,497 | 1791 (5·3) | 3 (0·3) | 39 (0·5) | 319 (2·8) | 1429 (10·5) | 411 (7·7) | 467 (4) | 913 (5·5) | |
| ARDS | 33,494 | 1501 (4·5) | 0 (0) | 31 (0·4) | 457 (4) | 1013 (7·4) | 484 (9) | 341 (2·9) | 676 (4·1) | |
| Severity of ARDS | 1452 | Mild | 278 (19·1) | 0 (0) | 8 (26·7) | 115 (25·8) | 155 (15·9) | 52 (11·7) | 61 (18·3) | 165 (24·6) |
| Moderate | 482 (33·2) | 0 (0) | 11 (36·7) | 177 (39·7) | 294 (30·1) | 147 (33) | 133 (39·8) | 202 (30·1) | ||
| Severe | 692 (47·7) | 0 (0) | 11 (36·7) | 154 (34·5) | 527 (54) | 247 (55·4) | 140 (41·9) | 305 (45·4) | ||
| Pneumothorax | 33,489 | 142 (0·4) | 0 (0) | 6 (0·1) | 25 (0·2) | 111 (0·8) | 48 (0·9) | 34 (0·3) | 60 (0·4) | |
| Pleural fluid | 33,488 | 994 (3) | 0 (0) | 17 (0·2) | 176 (1·5) | 801 (5·9) | 277 (5·2) | 230 (2) | 487 (3) | |
| Meningitis | 33,488 | 22 (0·1) | 0 (0) | 4 (0·1) | 9 (0·1) | 9 (0·1) | 12 (0·2) | 3 (0) | 7 (0) | |
| Seizure | 33,487 | 80 (0·2) | 3 (0·3) | 6 (0·1) | 24 (0·2) | 47 (0·3) | 29 (0·5) | 23 (0·2) | 28 (0·2) | |
| Stroke | 33,496 | 125 (0·4) | 0 (0) | 2 (0) | 25 (0·2) | 98 (0·7) | 39 (0·7) | 33 (0·3) | 53 (0·3) | |
| Deep vein thrombosis | 32,828 | 215 (0·7) | 0 (0) | 6 (0·1) | 51 (0·5) | 158 (1·2) | 53 (1·1) | 60 (0·5) | 102 (0·6) | |
| Myocarditis | 33,487 | 31 (0·1) | 1 (0·1) | 3 (0) | 12 (0·1) | 15 (0·1) | 14 (0·3) | 5 (0) | 12 (0·1) | |
| Arrythmia | 33,482 | 159 (0·5) | 1 (0·1) | 5 (0·1) | 30 (0·3) | 123 (0·9) | 47 (0·9) | 45 (0·4) | 67 (0·4) | |
| Cardiac ischemia | 33,487 | 56 (0·2) | 0 (0) | 1 (0) | 13 (0·1) | 42 (0·3) | 15 (0·3) | 20 (0·2) | 21 (0·1) | |
| Bacteraemia | 33,488 | 291 (0·9) | 2 (0·2) | 5 (0·1) | 72 (0·6) | 212 (1·6) | 80 (1·5) | 88 (0·8) | 123 (0·7) | |
| Gastrointestinal bleeding | 33,469 | 188 (0·6) | 0 (0) | 7 (0·1) | 30 (0·3) | 151 (1·1) | 52 (1) | 52 (0·4) | 84 (0·5) | |
| Clostridium difficile infection | 33,473 | 69 (0·2) | 0 (0) | 3 (0) | 11 (0·1) | 55 (0·4) | 17 (0·3) | 29 (0·2) | 23 (0·1) | |
| Pulmonary thromboembolism | 32,811 | 77 (0·2) | 0 (0) | 7 (0·1) | 21 (0·2) | 49 (0·4) | 27 (0·6) | 12 (0·1) | 38 (0·2) | |
Data are presented as n (%) unless otherwise indicated.
As the number of missing values differed for each parameter, the number of cases in each parameter's age/wave category was used as the denominator for calculating the percentage.
Abbreviations: BiPAP/CPAP, bilevel positive airway pressure/continuous positive airway pressure; ECMO, extracorporeal membrane oxygenation; HAP/VAP, hospital-acquired pneumonia/ventilator-associated pneumonia. IMV, invasive mechanical ventilation; IQR, interquartile range; RRT, renal replacement therapy;
If the patient received these treatments at any time during hospitalisation, the treatment was included in the analysis.
If the patient received more than one type of oxygen support during the hospitalisation, only one higher level of oxygen support was counted (i.e. ECMO>IMV>BIPAP/CPAP>high-flow nasal canula>other oxygen support).
Facilities for isolation purposes where no medical or nursing care was necessary.
Data were counted only for the patients who were discharged alive.
