Kidney transplant recipients (KTRs) are highly vulnerable to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and severe coronavirus disease 2019 (COVID-19). The 28-day case mortality for KTRs is 24%, and mounting evidence suggests poor immunogenicity and clinical effectiveness of vaccines in this group.1 , 2 Priority COVID-19 vaccination and booster dosing of close household contacts, that is, ring vaccination, has been suggested as an additional layer of protection for immunocompromised individuals.3 To address this concept, KTRs and their healthy cohabitants were concurrently vaccinated, and their immunity to SARS-CoV-2 was compared in a registered observational clinical trial (ACTRN12621000532808).
Forty-six kidney-alone transplant recipients from a South Australian transplant unit receiving immunosuppression with a calcineurin inhibitor, antimetabolite (mycophenolate mofetil or azathioprine), and steroid were included (Table 1 ). Each patient was paired with a healthy domestic cohabitant of similar age. Participants received 2 doses of either the ChAdOx1 (Oxford-AstraZeneca; n = 26 pairs) or the BNT162b2 (Pfizer-BioNTech; n = 20 pairs) vaccine as per Australian Government recommendations. Transplant recipients were predominantly male (n = 31 [67%]) and first-graft recipients (n = 40 [87%]), aged 60.8 ± 12.5 years, with transplant age 6.9 ± 7.2 years (see Table 1).
Table 1.
Participant characteristics
| Characteristic | Transplant (n = 46) | Cohabitants (n = 46) |
|---|---|---|
| Age, yr | 60.8 ± 12.5 | 59.2 ± 12.5 |
| Sex | F: 15; M: 31 | F: 31; M: 15 |
| Vaccine | ChAdOx1: 26 BNT162b2: 20 |
ChAdOx1: 25 BNT162b2: 21 |
| Cause of kidney failure | Glomerulonephritis: 17 (37%) Other: 12 (26%) Polycystic kidney disease: 11 (24%) Diabetes mellitus: 3 (6.5%) Hypertension/renovascular: 2 (4.5%) Unknown: 1 (2%) |
NA |
| Age of the transplanted kidney, yr | Range: 0.2–34.6 Mean: 6.9 ± 7.2 Median 5.0 |
NA |
| Graft number | First graft: 40 Second graft: 6 |
NA |
| Graft function | eGFR: 54.8 ± 18.5 ml/min/m2 | NA |
eGFR, estimated glomerular filtration rate; F, female; M, male; NA, not applicable.
Circulating anti–receptor-binding domain (RBD) IgG antibodies (Elecsys, Roche) and serological neutralization of live SARS-CoV-2 were measured after 2 doses (median 21 days; interquartile range 21–24 days) of the BNT162b2 or ChAdOx1 vaccine as correlates of protection from symptomatic COVID-19.4 The study was further designed to enable cohabitants to serve as a healthy control group against which the broader anti-spike IgG antibody and T-cell responses of KTRs over time were compared. There was no COVID-19 community transmission in South Australia at the time of the study, and SARS-CoV-2 infection was excluded by serology in all participants by an accredited pathology service (SA Pathology; SARS-CoV-2 nucleocapsid IgG, Elecsys, Roche).
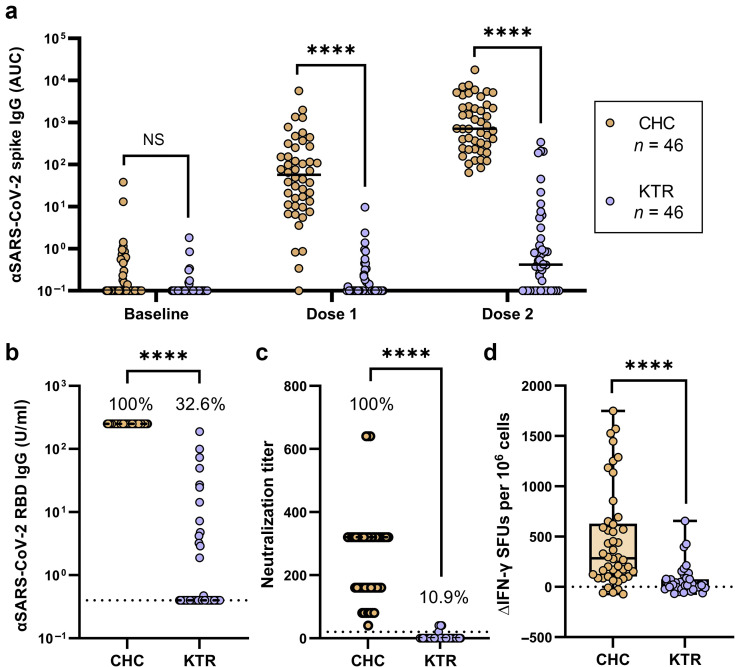
After the first vaccine dose, anti-spike IgG (>2 SDs above baseline) was detected in only 27.0% of transplant recipients and 83.3% of cohabitants. After 2 vaccine doses, 100% of cohabitants had detectable titers of anti-spike IgG compared with only 44% of transplant recipients (Figure 1 a). After the full course of COVID-19 immunization (2 doses), KTRs had a median anti-spike IgG titer >1000-fold lower than that of healthy cohabitants (area under the curve 0.83 versus 1454; Figure 1a).
