Abstract
Background
Most approved vaccines utilise a two-dose strategy. To enable larger groups of patients to receive the first dose, the UK government increased the gap between the two doses from three to twelve weeks. Here we report on the immunogenicity of the first dose, including effect of age and vitamin D status on these levels over an 8 week-period.
Methods
Blood samples were collected from healthcare workers (HCW) receiving their first BNT162b2 vaccine dose between January and February 2021. Antibody (Ab) production was measured, prior to and weekly for 4 weeks post immunization, and a final measurement was performed at 8 weeks. Serum vitamin D concentrations were also measured at baseline.
Findings
Immunization of 97 HCW induced an Ab response that peaked 3•2 weeks post immunization to decrease thereafter. Ab levels remained positive at 8 weeks. IgG peak concentration was negatively associated with age (β=-0•440, p<0.001). Response to immunization was also significantly affected by vitamin D status (p=0•022), on average 29•3% greater peak value in individuals with 25(OH)D>50nmol/L. No other variable showed significant effect.
Interpretation
The first dose of BNT162b2 produced Ab levels that remained positive after 8 weeks. Peak was greater in younger subjects and 25(OH)D>50nmol/L was beneficial. Booster campaigns should take into consideration vitamin D status which is at its highest following a period of sunshine exposure or following oral supplementation (400-1000IU daily).
Funding
Abbott Diagnostics Ltd supplied the kits used to quantify the anti-SARS -CoV-2 Spike IgG and technical support as well as provided financial support for sample collections.
Keywords: SARS-CoV-2, immunization, BNT162b2, Vitamin D, Age
Introduction
As SARS-CoV-2 continues to affect the world, large scale immunization programmes are having a significant impact on hospitalization rates and mortality. Pfizer/BioNTech BNT162b2, thereafter BNT162b2, was the first COVID-19 vaccine authorized for use in the UK[1]. The decision of the UK government to extend the recommended interval between the two doses from three to 12 weeks[2,3] was received with scepticism around the potential loss or degradation of the immune response, especially as trial data was based on the shorter interval of three weeks.
Ageing is known to be accompanied by dysregulation of immune system functions and a higher incidence with more severe outcomes of respiratory infections such as pneumonia [4] and an increased occurrence of cancers [5] and autoimmune diseases [6]. A number of publications have suggested a beneficial effect of vitamin D supplementation against severe COVID-19 symptoms [7] and supplementation was recommended by the UK government in specific high risk population groups [8]. Moreover, vitamin D, specifically its active form 1-alpha,25-dihydroxyvitamin D3, is known to modulate the innate and adaptive immune response [9,10] and vitamin D is often used as a candidate hormone in improving immune response [11,12].
In this study we followed the anti-SARS-CoV-2 Spike IgG production in a cohort of health care workers (HCW), over eight weeks after receiving the first dose of the BNT162b2 mRNA vaccine, using a newly developed and validated assay that can quantify the antibody concentrations. We investigated the relationship between IgG response post immunization and 25(OH)D concentrations, age and other demographics.
Methods
Study design and subject cohorts
All participants provided written informed consent. The study was approved by the Health Research Authority Health and Care Research Wales ethical committee (IRAS#292799).
We assessed the immune response generated after immunization with BNT162b2 (Pfizer Inc. [New York, USA] and BioNTech SE [Mainz, Germany]) in a cohort of 105 HCW immunized at the Norfolk and Norwich University Hospital (Norwich, UK) in January 2021. Serum samples were taken at baseline (within a week prior to immunization, Bo), followed by weekly (7±1 days) sampling for four weeks (W1 to W4) after the first vaccine dose was administered and at week eight (W8), prior to administration of the second dose. A questionnaire provided self-declared demographic characteristics (age, gender, ethnicity), clinical characteristics (weight, height, health issues, current medication), and the presence of COVID-19 symptoms within the last six months.
Biochemistry
Abbott Alinity i system Immunoassays (Abbott Park, IL, USA) were used to measure SARS-CoV-2 anti-spike IgG (quantitative) and anti-nucleocapsid IgG (qualitative) in serum. Quantitative results are reported in AU/mL and the positivity cut-off as per manufacturer's instruction is 50AU/mL. Qualitative results are positive for index results above 1.4, the index is calculated based on the optical density of the sample in reference to the optical density of the calibrator using the equation ODsample/ODcal. These assays were previously validated [13,14]. Baseline 25(OH)D was measured by LC-MS/MS and 1,25(OH)2D was measured by Immunoassay (DiaSorin, Dartford, UK).
