Abstract
Objective:
Given the higher rates of tobacco use along with increased mortality specific to lung cancer in rural settings, low-dose computed tomography (LDCT)-based lung cancer screening could be particularly beneficial to such populations. However, limited radiology facilities and increased geographical distance, combined with lower income and education along with reduced patient engagement, present heightened barriers to screening initiation and adherence.
Methods:
In collaboration with community leaders and stakeholders, we developed and implemented a community-based lung cancer screening program, including telephone-based navigation and tobacco cessation counseling support, serving 18 North Texas counties. Funding was available to support clinical services costs where needed. We collected data on LDCT referrals, orders, and completion.
Results:
To raise awareness for lung cancer screening, we leveraged our established collaborative network of more than 700 community partners. In the first year of operation, 107 medical providers referred 570 patients for lung cancer screening, of whom 488 (86%) were eligible for LDCT. The most common reasons for ineligibility were age (43%) and insufficient tobacco history (20%). Of 381 ordered LDCT, 334 (88%) were completed. Among screened patients, 61% were current smokers and 36% had insurance coverage for the procedure. Program cost per patient was $430.
Discussion:
Implementation, uptake, and completion of LDCT-based lung cancer screening is feasible in rural settings. Community outreach, health promotion, and algorithm-based navigation may support such efforts. Given low lung cancer screening rates nationally and heightened lung cancer risk in rural populations, similar programs in other regions may be particularly impactful.
Keywords: community, computed tomography (CT), navigation, stakeholder, tobacco
Summary Sentence:
Implementation, uptake, and completion of LDCT-based lung cancer screening is feasible in rural settings.
Introduction:
Lung cancer remains a highly lethal disease, where poor outcomes are primarily driven by late stage at diagnosis. While highly effective treatment options are available for localized disease, only one-third of patients present at these stages. With definitive therapy, five-year survival can exceed 80% for stage 1 lung cancer, compared to fewer than 10% for stage 4 cases.1,2 The National Lung Screening Trial (NLST) demonstrated a safe, feasible, and effective means to detect lung cancer at an early stage and improve clinical outcomes. Specifically, low-dose computed tomography (LDCT) reduced lung cancer-specific mortality by 20%3; other studies have replicated this benefit.4
With LDCT, the number needed to screen (NNS) to prevent one lung cancer death is 320, which compares favorably to other cancer screening modalities (>700 for mammography, 1,100 for Pap smear, and 800 for sigmoidoscopy).5–7. LDCT for high-risk individuals is now endorsed by the United States Preventative Services Task Force (USPSTF) and a covered benefit for Centers for Medicare and Medicaid Services (CMS), in addition to many private insurers. However, lack of awareness, limited screening facilities, and patient and provider concerns, have resulted in fewer than 5% of eligible individuals undergoing lung cancer screening.8–10
Lung cancer screening may be particularly beneficial in rural communities, as residents typically have higher rates of tobacco use, resulting in greater likelihood for smoking-attributable disease and death, and lung cancer mortality.11–14 Unfortunately, the screening process can be hindered by additional barriers to care in rural areas. These include higher poverty rates, lower income and educational levels, and inadequate health insurance.15 Similarly, rural populations face geographic barriers to complex care, with limited access and longer travel times to imaging and treatment centers.16,17 Medical providers in rural settings must also deal with heightened challenges in coordinating treatment, outreach, and specialty care, which may limit screening opportunities.18–20 At the system level, rural areas may lack integrated public health systems capable of vertical integration of care or providing multiple services required for multi-disciplinary screening programs.
Patient navigation programs have demonstrated success in addressing barriers in cancer screening. In breast, cervical, colorectal, and prostate cancer, navigation has resulted in increased screening initiation ranging from 11–17%, and increased screening adherence ranging 21–29%.21,22 The most profound effect of these efforts have occurred in medically underserved populations.22
Given these observations, we established the Lung Cancer Screening and Patient Navigation (LSPAN) program in May 2018 to provide comprehensive lung cancer screening with tobacco cessation counseling support to serve 18 rural and medically underserved counties across North Texas, targeting the estimated 107,000 residents with an elevated risk for lung cancer. At its foundation are three principal components: (1) community outreach and health promotion to bridge knowledge gaps regarding lung cancer screening among local providers and patients, in addition to fostering a virtual integrated provider network; (2) telephone-based patient navigation led by oncology-certified registered nurses using an algorithmic screening process with physician oversight; and (3) centralized reimbursement, where clinical service costs are supplemented with funding from the Cancer Prevention and Research Institute of Texas (CPRIT) for low-income, uninsured or underinsured patients. In this report we describe referral, ordering, and completion rates for LDCT in Year 1, encompassing May 1, 2018 – June 30, 2019.