Figure 1.
Immune response of kidney transplant recipients (KTRs; n = 46) and close household contacts (CHCs; n = 46) to 2 doses of coronavirus disease 2019 (COVID-19) vaccine. KTR and CHC were concurrently vaccinated with the BNT162b2 or ChAdOx1 vaccine. Blood samples collected at baseline, 3 weeks after dose 1, and 3 weeks after dose 2. (a) Log-scale longitudinal comparison of serum spike-specific antibody titers, reported as area under the curve (AUC) units. Circles represent AUC individual participant values, with mean value denoted by black bars. (b) Log-scale comparison of serum anti–severe acute respiratory syndrome coronavirus 2 (αSARS-CoV-2) receptor-binding domain (RBD) IgG titers 3 weeks after dose 2 in KTRs and CHCs. Results below the detection threshold are plotted as 0.4 U/ml and marked with a dashed line. The percentage of individuals above the detection limit is indicated. (c) Serological neutralization end point cutoff titers in KTRs and CHCs. Data points represent the highest serum dilution factor that yielded 50% inhibition of live virus (Wuhan) infection. Twenty was the minimum dilution tested for all samples, and the percentage of individuals achieving a neutralization titer of ≥20 is indicated. Individuals who did not achieve neutralization at a dilution factor of ≥20 were considered negative and ascribed a value of zero, marked with a dashed line. (d) Vaccine-induced interferon-γ (IFN-γ)–secreting T-cell response. Isolated peripheral blood mononuclear cells (PBMCs) were stimulated for 18 hours with spike glycoprotein-derived peptides and the frequency of IFN-γ–secreting cells measured as spot-forming units (SFUs) by enzyme-linked immunoabsorbent spot assay. SFUs at baseline were subtracted from SFUs 3 weeks after dose 2 to determine the change in spike-reactive, IFN-γ–secreting T-cell frequencies in response to vaccination. Differences between groups tested using the 2-tailed Mann-Whitney test. NS, not significant. ∗∗∗∗P < 0.0001. ΔIFN-γ, change in interferon-γ.
Where anti-spike IgG titer reflects the magnitude of antibody response to the vaccine, anti-RBD IgG and live virus neutralization are measures of functional immunity to SARS-CoV-2. Seroconversion of anti-RBD IgG (detection limit 0.4 U/ml) and serological neutralization were both achieved by 100% of cohabitants. In contrast, 10.9% of KTRs showed serum neutralizing activity and 32.6% had detectable titers of anti-RBD IgG (Figure 1b and c).
To evaluate the level of protection afforded these groups, an anti-RBD IgG titer of 100 U/ml and serological neutralization of 40 were used as benchmarks for a protective vaccine response on the basis of similar studies and accepted thresholds for influenza hemagglutination assays.4, 5, 6 All cohabitants met these thresholds, achieving anti-RBD IgG titers >100 U/ml and 50% live virus neutralization at serum dilutions of ≥1/40. Evidence of protective immunity was rarely achieved by transplant recipients, with 4.3% exceeding 100 U/ml of anti-RBD IgG and 8.7% achieving serological neutralization of ≥40.
Antiviral T-cell responses are important in viral clearance and for minimizing the severity of COVID-19, particularly in the absence of an effective neutralizing antibody response.7 Conserved SARS-CoV-2 epitopes recognized by T cells may also provide cross-protection against viral variants that evade antibody neutralization.8 Therefore, we evaluated the magnitude of spike-specific T-cell responses (reported by frequency of antigen-induced interferon-γ secretion) in KTRs and cohabitants by enzyme-linked immunosorbent spot (ELISpot) assay before vaccination and after 2 vaccine doses (Figure 1d). In line with previous reports (e.g., Anft et al. 9), preexisting T-cell immunity to SARS-CoV-2 spike was detected in both KTRs and cohabitants (35% and 60%, respectively). T-cell responses were increased upon vaccination in 49% of transplant recipients compared with 93% of cohabitants. The median increase from baseline in T-cell response of transplant recipients was 12.6-fold lower than that of cohabitants (22 spot-forming units per 106 cells versus 278 spot-forming units per 106 cells).
This study provides the first assessment of vaccine-induced SARS-CoV-2 immunity in KTRs compared with synchronously vaccinated controls. KTRs were found to have a profoundly impaired capacity to generate specific IgG after 1 and 2 vaccine doses. Recent studies have also observed reduced T-cell responses in KTRs3; however, significant preexisting T-cell reactivity has confounded interpretation. Subtracting baseline T-cell reactivity allowed us to accurately measure the vaccine-induced cellular response and revealed a significant impairment in the capacity of KTRs to form antiviral T-cell immunity.