Statistical analysis
All statistical analyses and graphical representation were performed using IBM SPSS Statistics 25.0.0.1 and/or GraphPad Prism version 9.0 (GraphPad Software, Inc., USA). Statistical significance was considered as a two-tailed P value <0.05 (*P<0.05; **P<0.01 and ***P<0.001). IgG concentrations over time were analyzed using a mixed model paired test. Non normally distributed variables were LN transformed. Increase (peak) and decrease of antibody (peak-W8) concentrations were analyzed by linear regression using age (use as continuous or categorical by deciles for visualisation purposes only), BMI (continuous or categorical), 1,25(OH)2D (continuous variable) and 25(OH)D (continuous or categorical variable) as predictors. The final model included age, BMI and 25(OH)D only. Because 25(OH)D has a greater stability than its active form 1,25(OH)2D, i.e. 3 weeks vs 2-3 days, circulating 25(OH)D serves as an indicator of vitamin D status. Guidelines on vitamin D as outlined by Royal Osteoporosis Society 2018[15] define circulating 25(OH)D<25nmol/L as deficient, 25(OH)D between 25 and 50nmol/L as inadequate (insufficient) and 25(OH)D>50nmol/L as adequate or replete. We used these groupings to analyse 25(OH)D as a categorical variable.
Results
Participants
A total of 105 HCW provided at least one blood sample for antibody testing prior to immunization (baseline, Bo). After excluding HCW who had a measurable antibody baseline (58.1 to 5496.9 AU/mL, n=6) and those who did not seroconvert after immunization (anti-Spike IgG concentration not reaching values greater than 22.0 AU/mL, n=2), the remaining 97 HCW (mean [SD] age, 40.9 [11.0] years; 76 [78.4%] women; mean [SD] BMI, 26.4 [5.8]; 87 (89.7%) white; mean [SD] 25(OH)D; 47.5 [30.1], mean [SD] 1,25(OH)2D; 108.2 [34.3] pmol/L) were included in the study. The baseline characteristics of the participants appear in Table 1 .
Table 1.
Population characteristics. Characteristics of the HCW at baseline. Two non-responders and six HCW with a positive anti-Spike IgG were excluded.
| Baseline | |
|---|---|
| N | 97 |
| Age in years, mean (SD) <30yrs (n) 31-40yrs (n) 41-50yrs (n) >50yrs (n) |
40.9 (11.0) 21 22 34 19 |
| Weight in kg, mean (SD) | 74.4 (18.6) |
| Height in cm, mean (SD) | 167.4 (8.4) |
| BMI, mean (SD) distribution: n (%) <25 25-30 >30 |
26.4 (5.8) 51 (52) 25 (25.5) 21 (21.4) |
| Race, n (%) | |
| White | 87 (89.7) |
| BAME | 9 (9.3) |
| Prefer not to answer | 1 (1.0) |
| Sex, n (%) | |
| Male | 21 (21.6) |
| Female | 76 (78.4) |
| Reported Prior COVID-19 symptoms or infection, n (%) | 1 (1.0) |
| Antibody levels, median (IQR) | |
