Abstract
Aging is the greatest independent risk factor for developing hypertension and cardiovascular-related diseases including systolic hypertension, vascular disease, ischemic events, arrhythmias, and heart failure. Age-related cardiovascular risk is associated with dysfunction of peripheral organ systems, such as the heart and vasculature, as well as an imbalance in the autonomic nervous system characterized by increased sympathetic and decreased parasympathetic neurotransmission. Given the increasing prevalence of aged individuals worldwide, it is critical to better understand mechanisms contributing to impaired cardiovascular autonomic control in this population. In this regard, the renin-angiotensin system has emerged as an important hormonal modulator of cardiovascular function in aging, in part through modulation of autonomic pathways controlling sympathetic and parasympathetic outflow to cardiovascular end organs. This review will summarize the role of the RAS in cardiovascular autonomic control during aging, with a focus on current knowledge of angiotensin II versus angiotensin-(1–7) pathways in both rodent models and humans, pharmacological treatment strategies targeting the renin-angiotensin system, and unanswered questions for future research.
Keywords: angiotensin, blood pressure, sympathetic, parasympathetic
Introduction
Aging is the greatest independent risk factor for the development of hypertension and cardiovascular disease (CVD).1 Importantly, CVD is the leading cause of death in people over age 65, which is the fastest growing age group worldwide.2 Several changes to the cardiovascular system occur in healthy aging that predispose this population to developing CVD (Figure 1). For example, aging leads to structural changes to the heart including cardiac fibrosis, left ventricular hypertrophy, and valve stenosis.1 Additionally, arterial plaques develop throughout the lifespan causing endothelial dysfunction, arterial stiffening, and atherosclerosis with aging. Collectively, these age-related changes in cardiovascular physiology lead to the development and progression of CVD including systolic hypertension, vascular disease, acute ischemic events, arrhythmias, and heart failure.1
Figure 1. Overview of Cardiovascular and Autonomic Changes in Aging.
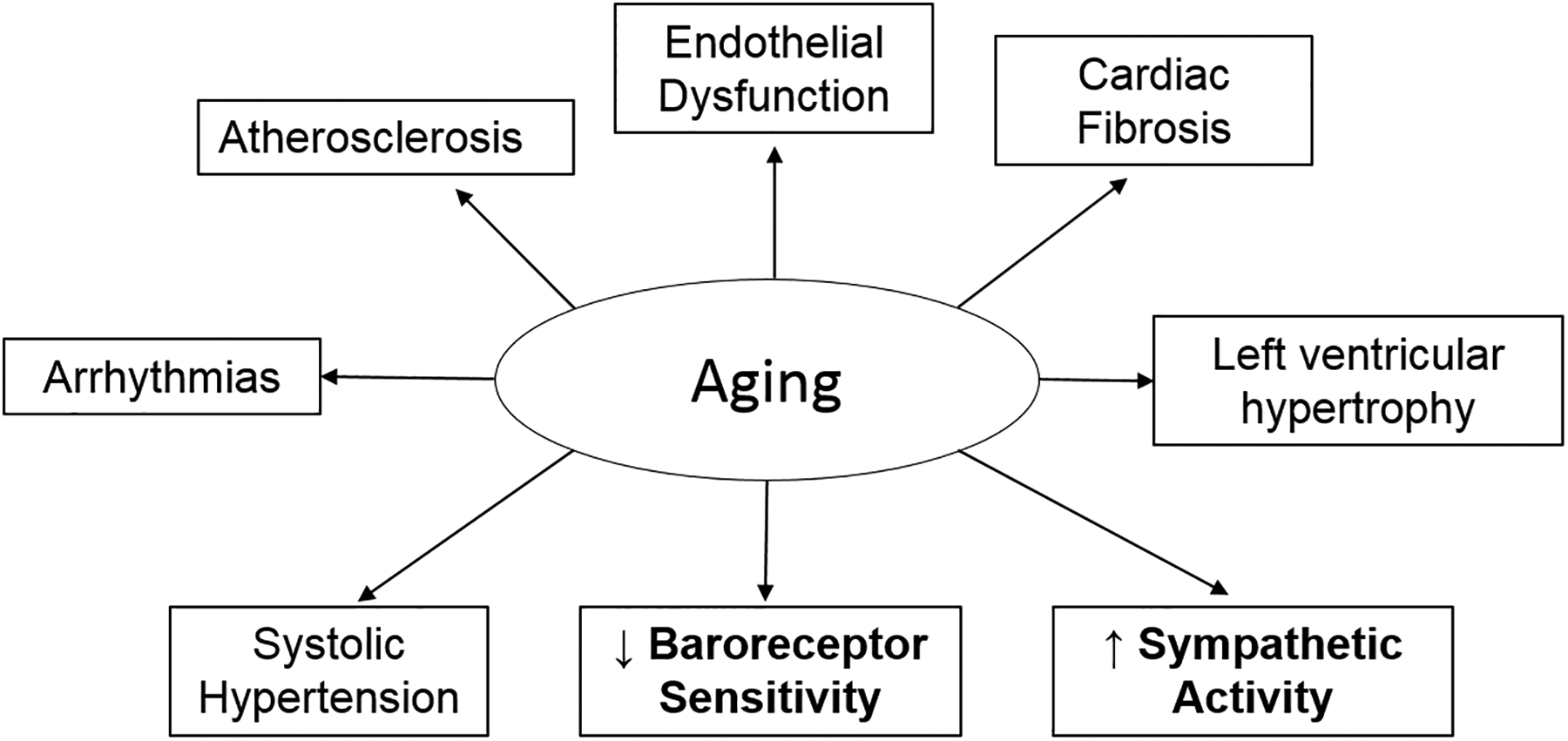
Healthy aging is associated with several changes to the cardiovascular system that predispose this population to developing cardiovascular disease. This review will focus on autonomic nervous system mechanisms (bolded), such as reduced baroreceptor sensitivity and increased sympathetic activity, that occur with aging. While not a focus, additional non-autonomic mechanisms occur during aging that impact cardiovascular control such as structural changes to the heart and blood vessels including cardiac fibrosis, left ventricular hypertrophy, endothelial damage, and atherosclerosis. All these changes can manifest clinically as isolated systolic hypertension and arrhythmias in aged individuals.
Changes in autonomic control of the cardiovascular system are also well recognized to contribute to age-related CVD.3 In terms of blood pressure regulation, aging is associated with isolated systolic hypertension, which is largely attributed to increases in arterial stiffness as well as increased sympathetic nervous system activity.4,5 Diastolic blood pressure and heart rate decrease after age 50 in humans due to large artery stiffening, reduced sinoatrial node firing, and decreased β-adrenergic receptor sensitivity.5,6 In rodent models, while systolic and mean blood pressure increase with age similar to humans, diastolic blood pressure and heart rate remain stable throughout the lifespan.7 Sympathetic traffic to the heart and vasculature also increases progressively with age,3 prior to hypertension and CVD onset.8 This chronically elevated sympathetic tone increases catecholamine release to desensitize and downregulate adrenergic receptors. Desensitization of α-adrenergic receptors impairs acute vasoconstriction in response to physical stimuli (e.g., postural changes), and increases the risk for orthostatic hypotension and syncope in elderly adults.9 High sympathetic tone also desensitizes β-adrenergic receptors in the heart and vessels to impair heart rate, cardiac contractility, and vasodilation thus increasing risk for hypertension and heart failure. This desensitization is evidenced clinically by decreased cardiac and vascular responses to infusion of adrenergic receptor agonists in small mechanistic studies in older men.10–12 In addition to increased sympathetic tone, aging is accompanied by reduced measures of cardiovascular parasympathetic tone including heart rate variability and the baroreflex sensitivity for control of heart rate.13 Overall, aging is associated with an imbalance in the sympathetic and parasympathetic arms of the autonomic nervous system, which predisposes to cardiovascular morbidity and mortality. As the aging population continues to increase, it is important to better understand mechanisms underlying age-related cardiovascular autonomic impairment to develop new therapies to reduce cardiovascular risk.
