Summary
Background:
Glucose concentrations during an oral glucose tolerance test (OGTT) have been used as biomarkers to differentiate type 2 diabetes risk phenotypes. No studies have examined changes in OGTT-glucose phenotypes following lifestyle intervention among high-risk youth.
Objective:
To examine changes in OGTT-glucose phenotypes following lifestyle intervention and to explore differences in insulin sensitivity and β-cell function among post-intervention phenotypes.
Methods:
Latino adolescents with obesity (n = 48, age 15.4 ± 1.0, BMI% 98.2 ± 1.4, female 56.3%) completed a 12-week lifestyle intervention that included weekly nutrition education and physical activity. At baseline and 12 weeks, youth completed a 2-h OGTT with glucose and insulin concentrations assessed at 0′, 30′, 60′, 90′ and 120′. Glucose concentrations during the OGTT were used to identify biomarkers, 1-h glucose, glucose response curve and time to glucose peak. Using these respective biomarkers, high-risk (1-h glucose ≥ 155 mg/dl, Monophasic, Late Peak) and lower-risk phenotypes (1-h glucose < 155 mg/dl, Biphasic, Early Peak) were categorized. Insulin sensitivity was estimated by whole-body insulin sensitivity index (WBISI) and β-cell function by oral disposition index (oDI).
Results:
Following intervention, the prevalence of Monophasic phenotypes decreased from 81% to 67% (p = 0.048) and 1-h glucose ≥ 155 mg/dl from 38% to 10% (p = 0.054). Although Late Peak phenotypes did not significantly change (from 58% to 29%, p = 0.200), Early Peak phenotypes at post-intervention demonstrated significantly higher WBISI compared to Late Peak (2.3 ± 0.1 vs 1.7 ± 0.2, p = 0.023).
Conclusions:
OGTT-glucose phenotypes improve following lifestyle intervention among high-risk youth. These findings further support their potential utility as clinical biomarkers to identify diabetes risk and risk reduction in youth.
Keywords: diabetes prevention, exercise, health behaviour, nutrition, physical activity
1 |. INTRODUCTION
There is increasing interest in glucose concentrations during an oral glucose tolerance test (OGTT) as biomarkers that differentiate type 2 diabetes risk among youth and adults.1–10 These biomarkers include 1-h glucose concentrations,2,3,11 the shape of the glucose response curve,1,7,12,13 and the time to glucose peak.3,5,9,14 Each biomarker has been characterized into high (1-h glucose ≥ 155 mg/dl, Monophasic/Incessant-Increase, Late Peak) or lower (1-h glucose ≤ 155 mg/dl, Biphasic, Early Peak) risk phenotypes, hereafter termed OGTT-glucose phenotypes. Cross-sectional studies have exhibited an increased risk of type 2 diabetes among high-risk OGTT-glucose phenotypes as demonstrated by significantly reduced insulin sensitivity and β-cell function,5,11 a blunted incretin response3 and glucose intolerance7 compared to respective lower-risk phenotypes among youth.
Few studies have prospectively examined changes in type 2 diabetes risk factors among OGTT-glucose phenotypes. In a study of Latino youth with obesity, 1-h glucose ≥ 155 mg/dl predicted β-cell deterioration after 8 years of follow-up, compared to 1-h glucose < 155 mg/dl phenotypes.11 Using data from the TODAY study (Treatment Options for Type 2 Diabetes in Adolescents and Youth), youth with type 2 diabetes who were characterized by Monophasic or Incessant-Increase phenotypes demonstrated greater glycemic failure rates (42.3% and 58.3%, respectively) compared to the Biphasic phenotype group (39.1%).1 Another longitudinal study among youth with obesity and polycystic ovary syndrome demonstrated that 40% of Late Peak youth developed impaired glucose tolerance (IGT) after a two-year follow-up period, whereas no youth with the Early Peak phenotype developed IGT.15 These prospective studies expand upon previous work and demonstrate that high-risk OGTT-glucose phenotypes may confer future type 2 diabetes risk.
Lifestyle intervention is the first-line approach to preventing type 2 diabetes16 and results in improvements in insulin sensitivity and β-cell function.17 However, the literature is void of studies that assess the effects of lifestyle intervention on OGTT-glucose phenotypes and whether lifestyle-induced transitions of high- to lower-risk phenotypes (or vice versa) are associated with improvements in type 2 diabetes risk factors. Therefore, the purpose of this study is twofold and includes (1) to assess the effects of lifestyle intervention on OGTT-glucose phenotypes among a high-risk population of adolescents and (2) to explore whether post-intervention OGTT-glucose phenotypes are differentiated by insulin sensitivity and β-cell function following lifestyle intervention.
2 |. METHODS
This study represents a secondary data analysis from a randomized controlled trial testing the efficacy of a 12-week lifestyle intervention among Latino adolescents with obesity. Details of the parent study as well as the primary outcomes have been previously published.18,19
2.1 |. Participants
Inclusion criteria for participation in the parent study were (1) self-identification as Latino, (2) ages 14–16 years at enrollment and (3) obesity as defined as body mass index (BMI) ≥95th percentile for age and sex or BMI ≥ 30 kg/m2. Exclusion criteria included (1) taking medication(s) or diagnosed with a condition that influences carbohydrate metabolism, physical activity, or cognition, (2) type 2 diabetes, (3) enrollment in a formal weight loss program within 6 months of the start of the study period, or (4) diagnosed with depression or any other condition that may impact quality of life. The classification of OGTT-derived phenotypes requires glucose concentrations from five time points during OGTT (described below); therefore, the current analysis focuses on youth enrolled in the intervention arm of the original study that had complete OGTT-glucose values at baseline and post-intervention (n = 48). A consort diagram of the original study can be found elsewhere.20 All youth provided written assent and a parent/guardian provided written informed consent. This study was approved by the Arizona State University (ASU) Bioscience Institutional Review Board.