| Abbott IgG (AU/mL) [cut-off=50AU/mL] | 0.6 (0.0-1.9) |
| Abbott IgG Index (S/C) [cut-off=1.4] | 0.03 (0.02-0.07) |
| 25(OH)D (nmol/L), median (IQR) distribution: %, median (IQR) <25nmol/L 25-50nmol/L >50nmol/L 1,25(OH)2D (pmol/L), mean (SD) |
37.0 (24.2-67.6) 27.9%, 20.4 (16.5-22.5) 38.1%, 35.7 (30.8-39.1) 33.0%, 72..8 (65.4-79.2) 108.2 (34.3) |
Antibody profile post immunization
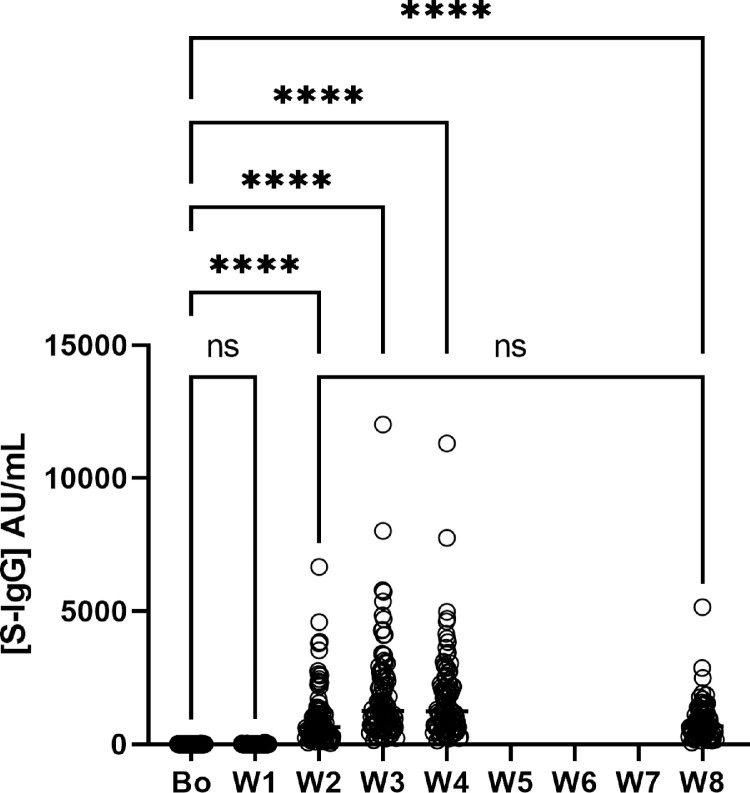
In all the remaining 97 HCW, the antibody concentration increased and peaked on average at 3.2 weeks (95% CI, 3.1-3.3). The concentrations of anti-Spike IgG did not change significantly at week 1 (p=0.3896) but increased sharply and significantly thereafter (Fig. 1 ) (p<0.0001). IgG peak concentrations were variable and ranged between 161-12020 AU/mL. Peaks were followed by a steady decrease in antibody concentrations over the following weeks (Table 2 ). Anti-nucleocapsid IgG were measured and not detected in any of these participants, confirming Ab response was not due to natural infection.
Fig. 1.
Anti-spike IgG concentration per week (median ± IQR) Graph showing the change in antibody concentration over time from baseline (pre-vaccination) to Week 4 then Week 8 values, with significant changes identified. ****(p<0•0001).
Table 2.
median and interquartile range of spike IgG concentrations over time.
| IgG] AU/mL | number | median [IQR] |
|---|---|---|
| Bo | 97 | 0.6 [0.0-1.9] |
| W1 | 97 | 1.3 [0.1-3.3] |
| W2 | 94 | 640.5 [295.5-1316.0] |
| W3 | 97 | 1263.0 [712.0-2555.0] |
| W4 | 91 | 1249.0 [659.8-2304.0] |
| W8 | 78 | 659.3 [338.4-1128] |
Association between antibody response and age and other characteristics
The group was composed of 79% females and 90% of participants were white, sex and ethnicity were therefore not included as criteria. BMI showed no association with the production of antibody (p=0.339) or with the decrease in antibody concentrations (p=0.574).
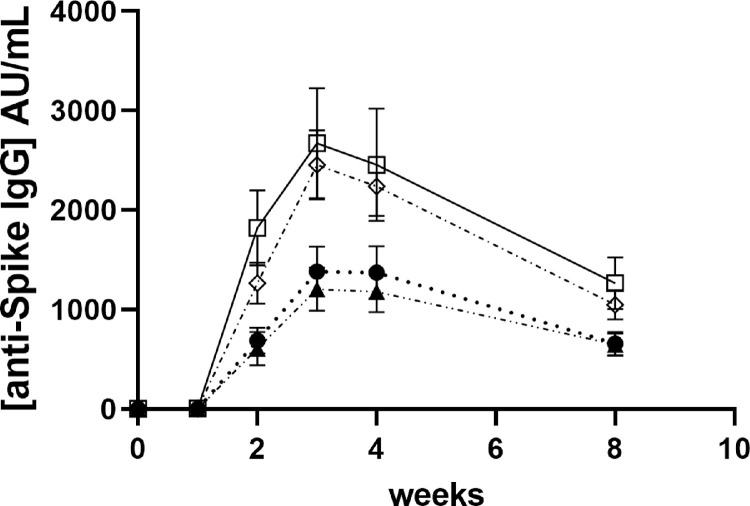
The peak in anti-Spike IgG was strongly negatively associated with age (continuous variable p<0.001). For visualisation purposes, we presented age by decile (Fig. 2 ). At W8, the concentrations of anti-Spike IgG had significantly decreased (p<0.001), on average by 53±11% (21-78%), to a median of 659.3 [338.4-1128.0] AU/mL. All subjects who demonstrated an Ab response following the first dose had positive IgG at W8, close to W2 levels (Wilcoxon p=0.729). Antibody concentrations also decreased in an age-dependent manner (p=0.002), the younger HCW with higher peaks decreasing faster to similar concentrations between ages (Kruskal-Wallis, p=0.703) at W8.
