Introduction
Biopsy deferral in men with a negative prostate multiparametric magnetic resonance imaging (mpMRI) is gaining popularity in clinical practice, as mpMRI becomes integrated into prostate cancer (PCa) diagnostic pathways.1 However, clinically significant (cs)PCa may be diagnosed at subsequent template biopsy in men with negative pre-biopsy mpMRI.2 This study evaluated the proportion of men with csPCa diagnosis at template biopsy following a negative mpMRI and evaluated prostate-specific antigen (PSA) density as a predictor of future positive template biopsy.
Methods
This institutional review board-approved, retrospective, single-center, cross-sectional study identified 104 consecutive men with negative mpMRI (Prostate Imaging Reporting and Data System [PI-RADS] score 1 or 2) performed before transrectal ultrasound (TRUS)-guided systematic template biopsy between January 1, 2013, and August 1, 2021. mpMRI technique (Table 1) and reporting was compliant with PI-RADS.3 Standard 12-sample TRUS-guided template biopsies were performed using 18-gauge side-cutting needles. Age, clinical indication, PSA, prostate volume (measured on magnetic resonance imaging [MRI] using ellipsoid volume calculation), PSA density (PSAD) (PSA/volume), and clinical stage were recorded. The proportion of men with csPCa (defined as International Society of Urogenital Pathology [ISUP] grade group ≥2 PCa) diagnosed at subsequent template biopsy was recorded. PSAD comparison between groups was performed using an independent t-test and empiric receiver operator characteristic (ROC) curves generated.
Table 1.
Multiparametric MRI techniquea performed during the study period
| Imaging plane | Field of view (mm) | Matrix size | Slice thickness/gap (mm) | TR/TE (msec) | Echo train length | Flip angle | Acceleration factor | Receiver bandwidth (Hz/Voxel) | Acquisition time (min) | Number of signals averaged | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T2 TSEb | Coronal | 220x220 | 320x256 | 4.0/0 | 3890– | 27–35 | 111 | N/A | 122 | 4 min | 1–2 |
| Sagittal | 3.0/0 | 5250/105– | 4 min | ||||||||
| Axial | 3.0/0 | 125 | 4 min | ||||||||
| DWIc | Axial | 220x220 | 128x80 | 4.0/0 | 4200/90 | 1 | 90 | 2 | 1950 | 5 min | 4–10 |
| T1 GREd dynamic contrast | Axial | 220x220 | 128x128 | 4.0/0 | 4.3/1.3 | N/A | 12 | 2 | 488 | 2 min | 1 |
Combined TRIO Tim (Siemens Healthcare) and Discovery 750W (General Electric, Milwaukee WI).
Integrated pelvic surface coils (16 channels) with activated spine coils (12 channels). Clinical 3 Tesla systems: TRIO Tim (Siemens Healthcare) and Discovery 750W (General Electric, Milwaukee WI).
Turbo/Fast Spin Echo.
DWI = Diffusion weighted imaging performed with spectral fat suppression echo planar imaging with tridirectional motion probing gradients and B values of 0, 500, 1000 mm2/sec with automatic apparent diffusion coefficient map generation derived.
1500 mm2/sec DWI acquired or calculated separately.
Dynamic fast spoiled 3D Gradient Recalled Echo performed with a temporal resolution of 9 seconds after injection of 0.1 mmol/kg of gadobutrol (Gadovist, Bayer Inc. Toronto, ON) at a rate of 3 mL/sec.
Results
A summary of patient clinical variables by indication is provided in Table 2. Considering any PCa, 40.4% (42/104) of cancers were diagnosed, with 58.1% (25/43) in biopsy-naive and 27.9% (17/61) in the previous negative template biopsy groups (p<0.01). Overall, 11.5% (12/104) of men had csPCa (ISUP ≥2) diagnosed at template biopsy — 20.9% (9/43) in the biopsy-naive group and 4.9% (3/61) in the previous negative template biopsy group (p<0.01) (Table 2).
Table 2.
Distribution of clinically significant prostate cancer diagnosis and clinical parameters by clinical indication in a cohort of 104 men with negative (PI-RADS score 1 or 2) pre-biopsy multiparametric MRI
| Pre-biopsy (N=104) | p | ||
|---|---|---|---|
|
| |||
| Biopsy-naïve (n=43) | Previous negative template biopsy (n=61) | ||
| Age (years) | 64±8 | 63±6 | 0.54 |
| Prostate volume (mL) | 53.5±23.7 | 75.6±42.8 | <0.01 |
| PSA (ng/mL) | 9.2±6.1 | 12.7±10.1 | 0.11 |
| PSAD (ng/mL) | 0.21±0.16 | 0.20±0.15 | 0.86 |
| Diagnosis at subsequent template biopsy | <0.01 | ||
| Benign | 41.8% (18/43) | 72.1% (/61) | |
| ISUP 1 | 37.2% (16/43) | 22.9% (14/61) | |
| ISUP 2 | 18.6% (8/43) | 1.6% (1/61) | |
| ISUP 3 | 0% (0/43) | 3.2% (2/61) | |
| ISUP 4 | 2.3% (1/43) | 0% (0/61) | |
| ISUP 5 | 0% (0/43) | 0% (0/61) | |
ISUP: International Society of Urogenital Pathology; PSA: prostate-specific antigen; PSAD: prostate-specific antigen density.
