Abstract
AIMS.
The V122I variant in transthyretin (TTR) is the most common amyloidogenic mutation worldwide. The aim of this study is to describe the cardiac phenotype and risk for adverse cardiovascular outcomes of young V122I TTR carriers in the general population.
METHODS AND RESULTS.
TTR genotypes were extracted from whole-exome sequence data in participants of the Dallas Heart Study. Participants with African ancestry, available V122I TTR genotypes (N=1,818), and either cardiac magnetic resonance imaging (CMR) (n=1,364), or long-term follow-up (n=1,532) were included. The prevalence of V122I TTR carriers (45±10 years) was 3.2% (n/N=59/1,818). V122I TTR carriers had higher baseline LV wall thickness (LVWT, 8.52±1.82 vs. 8.21±1.62 mm; adjusted P=0.038) than non-carriers, but no differences in other CMR measures (P>0.05 for all). Although carrier status was not associated with amino terminal pro-B-type natriuretic peptide (NT-proBNP) at baseline (P=0.79), V122I TTR carriers had a greater increase in NT-proBNP on follow-up than non-carriers (median [interquartile range] 28.5 [11.4-104.1] vs. 15.9 [0.0–43.0] pg/mL; adjusted P=0.018). V122I TTR carriers were at a higher adjusted risk of heart failure (HF) (HR 3.82, 95% CI 1.80-8.13, P<0.001), cardiovascular death (HR 2.65, 95% CI 1.14-6.15, P=0.023), and all-cause mortality (HR 1.95, 95% CI 1.08-3.51, P=0.026) in comparison with non-carriers.
CONCLUSION.
V122I TTR carrier status was associated with a greater increase in NT-proBNP, slightly greater LVWT, and a higher risk for HF, cardiovascular death, and all-cause mortality. These findings suggest the need to develop amyloidosis screening strategies for V122I TTR carriers.
Keywords: Cardiac amyloidosis, transthyretin, heart failure
Grapgical Abstract
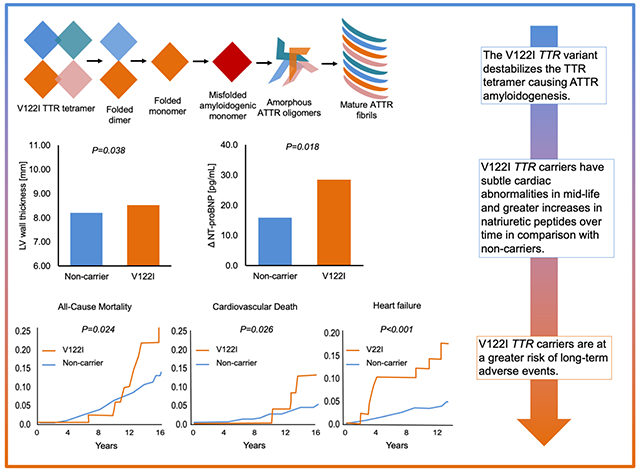
ONE-SENTENCE SUMMARY
Carriers of the V122I TTR mutation may have subtle differences in cardiac structure, greater changes in natriuretic peptides over time, and are at a higher risk of long-term events including heart failure and death.
LAY SUMMARY
The V122I variant in transthyretin (TTR) is the most common cause of hereditary cardiac amyloidosis worldwide – a disease where abnormal protein is deposited in the heart muscle, eventually leading to heart failure. However, early phases of the disease are not well known. 3.2% of individuals (45±10 years) with African ancestry in the Dallas Heart Study carried the V122I TTR variant. In comparison with non-carriers, V122I TTR carriers had subtle differences in cardiac structure, greater increases in biomarkers that indicate cardiac stress, and a higher risk of heart failure and death. This study highlights the need to develop amyloidosis screening strategies.
INTRODUCTION
Cardiac ATTR, whether hereditary due to a TTR genetic variant (ATTRv) or acquired (i.e. wild-type ATTR), is a progressive, infiltrative cardiomyopathy that leads to limitations in functional capacity, declines in quality of life, increased healthcare utilization, and higher risk of death.1 Fortunately, therapeutic options that have the potential to improve the outcomes of patients with either form of the disease are emerging rapidly.2, 3 In parallel, developments in noninvasive imaging techniques such as CMR imaging and bone scintigraphy have facilitated an increased recognition of ATTR.1, 4, 5
The valine-to-isoleucine substitution at position 122 (V122I; p.V142I) in the TTR protein causes tetrameric instability leading to amyloidogenesis,6 is the most common cause of ATTRv cardiomyopathy in the United States, and is nearly exclusive to individuals with African ancestry.7 Observations from epidemiologic studies have highlighted that carriers of the V122I TTR allele are at higher lifetime risk for HF than their non-carrier counterparts.8, 9
Although estimates suggest that penetrance of the V122I ATTRv phenotype increases with age,8 the overall penetrance of the V122I TTR allele remains unknown and may be related to other genetic and environmental interactions.10 As a result, the pre-symptomatic natural history is uncertain, and there is no consensus on age-appropriate screening strategies for asymptomatic V122I TTR carriers.11 The DHS, a large, multi-ethnic, population-based study with extensive clinical, genetic, imaging, and biomarker data, afforded the opportunity to test the hypotheses that young V122I TTR carriers, as compared with their non-carrier counterparts, may have both a distinct cardiac phenotype and increased risk for adverse cardiovascular outcomes.
METHODS
Study population
The DHS is a multiethnic probability-based population cohort study of adults in Dallas County, Texas that had intentional oversampling of self-identified Black individuals. Phase 1 of the DHS (DHS-1) was conducted between 2000 and 2002 and comprised of 3 visits: 1) initial home visit for collection of demographic, medical history, blood pressure, and anthropometric data (N=6,101); 2) second home visit for collection of blood and urine biospecimens (N=3,557); and 3) a final visit to the University of Texas Southwestern Medical Center for completion of detailed, protocolized, core lab imaging studies (N=2,971). Phase 2 of the DHS (DHS-2) was conducted between 2007 and 2009 and included alive and willing participants who underwent repeat cardiac imaging and fasting blood sample collection as previously described.12
The present analysis was restricted to DHS participants of genetically inferred African ancestry (defined below), who completed visit 2 of DHS-1 and had whole-exome sequencing data available through a collaboration with the Regeneron Genetics Center (Tarrytown, New York). The association between TTR V122I genotype and incident clinical outcomes was assessed in participants with long-term follow-up data (n=1,532, Figure 1). The association between TTR V122I genotype and cardiac phenotype was assessed in n=1,364 participants with CMR imaging data at baseline for DHS-1, and the association between TTR V122I genotype and changes in cardiac biomarkers, electrocardiogram parameters, and CMR data was assessed for n=872 participants with paired DHS-1 and DHS-2 data. This study complies with the Declaration of Helsinki, was approved by the University of Texas Southwestern Medical Center Institutional Review Board, and informed consent was obtained from all study subjects.
Figure 1.