Patients and Methods:
Study Setting:
The LSPAN service area spans more than 13,000 square miles in North Texas. Predominantly rural, the region is comprised of counties designated as Medically Underserved Areas or Primary Care Health Provider Shortage Areas, as defined by the U.S. Department of Health and Human Services.23 The median per capita income is $26,500, with 15% of the population living at or below the federal poverty level. Similarly, 20% of residents are uninsured and 27% are underinsured or enrolled in Medicare. 2019 population estimates define the population as predominately English-speaking, with country averages of 85% identifying as White, 11% as Hispanic, and only 2% as African American. Smoking prevalence is also higher across the region, ranging from 16 – 18%, compared to 14% for the U.S. adult population.24
Moncrief Cancer Institute (MCI), a non-profit, community-based affiliate of the National Cancer Institute (NCI)-designated Harold C. Simmons Comprehensive Cancer Center at UT Southwestern Medical Center, operates the program from Fort Worth, Texas, in Tarrant County. Using an integrated electronic medical record system (EMR) (EPIC Systems; Verona, WI), patient characteristics and outcomes are tracked electronically for all program LDCTs performed at one of three facilities: MCI (Tarrant County), Graham Regional Medical Center (Young County), or Comanche County Medical Center (Comanche County). Each screening site is outfitted with a 64-slice CT and staffed by board-certified radiologists using the Lung CT Screening Reporting & Data System (Lung-RADS®) quality assurance tool to ensure standardized LDCT reporting, interpretation, and management.24 Prior to implementation of this program, the participating centers did not have LDCT protocols or employ Lung-RADS templates for lung cancer screening.
Outreach and Health Promotion:
MCI uses a targeted outreach approach, which relies on locally sensitive context to facilitate active referrals from community partners such as community health clinics and federally qualified health centers (FQHCs). An outreach coordinator is assigned a designated region within MCI’s larger 35-county service area. Coordinators travel to their assigned counties to build partnerships and meet in-person with community partners. In addition to developing partnerships with providers, they collaborate with the LSPAN nurse manager to assist new providers with identifying screen-eligible patients and coordinating referrals.
Provider education emphasizes screening eligibility criteria the role of shared decision-making, while addressing concerns such as less favorable views of treatment for lung cancer as compared to other cancers.25 Education sessions are delivered to providers as part of the readiness assessment and contracting process. Participating providers are given a referral pad to document the shared decision-making process and ensure patient eligibility (see Supplemental Materials). Patient-level initiatives focus on educating the community about lung cancer screening, as well as the benefits of tobacco cessation. All materials are bilingual (English/Spanish) and appropriate for a fifth-grade reading level.
Service Delivery and Navigation:
The patient navigation team includes a nurse program manager and registered nurse navigators, who are trained in motivational interviewing, problem solving, and goal setting to guide patients through the lung cancer screening process in a culturally- and socioeconomically-appropriate manner. Trained through the MD Anderson Certified Tobacco Treatment Specialist certification training program,24 nurse navigators also provide telephone-based tobacco cessation counseling. To ensure consistency in delivery and documentation, telephone-scripts and standardized forms were developed and used, along with specific smart-phrases for encounter notes in the EMR.
Program eligibility mirrors CMS guidelines: ages 55 – 77 years, at least a 30 pack-year smoking history, and a quit date within the last 15 years if a former smoker.26 A nurse navigator contacts patients for intake and scheduling after receipt of the completed referral form. The navigator confirms patient eligibility and assesses quit readiness for active smokers, including a referral to tobacco cessation counseling when appropriate. Barriers to screening completion, such as transportation, are assessed and addressed during the intake process, with transportation assistance provided as needed.
Navigation to facilitate screening completion is telephone-based and algorithm-driven, with the nursing team operating under standing orders to guide patients through diagnostic evaluation and treatment, where appropriate (Figure 1). Once completed, screening results are communicated back to the patient; those with normal results (Lung-RADS 1–2) receiving instruction to repeat screening in one-year, while those with abnormal results (Lung-RADS 3–4) are contacted by the registered nurse navigator to review the results and discuss the follow- up care plan. Results are also communicated back to the referring provider.
Figure 1.
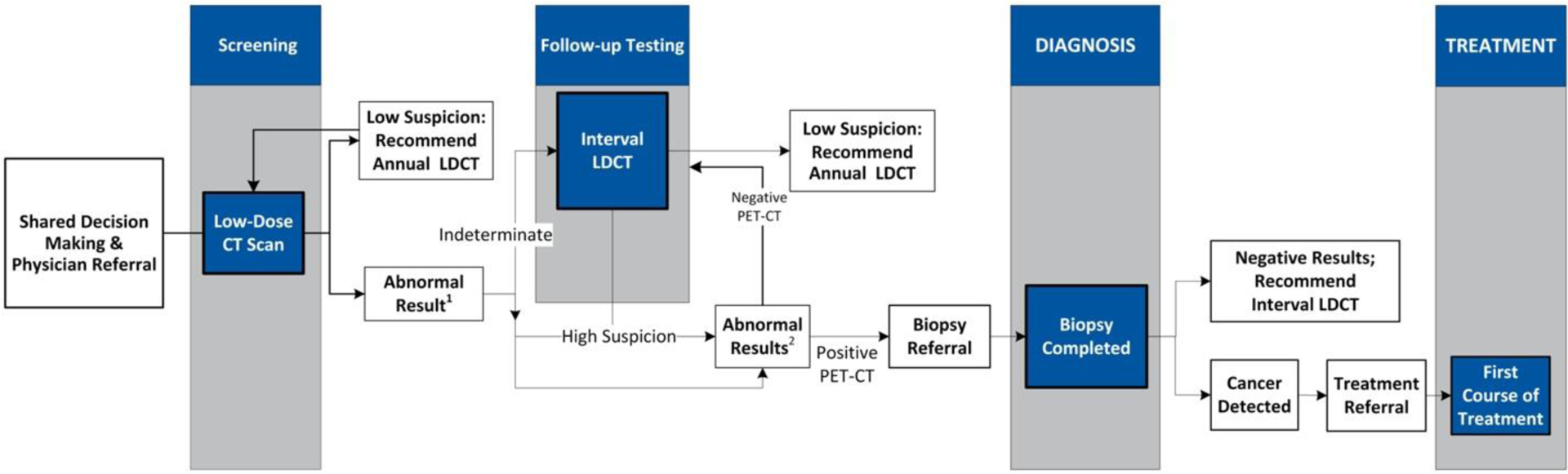
Lung Cancer Screening Algorithm
1Interval scan to evaluate indeterminate CT scan at interval < 12 months until there is resolution or findings.