Overall, 74·1% (n=24,835) of patients were discharged home from the hospitals, 11·9% (n=3,983) were transferred to a different hospital, 4·9% (n=1,656) were transferred to a non-medical facility such as hotels, 4·1% (n=1,386) were transferred to a long-term care facility, and 5% (n=1,671) died. The proportion of deaths increased dramatically among older patients (11·4% [1,559]).
Case fatality rate was highest and lowest during Wave 1 (7·3% [391]) and 2 (2·8% [324]), respectively. Case fatality rate was highest among patients who received ECMO (29·3% [43]), followed by those who received IMV (25·6% [369]), high-flow oxygen therapy (23·8% [168]), and BIPAP/CPAP (21·6% [51]). Most deaths were among older patients (93·3%, n = 1559/1671). In the old group, more than one-third of the patients who were treated with IMV, and about half of the patients who were treated with ECMO, died. Among the older patients, approximately 17% (n=1,989) experienced worsened self-care/walking ability and 11·5% (n=1,394) required oxygen upon discharge.
Bacterial pneumonia was the most common complication (5.3% [1,791]), followed by acute respiratory distress syndrome (ARDS) (4·5% [1,501]). Deep vein thrombosis and pulmonary embolism were diagnosed in 0·7% (n=215) and 0·2% (n=77) of the patients, respectively.
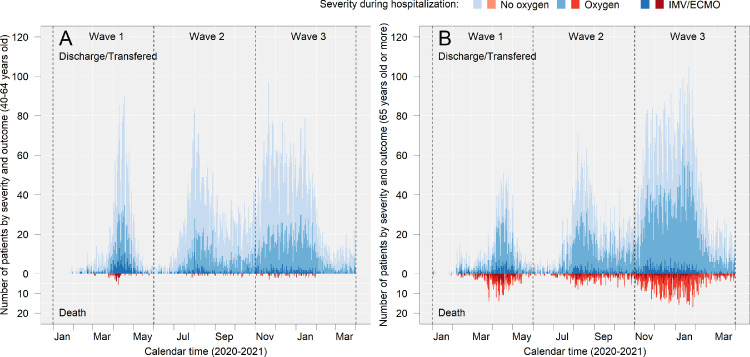
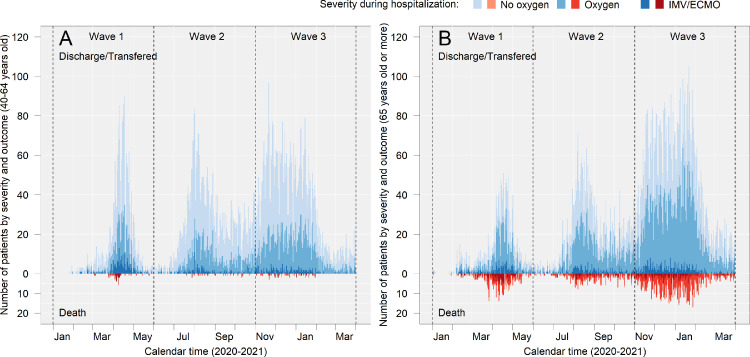
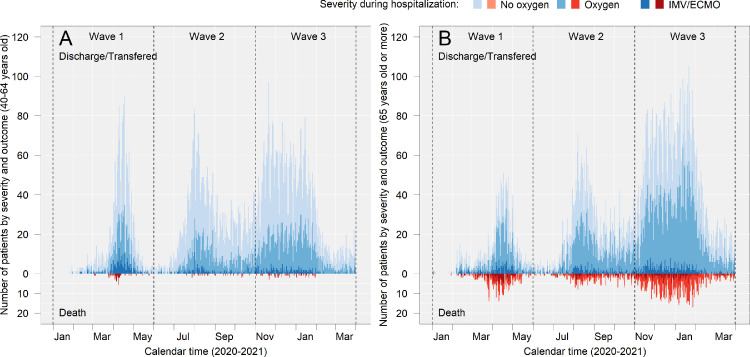
Epidemiologic curve of severity and death in middle-aged and older patients
The severity of disease (indicated by respiratory supports during hospitalisation) and deaths among middle-aged and older patients are shown in Figure 3, and related values are presented in Supplementary Table 2. Among middle-aged patients, IMV and/or ECMO were used more frequently during Wave 1 (10.5% [225]) than during waves 2 (3.7% [147]) and 3 (3.7% [198]), and death was most prominent during Wave 1(12% [27]) followed by Wave 3 (11.1% [22]) and Wave 2 (8.2% [12]) among patients who were treated with IMV and/or ECMO. Among older patients, the use of IMV and/or ECMO in both survivors and those who died was less frequent during Wave 3 (survivors: 4.2% [308], non-survivor: 16.5% [150], respectively) than during Wave 1 (9.9% [157] and 34.6% [121], respectively).
Figure 3.
Epidemiologic curve of severity and death in middle-aged and older patients.