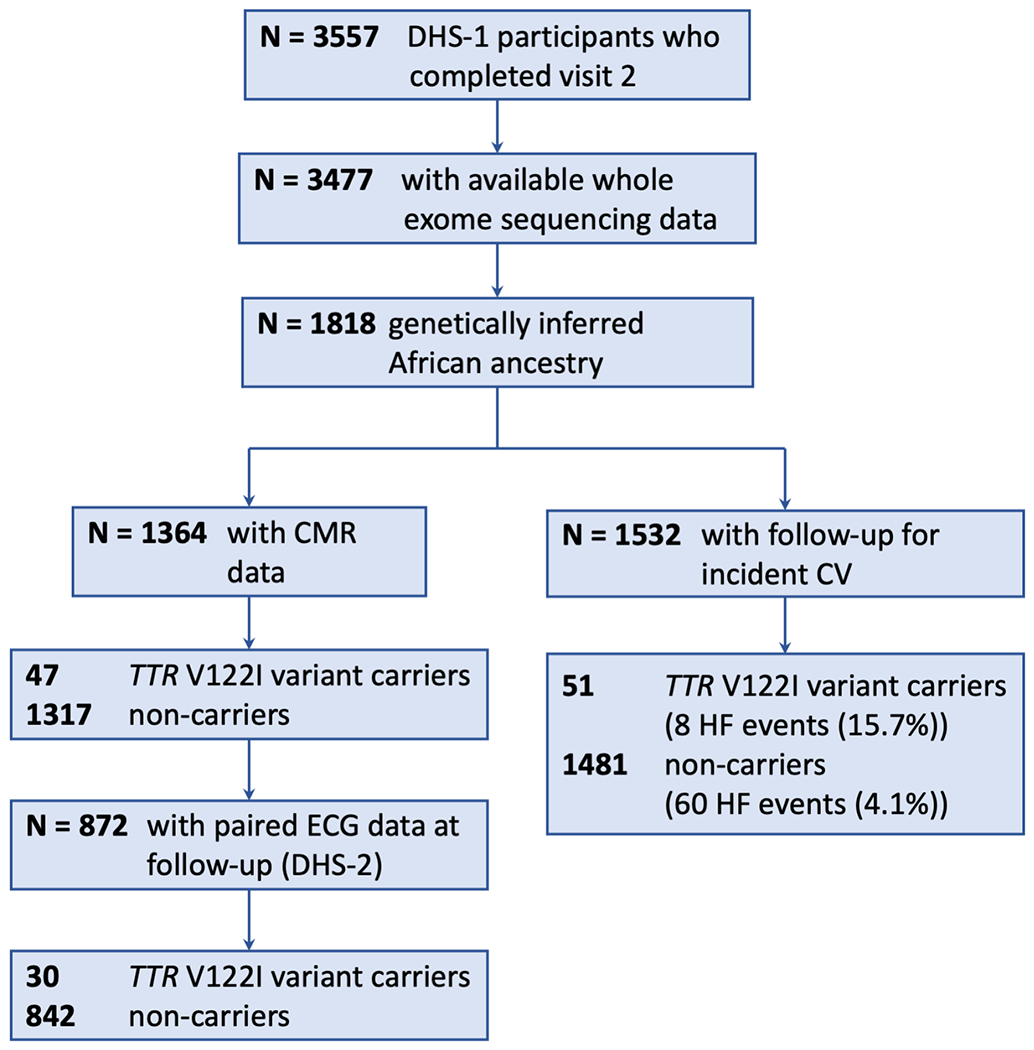
Consort Diagram. Of the 1,818 DHS participants of African ancestry, n=1,532 had follow-up CV data, n=1,364 had CMR imaging data, and n=1,205 had both. Abbreviations are DHS-1, Dallas Heart Study Phase 1; WES, whole exome sequencing; CMR, cardiac magnetic resonance imaging; ECG, electrocardiogram; and DHS-2, Dallas Heart Study Phase 2.
Imaging methods
CMR imaging was performed with a 1.5 Tesla system (Intera; Philips Medical Systems, Best, The Netherlands) at DHS-1. Follow-up imaging was performed a median of 7 years after baseline imaging at DHS-2. In DHS-2, CMR imaging was obtained using a 3.0 Tesla system (Achieva; Phillips Medical Systems, Best, The Netherlands). An accreditation phantom was imaged on the 1.5-T and 3-T systems, allowing baseline and follow-up mass and volume measurements to be normalized to this control. QMass software (Medis Medical Imaging Systems, Leiden, The Netherlands; version 6.2.3) was used to analyze the data. Short-axis scans were performed with breath hold and electrocardiographic-gated cine as previously described. 12, 13 LV measurements, LV mass, and LV wall thickness were then determined. Briefly, left ventricle LV measurements were determined by manually traced endocardial and epicardial contours. LV mass was calculated by multiplication of myocardial specific gravity (1.05 g/mL) with the difference between end-diastolic endocardial and epicardial contour. LV wall thickness was acquired from the short-axis images, with the exclusion of the most apical and basal slices. LV concentricity was defined as LV mass/LV end diastolic volume0.67. Myocardial contraction fraction was calculated as stroke volume/myocardial volume where myocardial volume was calculated as LV mass/1.05 g/mL.14 The interobserver difference, intraobserver difference, and interscan variability were previously described.12, 13 Some individuals failed to complete the CMR study for the following reasons: equipment failure (N=2), contraindication (N=48), refusal (N=6), claustrophobia (N=184), or scheduling conflicts (N=28). The comparison of characteristics of DHS-1 participants and TTR V122I carriers stratified by whether they had the CMR assessment is shown in Supplement 1.
ECG Acquisition
A twelve-lead ECG was performed for both DHS-1 and DHS-2 and measured using a Marquette Medical System (General Electric) with MAC 5000 hardware and software configuration, which measured rate, rhythm, intervals (PR, QRS, and QT), and voltages. Limb lead voltage was calculated as the sum of the entire QRS voltage of leads I, II, and III. The Sokolow voltage was calculated as the sum of the S wave in V1 plus the highest voltage R wave in V5 or V6.
Genetic sequencing
Genomic DNA was extracted from circulating leukocytes. DNA sample preparation and whole-exome sequencing were performed using standard methods, as previously described.15
Principal components of genetic ancestry were estimated in PLINK version 1.9b.16 African ancestry was inferred from genetic principal components based on a linear model trained on PC estimates from the 1000 genomes project known ancestry groups, as described previously.15 TTR genotypes were extracted from whole-exome sequence data. All V122I TTR carriers were of African ancestry based on principal component analysis. Genotype frequencies of the TTR V122I (rs76992529) variant were in Hardy-Weinberg proportions (P>0.05).
Measurement of Biomarkers
For both DHS-1 and DHS-2, blood samples were collected by venipuncture, centrifuged and the plasma component was stored in EDTA tubes. Samples were maintained at 4°C for less than 4 hours before undergoing centrifugation (1430 g for 15 minutes). Plasma was extracted and frozen at −70°C and stored until measurement was performed. Hs-cTnT and NT-proBNP measurements were obtained by previously established protocols 17, 18.
Variable definition
Participants self-reported their age, sex, and ethnicity. Hypertension was determined as mean systolic blood pressure ≥140 mmHg, mean diastolic pressure ≥90mmHg, or use of antihypertensive medications. Estimated glomerular filtration rate was calculated with the Modification of Diet and Renal Disease equation. Diabetes was defined as fasting glucose ≥126 mg/dL, non-fasting glucose ≥200, or use of antihyperglycemics.
Long-term Outcomes
The National Death Index was queried to determine participant mortality through December 31, 2016. Deaths were classified as cardiovascular according to International Classification of Diseases, Revision 10 codes 100-199 . Global cardiovascular disease, atherosclerotic cardiovascular disease, HF, myocardial infarction, and stroke were adjudicated through December 31, 2013. In the DHS, two overlapping approaches were used to capture nonfatal events: 1) a detailed health survey regarding interval cardiovascular events was administered by the Data Coordinating Center during annual calls to study subjects; and 2) for subjects providing informed consent (>90%), quarterly tracking was performed for hospital admissions using the Dallas–Fort Worth Hospital Council Data Initiative Database, which includes all hospital admission data for 70 out of 72 hospitals in the Dallas–Fort Worth area. The registry captures claims data from 96% of hospitals within a 75-mile radius in the metroplex (https://dfwhc.org).19 Two cardiologists, blinded to all study variables, separately adjudicated the data and hospital records. Follow-up data were available for n=1,532 individuals.