In this regard, the renin-angiotensin system (RAS) has emerged as an important hormonal mechanism contributing to aberrant cardiovascular autonomic control in aging. This review will summarize current understanding of the role of the RAS in cardiovascular autonomic control in aging, with a focus on angiotensin II versus angiotensin-(1–7) pathways in both rodent models and humans. Additionally, pharmacological strategies to target the RAS during aging will be discussed, as these therapies are commonly used to treat hypertension and CVD in elderly individuals. Importantly, this focus on aging builds on our recent review describing sites of interaction between the RAS and autonomic nervous system for cardiovascular control in younger models of CVD.14 While not a focus of this review, the RAS has been implicated in the regulation of numerous other physiological and pathophysiological processes during aging including glucose homeostasis, energy balance, neuroinflammation, cognition, mitochondrial redox balance, physical performance, renal disease, and osteoporosis.15–18
Angiotensin II Pathways in Cardiovascular Autonomic Control
Angiotensin II is an important hormonal contributor to homeostatic regulation of blood pressure, fluid and electrolyte balance, and control of the cardiovascular system via the brain and peripheral organs. Inappropriately elevated levels of angiotensin II, however, are implicated in the pathophysiology of numerous CVD-related states (e.g., hypertension, heart failure, atherosclerosis, stroke, obesity, diabetes). Angiotensin II has primary actions at type 1 receptors (AT1R), which are g-protein coupled receptors that facilitate increased blood pressure via neural, renal, vascular, and cardiac mechanisms.19,20 Specifically, AT1R activation promotes vasoconstriction, endothelial and cardiac dysfunction, release of aldosterone and vasopressin, oxidative stress, inflammation, sympathetic tone, and baroreflex dysfunction.21
As recently reviewed,14 chronic stimulation of AT1R by angiotensin II can also lead to hypertension in younger animal models via interactions with the autonomic nervous system. AT1R are abundant at each synaptic relay of the autonomic nervous system (e.g., preganglionic neurons, ganglia, nerve terminals, regulatory brain regions), with activation by angiotensin II increasing sympathetic and decreasing parasympathetic neurotransmission.22 Angiotensin II signaling can also affect adrenergic receptors through the sympathetic nervous system, to alter cardiovascular autonomic control. Angiotensin II desensitizes and downregulates adrenergic receptors, specifically β-adrenergic receptors in the heart, kidney, and blood vessels. For example, one study showed that exogenous angiotensin II downregulates β2-adrenergic receptor protein expression in human endothelial progenitor cells.23 Additionally, AT1R activation by angiotensin II affects β-arrestin binding to β2-adrenergic receptors to desensitize and downregulate these receptors in cultured kidney cell lines.24 Finally, angiotensin converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARBs), therapies which decrease both angiotensin II and sympathetic activity, can restore β-adrenergic receptor expression and sensitivity in cardiac tissue from rat models of hypertension independent of effects on blood pressure or cardiac hypertrophy.25 Overall, a handful of studies in cultured cells or isolated tissues provide evidence for crosstalk between Ang II AT1R and β-adrenergic receptor signaling. The functional importance of these interactions for cardiovascular autonomic regulation in vivo, however, remains unclear.
Angiotensin II also binds to type 2 receptors (AT2R) to oppose actions mediated by activation of AT1R; although these receptors have limited expression and affinity, particularly in adulthood.26 Activation of AT2R induces several cardioprotective effects including vasodilation and natriuresis through nitric oxide (NO) and prostaglandin pathways.27 Pharmacological blockade of AT1R with ARBs can increase angiotensin II binding to AT2R, which partially contributes to the antihypertensive effects of these drugs.28 AT2R are also expressed in cardiovascular autonomic pathways and promote sympathoinhibition. For example, overexpression of AT2R in the rostral ventrolateral medulla decreases blood pressure and norepinephrine release in normal rats.29 Furthermore, AT2R activation or overexpression in brain reduces sympathetic outflow in rat models of heart failure.29,30 In rat models of heart failure, hypertension and obesity, chronic AT2R stimulation or overexpression also improves the baroreflex sensitivity for control of both heart rate and renal sympathetic nerve activity,30–32 an important mechanism to restrain cardiovascular sympathetic outflow. Thus, accumulating data support that AT2R may provide a novel target to counteract Ang II actions at AT1R in animal models of CVD, at least in part by decreasing sympathetic and increasing parasympathetic neurotransmission. The translatability of these findings to humans remains unknown, although a few controlled clinical studies are registered on clinicaltrials.gov using AT2R agonists, such as compound 21, in diseases such as Covid-19 and idiopathic pulmonary fibrosis.
The sympathetic nervous system also regulates angiotensin II activity in a bidirectional manner, with high sympathetic tone increasing angiotensin II levels. Sympathetic innervation of β1-adrenergic receptors in the kidney stimulates renin release, which increases angiotensin II formation.14 As clinical evidence of the importance of this relationship, patients with pure autonomic failure and chronic sympathetic denervation have low and often undetectable levels of plasma renin activity.33 Furthermore, methods to suppress efferent sympathetic outflow, such as renal denervation or electrical activation of the carotid baroreflex, reduce circulating renin activity, angiotensin II and aldosterone levels in experimental animal models and in patients with resistant hypertension.34
Angiotensin II Pathways and Cardiovascular Autonomic Actions in Aging
During aging, there are several changes in the activity and responsiveness of the RAS that affect cardiovascular autonomic control (Figure 2; Table 1). In normotensive and hypertensive individuals, there is an age-related suppression of the systemic RAS. Specifically, renin formation and release decline in aging resulting in decreased plasma renin concentrations.35 Additionally, older individuals exhibit impaired renin responsiveness to physiological stimuli such as upright posture and sodium depletion as well as have increased prevalence of low renin hypertension.36,37 While not fully understood, mechanisms that have been proposed to reduce systemic RAS activity in the elderly include: reduced baroceptor-mediated renal renin release due to elevated arterial pressure;38 decreased renin production via reduced renal beta1-adrenergic receptor sensitivity;39 and more rarely reduced sympathetic innervation to attenuate renin release from renal juxtaglomerular cells in patients with autonomic impairment.33 Studies examining for circulating levels of prorenin, the renin precursor, and the prorenin receptor during aging are limited, with one report showing decreased plasma prorenin in hypertensive but not normotensive subjects, one showing increased soluble prorenin receptor in hypertensive subjects, and a few reporting no change in prorenin or soluble prorenin receptor levels, with age.40–44
Figure 2. Renin-Angiotensin and Autonomic Interactions in Aging for Cardiovascular Control.
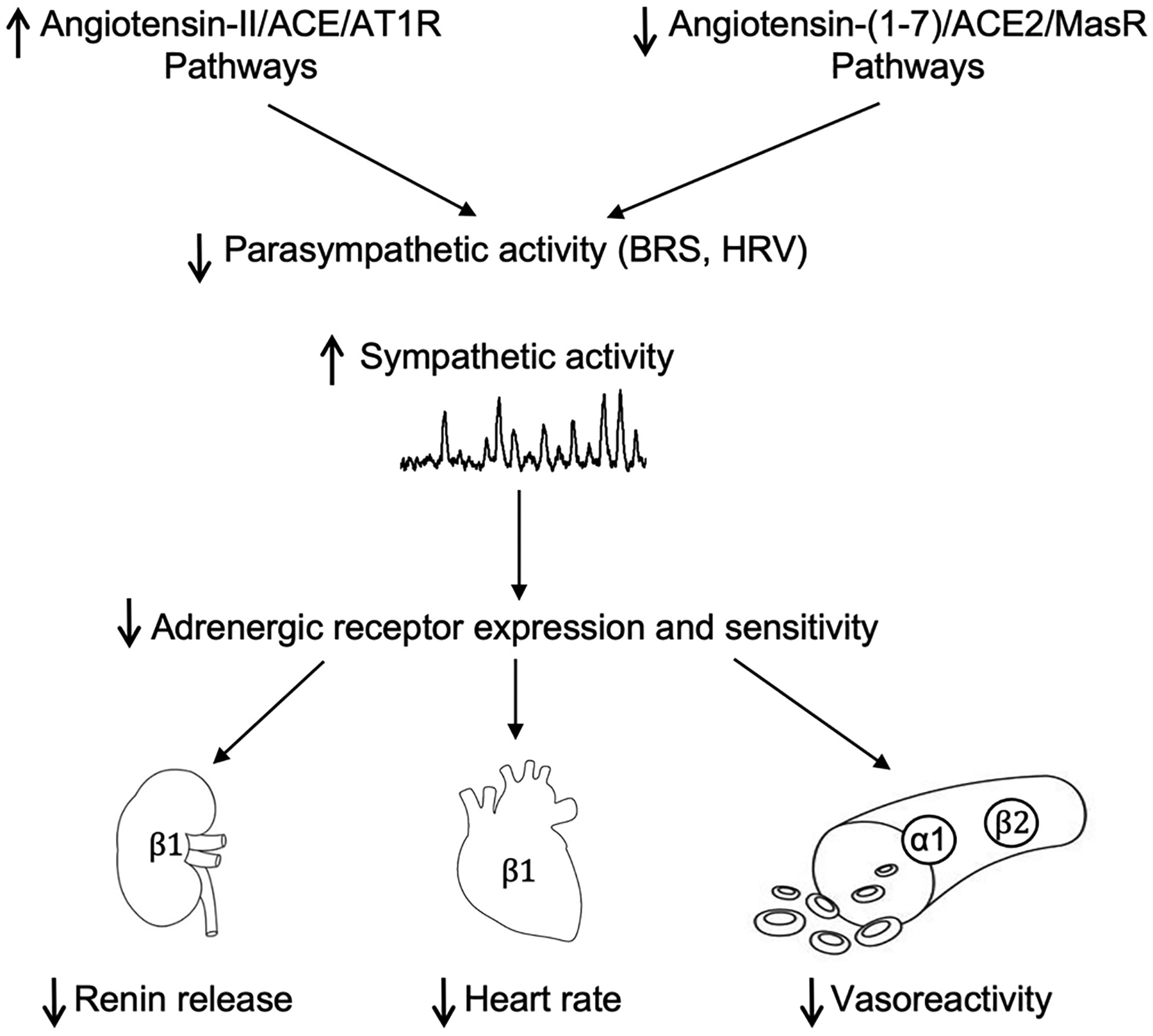
During aging, angiotensin II, angiotensin converting enzyme (ACE), and angiotensin type 1 receptor (AT1R) pathways are increased while angiotensin-(1–7), angiotensin converting enzyme 2 (ACE2), and mas receptor (MasR) are decreased. This imbalance in the renin-angiotensin system contributes to impaired measures of parasympathetic tone, such as baroreflex sensitivity (BRS) and heart rate variability (HRV). This reduced parasympathetic tone allows for unrestrained sympathetic activation, which can desensitize adrenergic receptors in the kidney, heart, and vasculature; these end organs are less responsive to sympathetic stimulation in aging.