(a) and (b) show the number of patients in each category in the middle and old age groups, respectively. The upward graphs (discharged/transferred) show the patients who were alive at the time of discharge, and the downward graphs (deaths) show the patients who died during hospitalisation. “Transferred” included patients who were transferred to either different hospital, non-medical facility, or long-term care facility. The outcome of patients who were transferred from the hospitals participating in the study at the time of admission are summarised in Supplementary Table 3. Detailed numbers in the figure are presented in Supplementary Table 2.
No oxygen: patients who were never supported with supplemental oxygen during hospitalisation.
Oxygen: patients who were supported with non-IMV or supplemental oxygen (including high-flow oxygen devices) during hospitalisation.
IMV/ECMO: Patients who were supported with invasive mechanical ventilation (IMV) or extracorporeal membrane oxygenation (ECMO).
History of drug administration during hospitalisation
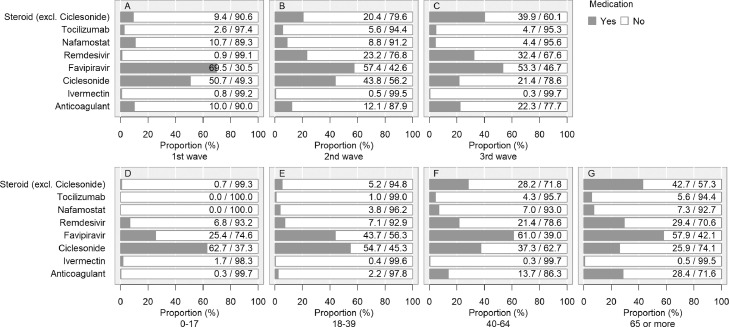
Use of steroids (excluding ciclesonide), remdesivir, and anticoagulants increased over time (ED of steroid: Wave 1 vs Wave 2: -11.0% [-12.4, -9.7], Wave 2 vs Wave 3: -19.5% [-20.7, -18.2]; remdesivir: Wave 1 vs Wave 2: -22.3% [-23.9, -20.8], Wave 2 vs Wave 3: -9.2% [-11.2, -7.3]; anticoagulant: Wave 1 vs Wave 2: -2.1% [-3.4, -0.9], Wave 2 vs Wave 3: -10.2% [-11.3, -9.1]) (Figure 4) (Table 3). In contrast, ciclesonide, favipiravir, and nafamostat use decreased over time. Tocilizumab use was most frequent in Wave 2, followed by Waves 3 and 1 (ED of Wave 2 vs Wave 3: 0.8% [-0.2, 1.8], Wave 1 vs Wave 2: -3.0% [-4.1, -1.9]). The use of most medications, except for favipiravir and ciclesonide, increased with age. Baricitinib was not administered to this cohort. Antimicrobials were administered in 23·3% (n=7,800) (under 18, 4·1% [45]; young, 7% [507]; middle age, 19·4% [2,211]; older patients, 36·9% [5,036]; Wave 1, 35·9% [1,916]; Wave 2, 18% [2,108]; and Wave 3, 22·9% [3,776]), and antifungal drugs in 0·9% (n=309) (under 18, 0% [0]; young, 0·2% [14]; middle age, 0·7% [81]; older patients, 1·6% [214]; Wave 1, 1·9% [101]; Wave 2, 0·7% [83]; and Wave 3, 0·8% [125]) of the cases.
Figure 4.
History of drug administration during hospitalisation.
Medications with antiviral effects against severe acute respiratory syndrome coronavirus 2, immunomodulatory effects against coronavirus disease (COVID-19), and/or immunosuppressive effects against COVID-19 were included. Ciclesonide is available only as an inhalant. Steroids (other than ciclesonide) were administered in 9,450 cases. For oral, intravenous, and inhalation administration, there were 5,279, 5,086, and 86 cases, respectively. There were 9,376 cases of either oral or intravenous administration. If more than one preparation was used for one patient, each was counted. Anticoagulation therapy included unfractionated heparin, low-molecular-weight heparin, fondaparinux, and oral anticoagulants (warfarin and direct oral anticoagulants: dabigatran, rivaroxaban, apixaban, and edoxaban) during hospitalisation. In this study, we did not distinguish between prophylactic and therapeutic administration of thromboembolism. We did not count concomitant therapies in the present analysis. The denominator is not the total number of patients, but the number of patients who were administered any drug to treat COVID-19 (n=15,880) for whom the data of each antiviral drug was not missing. For example, the proportion of patients administered favipiravir was calculated as: with favipiravir/(with favipiravir + without favipiravir).