2Go to PET-CT and/or subsequent biopsy to occur within weeks of screening result.
Centralized Reimbursement
Clinical services were provided to LSPAN program participants at no cost to the patient. While preventative services, including lung cancer screening, are typically covered by insurance without requiring deductibles, copayments or co-insurance, follow-up care required as a result of an abnormal screening (e.g., short-term repeat CT exams or other diagnostic procedures) may not be fully covered. In this program, clinical services are first billed to insurance, but MCI serves as the guarantor or secondary payor. As needed, CPRIT funds are used to cover co-pays and other uncovered costs associated with the screening process for underinsured patients, in addition to fully covering costs for uninsured patients. In this way, CPRIT funds fill gaps in coverage similar to Medicaid expansion in other states.
Data Analysis
Information on case characteristics and screening status (referrals, eligibility, LDCT orders, LDCT completion) was obtained from the program database. We calculated per-patient program cost by dividing the total annual personnel budget by the number of patients referred to the program. We compared age of individuals according to LDCT eligibility using Mood’s Median Test. We compared sex, race/ethnicity, and smoking status using Pearson’s Chi-Square test. Analyses were performed using R version 4.0.3 (2020-10-10).
Results:
In Year 1, the program received 570 patient referrals from 107 primary care providers, of which 488 (86%) were eligible per CMS guidelines (Figure 2). Median number of referrals per provider was 2 (range 1–76). Among those referred who were ineligible for screening (n = 82), 43% were outside the age range for screening, 20% did not meet the smoking pack-year requirement, and 15% had completed a chest CT within the preceding 12 months. Compared to ineligible referrals, eligible individuals had a trend toward being younger (median 62 vs 67 years; P=0.09). We also noted a significant difference in race/ethnicity according to eligibility (P<0.001). However, this result was driven by differences in the “unknown” category (55% of ineligible versus 19% of eligible), which reflects additional opportunities to collect race/ethnicity data from individuals entering the screening process. Among cases with available race/ethnicity data, eligible individuals were slightly less likely to be under-represented minorities than were eligible individuals (Black, 3% vs 7%; Hispanic, 3% vs 5%). There was no difference in sex or smoking status between eligible and ineligible referrals. Table 2 displays population characteristics through the entire lung screening process.