2.2 |. Lifestyle intervention
The 12-week intervention included weekly nutrition education and 3 days/week of physical activity. The nutrition education classes were delivered by bilingual health educators to youth and parents/guardians and targeted reductions in sugar-sweetened beverages and saturated fat and increases in fruit, vegetable and fiber intake. The physical activity sessions were delivered by certified trainers to groups of youth and designed to elicit an average heart rate of 150 beats per minute for each 60-minute session. Adherence to the lifestyle intervention was expressed as a percentage of nutrition education classes and physical activity sessions attended along with average heart rates during physical activity sessions, which were measured by Polar (Bethpage, NY) heart rate monitors.
2.3 |. Procedures
All participants arrived at the ASU clinical research unit after a 10–12 h fast. Height and weight were measured to the nearest 0.1 cm and 0.1 kg to calculate BMI and BMI% according to CDC Growth Charts.21 Pubertal growth stage was estimated by the pubertal development scale.22 Family history of type 2 diabetes including in utero exposure to gestational diabetes was measured by parental report. Body fat percent was estimated by bioelectric impedance scale (TBF-300A; Tanita Corporation of America, Arlington Heights, IL). A 75-g OGTT was administered with blood samples collected at 0′, 30′, 60′, 90′ and 120′ to measure insulin and glucose concentrations before and after the lifestyle intervention.
2.4 |. Biochemical assessment
Insulin was measured using the Stellux Insulin Chemiluminescence ELISA kit by ALPCO Diagnostics (Windham, NH). Glucose was measured by the cobas c111 analyser from Roche Diagnostics (Indianapolis, IN). Pre- and post-samples were used to analyse glucose and insulin concentrations were batched and measured in duplicate under identical conditions.
2.5 |. Classification of OGTT-glucose phenotypes
For the 1-h glucose biomarker, a 60′ glucose concentration of ≥155 mg/dl served as the threshold to differentiate high (≥155 mg/dl) and lower-risk phenotypes (<155 mg/dl).3 For the glucose response curve, biphasic phenotypes were classified as increasing to glucose peak before dropping by ≥4.5 mg/dl and increasing again by ≥4.5 mg/dl throughout the OGTT.7,23 Youth that did not meet the second rise in glucose by ≥4.5 mg/dl as with a biphasic shape were classified as Monophasic phenotypes. Youth that did not present an initial drop in glucose by ≥4.5 mg/dl were classified as Incessant-Increase phenotypes.12,13 Time to glucose peak phenotypes were differentiated by the time point of glucose peak with a 30′ peak classified as Early Peak and a peak any time after 30′ considered Late Peak.3,5
2.6 |. Type 2 diabetes risk factors
Insulin sensitivity was estimated by the whole-body insulin sensitivity index (WBISI), which is derived by the following equation24:
where I and G represent insulin and glucose concentrations at the respective sample collection time points listed in subscript text (in minutes). The WBISI has been validated against the gold standard and the hyperinsulinemic euglycemic clamp technique, among adults and youth with obesity.24,25 Insulinogenic index (IGI) was used to estimate insulin secretion (Δinsulin30′−0′/Δglucose30′−0′),26,27 and β-cell function was estimated by the oral disposition index (oDI) as WBISI × IGI.27
2.7 |. Analytical approach
Baseline differences between respective OGTT-glucose phenotypes were examined by independent t tests. Agreement of risk classification between respective high and lower risk OGTT-glucose phenotypes was examined by Cohen’s Kappa statistic (K), where <0 = Less than chance agreement, 0.01–0.20 = Slight agreement, 0.21–0.40 = Fair agreement, 0.41–0.60 = Moderate agreement, 0.61–0.80 = Substantial agreement and 0.81–0.99 = Almost perfect agreement.28 Changes in the prevalence of OGTT-glucose phenotypes (additionally grouped by high vs. lower risk) were examined using a chi-square statistical test. Youth were then classified into response phenotypes, which were based on the phenotype classification after the intervention. For example, an individual who maintained or transitioned into a Biphasic phenotype in response to the intervention was considered a Biphasic responder (i.e., Biphasic at post-intervention), and this example applied to all other response OGTT-glucose phenotypes examined. A paired t test was used to examine pre-/post-intervention changes in insulin sensitivity, β-cell function and glucose area under the curve (AUC) among all 48 youth. To analyse differences in insulin sensitivity and β-cell function after lifestyle intervention between OGTT-glucose response phenotypes, we applied an analysis of covariance (ANCOVA) model, adjusting for age, sex, BMI and the dependent variable (either WBISI or oDI) at baseline.29 SPSS Version 25 (Chicago, IL) was used with significance set at the 0.05 alpha level. Data are presented as mean ± SD or adjusted mean ± SE.
3 |. RESULTS
Comparisons of baseline characteristics between respective OGTT-glucose phenotypes are presented in Table 1. It should be noted that using the glucose response curve biomarker, only two youth were classified as Incessant-Increase phenotypes. Given that Monophasic and Incessant-Increase phenotypes are high-risk phenotypes and to maximize sub-sample sizes, the two Incessant-Increase phenotypes were combined with Monophasic phenotypes. Of the high-risk phenotypes, 38%, 81% and 58% of adolescents were classified as 1-h glucose ≥ 155 mg/dl, Monophasic and Late Peak, respectively. Agreement across OGTT-glucose phenotypes at baseline was fair for all comparisons: 1-h glucose and glucose response curve (κ = 0.243, p = 0.010), 1-h glucose and time to glucose peak (κ = 0.360, p = 0.007) and glucose response curve and time to glucose peak (κ = 0.302, p = 0.015).
TABLE 1.