Statistical Analysis
Baseline demographics, clinical characteristics, CMR, and ECG were presented as mean ± standard deviation or median (interquartile range, IQR) for continuous variables, where appropriate, and as number (percentage) for categorical variables. Statistical comparisons of these characteristics stratified by presence of the V122I TTR variant (non-carriers vs. carriers) were completed using the Wilcoxon rank-sum test for continuous variables, Fisher’s exact test for categorical variables, or Cochran-Armitage trend test for ordinal categorical variables. Changes in paired values of NT-proBNP, hs-cTnT, and ECG intervals were calculated by subtracting the DHS-1 value from the DHS-2 value of that parameter. A natural logarithm or an inverse normal transformation was applied to right-skewed variables prior to regression analysis. Multivariable linear regression models adjusted for age, sex, 2 principal components of ancestry, systolic blood pressure, height, and weight were performed to determine the independent association of the V122I TTR genotype (non-carriers vs. carriers) with CMR and ECG parameters at baseline and at follow-up. Logistic regression models adjusted for the same covariates were used to determine the association with categorical outcomes. To assess the association of TTR V122I genotype with changes in NT-proBNP, hs-cTnT, and ECG intervals, we modeled the DHS-2 value as the response variable, with adjustment for baseline value of each parameter, ancestry, age, gender, and systolic blood pressure. The Kaplan-Meier method was used to estimate cumulative incidence of fatal and non-fatal events stratified by V122I TTR carrier status, and log-rank test was used to compare the groups. Follow-up data for participants who did not experience the event of interest prior to the end of follow-up or those who died from other causes without experiencing the event of interest, were censored. Cox proportional hazards models were used to test the association between V122I TTR carrier status and incident outcomes. These models were adjusted for age, sex, systolic blood pressure, estimated glomerular filtration rate, and 2 principal components of ancestry. The proportional hazards assumption was checked by examining Schoenfeld residuals. In order to determine the influence of different baseline risk factors on the association between V122I TTR carrier status and outcomes, we repeated the analyses in subgroups stratified by sex, hypertension, and diabetes, and tested for interaction between V122I TTR genotype and these risk factors. All covariates in the multivariable models were selected a priori based on their capacity to influence the carrier status-risk relationship. In addition, four separate sensitivity analyses were performed to clarify the association between V122I TTR carrier status and HF: 1) additionally adjusting for a prior self-reported history of HF; 2) additionally adjusting for a prior self-reported history of cardiovascular disease; 3) excluding participants with a prior reported history of HF; and 4) excluding participants with a prior reported history of cardiovascular disease. Two-sided P-values <0.05 were considered statistically significant. P-values and 95% confidence intervals presented in this report have not been adjusted for multiplicity, and therefore inferences drawn from these statistics may not be reproducible. All statistical analyses were performed using R statistical software, version 3.6.0.
RESULTS
Among the 1,818 DHS participants of African ancestry, a total of 59 carried at least one copy of the TTR V122I variant (carrier frequency 3.2% [59/1,818]). Only one of the 59 individuals with the variant was homozygous for the 122I allele (allele frequency 1.65% [n/2N=60/3,636]). Similar estimates were obtained in the subset with CMR data (n=1,364, Table 1). The study consort diagram is highlighted in Figure 1.
Table 1.
Baseline Characteristics of African American DHS-1 Participants Stratified by TTR V122I Carrier Status
| Participants with baseline CMR data | Participants with event follow-up data | |||||
|---|---|---|---|---|---|---|
| Characteristic† | TTR V122I Non-carriers | TTR V122I Carriers | P-value | TTR V122I Non-carriers | TTR V122I Carriers | P-value |
| (N=1,317) | (N=47)* | (N=1,481) | (N=51)** | |||
| Age [years] | 45.1 ± 10 | 44 ± 9.2 | 0.43 | 44.8 ± 10.2 | 45.2 ± 9.4 | 0.89 |
| Female | 752 (57.1) | 28 (59.57) | 0.77 | 898 (60.63) | 33 (64.71) | 0.66 |
| Hypertension | 539 (40.9) | 18 (38.3) | 0.76 | 605 (40.9) | 21 (41.2) | 1 |
| Diabetes Mellitus | 179 (13.6) | 6 (12.8) | 1 | 203 (13.72) | 7 (13.73) | 1 |
| Current Smoking | 419 (31.9) | 17 (36.2) | 0.53 | 447 (30.2) | 18 (35.3) | 0.44 |
| Weight [kg] | 88.8 ± 22.5 | 86.4 ± 23.6 | 0.32 | 90.8 ± 23.7 | 87.7 ± 23.8 | 0.30 |
| Income | 0.45 | 0.57 | ||||
| < 16,000 | 323 (30.3) | 10 (25.6) | 353 (29.3) | 10 (24.4) | ||
| 16,000 - 29,999 | 286 (26.8) | 8 (20.5) | 317 (26.3) | 9 (22) | ||
| 30,000 - 49,999 | 268 (25.1) | 15 (38.5) | 306 (25.4) | 16 (39) | ||
| >= 50,000 | 189 (17.7) | 6 (15.4) | 229 (19) | 6 (14.6) | ||
| Education | 0.71 | 0.80 | ||||
| < High School | 220 (16.7) | 5 (10.6) | 241 (16.3) | 6 (11.8) | ||
| High School | 509 (38.6) | 22 (46.8) | 589 (39.8) | 23 (45.1) | ||
| Some college | 406 (30.8) | 13 (27.7) | 442 (29.8) | 15 (29.4) | ||
| College grad or higher | 182 (13.8) | 7 (14.9) | 209 (14.1) | 7 (13.7) | ||
| Body mass index [kg/m2] | 31.4 ± 7.9 | 30.8 ± 7.6 | 0.52 | 32 ± 8.2 | 31.5 ± 7.8 | 0.62 |
| Systolic blood pressure [mm Hg] | 129.9 ± 20.1 | 129.5 ± 26.2 | 0.25 | 129.5 ± 19.7 | 129.9 ± 26.2 | 0.33 |
| Diastolic blood pressure [mm Hg] | 81 ± 10.7 | 79.8 ± 13.1 | 0.15 | 80.9 ± 10.4 | 79.9 ± 13 | 0.16 |
| Creatinine [mg/dL] | 0.9 (0.8 – 1.0) | 0.9 (0.8 - 1.1) | 0.24 | 0.9 (0.8 - 1) | 0.9 (0.8 - 1.05) | 0.50 |
| eGFR‡ [mL/min] | 102 (88 - 116) | 100 (85 - 110) | 0.23 | 102 (88 - 116) | 101 (85 - 111) | 0.23 |
| NT-proBNP [pg/mL] | 26.1 (10.2 - 58.2) | 23.6 (8.4 - 54.1) | 0.79 | 24.5 (10.1 - 53.9) | 27.8 (12.4 - 59.1) | 0.57 |
| Log-NT-proBNP | 3.22 ± 1.42 | 3.2 ± 1.41 | 0.79 | 3.15 ± 1.37 | 3.31 ± 1.38 | 0.57 |
| hs-cTnT [ng/L] | 1.5 (1.5 - 4.2) | 1.5 (1.5 - 4.0) | 0.56 | 1.5 (1.5 - 3.94) | 1.5 (1.5 - 2.3) | 0.51 |
46 individuals are heterozygous and 1 individual is homozygous for the TTR V122I allele.
50 individuals are heterozygous and 1 individual is homozygous for the TTR V122I allele.
Presented as median [interquartile range] or as mean ± standard deviation for continuous variables where appropriate and N (%) for categorical variables. Groups were compared using Wilcoxon rank-sum test (continuous variables), Fisher exact test (categorical variables), or Cochran-Armitage trend test (ordinal categorical variables: income, education).
eGFR is estimated by the Modification of Diet in Renal Disease equation
The mean baseline age of the cohort was 45 ± 10 years. The baseline characteristics of participants (DHS-1) stratified by V122I TTR carrier status are shown in Table 1. There were no statistically significant differences in age, sex, prevalent hypertension, prevalent diabetes mellitus, or smoking status. In addition, there were no differences in anthropomorphic measures (weight and body mass index), serologic markers of renal function (creatinine and estimated glomerular filtration rate), or levels of natriuretic peptides (NTproBNP) and cardiac troponin (hs-cTnT). The follow-up characteristics of participants (DHS-2) stratified by V122I TTR carrier status are shown in Supplement 2.
