There are prevailing concerns about emergence of antibiotic resistant Neisseria gonorrhea (NG) throughout Africa1, 2-8. We evaluated test of cure positivity after NG patients treated with either ciprofloxacin or ceftriaxone in Kigali, the capital of Rwanda.
The Center for Family Health Research (CFHR) implemented a program for prospective diagnosis and treatment of STI and vaginal dysbioses between January 2016-August 2019. Radio announcements and pharmacists recruited symptomatic patients to seek services at CFHR where clinical data on symptoms were collected, genital exams performed, and patients were tested for HIV, syphilis, trichomoniasis, bacterial vaginosis, and vaginal candida. GeneXpert testing was used to diagnose NG and Chlamydia trachomatis.
Per the 2016 Rwandan National Guidelines for HIV and STI management9, patients diagnosed with NG were treated with ciprofloxacin (1g single dose tab for men, 500mg single dose tab for women), the first line recommendation at the time, and asked to return in 2-3 weeks for re-testing. Those who remained positive were treated with ceftriaxone (250mg IM single dose) and again asked to return in 2-3 weeks for retesting. Based on an interim review of the data presented here, the 2019 Rwandan National Guidelines10 were revised to recommend ceftriaxone (250mg IM single dose) as first line NG treatment. Thus, during the last months of the CFHR service delivery project, NG patients received ceftriaxone as first line treatment. We descriptively evaluated test of cure positivity among NG patients treated with ciprofloxacin or ceftriaxone who returned for follow-up testing.
All services were free and patients were not compensated. This program was determined to be non-research by the Rwandan National Ethics Committee and the Emory Institutional Review Board criteria.
Diagnosis and treatment of STI was provided during 1013 initial patient visits (not including test of cure follow-up visits) for men. and 579 initial patient visits for women. Eight hundred and eighty-nine NG diagnoses (87.8% of patient visits) were made among men and 197 NG diagnoses (16.8%) among women. Most patients did not return for test of cure testing.
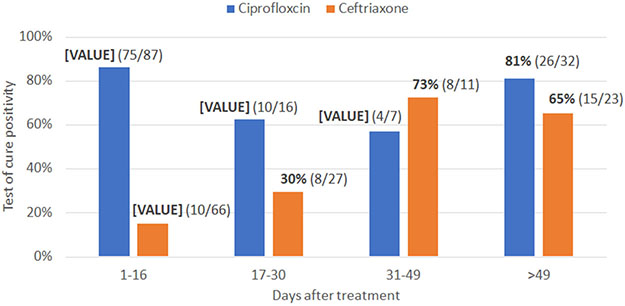
Figure 1 shows the results of repeat testing for NG after ciprofloxacin (n=142) or ceftriaxone (n=127) treatment stratified by the number of days between treatment and repeat testing. The proportion of patients who were positive for NG after ciprofloxacin treatment was 86% among those who came in ≤16 days after treatment. The proportion of patients who remained NG-positive ≤16 days after ceftriaxone treatment was 15%. That proportion increased as the number of days between treatment and testing increased. Possible reasons for positive tests after treatment are antibiotic resistance, reinfection from an untreated partner, and persistence of DNA detectable by GeneXpert for 2-3 weeks11. The latter two explanations would likely result in a similar proportion of NG-positive repeat tests regardless of treatment type. These results support that NG resistance to ciprofloxacin is prevalent.
Figure 1.
Test of cure positivity among patients treated for Neisseria gonorrhea with ciprofloxacin (n=142) or ceftriaxone (n=127)
Additionally, the high rate of repeat positives >49 days after ceftriaxone treatment suggests a high rate of reinfection. Index patients seen at CFHR were encouraged to recruit their sexual partners for testing. This resulted in about one-fifth of partners receiving treatment, though services were anonymous and partners were not linked to index cases.