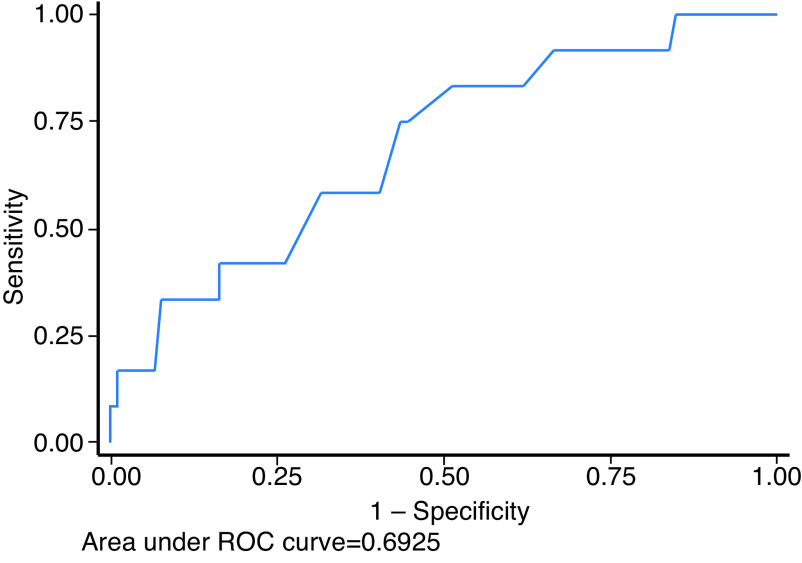
There was no difference in PSA or PSAD comparing the biopsy-naive to the previous negative template biopsy group (p>0.05), which were combined for all further analyses. Both PSA (p<0.01) and PSAD (p<0.01) were higher in men with csPCa diagnosed at subsequent template biopsy, but otherwise did not differ by indication (p=0.62) or diagnosis of any PCa (p>0.05). Area under the ROC curve for diagnosis of csPCa in the pre-biopsy group using PSA and PSAD were: 0.59 (95% confidence interval [CI] 0.0.42, 0.76) and 0.69 (0.0.53, 0.85) (Fig. 1). The optimal cutpoint for PSAD, derived by the method of Youden, was ≥0.18 ng/mL, with sensitivity and specificity of 75% and 57%, respectively. Patients were stratified into three groups by PSAD ≥0.10, ≥0.15, and ≥.0.20 ng/mL, as suggested previously,4 and diagnostic accuracy was calculated at each threshold (Table 3).
Fig. 1.
Empiric receiver operator characteristic (ROC) curve for diagnosis of clinically significant prostate cancer by prostate-specific antigen (PSA) density biopsy-naive men and those with previous negative template biopsy.
Table 3.
Diagnostic accuracy of PSAD in 104 pre-biopsy1 patients with negative MRI (PI-RADS score 1 or 2) using previously published PSAD thresholds to risk stratify men with eventual diagnosis of clinically significant (ISUP grade group ≥2, n=12) prostate cancer at subsequent template biopsy
| PSAD (ng/mL) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | Proportion of men with csPCa |
|---|---|---|---|---|---|
| 0.10 | 92% (62–100%) | 19% (11–28%) | 13% (7–22%) | 94% (73–100%) | 1/12 csPCa missed |
| 0.15 | 83% (52–98%) | 45% (34–55%) | 16% (8–28%) | 95% (84–99%) | 2/12 csPCa missed |
| 0.20 | 58% (28–85%) | 63% (52–73%) | 17% (7–32%) | 92% (82–97%) | 5/12 csPCa missed |
n=104, including biopsy-naive (n=44) and men with prior negative template biopsy (n=61).
CI: confidence interval; csPC: clinically significant prostate cancer; ISUP: International Society of Urogenital Pathology; NPV: negative predictive value; PPV: positive predictive value.
Discussion
In this study, PI-RADS score 1 or 2 plus PSAD was useful to predict eventual csPCa diagnosis in biopsy-naive men and those with previous negative template biopsy who under-went subsequent template biopsy. The optimal cutpoint of PSAD ≥0.18 yielded the highest accuracy for diagnosis of csPCa at subsequent template biopsy.