Table 1.
Changes in Renin-Angiotensin System Components with Aging
| Age-Related Change | References | Model | |
|---|---|---|---|
| Plasma Expression | |||
| Aldosterone | ↓ | 35,45 | rat, human |
| Angiotensin converting enzyme (ACE) | ↓ | 47 | human |
| Angiotensin converting enzyme (ACE2) | ↑ | 47 | human |
| Angiotensin-(1–7) | ↓ | 72 | human |
| Angiotensin II | ↓ | 47 | human |
| Prorenin | ↓, - | 40–44 | human |
| Renin | ↓ | 35, 36,37 | human |
| Cardiovascular Tissue Expression | |||
| ACE | ↑ | 52,53 | mouse, rat |
| ACE2 | ↓ | 52,72 | mouse |
| Angiotensin-(1–7) | ↑ | 45 | rat |
| Angiotensin-(1–7) mas receptor (MasR) | ↓ | 52,72 | mouse |
| Angiotensin II | ↑ | 45,50–52 | rat, mouse, non-human primate |
| Angiotensin II type 1 receptor (AT1R) | ↑ | 45,50–53 | rat, mouse, non-human primate |
| Angiotensin II type 2 receptor (AT2R) | ↓ | 50 | rat, mouse |
| Angiotensinogen | ↑ | 53 | rat |
| Prorenin receptor | ↑ | 52 | mouse |
| Renin | ↑ | 35 | rat, human |
Plasma aldosterone levels also decline during healthy aging in rodents and humans.35,45 As described in our prior review,14 aldosterone can activate either mineralocorticoid receptors or angiotensinergic signaling pathways within the brain to elevate sympathetic outflow and blood pressure in younger animal models of CVD. To our knowledge, there is no information on direct interactions of aldosterone with the autonomic nervous system during aging. Of potential relevance, a small controlled study showed that the mineralocorticoid receptor antagonist spironolactone effectively lowers blood pressure in elderly patients with stage I hypertension, in part by reducing sympathetic activity.46 This effect may not be specific to aldosterone, however, as spironolactone also blocks the actions of other hormones including angiotensin II and cortisol.
Similar to renin and aldosterone, circulating angiotensin II levels and angiotensin converting enzyme (ACE) activity decrease in healthy aged humans.47 Interestingly, acute intravenous angiotensin II infusion elicits exaggerated pressor responses and peripheral vasoconstriction in small trials in older healthy humans, perhaps reflecting increased AT1R sensitivity.48,49 Indeed, despite declines in systemic RAS activity, production of angiotensin II and AT1R expression and sensitivity increase in local RAS tissue systems during aging in animal models.45,50–52 In the heart of aged rats, angiotensinogen, ACE and AT1R are increased, independent of changes in the circulating RAS,53 which may contribute to age-related changes in cardiac structure and function. Similarly, renal expression of renin and ACE are increased during aging in rodents with enhanced renal responsiveness to angiotensin II, which may predispose to renal damage and fluid-electrolyte imbalances.35 Vascular changes are also evident with increased protein expression of the prorenin receptor, ACE, angiotensin II, and AT1R observed in the thoracic aorta of aged mice.52 Additionally, endothelial and renal AT2R expression decreases with age, thus potentially limiting the protective cardiovascular effects of angiotensin II.50 While AT2R is cardioprotective in young animals, one study showed AT2R agonism induced paradoxical vasoconstriction via oxidative stress in aging rats.50
In addition to peripheral tissues, a local RAS exists within the brain, which appears regulated independent from the circulating system. Altered brain RAS activity has been implicated in the autonomic imbalance observed during aging, to precipitate elevations in blood pressure. In particular, a chronic imbalance in which brain angiotensin II is increased and angiotensin-(1–7) is decreased has been observed in aging.54 In terms of autonomic effects, it is well established that angiotensin II, both endogenous to the brain and when given exogenously, increases cardiovascular sympathetic outflow and impairs the baroreceptor reflex control of heart rate in aged animal models. The precise mechanisms underlying the deleterious cardiovascular autonomic effects elicited by brain angiotensin II signaling, however, remain poorly understood.
These overall findings suggest that tissue angiotensin II production, ACE activity, and AT1R expression and sensitivity are increased in animal models during aging and contribute to age-related cardiovascular pathophysiology. In particular, activation of angiotensinergic signaling within brain may contribute to excess sympathetic outflow to cardiovascular end organs to elevate blood pressure and lead to the development and progression of hypertension. Additional research is needed to better understand the neural pathways and cellular mechanisms engaged by brain angiotensin II to elicit age-related changes in cardiovascular autonomic control (Table 2). The status of tissue angiotensin II pathways and their functionality during aging in humans is also largely unexplored, likely reflecting limitations in obtaining these samples.
Table 2.
Questions for Future Research on Renin-Angiotensin System: Autonomic Interactions for Cardiovascular Control in Aging
|
|
|
|
|
|
Ang-(1–7) Pathways in Cardiovascular Autonomic Control
Angiotensin-(1–7) is a more recently discovered RAS hormone that is formed from angiotensin II cleavage by angiotensin converting enzyme 2 (ACE2) or from angiotensin I cleavage by endopeptidases, such as neprilysin.14,55 Angiotensin-(1–7) binds to g-protein coupled mas receptors (MasR) to induce cardioprotective effects including vasodilation, endothelial NO release, enhanced baroreflex function, and reductions in sympathetic tone, cardiac hypertrophy, inflammation, oxidative stress, and fibrosis in animal models.56 In vivo studies support MasR as the primary receptor-mediated mechanism for angiotensin-(1–7) actions; however, MasR can heterodimerize with AT1R, AT2R, bradykinin B2 receptors, and dopamine D2 receptors in cellular models.56,57 While having minimal effects on cardiovascular function under normal conditions, angiotensin-(1–7) lowers blood pressure in younger rodent models of hypertension and CVD, in which circulating levels of the peptide appear deficient.58,59 The most studied acute mechanism for angiotensin-(1–7) depressor effects is peripheral vasodilation by activating endothelial nitric oxide synthase (eNOS) and stimulating endothelial NO release.60 Angiotensin-(1–7) also improves cardiac function, reduces cardiac fibrosis, and prevents cardiac remodeling following ischemia in younger rodent models of hypertension, obesity and metabolic syndrome.61–64
In addition, there is growing evidence that angiotensin-(1–7) interacts with autonomic nervous system pathways to regulate blood pressure.14,56 The mechanisms by which angiotensin-(1–7) interacts with the autonomic nervous system both centrally and peripherally, however, are less well understood. Studies support that depressor effects of angiotensin-(1–7) are associated with reduced peripheral and central measures of sympathetic tone in younger hypertensive rodents.65–69 Specifically, angiotensin-(1–7) has been shown to decrease norepinephrine release from the hypothalamus, kidney, and heart in rats.67,70 There is also evidence that angiotensin-(1–7) reduces norepinephrine synthesis and release from sympathetic nerve terminals innervating resistance arteries in hypertensive rats.67,69 Angiotensin-(1–7) also increases measures of parasympathetic tone, such as the baroreflex sensitivity for control of heart rate, in hypertensive rats.22,68 These autonomic effects of angiotensin-(1–7) are largely mediated by MasR, which are highly expressed in autonomic pathways similar to the distribution of angiotensin II receptors. For example, MasR antagonism in the midbrain prevents angiotensin-(1–7)-induced improvements in baroreflex control of sympathetic nerve activity and blood pressure.22,71 The sympathoinhibitory effects of angiotensin-(1–7) may also, however, be mediated in part through AT2R activation.67 Overall, these data suggest that angiotensin-(1–7) levels and MasR and ACE2 expression are reduced in younger rodent models of CVD, and that restoration of this hormone provides a novel target to restore the balance of the RAS to improve cardiovascular control.