CFHR has worked closely with the Rwanda Ministry of Health on research for improved HIV/STI and reproductive health care in government-run health centers for many years12-14. In 2019, the Rwanda National Guidelines10 were updated based on these findings and changed first line treatment for NG to ceftriaxone (250mg ceftriaxone IM single dose). These recommendations are in line with the World Health Organization (WHO)15 though notably do differ from the 2021 CDC NG treatment guidelines (which recommend 500mg ceftriaxone IM single dose for persons weighting <150kg)16.
Like many African countries which experience a disproportionate burden of global NG cases, Rwanda has a very limited NG surveillance program. The WHO is implementing a “Global Action Plan” for control of NG antimicrobial resistance17 including key recommendations to: discourage prescription and use of non-recommended antibiotics and non-adherence; strengthen surveillance systems; develop new NG treatments; and develop and support low-cost antimicrobial resistance diagnostics.
The importance of partner testing is highlighted by our data and emphasized in the 2019 Rwanda National Guidelines10. Partner notification involves identifying exposed sex partner(s) of index cases with STI; notifying them about their exposure; and offering testing, counselling, and treatment.
In conclusion, our findings confirm a high prevalence of ciprofloxacin-resistant NG in Rwanda. Systems to monitor antibiotic resistance are needed in the region. The high rates of NG reinfection observed in this study also suggest the need for more robust partner services.
Sources of funding.
This work was funded by the National Institutes of Health (NIAID R01 AI51231), the AIDS International Training and Research Program Fogarty International Center (D43 TW001042); and the Emory Center for AIDS Research (P30 AI050409). This work was partially funded by IAVI with the generous support of USAID, PEPFAR and other donors; a full list of IAVI donors is available at www.iavi.org. The contents of this manuscript are the responsibility of the authors and do not necessarily reflect the views of USAID or the US Government.
Footnotes
Conflicts of interest. None declared
Contributor Information
Kristin M. Wall, Rwanda Zambia HIV Research Group, Department of Pathology & Laboratory Medicine, School of Medicine and Hubert Department of Global Health and Department of Epidemiology, Rollins School of Public Health, Laney Graduate School, Emory University, Atlanta, Georgia, USA 30322.
Julien Nyombayire, Projet San Francisco, Rwanda Zambia HIV Research Group, Kigali, Rwanda.
Rachel Parker, Rwanda Zambia HIV Research Group, Department of Pathology & Laboratory Medicine, School of Medicine and Hubert Department of Global Health, Rollins School of Public Health, Laney Graduate School, Emory University, Atlanta, Georgia, USA 30322.
Rosine Ingabire, Projet San Francisco, Rwanda Zambia HIV Research Group, Kigali, Rwanda.
Jean Bizimana, Projet San Francisco, Rwanda Zambia HIV Research Group, Kigali, Rwanda.
Jeannine Mukamuyango, Projet San Francisco, Rwanda Zambia HIV Research Group, Kigali, Rwanda.
Amelia Mazzei, Projet San Francisco, Rwanda Zambia HIV Research Group, Kigali, Rwanda.
Matt A. Price, IAVI, NY, NY, University of California San Francisco, San Francisco, CA, USA 94115.
Marie Aimee Unyuzimana, Projet San Francisco, Rwanda Zambia HIV Research Group, Kigali, Rwanda.
Amanda Tichacek, Rwanda Zambia HIV Research Group, Department of Pathology & Laboratory Medicine, School of Medicine and Hubert Department of Global Health, Rollins School of Public Health, Laney Graduate School, Emory University, Atlanta, Georgia, USA 30322.
Susan Allen, Rwanda Zambia HIV Research Group, Department of Pathology & Laboratory Medicine, School of Medicine and Hubert Department of Global Health, Rollins School of Public Health, Laney Graduate School, Emory University, Atlanta, Georgia, USA 30322.
Etienne Karita, Projet San Francisco, Rwanda Zambia HIV Research Group, Kigali, Rwanda.
Data availability:
Data are available upon request to the first author.