Figure 2.
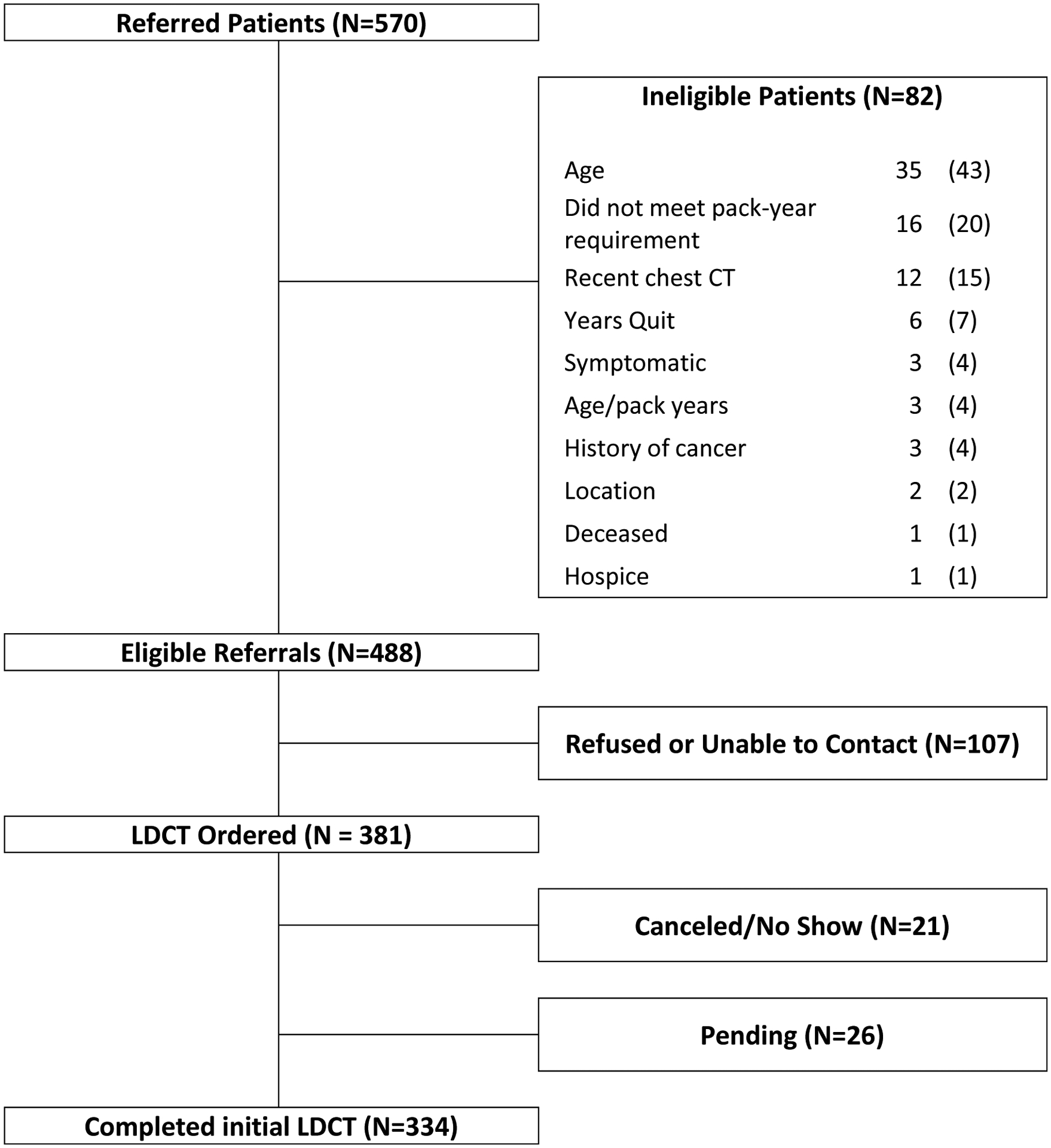
Patients referred to, eligible for, and completing low-dose computed tomography (LDCT)-based lung cancer screening.
Table 2.
Characteristics of patients throughout the lung cancer screening process
| Referrals n (%) or median (IQR) | Eligible n (%) or median (IQR) | Ordered n (%) or median (IQR) | Comple ted n (%) or median (IQR) | Pending n (%) or median (IQR) | Canceled/No Show n (%) or median (IQR) | |
|---|---|---|---|---|---|---|
| Age | 63 (59–68) | 62 (59–68) | 63 (59–68) | 63 (59–68) | 63 (59–67) | 60 (57–67) |
| Sex | ||||||
| Male | 315 (55) | 264 (54) | 206 (54) | 184 (55) | 17 (65) | 5 (24) |
| Female | 255 (45) | 224 (46) | 175 (46) | 150 (45) | 9 (35) | 16 (76) |
| Race-ethnicity | ||||||
| White (non-Hispanic) | 381 (67) | 354 (73) | 324 (85) | 283 (85) | 26 (100) | 15 (71) |
| Black | 19 (3) | 13 (3) | 13 (3) | 11 (3) | 0 (0) | 2 (10) |
| Hispanic | 21 (4) | 17 (3) | 17 (4) | 16 (5) | 0 (0) | 1 (5) |
| Other | 9 (2) | 9 (2) | 9 (2) | 9 (3) | 0 (0) | 0 (0) |
| Smoking status | ||||||
| Unknown | 140 (25) | 95 (19) | 18 (5) | 15 (4) | 0 (0) | 3 (14) |
| Current | 359 (63) | 309 (63) | 233 (61) | 206 (62) | 15 (58) | 12 (57) |
| Former | 211 (37) | 179 (37) | 148 (39) | 128 (38) | 11 (42) | 9 (43) |
IQR, interquartile range
At the time of this analysis, screening completion was 70%, with 74% of eligible referrals scheduled for LDCT. Median time between patient contact and LDCT completion was 12 days (interquartile range [IQR] 7–16 days). Among the remaining 26% not scheduled for LDCT, 60% could not be reached after three contact attempts, 24% actively declined, and 15% passively declined. Drop-off rates through eligibility, scheduling, and completion were similar across clinical sites (Table 1). The overall screened population was predominately male (55%) and non-Hispanic white (86%), with median of age 63 years. Relative to the LDCT arm of the NLST (N=26,722), however, participants were numerically more likely to be female (45% vs 41%), Hispanic (5% vs 2%), and an active smoker (61% vs 48%). Regarding healthcare coverage, 36% of individuals were insured, 49% were under- or uninsured, and 15% had unknown status.
Table 1.