Ang-(1–7) Pathways and Cardiovascular Autonomic Actions in Aging
While cardioprotective in younger animal models of CVD, there is limited research on levels and actions of angiotensin-(1–7) pathways during aging (Table 1). There is sparse and conflicting data on angiotensin-(1–7) levels in aging. For example, a small cross-sectional study showed lower circulating angiotensin-(1–7) in aged overweight and obese human subjects,72 while another study showed higher levels with aging in a rat model of metabolic syndrome.45 In another small cross-sectional study in healthy individuals, serum ACE2 activity appears higher in older women when compared with younger women, with no differences in other RAS enzyme activities.47 In contrast, MasR and ACE2 expression is decreased in the aorta of aged mice, which may diminish the vascular protective effects of Ang-(1–7) in aging.52,72 These disparate findings may reflect differences in species as well as in the analytical methods to measure RAS peptides and enzyme activities. Furthermore, while animal studies often focus on RAS levels in tissues, clinical studies typically assess circulating peptides and enzyme activities due to sampling limitations.
In terms of functional actions, one study found that angiotensin-(1–7) vasodilatory effects are impaired in aortic rings obtained from older versus younger female mice and restored by estrogen replacement, but with no male comparator.73 Chronic angiotensin-(1–7) treatment also decreases mean blood pressure to a greater extent in aged compared to young healthy rodents.74,75 This decrease in mean blood pressure was driven by changes in systolic blood pressure, as diastolic pressure and heart rate were unaffected.75 These depressor effects appear dependent on MasR in aging, with a greater contribution of AT2R-mediated effects in younger rodents.74 Additionally, the cardiovascular phenotype produced by genetic deletion of ACE2 in mice (e.g. systolic hypertension, cardiac autonomic imbalance, oxidative stress, vascular inflammation) appears associated with age, but may depend on the genetic background.54
Importantly, the depressor effects of angiotensin-(1–7) in aging are associated with decreased cardiovascular sympathetic tone. Our group recently published that chronic systemic angiotensin-(1–7) treatment decreases cardiac sympathetic tone in healthy aging mice.75 More specifically, we found that aged mice treated with angiotensin-(1–7) have reductions in the sympathetic component of heart rate variability as well as gene expression of tyrosine hydroxylase, the rate-limiting enzyme for catecholamine synthesis, in cardiac tissue.75 Additionally, low endogenous brain angiotensin-(1–7) levels contribute to reductions in the baroreflex sensitivity during aging in rodents. More specifically, low levels of angiotensin-(1–7) in the solitary tract nucleus of the dorsomedial medulla contribute to baroreflex impairment in aged rodents.53
Overall, these limited findings in rodent models suggest that circulating angiotensin-(1–7) levels and tissue ACE2 and MasR expression are reduced during healthy aging and associated with decreased parasympathetic and increased sympathetic tone (Figure 2; Table 1). Restoration of angiotensin-(1–7) may provide a novel target to maintain proper baroreceptor reflex function and decrease cardiovascular sympathetic tone, systolic hypertension, and age-related risk for CVD. Additional studies are needed to:confirm these findings in aged animal models of CVD, identify precise cellular and neural mechanisms involved, and determine the status of angiotensin-(1–7) pathways during aging and the potential impact of its restoration on cardiovascular regulation during aging in humans (Table 2).
RAS Therapies in Aging
ACE Inhibitors and Angiotensin Receptor Blockers
Pharmacological therapies blocking angiotensin II activity are widely used for the treatment of hypertension and CVD due to their antihypertensive and cardioprotective effects. The two main classes of drugs clinically used are ARBs and ACEi.76 ACEi prevent the conversion of angiotensin I to angiotensin II, whereas ARBs prevent angiotensin II from binding AT1R to limit downstream signaling effects. The beneficial effects of these therapies on blood pressure are partially attributed to autonomic mechanisms. ACEi and ARBs reduce central sympathetic neural discharge and norepinephrine spillover while improving norepinephrine tissue clearance at peripheral nerve terminals in essential hypertension.77 These therapies also improve the gain and set point of the arterial baroreflex for control of heart rate and sympathetic activity to preserve autonomic reflex control.78,79 A few initial studies have also shown that ACEi and ARBs increase β2-adrenergic receptor expression and sensitivity and eNOS expression in human endothelial progenitor cells and isolated cardiac tissue from hypertensive rats, which could contribute to the vasodilatory effects of these drugs.23,25 In addition to blocking angiotensin II activity, ACEi and ARBs are established to increase circulating angiotensin-(1–7) levels, which contributes to the beneficial cardiometabolic effects of these therapies in rodent models of CVD and obesity.80–83
In aged rodent models, ACEi and ARBs appear less effective at lowering blood pressure;35,84 however, data in preclinical models may not translate to clinical use. Given the well-recognized age-related decline in systemic RAS activity, some guidelines for hypertension pharmacotherapy, such as those provided by the National Institute for Health and Care Excellence in the United Kingdom, have recommended ACEi and ARBs as first line therapy in individuals less than 55 years of age, and calcium channel blockers for non-diabetic individuals aged 55 years and older. Clinically, however, several large trials support that ACEi and ARBs effectively lower blood pressure and decrease cardiovascular morbidity and mortality in older adults with CVD.74,85–87 This finding suggests that beneficial effects of these therapies are at least in part due to suppression of the local tissue RAS. In addition to cardioprotection, long-term RAS blockade extends lifespan and protects against age-related declines in physical performance and metabolic, cognitive, renal, and mitochondrial functions.53
Despite these protective effects, large population-based studies have shown that ACEi and ARBs may be less well tolerated in aging clinical populations. For example, the risk of angioedema and renal failure is greater with these treatments in older populations.88,89 In general, ACEi are reported to be limited in ~11% of patients by cough due to off-target production of kinins.89,90 In patients over 60, however, the incidence of dry cough with ACEi treatment is 32%; this may be a greater concern due to decreased lung function with aging.89 Elderly hypertensive patients also often have comorbid conditions including chronic kidney disease and autonomic disorders such as orthostatic hypotension, which may make use of angiotensin II blocking therapies more hazardous in aging.88 Therefore, there is need to develop alternative treatments targeting the RAS with a better risk profile for blood pressure lowering and cardioprotection in aging populations.
Direct Renin Inhibitors
Direct renin inhibitors offer a therapeutic approach to potentially achieve more complete RAS inhibition, by blocking the rate-limiting step for downstream RAS peptide formation. Aliskiren is a first-in-class nonpeptide orally active renin inhibitor that binds renin and non-proteolytically activated prorenin to prevent cleavage of angiotensinogen to angiotensin I, to ultimately reduce angiotensin II formation. The effects of aliskiren appear partially mediated by the autonomic nervous system as centrally administered aliskiren lowers blood pressure, in part by reducing renal sympathetic nerve activity and restoring arterial baroreflex function, in hypertensive rats.91 Aliskiren monotherapy has been shown to elicit sustained antihypertensive effects in large double-blind randomized controlled clinical trials;92 however, this drug has increased side effects compared with ACEi and ARBs as well as contraindications in patients with diabetes or renal impairment. In geriatric populations, aliskiren significantly reduces systolic blood pressure and is better tolerated than ACEi and other common antihypertensive drugs such as diuretics and calcium channel blockers.92–94 Despite promise in controlled clinical trials, aliskiren is not yet recommended as first line treatment for the elderly as data for safety and effectiveness in older patients in the real-world settings of clinical practice are still lacking;92 however, observational studies are currently ongoing to address this.