References
- 1.Ndowa FJ, Francis JM, Machiha A, Faye-Kette H, Fonkoua MC. Gonococcal antimicrobial resistance: perspectives from the African region. Sex Transm Infect. 2013;89 Suppl 4:iv11–15. [DOI] [PubMed] [Google Scholar]
- 2.Van Dyck E, Karita E, Abdellati S, et al. Antimicrobial susceptibilities of Neisseria gonorrhoeae in Kigali, Rwanda, and trends of resistance between 1986 and 2000. Sex Transm Dis. 2001;28(9):539–545. [DOI] [PubMed] [Google Scholar]
- 3.Moodley P, Sturm AW. Ciprofloxacin-resistant gonorrhoea in South Africa. Lancet (London, England). 2005;366(9492):1159. [DOI] [PubMed] [Google Scholar]
- 4.Maduna LD, Kock MM, van der Veer B, et al. Antimicrobial Resistance of Neisseria gonorrhoeae Isolates from High-Risk Men in Johannesburg, South Africa. Antimicrobial agents and chemotherapy. 2020;64(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juma M, Sankaradoss A, Ndombi R, et al. Antimicrobial Resistance Profiling and Phylogenetic Analysis of Neisseria gonorrhoeae Clinical Isolates From Kenya in a Resource-Limited Setting. Frontiers in microbiology. 2021;12:647565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mabonga E, Parkes-Ratanshi R, Riedel S, et al. Complete ciprofloxacin resistance in gonococcal isolates in an urban Ugandan clinic: findings from a cross-sectional study. International journal of STD & AIDS. 2019;30(3):256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Dyck E, Crabbé F, Nzila N, et al. Increasing resistance of Neisseria gonorrhoeae in west and central Africa. Consequence on therapy of gonococcal infection. Sex Transm Dis. 1997;24(1):32–37. [DOI] [PubMed] [Google Scholar]
- 8.Bogaerts J, Tello WM, Akingeneye J, Mukantabana V, Van Dyck E, Piot P. Effectiveness of norfloxacin and ofloxacin for treatment of gonorrhoea and decrease of in vitro susceptibility to quinolones over time in Rwanda. Genitourin Med. 1993;69(3):196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ministry of Health, Rwanda. Ro. National Guidelines for Prevention and Management of HIV and STIs. 2016. [Google Scholar]
- 10.Ministry of Health, Rwanda. Ro. NATIONAL GUIDELINES FOR AND MANAGEMENT OF VIRAL HEPATITIS B, C AND SEXUALLY TRANSMITTED INFECTIONS. 2019. [Google Scholar]
- 11.CDC. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae--2014. MMWR Recomm Rep. 2014;63(RR-02):1–19. [PMC free article] [PubMed] [Google Scholar]
- 12.Karita E, Nsanzimana S, Ndagije F, et al. Implementation and Operational Research: Evolution of Couples' Voluntary Counseling and Testing for HIV in Rwanda: From Research to Public Health Practice. Journal of acquired immune deficiency syndromes (1999). 2016;73(3):e51–e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazzei A, Ingabire R, Mukamuyango J, et al. Community health worker promotions increase uptake of long-acting reversible contraception in Rwanda. Reprod Health. 2019;16(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ingabire R, Nyombayire J, Hoagland A, et al. Evaluation of a multi-level intervention to improve postpartum intrauterine device services in Rwanda. Gates Open Res. 2018;2(38):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO. WHO Guidelines for the Treatment of Neisseria gonorrhoeae. 2016; http://apps.who.int/iris/bitstream/handle/10665/246114/9789241549691-eng.pdf?sequence=1. Accessed Sept 13, 2021. [PubMed]
- 16.CDC. Sexually Transmitted Infections Treatment Guidelines, 2021. 2021; https://www.cdc.gov/std/treatment-guidelines/STI-Guidelines-2021.pdf. Accessed Sept 13, 2021.
- 17.WHO. Multi-drug resistant gonorrhoea. 2020; https://www.who.int/news-room/fact-sheets/detail/multi-drug-resistant-gonorrhoea. Accessed Dec 15, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request to the first author.