Characteristics of transferred patients
A total of 1,306 patients were transferred from the institutions participating in the study at the time of admission. As for patients' demographics and characteristics on admission (Supplementary Table 3), compared to the whole cohort, there were more male patients (69.2% [n=904] vs 56.6% [18,985]) and more patients in Wave 1 (24.7% [322] vs 16% [5,372]) in the transferred cohort. There was also a higher median BMI (24.2 vs 23.3), a higher rate of immunodeficiency (4.2% [54] vs 2.2% [708]), and higher rate of severe disease on admission (68.5% [894] vs. 28.6% [9,587]) in the transferred cohort than in the whole cohort. The prevalence of any comorbidity was higher in transferred patients than the whole cohort (78.1% [1,020] vs 54.5% [18,295]), and for all individual comorbidities except leukaemia/lymphoma, the prevalence was higher in transferred patients. Regarding supportive care, outcome, and complications during hospitalisation, transferred patients had more ICU stays than the whole cohort (35.2% [458] vs. 7.2% [2,409]), and were more often treated by IMV (23.9% [312] vs 4.7%) and ECMO (3.4% [45] vs 0.4% [147]). In terms of the outcomes, discharge home was less common (51.4% [671] vs. 74.1% [24,835]) and death (11.9% [155] vs 5% [1,671]) was more common in the transferred cohort than in the whole cohort. Steroids, anticoagulants, remdesivir, tocilizumab, and nafamostat tended to be administered more frequently in transferred patients than in the whole cohort. In Waves 2 and 3, ciclesonide and favipiravir tended to be administered less frequently in the transferred cohort than in the whole cohort.
Evaluation of the effect of each wave on death during hospitalisation
After adjusting for other parameters that could interact with the risk of death and the transfer status on admission, compared to Wave 1, Wave 2 and Wave 3 rather decreased the risk of death (Table 4). The analysis was performed excluding patients transferred on admission (Supplementary Table 4-a). In this analysis, the trend of the results did not change from the analysis results that included transferred patients. The number of facilities that continuously participated in COVIREGI-JP from Wave 1 to Wave 3 was 234 (42.3% of 553 facilities). For each wave, 234 facilities accounted for about 60% of the total number of facilities (Wave 1: 234/378 [61.9%], Wave 2: 234/412 [56.8%], Wave 3: 234/408 [57.4%]). The number of patients (n=24806) from 234 institutions that had continuously participated in COVIREGI-JP accounted for 73.9% of the total patients (n=33554), and about 70% of the total for each wave (Wave 1: 3579/5372 [66.6%], Wave 2: 8771/11692 [75%], Wave 3: 12456/16490 [75.5%]). We performed the analysis to evaluate the effect of each wave on death, including only the patients from the facilities that continued to participate over three waves (Supplementary Table 4-b and 4-c). The results of the effect of each wave on death were similar to that of total participating facilities.
Table 4.
Evaluation of the effect of each wave on death during hospitalization.
| Risk ratio |
95%CI |
P value |
||||
|---|---|---|---|---|---|---|
| Parameters | Univariable | Multivariable | Univariable | Multivariable | Univariable | Multivariable |
| Effect of each wave | ||||||
| Wave 1 | Reference | |||||
| Wave 2 | 0.363 | 0.398 | [0.312-0.423] | [0.40-0.561] | < 0.001 | < 0.001 |
| Wave 3 | 0.783 | 0.473 | [0.694-0.886] | [0.325-0.487] | < 0.001 | < 0.001 |
| Other risk factors | ||||||
| Transfer from other facilities | 3.916 | 1.629 | [3.479-4.401] | [1.390-1.906] | < 0.001 | < 0.001 |
| Age | 1.092 | 1.103 | [1.088-1.097] | [1.096-1.110] | < 0.001 | < 0.001 |
| Male sex | 1.322 | 1.872 | [1.195-1.464] | [1.621-2.165] | < 0.001 | < 0.001 |
| Obesity (BMI>=25) | 0.678 | 1.098 | [0.593-0.773] | [0.940-1.281] | < 0.001 | 0.236 |
| Cardiovascular and peripheral vascular diseaseA | 4.989 | 1.521 | [4.394-5.654] | [1.277-1.805] | < 0.001 | < 0.001 |
| Chronic Respiratory DiseaseB | 4.728 | 1.675 | [4.055-5.492] | [1.367-2.043] | < 0.001 | < 0.001 |
| Liver disease | 2.281 | 2.459 | [1.793-2.865] | [1.780-3.347] | < 0.001 | < 0.001 |
| Diabetes mellitus | 2.377 | 1.388 | [2.132-2.647] | [1.199-1.605] | < 0.001 | < 0.001 |
| Cerebrovascular Disease | 3.965 | 1.370 | [3.482-4.504] | [1.455-1.632] | < 0.001 | < 0.001 |
| Hypertension | 2.291 | 0.790 | [2.075-2.530] | [0.688-0.906] | < 0.001 | < 0.001 |
| Severe condition at admission | 6.766 | 3.026 | [6.075-7.546] | [2.624-3.493] | < 0.001 | < 0.001 |
Abbreviations. BMI, body mass index; CI, confidence interval. A Cardiovascular and peripheral vascular disease includes myocardial infarction, congestive heart failure, and peripheral vascular disease. B Chronic respiratory disease was defined as pulmonary disease in patients who were dyspneic with slight activity and included chronic obstructive pulmonary disease.