Angiotensin-(1–7)
Targeting angiotensin-(1–7) directly may provide an ideal approach to mitigate hypertension and CVD in aging, while bypassing the known side effects of ACEi and ARBs. Despite evidence for cardioprotective effects in animal models, however, there are few clinical trials with angiotensin-(1–7). Angiotensin-(1–7) has been shown to improve NO-mediated vasodilation in the cutaneous microcirculation and isolated human coronary microvessels.95,96 Two studies have established safety, tolerability, and dosing for acute intravenous angiotensin-(1–7) infusion in younger healthy humans.97,98 A few small mechanistic studies have also shown that intra-arterial angiotensin-(1–7) infusion dilates forearm and renal vessels in patients with obesity or hypertension, but with inconsistent effects in younger healthy humans.99–101 In terms of aging, despite implications for angiotensin-(1–7) deficiency in CVD pathogenesis in aged animal models, there are no clinical trials to date investigating angiotensin-(1–7) effects in older clinical populations. A major challenge to conducting clinical research with angiotensin-(1–7) in humans is the short half-life of this peptide in the circulation. While approaches to chronically elevate angiotensin-(1–7) levels are in development and early phase clinical trials (e.g., oral formulations, stable analoges, ACE2 activators, MasR agonists), these are not yet widely available, thus currently limiting more long-term studies in humans.
Conclusions and Future Research Directions
The RAS has emerged as an important hormonal modulator of blood pressure in aging, in part through modulation of autonomic nervous system pathways controlling sympathetic and parasympathetic outflow to cardiovascular end organs. While the circulating RAS appears largely suppressed with aging, emerging evidence supports a role for increased angiotensin II and decreased angiotensin-(1–7) pathways in age-related sympathoexcitation and baroreflex dysfunction. Blockade of angiotensin II activity with ACEi or ARBs lowers blood pressure and provides cardioprotection in aged individuals, with effects of these therapies partially mediated via suppression of tissue angiotensin II signaling, autonomic mechanisms, and increased endogenous angiotensin-(1–7) levels. Additional therapies targeting the RAS, to restore the balance of angiotensin II versus angiotensin-(1–7), but with a more favorable side effect profile, would advance our ability to reduce cardiovascular risk in aging.
There are several unanswered questions remaining regarding the role of the RAS in cardiovascular autonomic regulation in aging that warrant additional research (Table 2). First, the precise neural pathways and cellular mechanisms engaged for RAS: autonomic interactions in aging are unknown, particularly related to aldosterone as well as more recently discovered angiotensin-(1–7)-ACE2-MasR pathways. Second, there is limited data on sex differences in RAS autonomic actions in aging. In this regard, future research should investigate if the cardiovascular autonomic pathways engaged by angiotensin II versus angiotensin-(1–7) differ in aged males versus females, as well as the impact of sex hormones, menopause, and hormone replacement therapy on these pathways. Emerging data in rodent models suggest that angiotensin-(1–7) provides added protection in females, although these studies are predominately in younger models.102,103 Postmenopausal women have higher sympathetic activity and blood pressure than males,104,105 as well as attenuated β2-adrenergic receptor-mediated vasodilation.106 Thus, it is possible that older females will be more responsive to the sympathoinhibitory and depressor effects of RAS therapies, including angiotensin-(1–7) treatment. Third, despite well-established differences in hypertension development and therapeutic approaches,107 there is limited information the impact of racial or ethnic influences on RAS: autonomic interactions or age-related changes in the RAS. African Americans are established to have lower plasma renin activity and aldosterone compared with Caucasians, which appear independent of blood pressure and age.107,108 While RAS blockers are less likely to be used as first line therapy in African Americans, emerging data suggests no evidence for reduced efficacy of these therapies perhaps due to a paradoxical increase in tissue RAS activation. Initial evidence also suggests increased sympathetic neurotransmission contributes to enhanced cardiovascular risk in African Americans,109 but the role of the RAS in this phenomenon is unknown. Finally, studies investigating age-related changes in RAS components and treatments have largely focused on healthy aging. Since healthy aging is atypical, additional studies are needed to investigate RAS actions and therapies, including potential autonomic mechanisms, in healthy versus diseased aging populations in both preclinical models and clinical trials.
Acknowledgements:
This work was supported by the National Institutes of Health [grant number R01 HL156986].
Abbreviations
- ACE
angiotensin converting enzyme
- ACE2
angiotensin converting enzyme 2
- ACEi
angiotensin converting enzyme inhibitors
- ARBs
angiotensin receptor blockers
- AT1R
angiotensin II type 1 receptors
- AT2R
angiotensin II type 2 receptors
- CVD
cardiovascular disease
- eNOS
endothelial nitric oxide synthase
- MasR
angiotensin-(1–7) mas receptors
- NO
nitric oxide
- RAS
renin-angiotensin system
Footnotes
Declarations of Interest: None
References Cited:
- 1.North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res. Apr 13 2012;110(8):1097–108. doi: 10.1161/CIRCRESAHA.111.246876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United Nations DoEaSA, Population Division. World Population Ageing 2019. 2020;(ST/ESA/SER.A/444)
- 3.Esler M, Hastings J, Lambert G, Kaye D, Jennings G, Seals DR. The influence of aging on the human sympathetic nervous system and brain norepinephrine turnover. Am J Physiol Regul Integr Comp Physiol. Mar 2002;282(3):R909–16. doi: 10.1152/ajpregu.00335.2001 [DOI] [PubMed] [Google Scholar]
- 4.Seals DR, Esler MD. Human ageing and the sympathoadrenal system. J Physiol. Nov 1 2000;528(Pt 3):407–17. doi: 10.1111/j.1469-7793.2000.00407.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franklin SS. Ageing and hypertension: the assessment of blood pressure indices in predicting coronary heart disease. J Hypertens Suppl. Dec 1999;17(5):S29–36. [PubMed] [Google Scholar]
- 6.Ferrari AU, Radaelli A, Centola M. Invited review: aging and the cardiovascular system. J Appl Physiol (1985). Dec 2003;95(6):2591–7. doi: 10.1152/japplphysiol.00601.2003 [DOI] [PubMed] [Google Scholar]
- 7.Barsha G, Denton KM, Mirabito Colafella KM. Sex- and age-related differences in arterial pressure and albuminuria in mice. Biol Sex Differ. 2016;7:57. doi: 10.1186/s13293-016-0110-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mancia G, Grassi G. The autonomic nervous system and hypertension. Circ Res. May 23 2014;114(11):1804–14. doi: 10.1161/CIRCRESAHA.114.302524 [DOI] [PubMed] [Google Scholar]
- 9.Arnold AC, Shibao C. Current concepts in orthostatic hypotension management. Curr Hypertens Rep. Aug 2013;15(4):304–12. doi: 10.1007/s11906-013-0362-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional alpha-adrenergic vasoconstriction in healthy men. Circulation. Sep 10 2002;106(11):1349–54. doi: 10.1161/01.cir.0000028819.64790.be [DOI] [PubMed] [Google Scholar]