The majority of SARS-CoV-2 transmission occurs between household contacts,S1 and this is likely to be exaggerated in transplant recipients because of practiced caution at avoiding infection in the community. Although epidemiological studies will be important for assessing the effect of vaccination on virus transmission to immunocompromised individuals, live virus neutralization is the current best in vitro correlate of protection from SARS-CoV-2 infection. In the study cohort, all cohabitants met the threshold for effective serological neutralization, as well as that set for anti-RBD IgG titer. By contrast, only 8.7% of KTRs met at least one of the thresholds. The choice of vaccine did not significantly influence the immune response in KTRs; however, superior IgG titers and serological neutralization were observed in household controls who received BNT162b2 (Supplementary Figures S1–S4).
In light of recent findings of poor real-world effectiveness of the BNT162b2 and ChAdOx1 vaccines in solid organ transplant recipients, these data provide strong support for ring vaccination of cohabitants to reduce the risk of SARS-CoV-2 infection.2 The effectiveness of ring vaccination in the real world is likely to depend on additional factors, including vaccination status of the whole household, including children as viable vaccination strategies for children are developed, and the emergence and spread of immune-evasive SARS-CoV-2 variants. With no forthcoming strategy to enhance vaccine immunogenicity in KTRs, and efforts underway to develop booster vaccines against the Omicron variant, priority booster vaccination of household contacts should be the preferred vaccination strategy to protect immunocompromised transplant recipients.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This study was funded by project grants from The Hospital Research Foundation Group, Adelaide, Australia.
Footnotes
Supplementary Methods.
Figure S1. Participant age by treatment and vaccine. Differences between groups tested using the 2-tailed Mann-Whitney test. NS, not significant. ∗∗∗∗P < 0.0001.
Figure S2. Anti–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike IgG. (A,B) Antibody titers in close household contacts and kidney transplant recipients at baseline, 3 weeks after dose 1, and 3 weeks after dose 2. (C,D) Antibody titers compared by vaccine received. Differences between groups tested using the 2-tailed Mann-Whitney test. NS, not significant. ∗P < 0.05; ∗∗∗P < 0.001; ∗∗∗∗P < 0.0001.
Figure S3. Anti–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) humoral immune response by vaccine. (A,C) Anti–SARS-CoV-2 receptor-binding domain (RBD) IgG titers in close household contacts (CHCs) and kidney transplant recipients (KTRs) 3 weeks after the second vaccine dose. (B,D) Serological live virus neutralization in CHCs and KTRs 3 weeks after the second vaccine dose. Differences between groups tested using the 2-tailed Mann-Whitney test. NS, not significant. ∗∗∗∗P < 0.0001.
Figure S4. Vaccine-induced antiviral T-cell response. (A,C) Spike-reactive interferon-γ (IFN-γ)–secreting T cells in close household contacts (CHCs) and kidney transplant recipients (KTRs) at baseline and 3 weeks after dose 2. (C,D) Change in IFN-γ spot-forming units (SFUs) between baseline and 3 weeks after the second vaccine dose in CHCs and KTRs compared by vaccine received. Differences between groups tested using the 2-tailed Mann-Whitney test. NS, not significant. ∗∗∗∗P < 0.0001.
Supplementary Reference.
Supplementary Material
References
- 1.Phanish M., Ster I.C., Ghazanfar A., et al. Systematic review and meta-analysis of COVID-19 and kidney transplant recipients, the South West London Kidney Transplant Network experience. Kidney Int Rep. 2021;6:574–585. doi: 10.1016/j.ekir.2020.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Callaghan C.J., Mumford L., Curtis R.M., et al. Real-world effectiveness of the Pfizer-BioNTech BNT162b2 and Oxford-AstraZeneca ChAdOx1-S vaccines against SARS-CoV-2 in solid organ and islet transplant recipients. Transplantation. 2022;106:436–446. doi: 10.1097/TP.0000000000004059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prendecki M., Thomson T., Clarke C.L., et al. Immunological responses to SARS-CoV-2 vaccines in kidney transplant recipients. Lancet. 2021;398:1482–1484. doi: 10.1016/S0140-6736(21)02096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khoury D.S., Cromer D., Reynaldi A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 5.Cox R. Correlates of protection to influenza virus, where do we go from here? Hum Vacc Immunother. 2013;9:405–408. doi: 10.4161/hv.22908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall V.G., Ferreira V.H., Ku T., et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385:1244–1246. doi: 10.1056/NEJMc2111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bange E.M., Han N.A., Wileyto P., et al. CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat Med. 2021;27:1280–1289. doi: 10.1038/s41591-021-01386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Bert N., Tan A.T., Kunasegaran K., et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 9.Anft M., Blazquez-Navarro A., Stervbo U., et al. Detection of pre-existing SARS-CoV-2-reactive T cells in unexposed renal transplant patients. J Nephrol. 2021;34:1025–1037. doi: 10.1007/s40620-021-01092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.