Discussion
We analysed a large cohort of mostly unvaccinated hospitalised Japanese patients with COVID-19. Our cohort included approximately 7% (n=33,554) of the total number of reported COVID-19 cases in Japan as of the end of March 2021 (n=469,713).14 A comparison of cases from Japan, with that from Western countries, and other Asian countries, based on available data by the end of March 2021, shows that the cumulative deaths per million people are lower in Japan than in Western countries, but higher than those of most Asian countries (e.g., Japan, 72·8; US, 1656·4; Germany, 912·9; United Kingdom, 1861·3; China 3·21; Singapore, 5·5; South Korea, 33.8; Philippines, 119.7).15 In comparison with Western countries, the case fatality rate for Japan (1.9) was similar to that of the U.S.(1.8), and lower than that for other countries (Germany, 2.7; United Kingdom, 2.9).15 In comparison with Asian countries, the case fatality rate for Japan was lower than that for China (5.1) but higher than that for Singapore (0.05), and similar to that for other countries (South Korea, 1.7; Philippines, 1.8).15 The proportion of people vaccinated against COVID-19 where data are available by the end of March 2021, are 0·1%, 0·05%, 5%, 6·6%, and 19% in Japan, Korea, Germany, the UK, and the US, respectively.5 Considering the lower proportion of people vaccinated in Japan by the end of March 2021 than Western countries, the case fatality rate among unvaccinated people in Japan was likely to be lower than those in Western countries. The case fatality rate in the inpatient cohort of 7·3% in Wave 1 in this report is lower than the case fatality rate (of over 15%) in China, the UK, and the US at the beginning of the epidemic.16, 17, 18 It is also lower than the 12% in the Philippines at the start of the epidemic19; however, it is higher than the 0.9% in Singapore.20 However, simple comparisons are difficult due to factors such as different times of infection, hospitalisation recommendation (i.e., denominator as hospitalised patients), effects of age and comorbidities, and medical resources. As previously discussed,2 despite the relatively high number of older patients, Japan has a low case fatality rate. This may be related to the low frequency of comorbidities, such as diabetes, obesity, and cardiovascular diseases. Moreover, the total number of cases did not surge to the point of causing a complete healthcare system meltdown. In addition, the present cohort included many patients with relatively mild disease due to the hospitalisation recommendation in Japan (Supplementary Table 5). For example, in a US cohort of 192,550 inpatients (with a case fatality rate of 13·6%) between March and August 2020, 28·9% (n=55,593) were admitted to ICU,21 compared to 7·2% (n=2,409) in this cohort. Furthermore, in a UK cohort of 20,133 inpatients between 6 February and 19 April 2020, 17% (n=3,001/18,183) were admitted to ICU and 10% (1,658/16,866) received IMV,16 while in this cohort only 4·3% (n=1,441) received IMV.
Age group comparisons revealed differences in epidemiologic characteristics. This indicates the need for tailored infection prevention and treatment approaches for each age group. The young patients tended to have a history of close contact with COVID-19 cases in the workplace. Furthermore, they engaged in high-risk behaviour, such as having meals with multiple non-family members or staying in crowded places. For the older patients, exposure to COVID-19 was mostly in the healthcare facility and at home.
Our previous analysis included 2,638 patients mainly from Wave 1, and the case fatality rate was as high as 7·5% (n=197).2 In the current cohort, more patients (n=5,372) were included from Wave 1. The resulting Wave 1 case fatality rate was 7·3% (n=391), which was slightly lower than the previously reported case fatality rate. Wave comparison revealed that each wave had different characteristics: Wave 1 had severe disease and the worst case fatality rate; Wave 2 had young patients and mild disease; and Wave 3 had older patients with comorbidities. The differences that were considered clinically significant were age (12 for ED of Waves 2 vs Wave 3), the severity of illness at admission (ED in Wave 2 were about 10% lower than those in Waves 1 and Wave 3), and hypertension (ED in Wave 2 were more than 10% lower than those in Waves 3 and 1). Other comorbidities also had lower EDs, especially in Wave 2, by more than a few percent than in Wave 3. In the multivariable analysis using a relative risk regression model in which the effects of other factors such as age and comorbidities were controlled, Wave 2 and Wave 3 had less risk of death than Wave 1. The median duration from symptom onset to hospitalisation was shortened from seven days during Wave 1 to four days during Wave 3. Furthermore, Wave 3 comprised a smaller percentage of patients who were on invasive or non-IMV or on ECMO at admission (Wave 1: 2·7% [143] vs Wave 3: 1·1% [174]).