The cardiac phenotypes of participants by CMR and ECG in DHS-1 and DHS-2, stratified by V122I TTR carrier status, are shown in Table 2 and Supplement 3, respectively. No differences were observed in these parameters by V122I TTR carrier status in unadjusted analyses for both DHS phases. After multivariable adjustment, carriers of the V122I TTR variant had higher LV wall thickness by approximately 0.3 mm (P=0.038) in DHS-1 and by approximately 0.6 mm in DHS-2 (P=0.009). However, this was not seen either with LV mass or LV concentricity. No other differences were apparent in markers of LV cavity size, left atrial size, and LV mass. The changes in CMR parameters from DHS-1 to DHS-2, stratified by V122I TTR carrier status are shown in Table 3. Carriers of the V122I TTR variant had a small increase in LV ejection fraction (P=0.047) and decrease in LV end systolic volume (P=0.035). No other differences were apparent for changes in markers of LV cavity size, left atrial size, and LV mass from DHS-1 to DHS-2.
Table 2.
Cardiac Phenotype of African American DHS-1 Participants Stratified by V122I TTR Carrier Status
| Non-carriers (N=1,317) | Carriers (N=47) | Unadjusted | Adjusted | |||
|---|---|---|---|---|---|---|
| Characteristic† | No. of people with data | Value | No. of people with data | Value | P-value | P-value |
| Cardiac magnetic resonance imaging | ||||||
| LV ejection fraction [%] | 1317 | 0.67 ± 0.07 | 47 | 0.66 ± 0.07 | 0.34 | 0.28 |
| LV end diastolic volume [mL] | 1317 | 124.6 ± 34.6 | 47 | 123.8 ± 30.1 | 0.99 | 0.67 |
| LV end diastolic volume by body surface area [ml/m2] | 1313 | 62.19 ± 15.33 | 47 | 63.43 ± 16.19 | 0.85 | 0.76 |
| LV end systolic volume [mL] | 1317 | 42 ± 23.6 | 47 | 42.4 ± 15.6 | 0.41 | 0.24 |
| LV end systolic volume by body surface area [ml/m2] | 1313 | 20.93 ± 11.23 | 47 | 21.92 ± 9.09 | 0.55 | 0.35 |
| Stroke volume [mL] | 1317 | 82.7 ± 17.6 | 47 | 81.4 ± 18.4 | 0.36 | 0.78 |
| Stroke volume by body surface area [ml/mm2] | 1313 | 41.25 ± 7.33 | 47 | 41.51 ± 8.8 | 0.74 | 0.83 |
| Myocardial contraction fraction | 1317 | 0.64 ± 0.16 | 47 | 0.62 ± 0.15 | 0.35 | 0.19 |
| Left atrial volume [mL] | 353 | 71.4 ± 19 | 13 | 75.3 ± 25.2 | 0.72 | 0.36 |
| Left atrial volume by body surface area [ml/m2] | 353 | 36.19 ± 7.88 | 13 | 38.37 ± 8.99 | 0.27 | 0.33 |
| LV mass [g] | 1317 | 142.3 ± 44.1 | 47 | 144.6 ± 45.3 | 0.80 | 0.18 |
| LV mass by body surface area [g/m2] | 1313 | 70.66 ± 18.93 | 47 | 73.42 ± 20.15 | 0.40 | 0.17 |
| LV wall thickness [mm] | 1312 | 8.21 ± 1.68 | 44 | 8.52 ± 1.82 | 0.51 | 0.038 |
| LV Concentricity [g/mL] | 1317 | 5.17 ± 0.5 | 47 | 5.2 ± 0.51 | 0.80 | 0.18 |
| LV hypertrophy by body surface area | 1316 | 230 (17.48) | 47 | 12 (25.53) | 0.17 | 0.14 |
| Electrocardiography | ||||||
| PR interval [ms] | 1304 | 161 ± 23.8 | 47 | 159.8 ± 25.3 | 0.77 | 0.81 |
| QRS interval [ms] | 1314 | 85.2 ± 11.7 | 47 | 84.8 ± 14.7 | 0.25 | 0.49 |
| QT interval [ms] | 1312 | 391.4 ± 32.1 | 47 | 392.9 ± 31.7 | 0.49 | 0.74 |
| LV hypertrophy | 1314 | 217 (16.51) | 47 | 12 (25.53) | 0.11 | 0.13 |
| Limb lead voltage [mm] | 1314 | 28.1 ± 7.9 | 47 | 29.5 ± 7.7 | 0.066 | 0.18 |
| Sokolow voltage [mm] | 1314 | 24.5 ± 8.3 | 47 | 26.3 ± 7.8 | 0.094 | 0.13 |
| Total voltage [mm] | 1314 | 1022 ± 255 | 47 | 1075 ± 260 | 0.13 | 0.14 |
| Limb lead voltage / LV mass [mm*m2/g] | 1310 | 0.41 ± 0.13 | 47 | 0.42 ± 0.12 | 0.39 | 0.81 |
| Sokolow voltage / LV mass [mm*m2g] | 1310 | 0.36 ± 0.12 | 47 | 0.37 ± 0.12 | 0.28 | 0.46 |
| Total voltage / LV mass [mm*m2g] | 1310 | 14.9 ± 3.7 | 47 | 15.2 ± 3.6 | 0.35 | 0.84 |
46 individuals are heterozygous and 1 individual is homozygous for the V122I allele.
Presented as mean ± standard deviation for continuous variables and N (%) for categorical variables. Unadjusted p-values were calculated using Wilcoxon rank-sum test (continuous variables) or Fisher exact test (categorical variables). Adjusted p-values were calculated using linear and logistic regression models (for continuous and categorical variables, respectively), including ancestry, age, sex, systolic BP, height and weight as covariates.
Table 3.
Change in Cardiac Biomarkers, Electrocardiogram, and Cardiac Magnetic Resonance Imaging Parameters from DHS-1 to DHS-2
| Non-carriers (N=842) | Carriers (N=30) | |||||
|---|---|---|---|---|---|---|
| Characteristic† | No. of people with data | Value | No. of people with data | Value | Unadjusted P-value | Adjusted P-value |
| Cardiac Biomarkers | ||||||
| ΔNT-proBNP [pg/mL] | 834 | 15.9 (0.0 - 43) | 30 | 28.5 (11.4 – 104.1) | 0.034 | 0.018 |
| Δhs-cTnT [ng/L] | 817 | 3.24 (0 - 6.31) | 29 | 1.33 (0 - 5.81) | 0.19 | 0.32 |
| Cardiac magnetic resonance imaging | ||||||
| ΔLV ejection fraction [%] | 586 | 0.01 (−0.03 - 0.05) | 22 | 0.03 (0 - 0.05) | 0.058 | 0.047 |
| ΔLV end diastolic volume [mL] | 586 | −1.65 (−11.53 - 7.74) | 22 | −5.57 (−10.95 - 5.37) | 0.70 | 0.60 |
| ΔLV end systolic volume [mL] | 586 | −1.43 (−7.03 - 4.16) | 22 | −4.86 (−9.24 - −1.21) | 0.044 | 0.035 |
| ΔStroke volume [mL] | 586 | 0.13 (−8.4 - 6.43) | 22 | 3.82 (−4.2 - 10.54) | 0.18 | 0.23 |
| ΔLeft atrial volume [mL] | 349 | 2.4 (−10.29 - 12.28) | 13 | 6.04 (−16.2 - 17.8) | 0.89 | 0.76 |
| ΔLV mass [g] | 586 | −2.65 (−12.3 - 9.2) | 22 | −0.97 (−4.88 - 6.71) | 0.57 | 0.55 |
| ΔLV wall thickness [mm] | 581 | 0 (−0.55 - 0.59) | 22 | 0.11 (−0.26 - 0.39) | 0.54 | 0.21 |
| ΔLV Concentricity [g/mL] | 586 | 0.43 (−0.13 - 1.04) | 22 | 0.58 (0.25 - 1.01) | 0.34 | 0.29 |
| Electrocardiography | ||||||
| ΔPR interval [ms] | 819 | 4 (−6 - 12) | 30 | 3 (−5.5 - 11) | 0.91 | 0.90 |
| ΔQRS interval [ms] | 842 | 2 (−2 - 6) | 30 | 2 (−2 - 6) | 0.66 | 0.66 |
| ΔQT interval [ms] | 841 | 4 (−16 - 26) | 30 | 6 (−7 - 27.5) | 0.40 | 0.25 |
| ΔLimb lead voltage [mm] | 841 | 3.6 (−0.1 - 7.9) | 30 | 2.6 (0 - 8.6) | 0.73 | 0.32 |
| ΔSokolow voltage [mm] | 841 | −2.2 (−5.4 - 0.9) | 30 | −2.3 (−5 - 0.9) | 0.74 | 0.91 |
| Δ Limb lead voltage / LV mass [mm/g] | 585 | 0.06 (0 - 0.15) | 22 | 0.08 (0.01 - 0.16) | 0.42 | 0.34 |
| Δ Sokolow voltage / LV mass [mm/g] | 585 | −0.02 (−0.07 - 0.03) | 22 | −0.03 (−0.07 - 0.02) | 0.70 | 0.89 |
1 individual is homozygous for die V122I allele.