Fig. 2.
Time-course of antibody production after immunization per age group Values represent mean ±SEM; IgG concentrations are presented for each age groups ≤30y (empty squares and full line); 31-40y (empty diamonds and dot-dashed line); 41-50y (black circles and dotted line) and >50y (black triangles and dash-double dot line), prior immunization (Bo) and for 8 weeks (W1, W2, W3, S4 and W8) after immunisation.
Association between antibody response and age vitamin D status
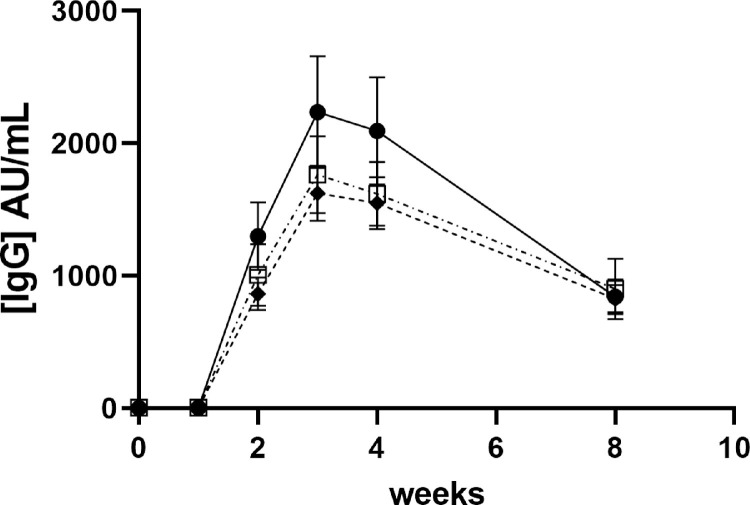
At baseline, only 34% of HCW had adequate 25(OH)D, 27.9% had deficient and 38.1% insufficient level. As a continuous variable, 25(OH)D showed association (p=0.019) with the production of Ab. However, there was a strong positive association between production of Ab and the categorised variable deficient/insufficient/replete (p=0.034) (Fig. 3 ) and an even stronger association when only using below and above 50nmol/L (p=0.013), mirroring clinical cut points. Loss of antibody after peak (peak – W8 concentrations) was also associated with 25(OH)D concentrations (p=0.040).
Fig. 3.
Time-course of antibody production after vaccination depending on 25(OH)D status Values represent mean ±SEM; IgG concentrations are presented for each 25(OH)D groups (≤25nmol/L (empty squares dash-dot line); 26-50nmol/L (black diamonds and dashed line); >50nmol/L (black circle and full line)) prior immunization (Bo) and for 8 weeks (W1, W2, W3, S4 and W8) after immunisation.
No association was observed between 1,25(OH)2D concentrations and peak IgG concentrations nor loss of IgG (p=0.104 peak; p=0.536 drop).
Discussion
In this study the initial dose of BNT162b2 triggered a serological response that is likely able to protect the recipients against COVID-19 infection by priming the immune system.
The older the subjects in this group the lower was the peak Ab response, however at W8, concentrations were similar between all ages. The age range of our subjects reflects the age of HCW currently employed with low incidence of very elderly subjects. Shroti et al., [16] reported an age dependent increase in the proportion of people who seroconvert over time. With a mean age of participants significantly higher than in our study, the study adds to the validity of our data suggesting that the effect of age is across the lifespan.