LDCT referrals, eligibility, ordering, and completion rates according to screening location.
| Referrals N | Eligible N (%a) | Ordered N (%b) | Completed N (%c) | Pending N (%c) | Canceled/No Show N (%c) | |
|---|---|---|---|---|---|---|
| MCI/UTSW | 332 | 284 (85) | 221 (78) | 194 (88) | 10 (5) | 17 (8) |
| Graham Regional Medical Center | 169 | 147 (87) | 110 (75) | 95 (86) | 12 (11) | 3 (3) |
| Comanche County Medical Center | 69 | 57 (83) | 50 (88) | 45 (90) | 4 (8) | 1 (2) |
| Total | 570 | 488 (86) | 381 (78) | 334 (88) | 26 (7) | 21 (6) |
LDCT, low-dose computed tomography; MCI, Moncrief Cancer Institute; UTSW, University of Texas Southwestern Medical Center
Among referred patients
Among eligible patients
Among ordered LDCT
Program operations included a medical director (10% effort), program manager (50% effort), evaluation professional (5% effort), one RN navigator (100% effort), a certified medical office assistant (50% effort), and an outreach coordinator (50% effort), for a total annual operating cost (salary + fringe) of approximately $245,000. Dividing this cost among the 570 referred patients, the per-patient cost was $430.
Discussion:
Based on the published literature,27,28 to our knowledge LSPAN is among the fastest growing and largest rural lung cancer screening programs in the United States. LSPAN participants program were more likely to be to be active smokers relative to the NLST population, reflecting a national trend of increased smoking prevalence in rural areas, particularly in the Southern U.S.29,30 Active tobacco use has direct relevance to lung cancer screening efforts because cost effectiveness for annual LDCT is seemingly strongly linked to achievable tobacco cessation.31 Furthermore, tobacco use is associated with decreased adherence for other cancer screening modalities. As an example, 12-month interval repeat mammography screening rates are 36% among active smokers, compared to 44% for former smokers with a quit date within the last 6 years and 58% for those with a quit date of more than 6 years prior.32 In the International Early Lung Cancer Action Program (I-ELCAP), former smokers also showed greater adherence to screening than active smokers.33
In addition to higher rates of smoking and lung cancer in rural communities,34 screening facilities are also geographically maldistributed.16,17,35 In Virginia, the majority of 37 accredited LDCT facilities are concentrated near Washington D.C., where only 10% of adults smoke. Conversely, in all of southwest Virginia, an Appalachian region where smoking rates exceed 30%, there are only two LDCT facilities.36 Similarly, rural residents meeting the eligibility criteria for lung cancer screening often report not receiving information or recommendations from a health care provider, in addition to lack of transportation as key barriers to screening.37
We designed the LSPAN program specifically to address these considerations. In collaboration with community partners, the program has increased screening awareness among the general public using similar measures successful in other rural settings.38 Additional education and guidance has been provided to clinicians and healthcare organizations on screening eligibility and procedures. A straightforward referral process, supported by telephone-based navigation, is designed to optimize feasibility and efficiency for patients and practitioners. In Year 1, the LSPAN outreach team established a referral network of more than 100 primary care providers in Year 1, echoing the success of stakeholder engagement efforts in other medically underserved lung cancer screening efforts.39 Similarly, provider education efforts also appear efficient, with more than 85% of referred patients meeting the complex eligibility criteria for screening LDCT.
Almost 15 percent of individuals referred for screening were ineligible, most commonly due to age, less commonly due to smoking history or recent chest CT scan. These individuals tended to be older and less likely to be Black or Hispanic. Educational efforts may help decrease ineligible referrals for LDCT.20 Additionally, recent changes to USPSTF lung cancer screening guidelines lowering minimum age and smoking history eligibility are expected to have a particular effect on non-white populations.40,41
Approximately one-fourth of eligible referrals did not complete screening. Although average time from referral to patient contact was three days, failure to contact the patient after three attempts accounted for more than half of these incomplete cases, a challenge noted in lung cancer screening programs for urban safety-net populations42. Reasons for active and passive refusals to undergo LDCT after referral merit further investigation, as it is unclear as to whether this behavior reflects patient health beliefs, logistical considerations, or other concerns. Notably, once an LDCT order was placed, adherence was almost 90%, suggesting such a procedure is feasible despite the distance needed to travel. This high adherence rate exceeds real-world reports across various cancer screening modalities and is comparable to that in the controlled clinical trial setting for the NLST.3,43,44 Patient navigation services may likely have contributed to the high completion rates, as such interventions have demonstrated efficacy in cervical, breast, and colorectal cancer screening.45 Prospective evaluation of the effect of patient navigation on adherence to lung cancer screening steps in medically underserved populations is currently underway.46
Our estimated per-patient cost of $430—based on the inaugural year of program operations—may change over time, as one might expect increased referrals and heightened program efficiency in future years. It is difficult to place this figure in a broader lung cancer screening context. Indeed, a recent systematic review of lung cancer screening navigation cost-effectiveness found no studies met inclusion criteria47. Beyond lung cancer, screening navigation costs appear to vary widely, from $195 per colorectal cancer screen in New Hampshire low-income adults48, to $275 across cancer types when navigation services start after detection of an abnormal finding49, to over $1,500 for each low-income individual in a French colorectal cancer screening program50.