- 11.Dinenno FA, Tanaka H, Stauffer BL, Seals DR. Reductions in basal limb blood flow and vascular conductance with human ageing: role for augmented alpha-adrenergic vasoconstriction. J Physiol. Nov 1 2001;536(Pt 3):977–83. doi: 10.1111/j.1469-7793.2001.00977.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross AJ, Gao Z, Pollock JP, Leuenberger UA, Sinoway LI, Muller MD. beta-Adrenergic receptor blockade impairs coronary exercise hyperemia in young men but not older men. Am J Physiol Heart Circ Physiol. Nov 15 2014;307(10):H1497–503. doi: 10.1152/ajpheart.00584.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monahan KD. Effect of aging on baroreflex function in humans. Am J Physiol Regul Integr Comp Physiol. Jul 2007;293(1):R3–R12. doi: 10.1152/ajpregu.00031.2007 [DOI] [PubMed] [Google Scholar]
- 14.Miller AJ, Arnold AC. The renin-angiotensin system in cardiovascular autonomic control: recent developments and clinical implications. Clin Auton Res. Apr 2019;29(2):231–243. doi: 10.1007/s10286-018-0572-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamo T, Akazawa H, Suzuki JI, Komuro I. Roles of renin-angiotensin system and Wnt pathway in aging-related phenotypes. Inflamm Regen. 2016;36:12. doi: 10.1186/s41232-016-0018-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. Jul 2010;2(7):247–57. doi: 10.1002/emmm.201000080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vajapey R, Rini D, Walston J, Abadir P. The impact of age-related dysregulation of the angiotensin system on mitochondrial redox balance. Front Physiol. 2014;5:439. doi: 10.3389/fphys.2014.00439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cosarderelioglu C, Nidadavolu LS, George CJ, et al. Brain Renin-Angiotensin System at the Intersect of Physical and Cognitive Frailty. Front Neurosci. 2020;14:586314. doi: 10.3389/fnins.2020.586314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Unger T The role of the renin-angiotensin system in the development of cardiovascular disease. Am J Cardiol. Jan 24 2002;89(2A):3A–9A; discussion 10A. doi: 10.1016/s0002-9149(01)02321-9 [DOI] [PubMed] [Google Scholar]
- 20.Santos RAS, Sampaio WO, Alzamora AC, et al. The ACE2/Angiotensin-(1–7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1–7). Physiol Rev. Jan 1 2018;98(1):505–553. doi: 10.1152/physrev.00023.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lavoie JL, Sigmund CD. Minireview: overview of the renin-angiotensin system--an endocrine and paracrine system. Endocrinology. Jun 2003;144(6):2179–83. doi: 10.1210/en.2003-0150 [DOI] [PubMed] [Google Scholar]
- 22.Averill DB, Diz DI. Angiotensin peptides and baroreflex control of sympathetic outflow: pathways and mechanisms of the medulla oblongata. Brain Res Bull. Jan 15 2000;51(2):119–28. doi: 10.1016/s0361-9230(99)00237-3 [DOI] [PubMed] [Google Scholar]
- 23.Lee SJ, Kim DY, Yun J, et al. Angiotensin II Attenuates the Bioactivities of Human Endothelial Progenitor Cells via Downregulation of beta2-Adrenergic Receptor. Stem Cells Int. 2018;2018:7453161. doi: 10.1155/2018/7453161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toth AD, Gyombolai P, Szalai B, Varnai P, Turu G, Hunyady L. Angiotensin type 1A receptor regulates beta-arrestin binding of the beta2-adrenergic receptor via heterodimerization. Mol Cell Endocrinol. Feb 15 2017;442:113–124. doi: 10.1016/j.mce.2016.11.027 [DOI] [PubMed] [Google Scholar]
- 25.Bohm M, Zolk O, Flesch M, et al. Effects of angiotensin II type 1 receptor blockade and angiotensin-converting enzyme inhibition on cardiac beta-adrenergic signal transduction. Hypertension. Mar 1998;31(3):747–54. doi: 10.1161/01.hyp.31.3.747 [DOI] [PubMed] [Google Scholar]
- 26.Lemarie CA, Schiffrin EL. The angiotensin II type 2 receptor in cardiovascular disease. J Renin Angiotensin Aldosterone Syst. Mar 2010;11(1):19–31. doi: 10.1177/1470320309347785 [DOI] [PubMed] [Google Scholar]
- 27.Carey RM, Wang ZQ, Siragy HM. Role of the angiotensin type 2 receptor in the regulation of blood pressure and renal function. Hypertension. Jan 2000;35(1 Pt 2):155–63. doi: 10.1161/01.hyp.35.1.155 [DOI] [PubMed] [Google Scholar]
- 28.Siragy H Angiotensin II receptor blockers: review of the binding characteristics. Am J Cardiol. Nov 18 1999;84(10A):3S–8S. doi: 10.1016/s0002-9149(99)00727-4 [DOI] [PubMed] [Google Scholar]
- 29.Gao L, Wang W, Wang W, Li H, Sumners C, Zucker IH. Effects of angiotensin type 2 receptor overexpression in the rostral ventrolateral medulla on blood pressure and urine excretion in normal rats. Hypertension. Feb 2008;51(2):521–7. doi: 10.1161/HYPERTENSIONAHA.107.101717 [DOI] [PubMed] [Google Scholar]
- 30.Gao J, Zucker IH, Gao L. Activation of central angiotensin type 2 receptors by compound 21 improves arterial baroreflex sensitivity in rats with heart failure. Am J Hypertens. Oct 2014;27(10):1248–56. doi: 10.1093/ajh/hpu044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Speretta GF, Ruchaya PJ, Delbin MA, et al. Importance of AT1 and AT2 receptors in the nucleus of the solitary tract in cardiovascular responses induced by a high-fat diet. Hypertens Res. Apr 2019;42(4):439–449. doi: 10.1038/s41440-018-0196-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruchaya PJ, Speretta GF, Blanch GT, et al. Overexpression of AT2R in the solitary-vagal complex improves baroreflex in the spontaneously hypertensive rat. Neuropeptides. Dec 2016;60:29–36. doi: 10.1016/j.npep.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 33.Biaggioni I, Garcia F, Inagami T, Haile V. Hyporeninemic normoaldosteronism in severe autonomic failure. J Clin Endocrinol Metab. Mar 1993;76(3):580–6. doi: 10.1210/jcem.76.3.7680352 [DOI] [PubMed] [Google Scholar]
- 34.Iliescu R, Lohmeier TE, Tudorancea I, Laffin L, Bakris GL. Renal denervation for the treatment of resistant hypertension: review and clinical perspective. Am J Physiol Renal Physiol. Oct 1 2015;309(7):F583–94. doi: 10.1152/ajprenal.00246.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoon HE, Choi BS. The renin-angiotensin system and aging in the kidney. Korean J Intern Med. May 2014;29(3):291–5. doi: 10.3904/kjim.2014.29.3.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weidmann P, De Myttenaere-Bursztein S, Maxwell MH, de Lima J. Effect on aging on plasma renin and aldosterone in normal man. Kidney Int. Nov 1975;8(5):325–33. doi: 10.1038/ki.1975.120 [DOI] [PubMed] [Google Scholar]
- 37.Mulkerrin E, Epstein FH, Clark BA. Aldosterone responses to hyperkalemia in healthy elderly humans. J Am Soc Nephrol. Nov 1995;6(5):1459–62. doi: 10.1681/ASN.V651459 [DOI] [PubMed] [Google Scholar]
- 38.Diz DI. Lewis K. Dahl memorial lecture: the renin-angiotensin system and aging. Hypertension. Jul 2008;52(1):37–43. doi: 10.1161/HYPERTENSIONAHA.107.108985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galbusera M, Garattini S, Remuzzi G, Mennini T. Catecholamine receptor binding in rat kidney: effect of aging. Kidney Int. Jun 1988;33(6):1073–7. doi: 10.1038/ki.1988.113 [DOI] [PubMed] [Google Scholar]
- 40.Nguyen G, Blanchard A, Curis E, et al. Plasma soluble (pro)renin receptor is independent of plasma renin, prorenin, and aldosterone concentrations but is affected by ethnicity. Hypertension. Feb 2014;63(2):297–302. doi: 10.1161/HYPERTENSIONAHA.113.02217 [DOI] [PubMed] [Google Scholar]
- 41.Tsunoda K, Abe K, Goto T, et al. Effect of age on the renin-angiotensin-aldosterone system in normal subjects: simultaneous measurement of active and inactive renin, renin substrate, and aldosterone in plasma. J Clin Endocrinol Metab. Feb 1986;62(2):384–9. doi: 10.1210/jcem-62-2-384 [DOI] [PubMed] [Google Scholar]
- 42.Nakamaru M, Ogihara T, Higaki J, et al. Effect of age on active and inactive plasma renin in normal subjects and in patients with essential hypertension. J Am Geriatr Soc. Aug 1981;29(8):379–82. doi: 10.1111/j.1532-5415.1981.tb01245.x [DOI] [PubMed] [Google Scholar]