Besides the opportunity of being admitted to hospital before the disease became severe, there were also differences in post-hospitalisation treatment between epidemic waves. The use of steroids was about four times more frequent during Wave 3 than during Wave 1, and the use of anticoagulants more than doubled. For steroids and remdesivir, ED increased by about 30% in the comparison between Waves 1 and Wave 3. Anticoagulants also showed a large difference in ED in Wave 2 and Wave 3, with an increase of ED about 10%.In terms of supportive care, the use of invasive supportive care, such as IMV and ECMO was lower during Wave 3 than during Wave 1, with ED of 3.9% for IMV and 1.3% for ECMO. Deaths following the use of high-flow therapy were most prominent during Wave 3 among the three epidemic waves.
Invasive supportive care may have been more reserved during Wave 3 than during Wave 1 due to various factors, such as the higher proportion of older patients during Wave 3; the increased number of COVID-19 patients, which strained medical institutions, compared to Wave 1; improved understanding of the disease; and the avoidance of invasive ventilator-associated complications through the use of high-flow oxygen therapy. Besides these factors, the fact that case fatality rate during Wave 3 was lower than that during Wave 1, despite the increase in age and comorbidity, may indicate an improvement in prognosis over time. This is also supported by the multivariable analysis results which adjusted for the confounding effects of age and comorbidity. Similar improvements in prognosis over the pandemic period have also been reported in other countries.22,23
The use of steroids, remdesivir, and anticoagulants tended to increase in the later waves. This was likely due to the widespread recognition of the evidence and the approval of remdesivir.24 As previously discussed,2 patients who were administered favipiravir might have been more likely to be included in our study cohort.
In order to determine the extent to which the data in this study are representative of Japan as a whole, first, we compared the number of infections and deaths in hospitalised patients enrolled in the COVIREGI-JP with those in Japan (i.e., national data), taking into account regional differences (Supplementary Table 6, the regions of Japan are shown on the map in Supplementary Figure 1). The coverage rate for infected patients in the COVIREGI-JP of 7·1% (n=33,554) varied by region, being extremely high in Hokuriku (24·8% [1,220]) and Chugoku (17·1% [1,680]), whereas it was lowest in Kanto (5·7% [14,229]), though the absolute number of cases was high. There was also a difference in the coverage rate depending on the epidemic wave, with Wave 1 covering 32·1% of patients (n=5,372), but dropped to 14% (n=11,692) in Wave 2. Wave 3 had 22 times more infected patients than Wave 1, when the rate dropped to 4·4% (n=16,490). The representativeness of our data might have decreased as the waves progressed. The trend of the highest case fatality rate in Wave 1 and the lowest in Wave 2 in our cohort was also observed in the infected population of Japan. The case fatality rate was higher in this study cohort than in the national data, probably because our cohort included more severe patients as they required hospitalisation. The ratio of case fatality rate between the national data and our data was as follows: 1·4 times (Wave 1), 2·8 times (Wave 2), and 2·9 times (Wave 3). As the wave progressed, patients in our cohort tended to have a higher case fatality rate ratio, suggesting that patients with more severe disease were selected in the later waves. The same trend was observed in each region; however in Hokkaido and Tokai, the case fatality rate in the national data was higher than that in our data in Wave 1, suggesting that there was a discrepancy between the overall regional situation and the trend of registered cases in COVIREGI-JP. The difference in case fatality rates between the nationwide and regional data, in most regions, showed increased or decreased patterns that were consistent between the two data sets (i.e., national data and COVIREGI-JP data) for each epidemic group. However, in some regions, there were partial divergence. The severity of the cases in COVIREGI-JP might not have been parallel to the severity of the total infected patients in these regions.
We also summarised the number of hospitals in Japan and those participating in the COVIREGI-JP (Supplementary Table 7). The number of COVIREGI-JP hospitals covered 6·7% (n=553) of the total number of hospitals in Japan, and in contrast to the gradual decrease in patient coverage, the number of facilities was highest in Wave 2 and was slightly increased in Wave 3 compared to Wave 1. Nearly half of the tertiary care facilities in Japan participated in the COVIREGI-JP, however, in Wave 3, the percentage of tertiary hospitals dropped to about 30% (n=94). This can be attributed to the fact that medical institutions other than tertiary hospitals treated COVID-19 inpatients due to the expansion of the number of infected patients, as well as that lower number of facilities that registered patients to COVIREGI-JP due to the increased burden of the workload triggered by more infected patients. As for the facility coverage rate, no regional trend similar to that of the number of patients was observed, and the rate was rather high in the Kanto region, where the coverage rate of the number of patients was low. Most of the regions were covered by 30% to 50% of tertiary medical institutions in each epidemic wave, however the number of facilities and tertiary medical institutions in Shikoku tended to be low. In summary, despite partial regional differences, the study cohort had a higher case fatality rate than that reported for total infected population in Japan. Although there was a tendency for the patient coverage rate to decrease in Wave 3, there was no significant increase or decrease in the facility coverage rate. The trend of increase or decrease in the case fatality rate in each wave showed a similar pattern to that of the overall nationwide infected population in Japan.