Presented as median [interquartile range] or as mean ± standard deviation for continuous variables where appropriate and N (%) for categorical variables. Unadjusted p-values were calculated using Wilcoxon rank-sum test. Adjusted p-values were calculated using linear regression models, using the value at DHS-2 as the response variable and adjusted for baseline value, ancestry, age, sex, and systolic blood pressure.
Similarly, there were no differences in ECG intervals, QRS voltage amplitude, or the ratios of QRS voltage amplitudes by LV mass by V122I TTR carrier status in unadjusted or adjusted analyses in DHS-1.
Of the study cohort, there were 872 participants with paired cardiac biomarker and ECG data. The changes in cardiac biomarkers and ECG parameters from DHS-1 to DHS-2 stratified by V122I TTR carrier status are shown in Table 3. Relative to non-carriers, V122I TTR carriers had nearly a 2-fold larger median increase in levels of NT-proBNP over time in both unadjusted and adjusted analyses (P=0.04 and P=0.01, respectively). After additional adjustment for body mass index, eGFR, and time between DHS-1 and DHS-2, the associations between V122I TTR carrier status and increased NT-proBNP persisted (β=0.35, P=0.026). In contrast, there were no differences in change in hs-cTnT or the ECG intervals, QRS voltage amplitudes, or the ratios of QRS voltage amplitudes by LV mass from DHS-1 and DHS-2 in both unadjusted and adjusted analyses (P>0.05 for all).
After 22,603 person-years of follow-up (through 2016, median duration of follow-up 15.3 years, IQR: 14.8-15.8 years), there were 195 all-cause deaths, and 77 cardiovascular deaths. After 18,495 person-years of follow-up for incident events (through 2013, median duration of follow-up 12.4 years, IQR: 11.9-12.8 years ), there were 151 incident atherosclerotic cardiovascular disease events, 67 incident HF events, and 58 myocardial infarctions. Kaplan-Meier estimates of cumulative incidence of fatal and non-fatal events are shown in the Figure 2. Of these, there was a significantly higher incidence of HF (Log-rank P<0.001), higher all-cause mortality (Log-rank P=0.026) and cardiovascular death (Log-rank P=0.024) for V122I TTR carriers in comparison with non-carriers. In the Cox proportional hazards models, there was no association between V122I TTR carrier status and atherosclerotic vascular disease, myocardial infarction or stroke in both unadjusted and adjusted analyses (P>0.05 for all). After multivariable adjustment, V122I TTR carriers were at a 3.8-fold higher risk of HF events (HR 3.82, 95% CI 1.80-8.13, P<0.001), 2.7 fold higher risk of cardiovascular death (HR 2.65, 95% CI 1.14-6.15, P=0.023), and 2-fold higher risk of all-cause mortality (HR 1.95, 95% CI 1.08-3.51, P=0.026) in comparison with non-carriers (Table 4). Additional adjustment for income and education did not alter the results (data not shown). In the four sensitivity analyses (Supplement 4), the independent association between V122I TTR carrier status and HF events remained largely consistent when additionally adjusting for prior HF (n events/N total=66/1,528) or prevalent cardiovascular disease (n events/N total=66/1,528) or excluding individuals with prior HF (n events/N total=51/1,463) or prior cardiovascular disease (n events/N total=42/1,382). In a sensitivity analysis restricted to participants who had complete longitudinal data with biomarkers, ECG, and CMR, results were qualitatively similar (Supplement 5).
Figure 2.
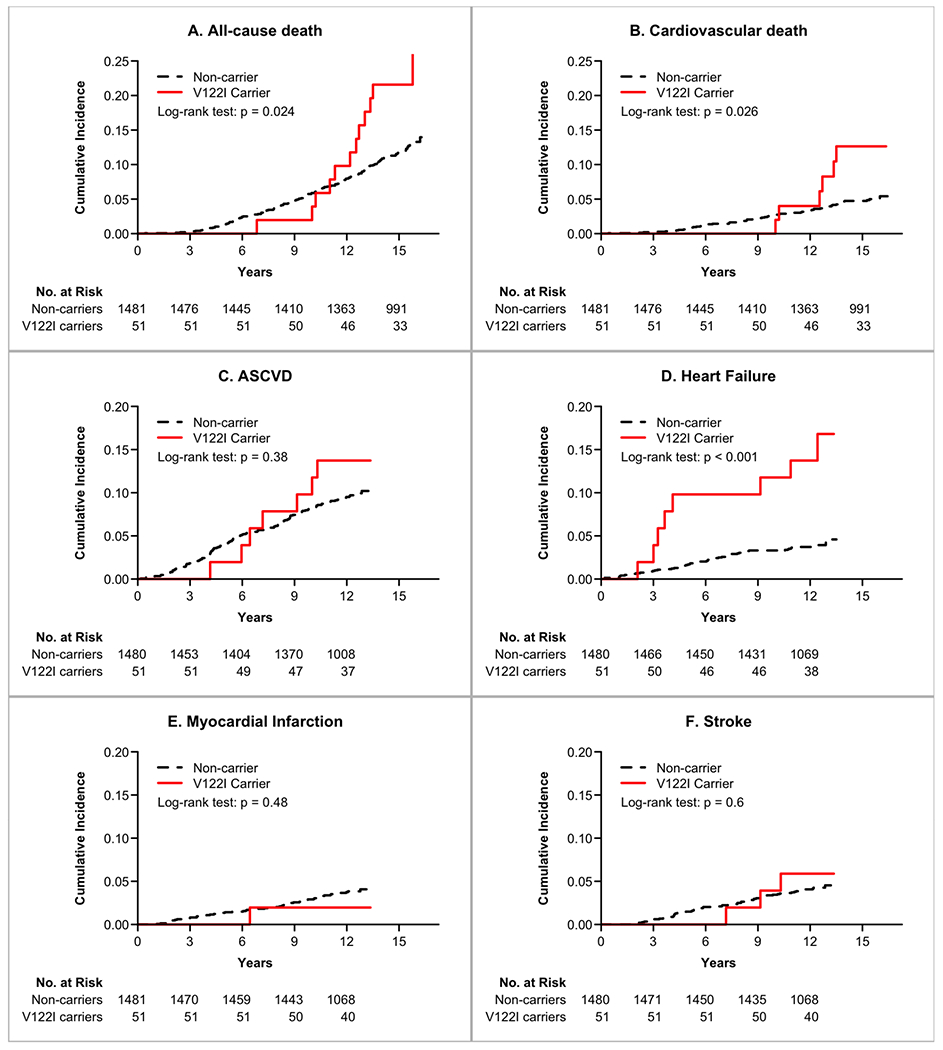
Kaplan-Meier Estimates of Cumulative Incidence of Fatal and Non-fatal Events Stratified by V122I TTR Carrier Status. Statistical comparisons are generated by the Log-rank test.
Table 4.