- 43.Trenkwalder P, James GD, Laragh JH, Sealey JE. Plasma renin activity and plasma prorenin are not suppressed in hypertensives surviving to old age. Am J Hypertens. Jul 1996;9(7):621–7. doi: 10.1016/0895-7061(96)00022-2 [DOI] [PubMed] [Google Scholar]
- 44.Morimoto S, Ando T, Niiyama M, et al. Serum soluble (pro)renin receptor levels in patients with essential hypertension. Hypertens Res. Jul 2014;37(7):642–8. doi: 10.1038/hr.2014.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rubio-Ruiz ME, Del Valle-Mondragon L, Castrejon-Tellez V, Carreon-Torres E, Diaz-Diaz E, Guarner-Lans V. Angiotensin II and 1–7 during aging in Metabolic Syndrome rats. Expression of AT1, AT2 and Mas receptors in abdominal white adipose tissue. Peptides. Jul 2014;57:101–8. doi: 10.1016/j.peptides.2014.04.021 [DOI] [PubMed] [Google Scholar]
- 46.Wray DW, Supiano MA. Impact of aldosterone receptor blockade compared with thiazide therapy on sympathetic nervous system function in geriatric hypertension. Hypertension. May 2010;55(5):1217–23. doi: 10.1161/HYPERTENSIONAHA.109.147058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernandez-Atucha A, Izagirre A, Fraile-Bermudez AB, et al. Sex differences in the aging pattern of renin-angiotensin system serum peptidases. Biol Sex Differ. 2017;8:5. doi: 10.1186/s13293-017-0128-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wray DW, Nishiyama SK, Harris RA, Richardson RS. Angiotensin II in the elderly: impact of angiotensin II type 1 receptor sensitivity on peripheral hemodynamics. Hypertension. Jun 2008;51(6):1611–6. doi: 10.1161/HYPERTENSIONAHA.108.111294 [DOI] [PubMed] [Google Scholar]
- 49.Takeda R, Morimoto S, Uchida K, Miyamori I, Hashiba T. Effect of age on plasma aldosterone response to exogenous angiotensin II in normotensive subjects. Acta Endocrinol (Copenh). Aug 1980;94(4):552–8. doi: 10.1530/acta.0.0940552 [DOI] [PubMed] [Google Scholar]
- 50.Carey RM. Angiotensin receptors and aging. Hypertension. Jul 2007;50(1):33–4. doi: 10.1161/HYPERTENSIONAHA.106.086587 [DOI] [PubMed] [Google Scholar]
- 51.Wang M, Takagi G, Asai K, et al. Aging increases aortic MMP-2 activity and angiotensin II in nonhuman primates. Hypertension. Jun 2003;41(6):1308–16. doi: 10.1161/01.HYP.0000073843.56046.45 [DOI] [PubMed] [Google Scholar]
- 52.Yoon HE, Kim EN, Kim MY, et al. Age-Associated Changes in the Vascular Renin-Angiotensin System in Mice. Oxid Med Cell Longev. 2016;2016:6731093. doi: 10.1155/2016/6731093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Diz DI, Varagic J, Groban L. Aging and the brain renin-angiotensin system: relevance to age-related decline in cardiac function. Future Cardiol. May 2008;4(3):237–45. doi: 10.2217/14796678.4.3.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arnold AC, Sakima A, Kasper SO, Vinsant S, Garcia-Espinosa MA, Diz DI. The brain renin-angiotensin system and cardiovascular responses to stress: insights from transgenic rats with low brain angiotensinogen. J Appl Physiol (1985). Dec 15 2012;113(12):1929–36. doi: 10.1152/japplphysiol.00569.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Santos RA, Simoes e Silva AC, Maric C, et al. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci U S A. Jul 8 2003;100(14):8258–63. doi: 10.1073/pnas.1432869100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Santos RA. Angiotensin-(1–7). Hypertension. Jun 2014;63(6):1138–47. doi: 10.1161/HYPERTENSIONAHA.113.01274 [DOI] [PubMed] [Google Scholar]
- 57.Bader M, Alenina N, Young D, Santos RAS, Touyz RM. The Meaning of Mas. Hypertension. Nov 2018;72(5):1072–1075. doi: 10.1161/HYPERTENSIONAHA.118.10918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giani JF, Mayer MA, Munoz MC, et al. Chronic infusion of angiotensin-(1–7) improves insulin resistance and hypertension induced by a high-fructose diet in rats. Am J Physiol Endocrinol Metab. Feb 2009;296(2):E262–71. doi: 10.1152/ajpendo.90678.2008 [DOI] [PubMed] [Google Scholar]
- 59.Sampaio WO, Nascimento AA, Santos RA. Systemic and regional hemodynamic effects of angiotensin-(1–7) in rats. Am J Physiol Heart Circ Physiol. Jun 2003;284(6):H1985–94. doi: 10.1152/ajpheart.01145.2002 [DOI] [PubMed] [Google Scholar]
- 60.Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM. Angiotensin-(1–7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension. Jan 2007;49(1):185–92. doi: 10.1161/01.HYP.0000251865.35728.2f [DOI] [PubMed] [Google Scholar]
- 61.Grobe JL, Mecca AP, Lingis M, et al. Prevention of angiotensin II-induced cardiac remodeling by angiotensin-(1–7). Am J Physiol Heart Circ Physiol. Feb 2007;292(2):H736–42. doi: 10.1152/ajpheart.00937.2006 [DOI] [PubMed] [Google Scholar]
- 62.Santiago NM, Guimaraes PS, Sirvente RA, et al. Lifetime overproduction of circulating Angiotensin-(1–7) attenuates deoxycorticosterone acetate-salt hypertension-induced cardiac dysfunction and remodeling. Hypertension. Apr 2010;55(4):889–96. doi: 10.1161/HYPERTENSIONAHA.110.149815 [DOI] [PubMed] [Google Scholar]
- 63.Guo L, Yin A, Zhang Q, Zhong T, O’Rourke ST, Sun C. Angiotensin-(1–7) attenuates angiotensin II-induced cardiac hypertrophy via a Sirt3-dependent mechanism. Am J Physiol Heart Circ Physiol. May 1 2017;312(5):H980–H991. doi: 10.1152/ajpheart.00768.2016 [DOI] [PubMed] [Google Scholar]
- 64.Giani JF, Munoz MC, Mayer MA, et al. Angiotensin-(1–7) improves cardiac remodeling and inhibits growth-promoting pathways in the heart of fructose-fed rats. Am J Physiol Heart Circ Physiol. Mar 2010;298(3):H1003–13. doi: 10.1152/ajpheart.00803.2009 [DOI] [PubMed] [Google Scholar]
- 65.Guimaraes PS, Oliveira MF, Braga JF, et al. Increasing angiotensin-(1–7) levels in the brain attenuates metabolic syndrome-related risks in fructose-fed rats. Hypertension. May 2014;63(5):1078–85. doi: 10.1161/HYPERTENSIONAHA.113.01847 [DOI] [PubMed] [Google Scholar]
- 66.Guimaraes PS, Santiago NM, Xavier CH, et al. Chronic infusion of angiotensin-(1–7) into the lateral ventricle of the brain attenuates hypertension in DOCA-salt rats. Am J Physiol Heart Circ Physiol. Aug 1 2012;303(3):H393–400. doi: 10.1152/ajpheart.00075.2012 [DOI] [PubMed] [Google Scholar]
- 67.Gironacci MM, Valera MS, Yujnovsky I, Pena C. Angiotensin-(1–7) inhibitory mechanism of norepinephrine release in hypertensive rats. Hypertension. Nov 2004;44(5):783–7. doi: 10.1161/01.HYP.0000143850.73831.9d [DOI] [PubMed] [Google Scholar]
- 68.Britto RR, Santos RA, Fagundes-Moura CR, Khosla MC, Campagnole-Santos MJ. Role of angiotensin-(1–7) in the modulation of the baroreflex in renovascular hypertensive rats. Hypertension. Sep 1997;30(3 Pt 2):549–56. doi: 10.1161/01.hyp.30.3.549 [DOI] [PubMed] [Google Scholar]
- 69.Byku M, Macarthur H, Westfall TC. Inhibitory effects of angiotensin-(1–7) on the nerve stimulation-induced release of norepinephrine and neuropeptide Y from the mesenteric arterial bed. Am J Physiol Heart Circ Physiol. Feb 2010;298(2):H457–65. doi: 10.1152/ajpheart.00400.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gironacci MM, Adler-Graschinsky E, Pena C, Enero MA. Effects of angiotensin II and angiotensin-(1–7) on the release of [3H]norepinephrine from rat atria. Hypertension. Oct 1994;24(4):457–60. doi: 10.1161/01.hyp.24.4.457 [DOI] [PubMed] [Google Scholar]
- 71.Becker LK, Etelvino GM, Walther T, Santos RA, Campagnole-Santos MJ. Immunofluorescence localization of the receptor Mas in cardiovascular-related areas of the rat brain. Am J Physiol Heart Circ Physiol. Sep 2007;293(3):H1416–24. doi: 10.1152/ajpheart.00141.2007 [DOI] [PubMed] [Google Scholar]
- 72.Vargas-Castillo A, Tobon-Cornejo S, Del Valle-Mondragon L, et al. Angiotensin-(1–7) induces beige fat thermogenesis through the Mas receptor. Metabolism. Feb 2020;103:154048. doi: 10.1016/j.metabol.2019.154048 [DOI] [PubMed] [Google Scholar]