The study had some limitations. The hospitalisation recommendations changed during the study period;4 therefore, patient background and severity of illness were not uniform throughout the study period. The changes in hospitalisation recommendations of the Ministry of Health, Labour and Welfare are summarised in Supplementary Table 5. In the early stages of the epidemic, hospitalisation was recommended for almost all patients. In the middle of Wave 1, the recommendation for recuperation at accommodation facilities or home was issued, and hospitalisation was recommended in principle for the elderly and patients with underlying diseases. At the end of Wave 2, these standards were revised, and the hospitalisation recommendation became more stringent. However, these recommendations were not compulsory, and hospitalisation at the physicians’ discretion might have been adopted, especially when the number of infected people was not significantly high. With regard to diagnosis, the number of tests conducted in Japan was low at the beginning of the epidemic and they increased over time.25 Thus, especially in Wave 1, asymptomatic or mildly ill patients may have remained undiagnosed or the time from infection to diagnosis might have been long. This is probably one of the reasons why the time from onset to hospitalisation was longer in Wave 1 than Waves 2 and 3 and might have contributed to the severity of the disease at the time of hospitalisation in Wave 1, even if the hospitalisation recommendation was less restrictive. These multiple factors may have resulted in more hospitalisations of young people with mild illnesses in Wave 2 than in other waves.
The outcomes of patients who required prolonged hospitalisation might not have been reflected, especially during Wave 3. Overall, 1,943 patients (5·5%) were excluded from the study because of non-fixed data. In many cases, information such as outcome at discharge, and supportive care, history of drug administration, and complications during hospitalisation were not fixed. If the data were available, this might change the numbers including the outcome especially during Wave 3. In addition, we defined obesity based on BMI, but it was missing in 6,374 (19%) cases, which may have led to underestimation or overestimation of obesity. On the other hand, missing data for important parameters such as age, sex, comorbidity (other than obesity), oxygen support, and outcome were low, as shown below, and we believe that the impact on the analysis was minimal (missing data for age: 12 [0·04%], sex: 3 [0·01%], comorbidity: 0, oxygen support: 9 [0·03%], outcome: 24 [0·07%]). Since the first case of the alpha variant was reported in December 2020, there were 678 cases of variants reported in Japan (mostly the alpha variant) as of the end of March 2021,26 although these might have been underreported. Unfortunately, there is no information on the variants in this study. However, the impact was considered to be minimal. The other limitations due to the nature of the registry, such as the potential of selection bias and accuracy of data entered at each facility, were previously described.2 As mentioned earlier, based on the rate of vaccination in Japan as a whole, almost all the patients in the present cohort are considered to be unvaccinated; however, detailed data on vaccination history were not available for this analysis. Because the study included patients who were transferred from other hospitals if the selection criteria were met, a patient may have been enrolled more than once for different time periods if they were transferred during a single SARS-CoV-2 infection episode. While this would enable the collection of accurate data on the outcome of each patient's hospitalisation episode, it might have led to an overrepresentation of some parameters, such as demographic data of patients who were transferred, especially for rare events. A total of 1,306 patients were transferred from the institutions participating in the study at the time of admission. Unfortunately, the reason for the transfer was unknown. The summarised characteristics of these patients suggest that there were more severe cases with higher case fatality rate and more frequent invasive treatment (e.g. IMV or ECMO) than whole cohort. If these cases were included only once in the whole cohort, then the case fatality rate might have been slightly higher. We did not adjust for the drug administration or supportive care in the multivariable analysis, to evaluate the effect of each wave on outcome. As the outcome was better in Wave 3 than in Wave 1 after adjusting for the effects of age and comorbidities, the change of the drug administration and/or supportive care might have affected the outcome.
In conclusion, although the overall case fatality rate remained relatively low, the case fatality rate was approximately twice as high among older patients and varied with the epidemic wave. After adjusting for age and comorbidities, the risk of death was highest in Wave 1. However, it seems difficult to keep the case fatality rate low during waves that include a large number of older patients. Changes in COVID-19 medications and supportive care were observed over time in Japan. These findings present important basic data for understanding future changes due to increased vaccination rates and variants.
Declaration of interests
The authors declare no conflicts of interest.
Acknowledgments
Contributors
Conception/design of the work: NM, KH, YA, ST, and SS.; acquisition of data: NM, MT, SS, HO, KK, AT, KS, MS; analysis of data: YA and ST; drafting of the manuscript: KH. All authors were involved in data interpretation and review of the final manuscript.
Acknowledgements
We thank all the participating facilities for their care of COVID-19 patients and their cooperation in entering data into the registry.
Data sharing statement
Data are available upon reasonable request. Data on an individual level is shared with limitation to participating healthcare facilities through applications to COVIREGI-JP.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Footnotes
Previous presentation: Part of these data was presented at Chasing the Sun: COVID-19, Beyond the Horizon, IDWeek 2021 on September 30, 2021.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanwpc.2022.100421.