Numerous factors support the generalizability of our program to other settings. With USPSTF endorsement, LDCT-based lung cancer screening is eligible for reimbursement by Medicare, Medicaid, Affordable Care Act plans, and commercial insurance carriers. Providing this service to indigent populations may be easier elsewhere than in Texas; depending on the state, rates of Medicaid enrollment may be up to four times that of Texas, which has one of the most limited programs in the nation51. Over time, LDCT referrals may increase nationwide in concert with growth in pay-for-performance healthcare models, which frequently incorporate screening and prevention efforts52. Similarly, patient navigation is now broadly recognized to improve patient outcomes and satisfaction, while imparting financial benefits53. Telephone- and web-based interpreter services allow delivery of navigation across languages. Perhaps the greatest impediment to replicating our operation is the challenge of establishing trust and referral patterns, for which we capitalized on existing rural cancer screening and survivorship programs54.
Other limitations of this study include a sociocultural and geographic context which may be distinct from other rural regions. Specifically, the LSPAN population is largely non-Hispanic white and English speaking. We also recognize dedicated funding for LDCT performance may not be available at other sites, although Medicaid expansion in other states has resulted in a lower proportion of individuals without healthcare coverage compared to Texas. Given our relatively small sample size and limited clinical follow-up, cost effectiveness of the program cannot yet be determined. For some case characteristics, such as race/ethnicity, a substantial proportion of cases had missing data. Importantly, because there were no formal lung cancer screening efforts in this geographic region prior to implementation of this program, the relative impact of the program’s centralized LDCT ordering and navigation on parameters such as screening adherence and follow-up cannot be determined. Finally, as previously noted, we cannot determine the overall reach and penetration of our program, as sufficient tobacco history data is not available at the population level. That is, we do not know the number or proportion of potentially eligible individuals who were not referred for screening. Even taking a highly conservative approach, based on an estimate of more than 100,000 smokers in the region served by our program, it seems likely that only a small proportion of potentially eligible patients have been reached thus far.
In conclusion, implementation, uptake, and completion of LDCT-based lung cancer screening is feasible in rural settings. Community outreach, health promotion, and navigation may support such efforts. Given low lung cancer screening rates nationally and heightened lung cancer risk in rural populations, similar programs in other regions may be particularly impactful.
Supplementary Material
Take Home Points:
Implementation, uptake, and completion of LDCT screening for lung cancer is feasible in a rural setting, particularly when supported by community outreach, health promotion, and algorithm-based navigation.
Provider education emphasizing eligibility criteria for screening and the importance of shared decision-making to address the risks and benefits, coupled with standardized counseling and referral tools, can support efficient and effective collaboration as demonstrated by eligibility and completion rates for Year 1.
Patient engagement remains high once active in the program, with almost 90% of ordered LDCT completed.
Funding:
This work was supported by The Cancer Prevention and Research Institute of Texas (CPRIT) prevention awards (PP180025 - PI: Argenbright; PP190052 - PI: Gerber), and by National Cancer Institute (NCI) Midcareer Investigator Award in Patient-Oriented Research (K24 CA201543-01 - PI: Gerber).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: The authors declare no conflict of interest.
Data Statement:
The authors declare they had full access to all of the data in this study and the authors take complete responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Shewale JB, Corsini EM, Correa AM, et al. Time trends and predictors of survival in surgically resected early-stage non-small cell lung cancer patients. J Surg Oncol. 2020. [DOI] [PubMed] [Google Scholar]
- 2.Cetin K, Ettinger DS, Hei YJ, O’Malley CD. Survival by histologic subtype in stage IV nonsmall cell lung cancer based on data from the Surveillance, Epidemiology and End Results Program. Clin Epidemiol. 2011;3:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Lung Screening Trial Research T, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med. 2020;382(6):503–513. [DOI] [PubMed] [Google Scholar]
- 5.Gerber DE. Should family physicians routinely screen for lung cancer in high-risk populations? Yes: CT-based screening is complex but worthwhile. Am Fam Physician. 2014;90(2):73B–74B. [PubMed] [Google Scholar]