Data regarding biopsy deferral in at-risk men without a prior PCa diagnosis (including biopsy-naive men and those with previous negative biopsy) informed by negative mpMRI and PSAD are favorable. Previous studies have shown an eventual clinically significant PCa diagnosis rate in this population ranging from 4.4–17%,2,5 and have consistently shown that PSAD differs between those men with an eventual csPCa diagnosis and those with negative results at subsequent template biopsy. A recent study by Deniffel et al demonstrated that a PI-RADS plus PSAD ≥0.1 ng/mL strategy could safely reduce unnecessary biopsy without missing csPCa, outperforming other MRI-based risk models and did not require calibration.6
To our knowledge, the optimal PSAD level to optimally define a patient with negative pre-biopsy mpMRI who may benefit from template biopsy has not been derived. A 2020 review, which included 3006 biopsy-naive men in five studies, showed that MRI-negative men with low-risk PSAD (≤0.10 ng/mL) have a 3% risk of csPCa, those with low-intermediate risk PSAD (≤0.15 ng/mL) have a 7% risk of csPCa, and those with high-risk PSAD (≥0.20 ng/mL) have an 18% risk of csPCa.4 Our data are strikingly similar to this review, suggesting that with a PSAD <0.1, biopsy can be avoided, and with a PSAD>0.2, biopsy should be considered.
Our study has limitations. Our cohort consisted of men having pre-biopsy MRI with or without a preceding negative template biopsy and the mixed indications may be considered a limitation. Our sample is relatively small, consisting of approximately 104 men split relatively evenly into biopsy-naive and previous negative template biopsy cohorts. Not all patients with negative MRI undergo template biopsy at our institution and our results could be biased by those men who were referred for template biopsy compared to a consecutive cross-section of men with negative MRI.
Conclusions
Our results support a PI-RADS score 1 or 2 plus PSAD level strategy in biopsy-naive men and in those with previous negative template biopsy, which may predict which men may benefit from a template biopsy after negative MRI. The optimal PSAD level that should be used clinically with PI-RADS scoring in this clinical context requires further study.
Footnotes
Competing interests: Dr. Cagiannos has been an advisor for Abbvie; and has received grants and/or honoraria from Abbvie, Astellas, Bayer, and Ferring. Dr. Lavallée has participated in advisory boards for Astellas, Bayer, Ferring, Janssen, Knight, and Sanofi; and has received an educational grant (unrelated to current work) from Sanofi. Dr. Morash has participated in advisory boards for Abbvie, Amgen, Astellas, Bayer, Ferring, Janssen, Sanofi, and TerSera. The remaining authors do not report any competing personal or financial interests related to this work.
This paper has been peer-reviewed.
References
- 1.Haider MA, Brown J, Chin J, et al. Program in evidence-based Care Guideline No.: 27-2 Version 2. Ontario Health Cancer Care Ontario; 2021. [Accessed Oct 18, 2021]. Multiparametric magnetic resonance imaging in the diagnosis of clinically significant prostate cancer. Available at: https://www.cancercareontario.ca/sites/ccocancercare/files/guidelines/summary/pebc27-2v2s.pdf. [Google Scholar]
- 2.Buisset J, Norris JM, Puech P, et al. Negative pre-biopsy magnetic resonance imaging and risk of significant prostate cancer: Baseline and long-term followup results. J Urol. 2021;205:725–31. doi: 10.1097/JU.0000000000001414. [DOI] [PubMed] [Google Scholar]
- 3.Purysko A, Rosenkrantz AB, Barentsz JO, et al. PI-RADS Version 2: A pictorial update. Radiographics. 2016;36:1354–72. doi: 10.1148/rg.2016150234. [DOI] [PubMed] [Google Scholar]
- 4.Schoots I, Padhani A. Risk-adapted biopsy decision based on prostate MRI and PSA-density for enhanced biopsy avoidance in first prostate cancer diagnostic workup. BJU Int. 2021;127:175–8. doi: 10.1111/bju.15277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norris JM, Carmona Echeverria LM, Bott SRJ, et al. What type of prostate cancer is systematically overlooked by multiparametric magnetic resonance imaging? An analysis from the PROMIS Cohort. Eur Urol. 2020;78:163–70. doi: 10.1016/j.eururo.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deniffel D, Healy GM, Dong X, et al. Avoiding unnecessary biopsy: MRI-based risk models vs. a PI-RADS and PSA density strategy for clinically significant prostate cancer. Radiology. 2021;300:369–79. doi: 10.1148/radiol.2021204112. [DOI] [PubMed] [Google Scholar]