Baseline characteristics across OGTT-glucose phenotypes among Latino youth (n = 48)
| 1-h Glucose |
Glucose response curve |
Time to glucose peak |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | 1-h glucose < 155 (n = 30) | 1-h glucose ≥ 155 (n = 18) | p value | Biphasic (n = 9) | Monophasic (n = 39) | p value | Early peak (n = 20) | Late peak (n = 28) | p value |
| Age, years | 15.3 ± 1.0 | 15.4 ± 1.0 | 0.779 | 15.2 ± 1.1 | 15.4 ± 1.0 | 0.665 | 15.2 ± 1.1 | 15.5 ± 0.9 | 0.428 |
| Female, N (%) | 20 (66.7%) | 7 (38.9%) | 0.060 | 6 (66.7%) | 21 (53.8%) | 0.485 | 11 (55.0%) | 16 (57.1%) | 0.762 |
| Prediabetes, N (%) | 2 (22.2%) | 11 (39.3%) | 0.716 | 5 (16.7%) | 8 (44.4%) | 0.040 | 3 (15.0%) | 10 (35.7%) | 0.111 |
| Family history, N (%) | 13 (43.3%) | 5 (27.8%) | 0.281 | 3 (33.3%) | 15 (38.5%) | 0.775 | 7 (35.0%) | 11 (65.0%) | 0.883 |
| PDS | 2.7 ± 0.5 | 2.6 ± 0.4 | 0.418 | 2.8 ± 0.5 | 2.6 ± 0.5 | 0.346 | 2.7 ± 0.5 | 2.7 ± 0.5 | 0.915 |
| BMI, kg/m2 | 34.0 ± 4.9 | 35.5 ± 4.6 | 0.318 | 33.0 ± 2.7 | 34.9 ± 5.1 | 0.271 | 35.5 ± 4.3 | 33.9 ± 5.0 | 0.247 |
| BMI% | 98.0 ± 1.5 | 98.6 ± 0.9 | 0.071 | 97.9 ± 1.4 | 98.3 ± 1.4 | 0.459 | 98.6 ± 0.9 | 97.9 ± 1.6 | 0.083 |
| WC, cm | 106.9 ± 10.4 | 111.7 ± 13.7 | 0.181 | 106.4 ± 7.0 | 109.3 ± 12.7 | 0.520 | 112.3 ± 11.5 | 106.2 ± 11.6 | 0.076 |
| G0, mg/dl | 91.8 ± 5.4 | 94.7 ± 6.8 | 0.104 | 92.5 ± 7.2 | 93.0 ± 5.8 | 0.844 | 94.6 ± 4.3 | 91.6 ± 6.8 | 0.091 |
| G120, mg/dl | 114.7 ± 17.7 | 132.3 ± 20.7 | 0.003 | 117.3 ± 17.5 | 122.2 ± 21.3 | 0.528 | 112.3 ± 19.4 | 127.7 ± 19.1 | 0.009 |
| HOMA-IR | 4.9 ± 2.6 | 6.0 ± 3.1 | 0.161 | 5.1 ± 2.8 | 5.4 ± 2.8 | 0.773 | 5.9 ±2.9 | 4.9 ± 2.7 | 0.223 |
| WBISI | 2.1 ± 1.2 | 1.3 ± 0.6 | 0.012 | 2.0 ± 1.2 | 1.7 ± 1.0 | 0.524 | 1.7 ± 1.0 | 1.8 ± 1.1 | 0.737 |
| oDI | 8.7 ± 5.5 | 3.2 ± 1.2 | <0.001 | 11.2 ± 5.6 | 5.6 ± 3.6 | 0.002 | 6.9 ± 3.5 | 6.5 ± 6.1 | 0.832 |
Note: Data presented as Mean ± SD.
Abbreviations: G0, fasting glucose; G120, 2-h glucose during OGTT; HOMA-IR, homeostatic model assessment of insulin resistance; oDI, oral disposition index; PDS, pubertal development scale; WBISI, whole-body insulin sensitivity index; WC, waist circumference.
Comparisons of insulin sensitivity and β-cell function between respective OGTT-glucose phenotypes are listed in Table 1. The 1-h glucose ≥ 155 mg/dl phenotype presented with 38.1% lower insulin sensitivity and 63.2% lower β-cell function (p < 0.05) compared to 1-h glucose < 155 mg/dl phenotype. For the glucose response curve biomarker, Monophasic phenotypes demonstrated 50.0% lower β-cell function (p < 0.05), with no difference in insulin sensitivity. For the time to glucose peak biomarker, Late versus Early Peak phenotypes were similar in insulin sensitivity and β-cell function at baseline.
Changes in the prevalence of OGTT-glucose phenotypes in response to lifestyle intervention are presented in Figure 1. There were significant reductions in the prevalence of Monophasic phenotypes (p = 0.048) and a trend for reductions in the prevalence of 1-h glucose ≥ 155 mg/dl (p = 0.054) following lifestyle intervention. The prevalence of Late Peak phenotypes after lifestyle intervention was reduced, but was not significant (p = 0.200).
FIGURE 1.