- 6.Hendrick RE, Helvie MA. Mammography screening: a new estimate of number needed to screen to prevent one breast cancer death. AJR Am J Roentgenol. 2012;198(3):723–728. [DOI] [PubMed] [Google Scholar]
- 7.Zhu CS, Pinsky PF, Kramer BS, et al. The prostate, lung, colorectal, and ovarian cancer screening trial and its associated research resource. J Natl Cancer Inst. 2013;105(22):1684–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okereke IC, Nishi S, Zhou J, Goodwin JS. Trends in lung cancer screening in the United States, 2016–2017. J Thorac Dis. 2019;11(3):873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raju S, Khawaja A, Han X, Wang X, Mazzone PJ. Lung Cancer Screening: Characteristics of Nonparticipants and Potential Screening Barriers. Clin Lung Cancer. 2020. [DOI] [PubMed] [Google Scholar]
- 10.Wang GX, Baggett TP, Pandharipande PV, et al. Barriers to Lung Cancer Screening Engagement from the Patient and Provider Perspective. Radiology. 2019;290(2):278–287. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins WD, Matthews AK, Bailey A, et al. Rural areas are disproportionately impacted by smoking and lung cancer. Prev Med Rep. 2018;10:200–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zahnd WE, James AS, Jenkins WD, et al. Rural-Urban Differences in Cancer Incidence and Trends in the United States. Cancer Epidemiol Biomarkers Prev. 2018;27(11):1265–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doogan NJ, Roberts ME, Wewers ME, et al. A growing geographic disparity: Rural and urban cigarette smoking trends in the United States. Prev Med. 2017;104:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atkins GT, Kim T, Munson J. Residence in Rural Areas of the United States and Lung Cancer Mortality. Disease Incidence, Treatment Disparities, and Stage-Specific Survival. Ann Am Thorac Soc. 2017;14(3):403–411. [DOI] [PubMed] [Google Scholar]
- 15.Rosenthal TC, Fox C. Access to health care for the rural elderly. JAMA. 2000;284(16):2034–2036. [DOI] [PubMed] [Google Scholar]
- 16.Eberth JM, Bozorgi P, Lebron LM, et al. Geographic Availability of Low-Dose Computed Tomography for Lung Cancer Screening in the United States, 2017. Prev Chronic Dis. 2018;15:E119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tailor TD, Tong BC, Gao J, Choudhury KR, Rubin GD. A Geospatial Analysis of Factors Affecting Access to CT Facilities: Implications for Lung Cancer Screening. J Am Coll Radiol. 2019;16(12):1663–1668. [DOI] [PubMed] [Google Scholar]
- 18.Eberth JM, McDonnell KK, Sercy E, et al. A national survey of primary care physicians: Perceptions and practices of low-dose CT lung cancer screening. Prev Med Rep. 2018;11:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Odahowski CL, Zahnd WE, Eberth JM. Challenges and Opportunities for Lung Cancer Screening in Rural America. J Am Coll Radiol. 2019;16(4 Pt B):590–595. [DOI] [PubMed] [Google Scholar]
- 20.Raz DJ, Wu GX, Consunji M, et al. The Effect of Primary Care Physician Knowledge of Lung Cancer Screening Guidelines on Perceptions and Utilization of Low-Dose Computed Tomography. Clin Lung Cancer. 2018;19(1):51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wells KJ, Battaglia TA, Dudley DJ, et al. Patient navigation: state of the art or is it science? Cancer. 2008;113(8):1999–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neal CD, Weaver DT, Raphel TJ, et al. Patient Navigation to Improve Cancer Screening in Underserved Populations: Reported Experiences, Opportunities, and Challenges. J Am Coll Radiol. 2018;15(11):1565–1572. [DOI] [PubMed] [Google Scholar]
- 23.Negotiated rulemaking committee on the designation of medically underserved populations and health professional shortage areas: final report to the secretary. http://www.hrsa.gov/advisorycommittees/shortage/nrmcfinalreport.pdf. Published 2011. Accessed May 11, 2020.
- 24.https://www.mdanderson.org/education-training/professional-education/continuing-education-review-courses/certified-tobacco-treatment-training-program.html. Accessed May 11, 2020.
- 25.Wassenaar TR, Eickhoff JC, Jarzemsky DR, Smith SS, Larson ML, Schiller JH. Differences in primary care clinicians’ approach to non-small cell lung cancer patients compared with breast cancer. J Thorac Oncol. 2007;2(8):722–728. [DOI] [PubMed] [Google Scholar]
- 26.Decision Memo for Screening for Lung Cancer with Low Dose Computed Tomography (LDCT) (CAG-00439N). https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274. Published 2015. Accessed May 11, 2020.
- 27.Cardarelli R, Madabhushi V, Bledsoe K, Weaver A. Lung cancer screening in Appalachian Kentucky: The impact of Lung-RADS on subsequent testing and cancer identification. J Clin Transl Sci. 2019;4(5):468–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Headrick JR Jr., Morin O, Miller AD, Hill L, Smith J. Mobile Lung Screening: Should We All Get on the Bus? Ann Thorac Surg. 2020;110(4):1147–1152. [DOI] [PubMed] [Google Scholar]
- 29.https://www.ers.usda.gov/topics/rural-economy-population/rural-poverty-well-being/ Accessed November 11, 2020.
- 30.https://www.cdc.gov/tobacco/disparities/geographic/index.htm. Accessed November 11, 2020.