Appendix. Supplementary materials
References
- 1.Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus Pandemic (COVID-19) – Japan: coronavirus pandemic country profile. Published online at OurWorldInData.org. 2020. Retrieved from: https://ourworldindata.org/coronavirus/country/japan?country=∼JPN. Accessed 10 December 2021.
- 2.Matsunaga N, Hayakawa K, Terada M, et al. Clinical epidemiology of hospitalized patients with COVID-19 in Japan: report of the COVID-19 REGISTRY JAPAN. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinical Practice Guideline Review Committee. COVID-19 clinical practice guideline ver 6.0 2021. April 1, 2020. Available at: https://www.mhlw.go.jp/content/000851082.pdf. Accessed 10 December 2021.
- 4.Yamada G, Hayakawa K, Matsunaga N, et al. Predicting respiratory failure for COVID-19 patients in Japan: a simple clinical score for evaluating the need for hospitalisation. Epidemiol Infect. 2021;149:e175. doi: 10.1017/S0950268821001837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ritchie H, Mathieu E, Rodés-Guirao L,, et al. Coronavirus Pandemic (COVID-19) – Japan: Coronavirus (COVID-19) Vaccinations. Published online at OurWorldInData.org. 2020. Retrieved from: https://ourworldindata.org/covid-vaccinations?country=∼JPN. Accessed 10 December 2021.
- 6.World Health Organization. Mean body mass index trends among adults, age-standardized (kg/m²). Estimates by country. 2017. Available at: https://apps.who.int/gho/data/view.main.CTRY12461?lang=en. Accessed 10 December 2021.
- 7.Ministry of Health, Labour and Welfare. Notification of physicians and veterinarians under the Infectious Diseases Act. Available at: https://www.mhlw.go.jp/bunya/kenkou/kekkaku-kansenshou11/01-shitei-01.html. Accessed 10 December 2021.
- 8.National Center for Global Health and Medicine. COVID-19 REGISTRY JAPAN. Available at: https://covid-registry.ncgm.go.jp/. Accessed 10 December 2021.
- 9.International Severe Acute Respiratory and Emerging Infection Consortium. COVID-19 clinical research resources. COVID-19 Clinical Research Resources. Available at: https://isaric.tghn.org/covid-19-clinical-research-resources/. Accessed 10 December 2021.
- 10.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saito S, Asai Y, Matsunaga N, et al. First and second COVID-19 waves in Japan: a comparison of disease severity and characteristics. J Infect. 2021;82:84–123. doi: 10.1016/j.jinf.2020.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19 – —final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terada M, Ohtsu H, Saito S, et al. Risk factors for severity on admission and the disease progression during hospitalisation in a large cohort of patients with COVID-19 in Japan. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-047007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ministry of Health, Labour and Welfare. Current status of the novel coronavirus infection and the response of the MHLW. March 31,2021 Available at: https://www.mhlw.go.jp/stf/newpage_17801.html. Accessed 10 December 2021.
- 15.Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus (COVID-19) Deaths. Published online at OurWorldInData.org. 2020. Retrieved from: https://ourworldindata.org/covid-deaths. Accessed 10 December 2021.
- 16.Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agrupis KA, Smith C, Suzuki S, et al. Epidemiological and clinical characteristics of the first 500 confirmed COVID-19 inpatients in a tertiary infectious disease referral hospital in Manila, Philippines. Trop Med Health. 2021;49:48. doi: 10.1186/s41182-021-00340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan JB, Cook MJ, Logan P, Rozanova L, Wilder-Smith A. Singapore's Pandemic Preparedness: An Overview of the First Wave of COVID-19. Int J Environ Res Public Health. 2020;18:252. doi: 10.3390/ijerph18010252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen NT, Chinn J, Nahmias J, et al. Outcomes and mortality among adults hospitalized with COVID-19 at US medical centers. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dennis JM, McGovern AP, Vollmer SJ, Mateen BA. Improving survival of critical care patients with coronavirus disease 2019 in England: a national cohort study, March to June 2020. Crit Care Med. 2021;49:209–214. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47:60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saito S, Hayakawa K, Mikami A, et al. Investigator initiated clinical trial of remdesivir for the treatment of COVID-19 in Japan. Glob Health Med. 2021;3:62–66. doi: 10.35772/ghm.2020.01106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ministry of Health, Labour and Welfare. Situation report. No. of PCR tests conducted. Available at: https://www.mhlw.go.jp/stf/covid-19/kokunainohasseijoukyou_00006.html. Accessed 10 December 2021.
- 26.Ministry of Health, Labour and Welfare. Number of confirmed variants strains (genome analysis) by prefecture. March 31, 2021. Available at: https://www.mhlw.go.jp/content/10900000/000763676.pdf. Accessed 10 December 2021.
- 27.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28.Expert Meeting on Control of the Novel Coronavirus Disease Control. Analysis and recommendations of the response to the novel coronavirus (COVID-19). April 1, 2020. Available at: https://www.mhlw.go.jp/content/10900000/000620826.pdf. Accessed 10 December 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.