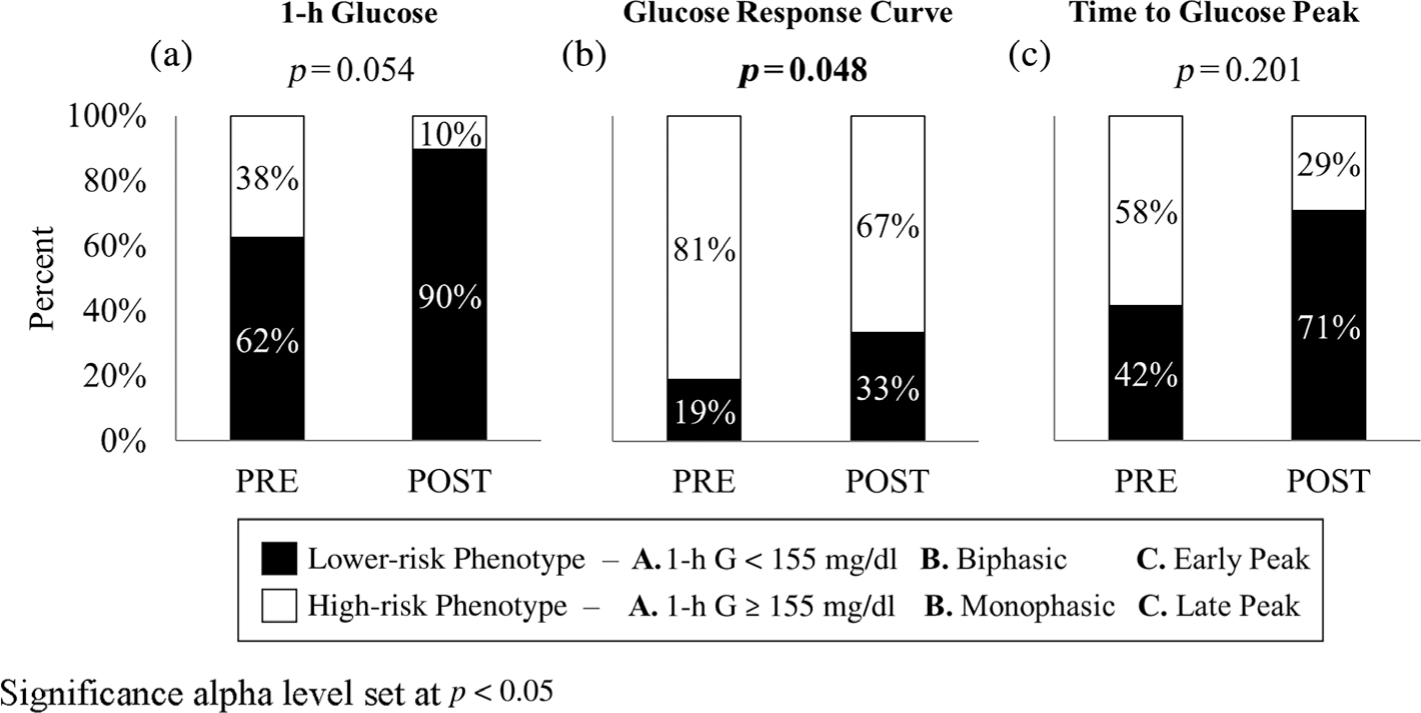
Prevalence of respective high and lower-risk OGTT-glucose phenotypes before (PRE) and after (POST) lifestyle intervention. Significance alpha level set at p < 0.05
As a whole, the intervention led to significant increases in insulin sensitivity (from 1.7 ± 0.2 to 2.1 ± 0.1, p = 0.015) and β-cell function (from 6.7 ± 0.7 to 10.0 ± 1.3, p = 0.006), and reductions in glucose AUC (from 16122.0 ± 320.4 to 14973.4 ± 291.6 mg h/dl, p = 0.002) among the 48 participants (Figure 2). Differences in insulin sensitivity and β-cell function between each respective OGTT-glucose response phenotype after lifestyle intervention are plotted in Figure 3. The most robust finding was that Early Peak response phenotypes demonstrated 35.3% (p = 0.023) greater insulin sensitivity after lifestyle intervention compared to Late Peak response phenotypes. No other differences in insulin sensitivity or β-cell function were found after lifestyle intervention between the respective OGTT-glucose phenotype comparisons. Within-group effects across all potential transition phenotypes are described in Table S1.
FIGURE 2.
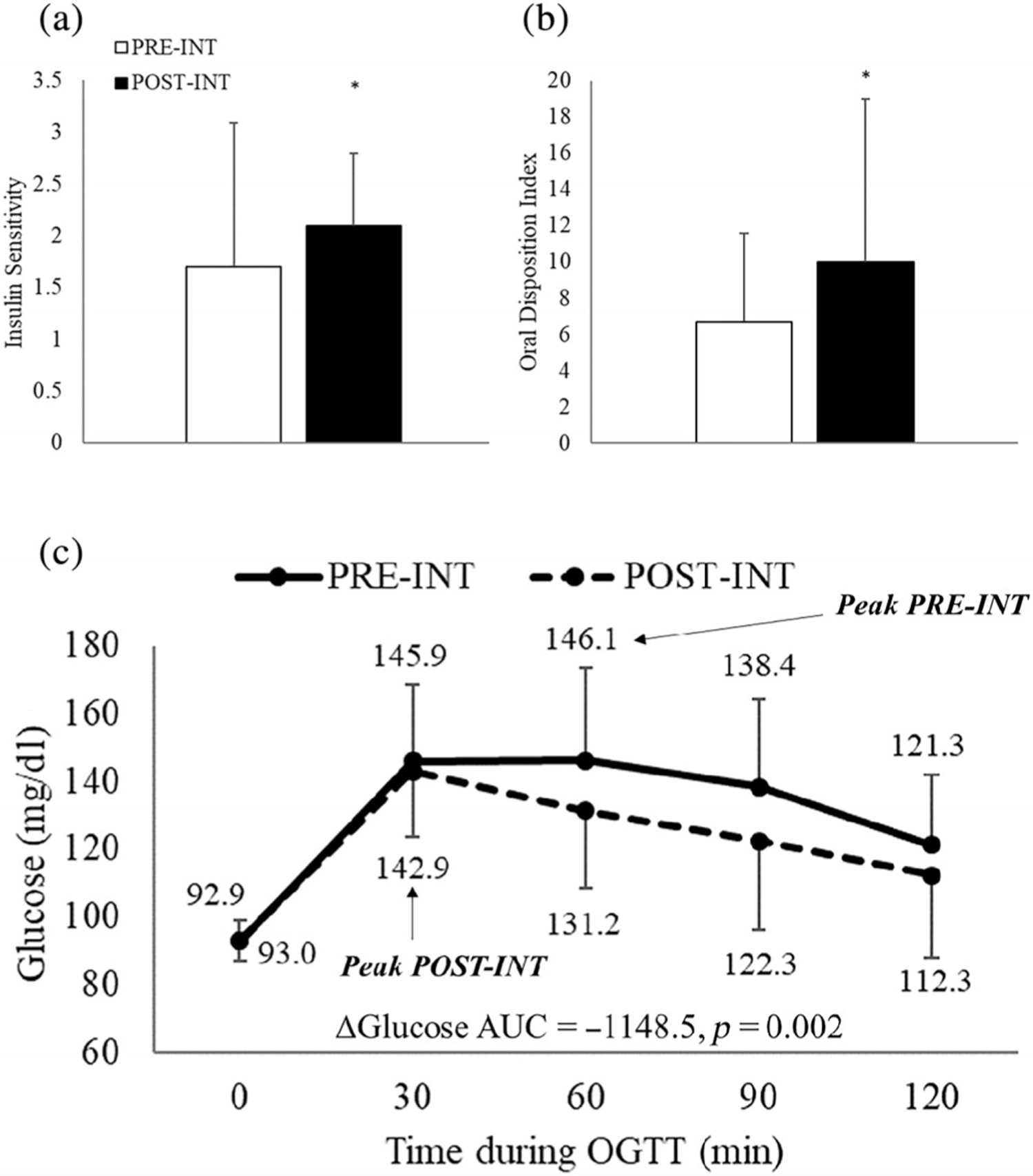
Change in WBISI (Panel A), oDI (Panel B) and glucose AUC (Panel C) among the entire cohort of analysis (n = 48)
FIGURE 3.