Association of the TTR V122I Carrier Status with Mortality and Cardiovascular outcomes
| Outcomes | N event/N Total, non-carriers | N event/N Total, V122I carriers | Hazard Ratio (95% CI) | Unadjusted P-Value | N event/N Total, non-carriers | N event/N Total, V122I carriers | Adjusted Hazard Ratio (95% CI) | Adjusted P-Value |
|---|---|---|---|---|---|---|---|---|
| All-cause mortality | 183/1481 | 12/51 | 1.93 (1.08 - 3.47) | 0.027 | 182/1478 | 12/51 | 1.95 (1.08 - 3.51) | 0.026 |
| Cardiovascular death | 71/1481 | 6/51 | 2.49 (1.08 - 5.74) | 0.032 | 70/1478 | 6/51 | 2.65 (1.14 - 6.15) | 0.023 |
| Atherosclerotic cardiovascular disease | 144/1480 | 7/51 | 1.4 (0.66 - 2.99) | 0.38 | 144/1477 | 7/51 | 1.1 (0.51 - 2.4) | 0.8 |
| Heart failure | 59/1480 | 8/51 | 4.09 (1.95 - 8.56) | <0.001 | 58/1477 | 8/51 | 3.82 (1.8 - 8.13) | <0.001 |
| Myocardial infarction | 57/1481 | 1/51 | 0.5 (0.07 - 3.62) | 0.49 | 57/1478 | 1/51 | 0.43 (0.06 - 3.15) | 0.41 |
| Stroke | 63/1480 | 3/51 | 1.36 (0.43 - 4.35) | 0.6 | 63/1477 | 3/51 | 1.11 (0.34 - 3.59) | 0.86 |
Hazard ratio for the comparison of carriers of the V122I variant versus non-carriers.
Multivariable models are adjusted for baseline age, sex, BMI, systolic blood pressure, eGFR, and 2 principal components of ancestry.
All-cause mortality and cardiovascular death were followed till December 31, 2016 and all other outcomes were followed until December 31, 2013.
At baseline, V122I TTR carriers who experienced HF were older and had lower eGFR, higher NT-proBNP and hs-cTnT, larger LV volumes and mass, and longer QRS intervals, with a trend towards lower indexed ECG voltages to LV mass, as compared with non-carriers (Supplement 6).
In a separate analysis, no heterogeneity was observed for the association between V122I carrier status and incident HF when stratified by sex, prevalent hypertension, or prevalent diabetes (P-interaction>0.05 for all, Supplement 7).
DISCUSSION
This analysis from the DHS - a young, population-based cohort with detailed imaging and biomarker phenotyping - has several important observations that may clarify the natural history of V122I TTR carriers. First, V122I TTR carrier status was associated with increased risk for incident HF, even though carriers only had subtle cardiac phenotypic differences versus non-carriers at baseline. This was coupled with a higher risk for all-cause mortality and cardiovascular death. Second, V122I carrier status was associated with a larger increase in NT-proBNP levels over time than was seen in non-carriers. In addition, V122I TTR carriers both at DHS-1 and DHS-2 had a slightly increased LV wall thickness, but not LV mass or concentricity. Carrier status was also associated with a small increase in LV ejection fraction and decrease in LV end systolic volume from DHS-1 to DHS-2, but no other differences in cardiac phenotype by CMR or ECG were observed compared with non-carriers in this cohort. These observations highlight a clear unmet need to better understand the natural history of pre-symptomatic amyloid progression in V122I TTR carriers.
V122I TTR carrier status is associated with a lifelong risk for incident HF – suggestive of downstream effects of progressive ATTRv amyloid cardiomyopathy. Observations from DHS are consistent with data from other cohorts and confirm the genotypic prevalence of the V122I TTR genotype (~3-4% of all African Americans) and the association between V122I carrier status and HF risk.8, 9, 20 In particular, data from the Arteriosclerosis Risk in Communities and Cardiovascular Health Studies (ARIC),8 highlighted the association of V122I TTR carrier status and higher long-term HF risk. Findings from the present study may add to those observations in 4 ways: 1) the V122I TTR variant was associated with an increased risk for not only HF but also all-cause mortality and cardiovascular death; 2) there was a suggestion of a subtle increase in LV wall thickness at baseline, also seen at DHS-2, but otherwise no obvious baseline subclinical ATTRv phenotype; 3) over time, there was a small but significantly greater increase in natriuretic peptide level, decline in end-systolic volume and increase in LV ejection fraction; and 4) the association with incident HF was observed among participants at a younger average age, and did not appear to vary according to sex, hypertension, or diabetes.
At the same time, we noted some differences from previous studies. The estimated relative hazard for incident HF was numerically higher in DHS (>3 in DHS vs 1.5 in ARIC) and we observed a higher risk of all-cause and cardiovascular death among TTR V122I carriers. While the reasons for these differences are uncertain, in comparison with data from ARIC,8 V122I TTR carriers in the DHS were younger and had less prevalent diabetes and hypertension. In lower risk populations with fewer competing comorbidities, the relative risk of both incident HF and death associated with V122I TTR carrier status may be larger, due to lower rates of HF and death among non-carriers. Indeed, the absolute risk difference for HF between carriers and non-carriers was comparable between the two studies. It is also possible the higher point estimates for incident HF in the DHS may result from sampling variation since the confidence intervals overlap between the two cohorts, highlighting the need for additional studies with larger samples sizes to derive more precise estimates. Nevertheless, the strong pathological phenotype for carriers who developed subsequent HF in the DHS provides some evidence that midlife structural and biomarker abnormalities precede clinical HF in V122I TTR carriers and underscore this variant’s importance over an individual’s lifespan.
Data from the DHS may inform the potential screening strategies for ATTR in V122I TTR carriers. Similar to prior observations in ARIC,8 we observed that V122I carriers had a subtle increase in LV wall thickness compared with non-carriers. A potentially important difference between the two studies was that the mean age of DHS subjects at baseline was 45 years, whereas the subset of ARIC participants in which this observation was made was approximately 60-80 years old. One hypothesis for this observation is that the increased LV wall thickening represents early ATTRv amyloid deposition. Indeed, CMR studies have demonstrated associations of quantifiable amyloid deposition with increased LV wall thickness.5 though gadolinium was not administered in DHS-1 or DHS-2. However, additional study of this important issue is needed before accepting this hypothesis, especially since the excess wall thickening was evident only after adjustment in multivariable analysis, was of small magnitude, and neither LV mass nor LV concentricity was associated with carrier status. V122I TTR carriers had a slight increase in LV ejection fraction and decrease in LV end systolic volume from DHS-1 to DHS-2. These findings were unexpected, but directionally consistent with greater LV end diastolic volumes observed in similarly aged V122I TTR carriers in the Coronary Artery Risk Development in Young Adults (CARDIA) study.21 The implications of these observations are limited by small sample size, however data from the DHS and CARDIA suggest the subclinical cardiac ATTR phenotype may experience a phase of eccentric remodeling. Whether contemporary CMR studies with gadolinium,5 bone scintigraphy,22 or histologic assessments could detect evidence of ATTR deposition at this degree of LV thickening is also unknown. Nevertheless, the similarities between V122I TTR carriers and non-carriers in this age range emphasize the importance of genetic testing and argue for the inclusion of V122I TTR into genome first screening programs.