Circulating 25(OH)D concentrations are used as marker of vitamin D status. They are at their lowest during winter and spring seasons, lower in BAME and lower at higher latitudes. Across the UK, the expected prevalence of deficiency (<25nmol/L) in winter is 23.1% [17] and 10% in Caucasian women [18]. Almost a third (27.9%) of our group comprising close to 90% Caucasian women was 25(OH)D deficient, higher than the expected prevalence for this demographic. Repetitive lockdowns may have had a detrimental effect on the vitamin D status and may have other consequences for the health of the population (osteoporosis, other bone related disorders). There was a significantly higher Ab response observed in subjects with 25(OH)D >50nmol/L. The optimal concentration for 25(OH)D, leading to optimal 1,25(OH)2D and the best Ab response remains unclear in this study and would require larger numbers of participants studied to demonstrate definitive effects. A number of trials have shown variable effects of vitamin D and the vitamin D pathway polymorphism in improving vaccine effect for infectious diseases such as influenza (A/H1N1, A/H3N2) hepatitis B, measles, rubella, tuberculosis, pneumococcal, and meningococcal disease (for review see [19,20]). It is also important to remember that vitamin D has known effects on the immune system which are beyond the production of Ab [9,21]. High dose vitamin D treatment studies, commenced when COVID-19 infection was already established, have had variable success [22,23]. Our data would suggest that it is important to have good vitamin D status prior to COVID-19 infection or immunization to prime the immune response to be ready to combat the virus once exposure occurs. Once infected, high dose therapy may be relatively ineffective. From a Public Health point of view, a booster immunization programme would be best planned when the population's vitamin D is at its highest following a period of sunshine exposure (end of Summer or early Autumn) or after supplementation with a minimum daily dose of 400-1000 IU D3.
The strengths of this dataset include the use of a highly performing, quantitative assay to measure anti-spike IgG across a wide range of concentrations (21-40,000AU/mL) and traceable to the WHO International Standard for anti-SARS-CoV-2 immunoglobulin, allowing robust determination of differences in concentrations over time. This is the first study describing an effect of vitamin D on the response to immunization against SARS-CoV-2. The potential implications of the observed beneficial effect of replete vitamin D [25(OH)D>50nmol/L] status is to ensure booster immunisation programs are planned when the population's vitamin D is at its highest following a period of sunshine exposure or supplementation.
Limitations
This is a single-centre study with a limited number of participants and is strongly biased towards female and white participants which might limit the generalizability of the findings.
Conclusions
Amongst HCW in a single UK centre, antibody profile after a first injection of BNT162b2 was associated with age and vitamin D status at baseline. Younger people were reaching higher peaks but decreasing to similar levels as older after 8 weeks. Vitamin D was also beneficial to the production of antibodies.
Criteria for authorship
Isabelle Piec: Sample collection, data collection, data analysis, literature search, data interpretation, writing original draft and revisions.
Laura Cook: Data collection and manuscript revision
Samir Dervisevic: Conceptualisation, methodology, interpretation, reviewing and editing
William D Fraser: Conceptualisation, methodology, resource, funding acquisition, interpretation, reviewing and editing
Scott Ruetten: Resources, reviewing and editing
Marvin Berman: Resources, reviewing and editing
Emma English: Conceptualisation, methodology, resource, supervision, funding acquisition, interpretation, reviewing and editing
W Garry John: Conceptualisation, methodology, resource, supervision, funding acquisition, interpretation, reviewing and editing
Declaration of Competing Interest
Two of the authors (SR and MB) are employees of Abbott Diagnostics Ltd who supplied the kits used to quantify the anti-SARS -CoV-2 Spike IgG and technical support as well as provided financial support for sample collections. All other authors have no conflict of interest.
Acknowledgements
The authors would like to thank Lucy Browne and Cameron Cornwell for they help with sampling our HCW. We also would like to thank Rachel Dunn for the analysis of 25(OH)D and Lee Shepstone for his advice on statistics. Finally, we wish to thank Abbott Diagnostics Ltd for their continuous technical support and providing the kits necessary to perform this study.