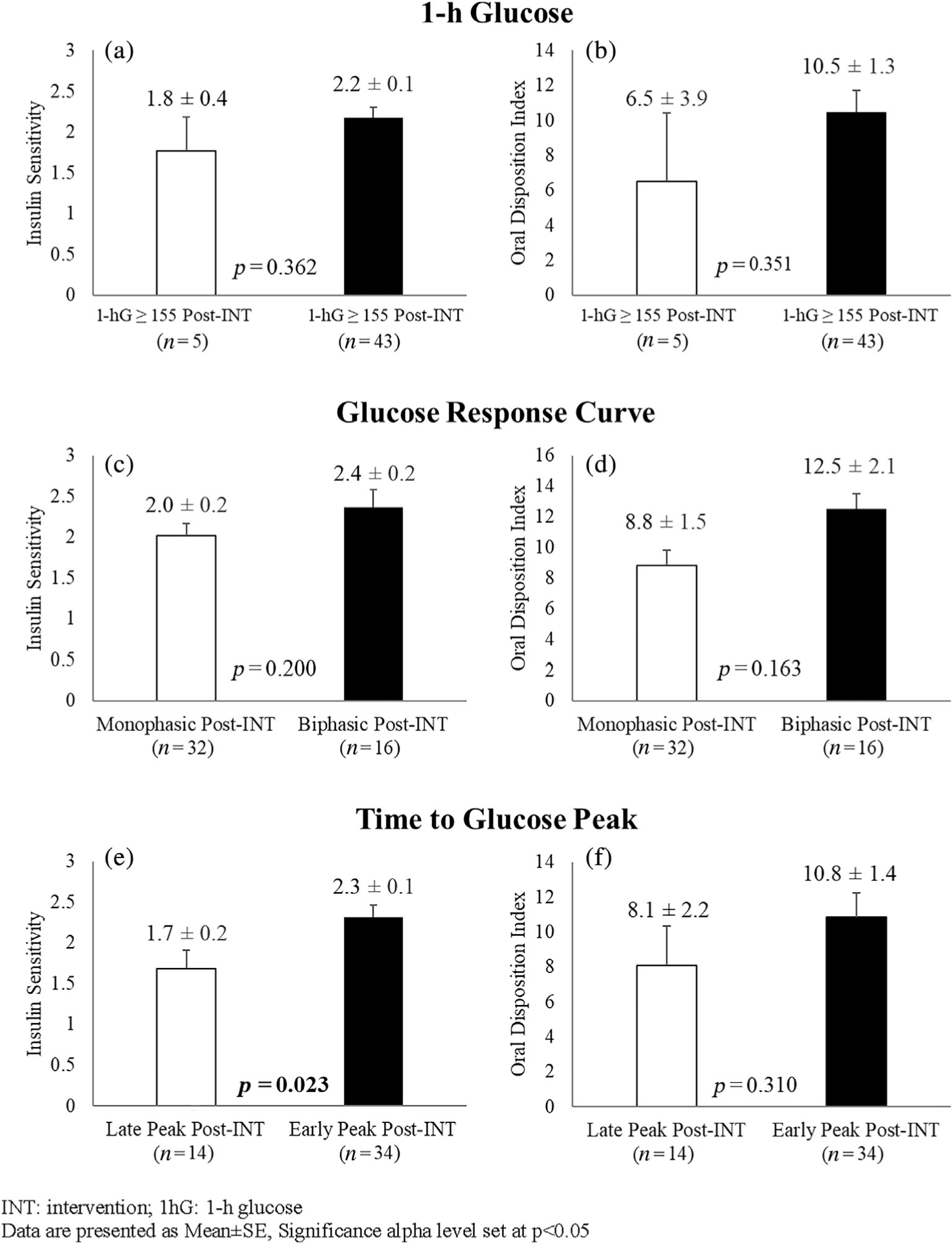
Differences in insulin sensitivity by WBISI (Panels A, C, E) and β-cell function by oDI (Panels B, D, F) between respective OGTT-glucose phenotypes after intervention (Post-INT). INT, intervention; 1hG, 1-h glucose. Data are presented as mean ± SE, significance alpha level set at p < 0.05
Attendance was not significantly different between respective glucose response curve (Monophasic at post-intervention: 85.6%, Biphasic at post-intervention: 86.8%, p = 0.809) and time to glucose peak response phenotypes (Late Peak at post-intervention: 87.4%, Early Peak at post-intervention: 82.5%, p = 0.324), but was significantly greater among 1-h glucose < 155 mg/dl compared to 1-h glucose ≥ 155 mg/dl response phenotypes (87.9% vs 69.7%, p = 0.011). Average heart rates during the exercise portion of the intervention were not significantly different between any respective OGTT-glucose response phenotype (1-h glucose ≥ 155 mg/dl at post-intervention: 154.1 beats/min, 1-h glucose < 155 mg/dl at post-intervention: 153.7 beats/min, p = 0.921), (Monophasic at post-intervention: 152.0 beats/min, Biphasic at post-intervention: 157.1 beats/min, p = 0.058) and (Late Peak at post-intervention: 152.8 beats/min, Early Peak at post-intervention: 154.1 beats/min, p = 0.630).