In comparison with wild-type ATTR , V122I ATTRv is more aggressive with shorter time from symptom onset until clinical presentation, increased natriuretic peptides over time, worse functional capacity, and increased risk of mortality.1 Therefore, efforts to investigate optimal timing for implementing novel ATTR treatments are critical to alter the natural history of the disease.2, 3 In parallel with individuals with manifest V122I ATTRv,1 V122I TTR carriers in the DHS were observed to have a modest increase in natriuretic peptides over time (from DHS-1 to DHS-2). Because natriuretic peptides are associated with ATTRv disease progression and prognosis,23, 24 this observation may support the potential utility of performing serial measurements of NT-proBNP levels in V122I TTR carriers. In contrast, there were no such trends with hs-cTnT or ECG parameters over time that distinguished V122I TTR carriers from non-carriers. Two potential explanations for this include that ECG abnormalities may inconsistently distinguish those with clinical ATTRv from individuals without ATTR and that elevations in cardiac troponins are later manifestations of the disease.25, 26
This study had several limitations. First, the number of participants with V122I TTR in our study was small (n=59) and, although the groups overlapped, the individuals with CMR imaging were not an exact subset of those with follow-up data. We cannot exclude the potential for bias related to incomplete MRI and biomarker follow up measures. Apart from body mass index and weight, however, there were no differences in characteristics at DHS-1 for those who had a CMR assessment and those who did not (Supplement 1). Furthermore, the duration of time between MRI assessments was shorter (median duration of follow-up 7.0 years, IQR 6.5-7.5 years) than the duration of follow-up from DHS-1 for non-fatal events (median duration of follow-up 12.4 years, IQR 11.9-12.8 years) and fatal events (median duration of follow-up 15.3 years, IQR 14.8-15.8 years) which may explain why there were more subtle differences in cardiac and biomarkers phenotypes when compared with the high relative risk of long-term outcomes. Second, there were no amyloid-specific diagnostic modalities, such as tissue biopsy, contrast enhanced CMR with parametric mapping, or bone scintigraphy employed in the DHS protocols – a limitation shared with ARIC and CARDIA.8, 21 As such, we can only hypothesize that the observed association between the V122I TTR genotype with HF and fatal outcomes is mediated by ATTRv infiltration. Furthermore, musculoskeletal syndromes which can occur years prior to ATTRv disease onset, such as carpal tunnel syndrome, lumbar canal stenosis, and biceps tendon rupture, were not recorded in the DHS. Yet, our findings are directionally consistent with prior observations from population-based cohorts linking the V122I TTR to cardiac phenotype differences and a higher risk of incident HF in comparison to non-carriers.8, 9 Furthermore, the consistency in the sensitivity analyses, as well as the lack of association between V122I TTR carrier status with falsification endpoints (atherosclerotic cardiovascular disease, myocardial infarction, and stroke), support the primary conclusions of our study. Third, we cannot exclude the influence of selection bias for participants with and without follow up testing in DHS-2. While the present analysis leverages a specific genetic variant in persons with African ancestry, these data inform a broader understanding of the natural history of carriers of other far less common TTR variants present in other ethnic groups.
CONCLUSION
In individuals with African ancestry in the DHS, the V122I TTR carrier status was associated with a larger increase in NT-proBNP over time and a significantly increased risk for incident HF, all-cause mortality, and cardiovascular death. V122I TTR was also associated with increased wall thickness in adjusted models. In total, these findings inform the natural history of V122I ATTRv and raise the question of the need of ATTRv screening strategies in V122I TTR carriers at younger ages than conventionally considered.
Supplementary Material
3 BRIEF BULLET POINTS.
Carriers of the V122I TTR mutation may have subtle differences in cardiac structure, greater changes in natriuretic peptides over time, and are at a higher risk of long-term events including heart failure and death.
Although many of the LV imaging parameters in this study were subtle, contemporary CMR studies with gadolinium, bone scintigraphy, or histologic assessments are needed to better understand whether these observations are evidence of ATTR deposition in V122I TTR carriers.
Carriers of the V122I TTR should be made aware that screening for ATTRv may be considered.
PROPOSED TWEET.
Data from individuals with African ancestry in Dallas Heart Study suggest that, in comparison with non-carriers, carriers of the V122I TTR mutation in mid-life may have subtle differences in cardiac structure, greater changes in NT-proBNP over time, and are at a higher risk of long-term events including heart failure and death.
HIGHLIGHTS.
The prevalence of the V122I TTR variant in individuals with African ancestry is 3.2%
In mid-life, carriers of the V122I TTR have subtle cardiac structural differences in comparison with non-carriers
Over time, carriers of V122I have a greater increase in NT-proBNP in comparison with non-carriers.
In comparison with non-carriers, carriers of the V122I TTR are at a higher long-term risk of heart failure and death
ACKNOWLEDGEMENTS
Whole-exome sequencing was performed in collaboration with Regeneron Genetics Center; individual scientific contributions by Regeneron Genetics Center personnel are listed in the Supplement.
FUNDING
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001105.
DISCLOSURES
JLG has received consulting fees from Pfizer, Inc, Eidos Therapeutics, and Alynlam Pharmaceuticals; and research funding from Eidos Therapeutics and the Texas Health Resources Clinical Scholars fund. JK has received research funding from Regeneron Pharmaceuticals, Inc. JAd has received grant support from Roche Diagnostics and Abbott Diagnostics, and consulting income from Siemens Health Care Diagnostics, Ortho Clinical Diagnostics, Quidel Inc, and Regeneron. AP has received research funding from the National Institute of Aging, Gilead Sciences, and Applied Therapeutics. JB has reported relevant grant support from Roche Diagnostics, Abbott diagnostics, and the National Instituted of Health. JB has also reported consulting income from Abbott and the Cooper Institute. JO, JR, and AB are employed by Regeneron.
ABBREVIATIONS
- ATTR
transthyretin amyloidosis
- ATTRv
variant transthyretin amyloidosis
- CMR
cardiac magnetic resonance
- TTR
transthyretin
- HF
heart failure
- DHS
Dallas Heart Study
- LV
left ventricle
- hs-cTnT
high sensitivity cardiac troponin T
- NT-proBNP
amino terminal pro-B-type natriuretic peptide
- eGFR
estimated glomerular filtration rate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lane T, Fontana M, Martinez-Naharro A, Quarta CC, Whelan CJ, Petrie A, Rowczenio DM, Gilbertson JA, Hutt DF, Rezk T, Strehina SG, Caringal-Galima J, Manwani R, Sharpley FA, Wechalekar AD, Lachmann HJ, Mahmood S, Sachchithanantham S, Drage EPS, Jenner HD, McDonald R, Bertolli O, Calleja A, Hawkins PN, Gillmore JD. Natural History, Quality of Life, and Outcome in Cardiac Transthyretin Amyloidosis. Circulation 2019;140(1):16–26. [DOI] [PubMed] [Google Scholar]
- 2.Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, Drachman BM, Shah SJ, Hanna M, Judge DP, Barsdorf AI, Huber P, Patterson TA, Riley S, Schumacher J, Stewart M, Sultan MB, Rapezzi C, Investigators A-AS. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N Engl J Med 2018;379(11):1007–1016. [DOI] [PubMed] [Google Scholar]