- 31.McMahon PM, Kong CY, Bouzan C, et al. Cost-effectiveness of computed tomography screening for lung cancer in the United States. J Thorac Oncol. 2011;6(11):1841–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rakowski W, Breen N, Meissner H, et al. Prevalence and correlates of repeat mammography among women aged 55–79 in the Year 2000 National Health Interview Survey. Prev Med. 2004;39(1):1–10. [DOI] [PubMed] [Google Scholar]
- 33.Montes U, Seijo LM, Campo A, Alcaide AB, Bastarrika G, Zulueta JJ. Factors determining early adherence to a lung cancer screening protocol. Eur Respir J. 2007;30(3):532–537. [DOI] [PubMed] [Google Scholar]
- 34.Blake KD, Moss JL, Gaysynsky A, Srinivasan S, Croyle RT. Making the Case for Investment in Rural Cancer Control: An Analysis of Rural Cancer Incidence, Mortality, and Funding Trends. Cancer Epidemiol Biomarkers Prev. 2017;26(7):992–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hasson RM, Fay KA, Phillips JD, Millington TM, Finley DJ. Rural barriers to early lung cancer detection: Exploring access to lung cancer screening programs in New Hampshire and Vermont. Am J Surg. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin AN, Hassinger TE, Kozower BD, Camacho F, Anderson RT, Yao N. Disparities in Lung Cancer Screening Availability: Lessons From Southwest Virginia. Ann Thorac Surg. 2019;108(2):412–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schiffelbein JE, Carluzzo KL, Hasson RM, Alford-Teaster JA, Imset I, Onega T. Barriers, Facilitators, and Suggested Interventions for Lung Cancer Screening Among a Rural Screening-Eligible Population. J Prim Care Community Health. 2020;11:2150132720930544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Springer SM, McFall A, Hager P, Percy-Laury A, Vinson CA. Lung cancer screening: an emerging cancer control issue presents opportunities for an awareness campaign in rural Michigan. Cancer Causes Control. 2018;29(12):1257–1263. [DOI] [PubMed] [Google Scholar]
- 39.Lee SJC, Hamann HA, Browning T, et al. Stakeholder engagement to initiate lung cancer screening in an urban safety-net health system. Healthc (Amst). 2020;8(1):100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meza R, Jeon J, Toumazis I, et al. Evaluation of the Benefits and Harms of Lung Cancer Screening With Low-Dose Computed Tomography: Modeling Study for the US Preventive Services Task Force. JAMA. 2021;325(10):988–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ritzwoller DP, Meza R, Carroll NM, et al. Evaluation of Population-Level Changes Associated With the 2021 US Preventive Services Task Force Lung Cancer Screening Recommendations in Community-Based Health Care Systems. JAMA Netw Open. 2021;4(10):e2128176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerber DE, Hamann HA, Chavez C, et al. Tracking the Nonenrolled: Lung Cancer Screening Patterns Among Individuals not Accrued to a Clinical Trial. Clin Lung Cancer. 2020;21(4):326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burhansstipanov L, Dignan MB, Schumacher A, Krebs LU, Alfonsi G, Apodaca CC. Breast screening navigator programs within three settings that assist underserved women. J Cancer Educ. 2010;25(2):247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christie J, Itzkowitz S, Lihau-Nkanza I, Castillo A, Redd W, Jandorf L. A randomized controlled trial using patient navigation to increase colonoscopy screening among low-income minorities. J Natl Med Assoc. 2008;100(3):278–284. [DOI] [PubMed] [Google Scholar]
- 45.Paskett ED, Harrop JP, Wells KJ. Patient navigation: an update on the state of the science. CA Cancer J Clin. 2011;61(4):237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerber DE, Hamann HA, Santini NO, et al. Patient navigation for lung cancer screening in an urban safety-net system: Protocol for a pragmatic randomized clinical trial. Contemp Clin Trials. 2017;60:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kass B, Dornquast C, Rieckmann N, Goerling U, Holmberg C, Reinhold T. Cost-effectiveness of patient navigation for lung cancer ? a systematic review [version 2; peer review: 1 approved]. F1000Research. 2021;10(314). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rice K, Sharma K, Li C, Butterly L, Gersten J, DeGroff A. Cost-effectiveness of a patient navigation intervention to increase colonoscopy screening among low-income adults in New Hampshire. Cancer. 2019;125(4):601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bensink ME, Ramsey SD, Battaglia T, et al. Costs and outcomes evaluation of patient navigation after abnormal cancer screening: evidence from the Patient Navigation Research Program. Cancer. 2014;120(4):570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Mil R, Guillaume E, Guittet L, et al. Cost-Effectiveness Analysis of a Navigation Program for Colorectal Cancer Screening to Reduce Social Health Inequalities: A French Cluster Randomized Controlled Trial. Value Health. 2018;21(6):685–691. [DOI] [PubMed] [Google Scholar]
- 51.https://www.medicaid.gov/state-overviews/scorecard/percentage-of-population-enrolled-medicaid-or-chip-state/index.html. Accessed November 5, 2021.
- 52.Rosenthal MB, Frank RG, Li Z, Epstein AM. Early experience with pay-for-performance: from concept to practice. JAMA. 2005;294(14):1788–1793. [DOI] [PubMed] [Google Scholar]
- 53.Kline RM, Rocque GB, Rohan EA, et al. Patient Navigation in Cancer: The Business Case to Support Clinical Needs. J Oncol Pract. 2019;15(11):585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee SJC, Higashi RT, Inrig SJ, et al. County-level outcomes of a rural breast cancer screening outreach strategy: a decentralized hub-and-spoke model (BSPAN2). Transl Behav Med. 2017;7(2):349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare they had full access to all of the data in this study and the authors take complete responsibility for the integrity of the data and the accuracy of the data analysis.