4 |. DISCUSSION
Previous studies have established a differential risk for type 2 diabetes between respective OGTT-glucose phenotypes among youth.1,3,7,11–13 Our findings add novelty to the body of knowledge in this field and suggest that higher risk OGTT-glucose phenotypes, namely, Monophasic and 1-h glucose ≥ 155 mg/dl, may be responsive to lifestyle intervention among a high-risk youth population. Furthermore, transitioning into an Early Peak phenotype after lifestyle intervention may be associated with increases in insulin sensitivity.
Studies among youth demonstrate that 1-h glucose ≥ 155 mg/dl and Monophasic glucose response curve at baseline are prospectively associated with worsening type 2 diabetes profiles (e.g., β-cell deterioration, glycemic failure) compared to their healthier phenotype counterparts.1,11 The present investigation extends these findings and suggests that these phenotypes can improve among high-risk youth in response to lifestyle intervention. However, the mechanisms that mediate changes and the clinical significance of transitioning from a high-risk phenotype to a lower-risk phenotype have yet to be elucidated, particularly since we found no significant differences in insulin sensitivity and β-cell function between respective glucose response curve (Biphasic vs. Monophasic) and 1-h glucose (1-h glucose < 155 mg/dl versus 1-h glucose ≥ 155 mg/dl) response phenotypes following lifestyle intervention. It is important to consider that the 1-h glucose ≥ 155 mg/dl group at post-intervention only included five adolescents, and thus, interpretations should be made with caution.
It is expected that changes in insulin sensitivity and β-cell function would contribute to changes in OGTT-glucose phenotypes given that these factors contribute largely to the regulation of glucose in the post-prandial state.30 Reductions in type 2 diabetes incidence in the Diabetes Prevention Program were associated with enhancements in β-cell function supported by improvements in insulin secretion relative to insulin sensitivity among high-risk adults.17 The Yale Bright Bodies Healthy Lifestyle Program was an adapted DPP for youth with IGT that demonstrated significant increases in insulin sensitivity but no significant changes in insulin secretion, or β-cell function compared to a standard clinical care group.31 Our study exhibited differential effects on insulin sensitivity only among one of the respective phenotype comparisons with no differences in insulin secretion (data not shown) or β-cell function following lifestyle intervention. Studies that assess β-cell function relative to insulin sensitivity in response to lifestyle intervention in youth are scarce.32 Therefore, it is difficult to speculate whether improvements in β-cell function after lifestyle intervention are predominantly due to changes in insulin secretion or insulin sensitivity among high-risk youth. It is possible that reductions in type 2 diabetes risk occur through increases in insulin sensitivity and that more aggressive interventions may be needed to improve β-cell function measures in youth. Furthermore, other factors related to glucose regulation during an OGTT, such as endogenous glucose production,33 the incretin response,3,8 glucose retention in the splanchnic beds34 and sex,35 may contribute to changes in OGTT-glucose phenotypes. Future research is warranted to understand the mechanisms responsible for inducing changes in OGTT-glucose phenotypes in response to intervention.
Although we found no significant changes in the prevalence of time to glucose peak phenotypes following lifestyle intervention, insulin sensitivity was significantly increased among Early Peak responders compared to Late Peak responders. Previous work has demonstrated significantly increased prevalence of T-risk allele TCF7L2 rs7903146 variants among high-risk youth with Late Peak phenotypes compared to Early Peak phenotypes.15 The TCF7L2 rs7903146 variant has been negatively associated with insulin sensitivity and an increased risk for type 2 diabetes among high-risk youth.36 It is plausible that the time to glucose peak biomarker is not responsive to detecting changes in respective high- and lower-risk phenotypes following lifestyle intervention. A previous study among adolescent girls with obesity or overweight identified hepatic steatosis and inflammation as differentiating Early and Late Peak phenotypes.3 Given that both liver fat37 and inflammatory processes38 are associated with insulin resistance, these factors may have contributed to the observed differential response in insulin sensitivity between Early and Late Peak response phenotypes. Furthermore, Late Peak phenotypes have been characterized by a blunted incretin response as measured by lower glucagon-like peptide-1 (GLP-1) compared to Early Peak phenotypes.3 GLP-1 is known to influence insulin dynamics and post-prandial glucose regulation39 and is responsive to lifestyle changes.40,41 Therefore, it stands to reason that other factors not measured in the current study such as liver fat, inflammation, incretins and key genetic risk factors for type 2 diabetes may differentiate Early Peak and Late Peak response phenotypes. Further examination is warranted to understand the mechanisms that may differentiate the response of insulin sensitivity among Early Peak response phenotypes compared to Late Peak.
This study is the first to assess changes in OGTT-glucose phenotypes in response to lifestyle intervention among a high-risk youth population and adds to the growing body of knowledge about the utility of glucose phenotypes to identify type 2 diabetes risk. Our findings provide novel information to suggest that these phenotypes are malleable to a lifestyle intervention and therefore may support their utility as clinical biomarkers to assess diabetes risk reduction. Despite these strengths, we acknowledge that our study is not without limitations. This study was a secondary analysis and thus was not powered to detect significant changes in OGTT-glucose phenotypes prevalence or changes in type 2 diabetes risk factors between response phenotypes. Time to glucose peak phenotypes are classified by a 5-sample OGTT with 30′ sampling intervals, thereby limiting more precise glucose peak phenotyping methods that include more frequent sampling. Furthermore, time to insulin peak and area under the curve are differentiated by insulin sensitivity and β-cell function (relative to insulin sensitivity)42 and warrant future research. This study was conducted among Latino adolescents with obesity, and thus, the findings may not be generalizable to other populations. Our measures to estimate insulin sensitivity and β-cell function were derived from the OGTT and are not the gold standard in the field.25,27 Therefore, autocorrelation may influence our findings since biomarkers and risk factors examined used the insulin and glucose values from the same OGTT. Future research is needed to confirm these results using gold-standard measures of insulin sensitivity and β-cell function.