- 3.Judge DP, Heitner SB, Falk RH, Maurer MS, Shah SJ, Witteles RM, Grogan M, Selby VN, Jacoby D, Hanna M, Nativi-Nicolau J, Patel J, Rao S, Sinha U, Turtle CW, Fox JC. Transthyretin Stabilization by AG10 in Symptomatic Transthyretin Amyloid Cardiomyopathy. J Am Coll Cardiol 2019;74(3):285–295. [DOI] [PubMed] [Google Scholar]
- 4.Bokhari S, Castano A, Pozniakoff T, Deslisle S, Latif F, Maurer MS. (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging 2013;6(2):195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Syed IS, Glockner JF, Feng D, Araoz PA, Martinez MW, Edwards WD, Gertz MA, Dispenzieri A, Oh JK, Bellavia D, Tajik AJ, Grogan M. Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis. JACC Cardiovasc Imaging 2010;3(2):155–64. [DOI] [PubMed] [Google Scholar]
- 6.Jiang X, Buxbaum JN, Kelly JW. The V122I cardiomyopathy variant of transthyretin increases the velocity of rate-limiting tetramer dissociation, resulting in accelerated amyloidosis. Proc Natl Acad Sci U S A 2001;98(26):14943–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maurer MS, Hanna M, Grogan M, Dispenzieri A, Witteles R, Drachman B, Judge DP, Lenihan DJ, Gottlieb SS, Shah SJ, Steidley DE, Ventura H, Murali S, Silver MA, Jacoby D, Fedson S, Hummel SL, Kristen AV, Damy T, Plante-Bordeneuve V, Coelho T, Mundayat R, Suhr OB, Waddington Cruz M, Rapezzi C, Investigators T. Genotype and Phenotype of Transthyretin Cardiac Amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey). J Am Coll Cardiol 2016;68(2):161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quarta CC, Buxbaum JN, Shah AM, Falk RH, Claggett B, Kitzman DW, Mosley TH, Butler KR, Boerwinkle E, Solomon SD. The amyloidogenic V122I transthyretin variant in elderly black Americans. N Engl J Med 2015;372(1):21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damrauer SM, Chaudhary K, Cho JH, Liang LW, Argulian E, Chan L, Dobbyn A, Guerraty MA, Judy R, Kay J, Kember RL, Levin MG, Saha A, Van Vleck T, Verma SS, Weaver J, Abul-Husn NS, Baras A, Chirinos JA, Drachman B, Kenny EE, Loos RJF, Narula J, Overton J, Reid J, Ritchie M, Sirugo G, Nadkarni G, Rader DJ, Do R. Association of the V122I Hereditary Transthyretin Amyloidosis Genetic Variant With Heart Failure Among Individuals of African or Hispanic/Latino Ancestry. JAMA 2019;322(22):2191–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soares ML, Coelho T, Sousa A, Batalov S, Conceicao I, Sales-Luis ML, Ritchie MD, Williams SM, Nievergelt CM, Schork NJ, Saraiva MJ, Buxbaum JN. Susceptibility and modifier genes in Portuguese transthyretin V30M amyloid polyneuropathy: complexity in a single-gene disease. Hum Mol Genet 2005;14(4):543–53. [DOI] [PubMed] [Google Scholar]
- 11.Dorbala S, Ando Y, Bokhari S, Dispenzieri A, Falk RH, Ferrari VA, Fontana M, Gheysens O, Gillmore JD, Glaudemans A, Hanna MA, Hazenberg BPC, Kristen AV, Kwong RY, Maurer MS, Merlini G, Miller EJ, Moon JC, Murthy VL, Quarta CC, Rapezzi C, Ruberg FL, Shah SJ, Slart R, Verberne HJ, Bourque JM. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI Expert Consensus Recommendations for Multimodality Imaging in Cardiac Amyloidosis: Part 1 of 2-Evidence Base and Standardized Methods of Imaging. J Card Fail 2019;25(11):e1–e39. [DOI] [PubMed] [Google Scholar]
- 12.Garg S, de Lemos JA, Matulevicius SA, Ayers C, Pandey A, Neeland IJ, Berry JD, McColl R, Maroules C, Peshock RM, Drazner MH. Association of Concentric Left Ventricular Hypertrophy With Subsequent Change in Left Ventricular End-Diastolic Volume: The Dallas Heart Study. Circ Heart Fail 2017;10(8). [DOI] [PubMed] [Google Scholar]
- 13.Drazner MH, Dries DL, Peshock RM, Cooper RS, Klassen C, Kazi F, Willett D, Victor RG. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas Heart Study. Hypertension 2005;46(1):124–9. [DOI] [PubMed] [Google Scholar]
- 14.Abdalla M, Akwo EA, Bluemke DA, Lima JAC, Shimbo D, Maurer MS, Bertoni AG. Association between reduced myocardial contraction fraction and cardiovascular disease outcomes: The Multi-Ethnic Study of Atherosclerosis. Int J Cardiol 2019;293:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dewey FE, Murray MF, Overton JD, Habegger L, Leader JB, Fetterolf SN, O’Dushlaine C, Van Hout CV, Staples J, Gonzaga-Jauregui C, Metpally R, Pendergrass SA, Giovanni MA, Kirchner HL, Balasubramanian S, Abul-Husn NS, Hartzel DN, Lavage DR, Kost KA, Packer JS, Lopez AE, Penn J, Mukherjee S, Gosalia N, Kanagaraj M, Li AH, Mitnaul LJ, Adams LJ, Person TN, Praveen K, Marcketta A, Lebo MS, Austin-Tse CA, Mason-Suares HM, Bruse S, Mellis S, Phillips R, Stahl N, Murphy A, Economides A, Skelding KA, Still CD, Elmore JR, Borecki IB, Yancopoulos GD, Davis FD, Faucett WA, Gottesman O, Ritchie MD, Shuldiner AR, Reid JG, Ledbetter DH, Baras A, Carey DJ. Distribution and clinical impact of functional variants in 50,726 whole-exome sequences from the DiscovEHR study. Science 2016;354(6319). [DOI] [PubMed] [Google Scholar]
- 16.Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 2015;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Lemos JA, McGuire DK, Khera A, Das SR, Murphy SA, Omland T, Drazner MH. Screening the population for left ventricular hypertrophy and left ventricular systolic dysfunction using natriuretic peptides: results from the Dallas Heart Study. Am Heart J 2009;157(4):746–53 e2. [DOI] [PubMed] [Google Scholar]
- 18.deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, Seliger SL. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA 2010;304(22):2494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Lemos JA, Ayers CR, Levine BD, deFilippi CR, Wang TJ, Hundley WG, Berry JD, Seliger SL, McGuire DK, Ouyang P, Drazner MH, Budoff M, Greenland P, Ballantyne CM, Khera A. Multimodality Strategy for Cardiovascular Risk Assessment: Performance in 2 Population-Based Cohorts. Circulation 2017;135(22):2119–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobson DR, Alexander AA, Tagoe C, Buxbaum JN. Prevalence of the amyloidogenic transthyretin (TTR) V122I allele in 14 333 African-Americans. Amyloid 2015;22(3):171–4. [DOI] [PubMed] [Google Scholar]
- 21.Sinha A, Zheng Y, Nannini D, Qu Y, Hou L, Shah SJ, Yancy CW, McNally EM, Fornage M, Lima J, Lloyd-Jones DM, Rasmussen-Torvik LJ, Khan SS. Association of the V122I Transthyretin Amyloidosis Genetic Variant With Cardiac Structure and Function in Middle-aged Black Adults: Coronary Artery Risk Development in Young Adults (CARDIA) Study. JAMA Cardiol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, Wechalekar AD, Berk JL, Quarta CC, Grogan M, Lachmann HJ, Bokhari S, Castano A, Dorbala S, Johnson GB, Glaudemans AW, Rezk T, Fontana M, Palladini G, Milani P, Guidalotti PL, Flatman K, Lane T, Vonberg FW, Whelan CJ, Moon JC, Ruberg FL, Miller EJ, Hutt DF, Hazenberg BP, Rapezzi C, Hawkins PN. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation 2016; 133(24) :2404–12. [DOI] [PubMed] [Google Scholar]
- 23.Gillmore JD, Damy T, Fontana M, Hutchinson M, Lachmann HJ, Martinez-Naharro A, Quarta CC, Rezk T, Whelan CJ, Gonzalez-Lopez E, Lane T, Gilbertson JA, Rowczenio D, Petrie A, Hawkins PN. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J 2018;39(30):2799–2806. [DOI] [PubMed] [Google Scholar]
- 24.Kristen AV, Maurer MS, Rapezzi C, Mundayat R, Suhr OB, Damy T, investigators T. Impact of genotype and phenotype on cardiac biomarkers in patients with transthyretin amyloidosis - Report from the Transthyretin Amyloidosis Outcome Survey (THAOS). PLoS One 2017;12(4):e0173086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sperry BW, Vranian MN, Hachamovitch R, Joshi H, McCarthy M, Ikram A, Hanna M. Are classic predictors of voltage valid in cardiac amyloidosis? A contemporary analysis of electrocardiographic findings. Int J Cardiol 2016;214:477–81. [DOI] [PubMed] [Google Scholar]
- 26.Grogan M, Scott CG, Kyle RA, Zeldenrust SR, Gertz MA, Lin G, Klarich KW, Miller WL, Maleszewski JJ, Dispenzieri A. Natural History of Wild-Type Transthyretin Cardiac Amyloidosis and Risk Stratification Using a Novel Staging System. J Am Coll Cardiol 2016;68(10):1014–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