References
- 1.Walsh EE, Frenck RW, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deaprtment of Health and Social Care. Optimising the covid-19 vaccination programme for maximum short-term impact. 2022https://www.gov.uk/government/publications/prioritising-the-first-covid-19-vaccine-dose-jcvi-statement/optimising-the-covid-19-vaccination-programme-for-maximum-short-term-impact
- 3.Pfizer-BioNTech covid-19 vaccine (BNT162, PF-07302048). Vaccines and related biological products advisory committee briefing document, 10 Dec 2020. https://www.fda.gov/media/144246/download
- 4.Fleming DM, Elliot AJ. The impact of influenza on the health and health care utilisation of elderly people. Vaccine. Jul 2005;23(Suppl 1):S1–S9. doi: 10.1016/j.vaccine.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 5.White MC, Holman DM, Boehm JE, Peipins LA, Grossman M, Henley SJ. Age and cancer risk: a potentially modifiable relationship. Am J Prev Med. 2014;46(3 Suppl 1):S7–S15. doi: 10.1016/j.amepre.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watad A, Bragazzi NL, Adawi M, et al. Autoimmunity in the elderly: insights from basic science and clinics - a mini-review. Gerontology. 2017;63(6):515–523. doi: 10.1159/000478012. [DOI] [PubMed] [Google Scholar]
- 7.Kumar R, Rathi H, Haq A, Wimalawansa SJ, Sharma A. Elsevier B.V.; 2021. Putative roles of vitamin D in modulating immune response and immunopathology associated with COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nice. COVID-19 rapid guideline: vitamin D. 2020. [PubMed]
- 9.Bouillon R, Marcocci C, Carmeliet G, et al. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. 2019. p. 1109-1151. [DOI] [PMC free article] [PubMed]
- 10.Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2008;324(1):23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 11.Rosenblatt J, Bissonnette A, Ahmad R, et al. Immunomodulatory effects of vitamin D: implications for GVHD. Bone Marrow Transplant. Sep 2010;45(9):1463–1468. doi: 10.1038/bmt.2009.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avenell A, Cook JA, Maclennan GS, Macpherson GC. Vitamin D supplementation to prevent infections: a sub-study of a randomised placebo-controlled trial in older people (RECORD trial, ISRCTN 51647438) Age Ageing. Sep 2007;36(5):574–577. doi: 10.1093/ageing/afm091. [DOI] [PubMed] [Google Scholar]
- 13.Piec I, English E, Thomas MA, Dervisevic S, Fraser WD, John WG. Performance of SARS-CoV-2 serology tests: Are they good enough? PLoS One. 2021;16(2) doi: 10.1371/journal.pone.0245914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.English E, Cook L, Piec I, Dervisevic S, Fraser WD, John WG. Performance of the Abbott SARS-CoV-2 IgG II Quantitative antibody assay including the new Variants of Concern (VOC 202012/V1 (UK) and VOC 202012/V2 (South Africa)): and first steps towards global harmonization of COVID-19 antibody methods. J Clin Microbiol. 2021 doi: 10.1128/JCM.00288-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.VitaminD and bone health: a practical clinical guideline for patient management (2020).
- 16.Shrotri M, Fragaszy E, Geismar C, et al. Spike-antibody responses following first and second doses of ChAdOx1 and BNT162b2 vaccines by age, gender, and clinical factors - a prospective community cohort study (Virus Watch) medRxiv. 2021 doi: 10.1101/2021.05.12.21257102. 2021.05.12.21257102. [DOI] [Google Scholar]
- 17.Lin L-Y, Smeeth L, Langan S, Warren-Gash C. Distribution of vitamin D status in the UK: a cross-sectional analysis of UK Biobank. BMJ Open. 2021;11(1) doi: 10.1136/bmjopen-2020-038503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darling AL, Hart KH, Macdonald HM, et al. Vitamin D deficiency in UK South Asian Women of childbearing age: a comparative longitudinal investigation with UK Caucasian women. Osteoporos Int. Feb 2013;24(2):477–488. doi: 10.1007/s00198-012-1973-2. [DOI] [PubMed] [Google Scholar]
- 19.Sadarangani SP, Whitaker JA, Poland GA. Let there be light": the role of vitamin D in the immune response to vaccines. Expert Rev Vaccines. 2015;14(11):1427–1440. doi: 10.1586/14760584.2015.1082426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goncalves-Mendes N, Talvas J, Dualé C, et al. Impact of vitamin D supplementation on influenza vaccine response and immune functions in deficient elderly persons: a randomized placebo-controlled trial. Front Immunol. 2019;10:65. doi: 10.3389/fimmu.2019.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cantorna MT, Snyder L, Lin Y-D, Yang L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients. 2015;7(4):3011–3021. doi: 10.3390/nu7043011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah K, Saxena D, Mavalankar D. Vitamin D supplementation, COVID-19 and disease severity: a meta-analysis. QJM: Int J Med. 2021;114(3):175–181. doi: 10.1093/qjmed/hcab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grant WB, Lahore H, McDonnell SL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. Apr 2020;12(4) doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]