In conclusion, OGTT-glucose phenotypes may be responsive to lifestyle intervention among Latino adolescents with obesity. Furthermore, maintaining or transitioning into an Early Peak phenotype may be associated with increases in insulin sensitivity. Future larger trials are warranted to examine potential predictors of response and understand mechanisms that may explain the response of OGTT-glucose phenotypes. Such research may support the use of OGTT-glucose phenotypes as clinical biomarkers to assess risk and risk reduction among high-risk youth populations.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to all research participants and families who devoted their time and energy to making this research possible. This research was supported by funding from the National Institutes of Health/National Institute on Minority Health and Health Disparities (NIH/NIMHD), awards P20MD002316 and U54MD002316. Drs. Shaibi and Olson were supported by a grant from NIH, National Institute of Diabetes Digestive and Kidney Disease, NIDDK, (R01DK107579) and Peña through a NIDDK Diversity F31 grant (F31DK125037). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMHD or the NIH.
Funding information
National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Numbers: F31DK125037, R01DK107579; National Institute on Minority Health and Health Disparities, Grant/Award Numbers: P20MD002316, U54MD002316
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest to disclose.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
REFERENCES
- 1.Arslanian S, El Ghormli L, Young Kim J, et al. The shape of the glucose response curve during an Oral glucose tolerance test: forerunner of heightened glycemic failure rates and accelerated decline in β-cell function in TODAY. Diabetes Care. 2019;42(1):164–172. doi: 10.2337/dc18-1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kasturi K, Onuzuruike AU, Kunnam S, Shomaker LB, Yanovski JA, Chung ST. Two- vs one-hour glucose tolerance testing: predicting prediabetes in adolescent girls with obesity. Pediatr Diabetes. 2019; 20(2):154–159. doi: 10.1111/pedi.12803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cree-Green M, Xie D, Rahat H, et al. Oral glucose tolerance test glucose peak time is Most predictive of Prediabetes and hepatic Steatosis in obese girls. J Endocr Soc. 2018;2(6):547–562. doi: 10.1210/js.2018-00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng X, Yang N, Li Y, et al. The shape of the glucose response curve during an oral glucose tolerance test heralds β-cell function in a large Chinese population. BMC Endocr Disord. 2019;19(1):119. doi: 10.1186/s12902-019-0446-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung ST, Ha J, Onuzuruike AU, et al. Time to glucose peak during an oral glucose tolerance test identifies prediabetes risk. Clin Endocrinol. 2017;87(5):484–491. doi: 10.1111/cen.13416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bervoets L, Mewis A, Massa G. The shape of the plasma glucose curve during an oral glucose tolerance test as an indicator of Beta cell function and insulin sensitivity in end-pubertal obese girls. Horm Metab Res. 2015;47(6):445–451. doi: 10.1055/s-0034-1395551 [DOI] [PubMed] [Google Scholar]
- 7.Kim JY, Coletta DK, Mandarino LJ, Shaibi GQ. Glucose response curve and type 2 diabetes risk in Latino adolescents. Diabetes Care. 2012;35(9):1925–1930. doi: 10.2337/dc11-2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JY, Tfayli H, Bacha F, et al. β-Cell function, incretin response, and insulin sensitivity of glucose and fat metabolism in obese youth: relationship to OGTT-time-to-glucose-peak. Pediatr Diabetes. 2020;21(1): 18–27. doi: 10.1111/pedi.12940 [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Zhao X, Zhou R, et al. Delay in glucose peak time during the oral glucose tolerance test as an indicator of insulin resistance and insulin secretion in type 2 diabetes patients. J Diabetes Investig. 2018; 9(6):1288–1295. doi: 10.1111/jdi.12834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marathe CS, Horowitz M, Trahair LG, et al. Relationships of early and late glycemic responses with gastric emptying during an Oral glucose tolerance test. J Clin Endocrinol Metab. 2015;100(9):3565–3571. doi: 10.1210/JC.2015-2482 [DOI] [PubMed] [Google Scholar]
- 11.Kim JY, Goran MI, Toledo-Corral CM, Weigensberg MJ, Choi M, Shaibi GQ. One-hour glucose during an oral glucose challenge prospectively predicts β-cell deterioration and prediabetes in obese Hispanic youth. Diabetes Care. 2013;36(6):1681–1686. doi: 10.2337/dc12-1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arslanian SA, El Ghormli L, Young Kim J, et al. OGTT glucose response curves, insulin sensitivity, and β-cell function in RISE: comparison between youth and adults at randomization and in response to interventions to preserve β-cell function. Diabetes Care. 2021;44:817–825. doi: 10.2337/dc20-2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JY, Michaliszyn SF, Nasr A, et al. The shape of the glucose response curve during an Oral glucose tolerance test heralds biomarkers of type 2 diabetes risk in obese youth. Diabetes Care. 2016;39(8):1431–1439. doi: 10.2337/dc16-0352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin YC, Chen HS. Longer time to peak glucose during the oral glucose tolerance test increases cardiovascular risk score and diabetes prevalence. PLoS One. 2017;12(12):e0189047. doi: 10.1371/journal.pone.0189047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galderisi A, Tricò D, Dalla Man C, et al. Metabolic and genetic determinants of glucose shape after Oral challenge in obese youths: a longitudinal study. J Clin Endocrinol Metab. 2020;105(2):534–542. doi: 10.1210/clinem/dgz207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitabchi AE, Temprosa M, Knowler WC, et al. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes. 2005;54(8):2404–2414. doi: 10.2337/diabetes.54.8.2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams AN, Konopken YP, Keller CS, et al. Culturally-grounded diabetes prevention program for obese Latino youth: rationale, design, and methods. Contemp Clin Trials. 2017;03(54):68–76. doi: 10.1016/j.cct.2017.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soltero EG, Konopken YP, Olson ML, et al. Preventing diabetes in obese Latino youth with prediabetes: a study protocol for a randomized controlled trial. BMC Public Health. 2017;17(1):261. doi: 10.1186/s12889-017-4174-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soltero EG, Olson ML, Williams AN, et al. Effects of a community-based diabetes prevention program for Latino youth with obesity: a randomized controlled trial. Obesity. 2018;26(12):1856–1865. doi: 10.1002/oby.22300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat. 2002;246:1–190. [PubMed] [Google Scholar]
- 22.Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. J Adolesc Health. 1993;14(3):190–195. doi: 10.1016/1054-139x(93)90004-9 [DOI] [PubMed] [Google Scholar]
- 23.Tschritter O, Fritsche A, Shirkavand F, Machicao F, Häring H, Stumvoll M. Assessing the shape of the glucose curve during an oral glucose tolerance test. Diabetes Care. 2003;26(4):1026–1033. doi: 10.2337/diacare.26.4.1026 [DOI] [PubMed] [Google Scholar]
- 24.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. [DOI] [PubMed] [Google Scholar]
- 25.Yeckel CW, Weiss R, Dziura J, et al. Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. J Clin Endocrinol Metab. 2004;89(3):1096–1101. doi: 10.1210/jc.2003-031503 [DOI] [PubMed] [Google Scholar]
- 26.Uwaifo GI, Fallon EM, Chin J, Elberg J, Parikh SJ, Yanovski JA. Indices of insulin action, disposal, and secretion derived from fasting samples and clamps in normal glucose-tolerant black and white children. Diabetes Care. 2002;25(11):2081–2087. doi: 10.2337/diacare.25.11.2081 [DOI] [PubMed] [Google Scholar]
- 27.Caprio S The oral disposition index: a valuable estimate of β-cell function in obese youth. J Pediatr. 2012;161(1):3–4. doi: 10.1016/j.jpeds.2012.02.013 [DOI] [PubMed] [Google Scholar]
- 28.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37(5):360–363. [PubMed] [Google Scholar]
- 29.Liu S, Rovine MJ, Molenaar PCM. Selecting a linear mixed model for longitudinal data: repeated measures analysis of variance, covariance pattern model, and growth curve approaches. Psychol Methods. 2012;17:15–30. [DOI] [PubMed] [Google Scholar]
- 30.Cerf ME. Beta cell dysfunction and insulin resistance. Front Endocrinol. 2013;4:37. doi: 10.3389/fendo.2013.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savoye M, Caprio S, Dziura J, et al. Reversal of early abnormalities in glucose metabolism in obese youth: results of an intensive lifestyle randomized controlled trial. Diabetes Care. 2014;37(2):317–324. doi: 10.2337/dc13-1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JY, Jeon JY. Role of exercise on insulin sensitivity and beta-cell function: is exercise sufficient for the prevention of youth-onset type 2 diabetes? Ann Pediatr Endocrinol Metab. 2020;25(4):208–216. doi: 10.6065/apem.2040140.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lund A, Bagger JI, Christensen M, et al. Higher endogenous glucose production during OGTT vs Isoglycemic intravenous glucose infusion. J Clin Endocrinol Metab. 2016;101(11):4377–4384. doi: 10.1210/jc.2016-1948 [DOI] [PubMed] [Google Scholar]
- 34.Waldhäusl WK, Gasić S, Bratusch-Marrain P, Nowotny P. The 75-g oral glucose tolerance test: effect on splanchnic metabolism of substrates and pancreatic hormone release in healthy man. Diabetologia. 1983;25(6):489–495. doi: 10.1007/BF00284457 [DOI] [PubMed] [Google Scholar]
- 35.Anderwald C, Gastaldelli A, Tura A, et al. Mechanism and effects of glucose absorption during an oral glucose tolerance test among females and males. J Clin Endocrinol Metab. 2011;96(2):515–524. doi: 10.1210/jc.2010-1398 [DOI] [PubMed] [Google Scholar]
- 36.Cropano C, Santoro N, Groop L, et al. The rs7903146 variant in the TCF7L2 gene increases the risk of prediabetes/type 2 diabetes in obese adolescents by impairing beta-cell function and hepatic insulin sensitivity. Diabetes Care. 2017;40(8):1082–1089. doi: 10.2337/dc17-0290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thamer C, Machann J, Stefan N, et al. High visceral fat mass and high liver fat are associated with resistance to lifestyle intervention. Obesity. 2007;15(2):531–538. doi: 10.1038/oby.2007.568 [DOI] [PubMed] [Google Scholar]
- 38.Aguilar MJ, González-Jiménez E, Antelo A, Perona JS. Insulin resistance and inflammation markers: correlations in obese adolescents. J Clin Nurs. 2013;22(13–14):2002–2010. doi: 10.1111/jocn.12034 [DOI] [PubMed] [Google Scholar]
- 39.Nauck MA, Meier JJ. The incretin effect in healthy individuals and those with type 2 diabetes: physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol. 2016;4(6):525–536. doi: 10.1016/S2213-8587(15)00482-9 [DOI] [PubMed] [Google Scholar]
- 40.Keller J, Kahlhöfer J, Peter A, Bosy-Westphal A. Effects of low versus high glycemic index sugar-sweetened beverages on postprandial vasodilatation and inactivity-induced impairment of glucose metabolism in healthy men. Nutrients. 2016;8(12):802. doi: 10.3390/nu8120802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiss EP, Albert SG, Reeds DN, et al. Calorie restriction and matched weight loss from exercise: independent and additive effects on Glucoregulation and the Incretin system in overweight women and men. Diabetes Care. 2015;38(7):1253–1262. doi: 10.2337/dc14-2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayashi T, Boyko EJ, Sato KK, et al. Patterns of insulin concentration during the OGTT predict the risk of type 2 diabetes in Japanese Americans. Diabetes Care. 2013;36(5):1229–1235. doi: 10.2337/dc12-0246 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