- 73.Costa-Fraga FP, Goncalves GK, Souza-Neto FP, et al. Age-related changes in vascular responses to angiotensin-(1–7) in female mice. J Renin Angiotensin Aldosterone Syst. Jul-Sep 2018;19(3):1470320318789332. doi: 10.1177/1470320318789332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bosnyak S, Widdop RE, Denton KM, Jones ES. Differential mechanisms of ang (1–7)-mediated vasodepressor effect in adult and aged candesartan-treated rats. Int J Hypertens. 2012;2012:192567. doi: 10.1155/2012/192567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miller AJ, Bingaman SS, Mehay D, Medina D, Arnold AC. Angiotensin-(1–7) Improves Integrated Cardiometabolic Function in Aged Mice. Int J Mol Sci. Jul 20 2020;21(14): 5131. doi: 10.3390/ijms21145131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Robles NR, Cerezo I, Hernandez-Gallego R. Renin-angiotensin system blocking drugs. J Cardiovasc Pharmacol Ther. Jan 2014;19(1):14–33. doi: 10.1177/1074248413501018 [DOI] [PubMed] [Google Scholar]
- 77.Grassi G Sympathomodulatory Effects of Antihypertensive Drug Treatment. Am J Hypertens. Jun 2016;29(6):665–75. doi: 10.1093/ajh/hpw012 [DOI] [PubMed] [Google Scholar]
- 78.Heringer-Walther S, Batista EN, Walther T, Khosla MC, Santos RA, Campagnole-Santos MJ. Baroreflex improvement in shr after ace inhibition involves angiotensin-(1–7). Hypertension. May 2001;37(5):1309–14. doi: 10.1161/01.hyp.37.5.1309 [DOI] [PubMed] [Google Scholar]
- 79.Grassi G, Cattaneo BM, Seravalle G, et al. Effects of chronic ACE inhibition on sympathetic nerve traffic and baroreflex control of circulation in heart failure. Circulation. Aug 19 1997;96(4):1173–9. doi: 10.1161/01.cir.96.4.1173 [DOI] [PubMed] [Google Scholar]
- 80.Loloi J, Miller AJ, Bingaman SS, Silberman Y, Arnold AC. Angiotensin-(1–7) contributes to insulin-sensitizing effects of angiotensin-converting enzyme inhibition in obese mice. Am J Physiol Endocrinol Metab. Dec 1 2018;315(6):E1204–E1211. doi: 10.1152/ajpendo.00281.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yousif MH, Dhaunsi GS, Makki BM, Qabazard BA, Akhtar S, Benter IF. Characterization of Angiotensin-(1–7) effects on the cardiovascular system in an experimental model of type-1 diabetes. Pharmacol Res. Sep 2012;66(3):269–75. doi: 10.1016/j.phrs.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 82.Kucharewicz I, Pawlak R, Matys T, Pawlak D, Buczko W. Antithrombotic effect of captopril and losartan is mediated by angiotensin-(1–7). Hypertension. Nov 2002;40(5):774–9. doi: 10.1161/01.hyp.0000035396.27909.40 [DOI] [PubMed] [Google Scholar]
- 83.Benter IF, Yousif MH, Al-Saleh FM, Raghupathy R, Chappell MC, Diz DI. Angiotensin-(1–7) blockade attenuates captopril- or hydralazine-induced cardiovascular protection in spontaneously hypertensive rats treated with NG-nitro-L-arginine methyl ester. J Cardiovasc Pharmacol. May 2011;57(5):559–67. doi: 10.1097/FJC.0b013e31821324b6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Basso N, Paglia N, Stella I, et al. Protective effect of the inhibition of the renin-angiotensin system on aging. Regul Pept. Jun 30 2005;128(3):247–52. doi: 10.1016/j.regpep.2004.12.027 [DOI] [PubMed] [Google Scholar]
- 85.Arnold AC, Gallagher PE, Diz DI. Brain renin-angiotensin system in the nexus of hypertension and aging. Hypertens Res. Jan 2013;36(1):5–13. doi: 10.1038/hr.2012.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thomas GN, Chan P, Tomlinson B. The role of angiotensin II type 1 receptor antagonists in elderly patients with hypertension. Drugs Aging. 2006;23(2):131–55. doi: 10.2165/00002512-200623020-00004 [DOI] [PubMed] [Google Scholar]
- 87.Rich MW. Are angiotensin-converting enzyme inhibitors indicated for the routine treatment of elderly heart failure patients? J Gerontol A Biol Sci Med Sci. Jul 2004;59(7):713–5. doi: 10.1093/gerona/59.7.m713 [DOI] [PubMed] [Google Scholar]
- 88.Turgut F, Balogun RA, Abdel-Rahman EM. Renin-angiotensin-aldosterone system blockade effects on the kidney in the elderly: benefits and limitations. Clin J Am Soc Nephrol. Jul 2010;5(7):1330–9. doi: 10.2215/CJN.08611209 [DOI] [PubMed] [Google Scholar]
- 89.Alharbi FF, Kholod AAV, Souverein PC, et al. The impact of age and sex on the reporting of cough and angioedema with renin-angiotensin system inhibitors: a case/noncase study in VigiBase. Fundam Clin Pharmacol. Dec 2017;31(6):676–684. doi: 10.1111/fcp.12313 [DOI] [PubMed] [Google Scholar]
- 90.Bangalore S, Kumar S, Messerli FH. Angiotensin-converting enzyme inhibitor associated cough: deceptive information from the Physicians’ Desk Reference. Am J Med. Nov 2010;123(11):1016–30. doi: 10.1016/j.amjmed.2010.06.014 [DOI] [PubMed] [Google Scholar]
- 91.Huang BS, White RA, Bi L, Leenen FH. Central infusion of aliskiren prevents sympathetic hyperactivity and hypertension in Dahl salt-sensitive rats on high salt intake. Am J Physiol Regul Integr Comp Physiol. Apr 2012;302(7):R825–32. doi: 10.1152/ajpregu.00368.2011 [DOI] [PubMed] [Google Scholar]
- 92.Pantzaris ND, Karanikolas E, Tsiotsios K, Velissaris D. Renin Inhibition with Aliskiren: A Decade of Clinical Experience. J Clin Med. Jun 9 2017;6(6)doi: 10.3390/jcm6060061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Duprez DA, Munger MA, Botha J, Keefe DL, Charney AN. Aliskiren for geriatric lowering of systolic hypertension: a randomized controlled trial. J Hum Hypertens. Sep 2010;24(9):600–8. doi: 10.1038/jhh.2009.107 [DOI] [PubMed] [Google Scholar]
- 94.Teo KK, Pfeffer M, Mancia G, et al. Aliskiren alone or with other antihypertensives in the elderly with borderline and stage 1 hypertension: the APOLLO trial. Eur Heart J. Jul 2014;35(26):1743–51. doi: 10.1093/eurheartj/ehu079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Durand MJ, Zinkevich NS, Riedel M, et al. Vascular Actions of Angiotensin 1–7 in the Human Microcirculation: Novel Role for Telomerase. Arterioscler Thromb Vasc Biol. Jun 2016;36(6):1254–62. doi: 10.1161/ATVBAHA.116.307518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stanhewicz AE, Alexander LM. Local angiotensin-(1–7) administration improves microvascular endothelial function in women who have had preeclampsia. Am J Physiol Regul Integr Comp Physiol. Jan 1 2020;318(1):R148–R155. doi: 10.1152/ajpregu.00221.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nussberger J, Brunner DB, Nyfeler JA, Linder L, Brunner HR. Measurement of immunoreactive angiotensin-(1–7) heptapeptide in human blood. Clin Chem. Apr 2001;47(4):726–9. [PubMed] [Google Scholar]
- 98.Plovsing RR, Wamberg C, Sandgaard NC, et al. Effects of truncated angiotensins in humans after double blockade of the renin system. Am J Physiol Regul Integr Comp Physiol. Nov 2003;285(5):R981–91. doi: 10.1152/ajpregu.00263.2003 [DOI] [PubMed] [Google Scholar]
- 99.Sasaki S, Higashi Y, Nakagawa K, Matsuura H, Kajiyama G, Oshima T. Effects of angiotensin-(1–7) on forearm circulation in normotensive subjects and patients with essential hypertension. Hypertension. Jul 2001;38(1):90–4. [DOI] [PubMed] [Google Scholar]
- 100.Schindler C, Bramlage P, Kirch W, Ferrario CM. Role of the vasodilator peptide angiotensin-(1–7) in cardiovascular drug therapy. Vasc Health Risk Manag. 2007;3(1):125–37. [PMC free article] [PubMed] [Google Scholar]
- 101.van Twist DJ, Houben AJ, de Haan MW, Mostard GJ, Kroon AA, de Leeuw PW. Angiotensin-(1–7)-induced renal vasodilation in hypertensive humans is attenuated by low sodium intake and angiotensin II co-infusion. Hypertension. Oct 2013;62(4):789–93. doi: 10.1161/HYPERTENSIONAHA.113.01814 [DOI] [PubMed] [Google Scholar]
- 102.White MC, Miller AJ, Loloi J, et al. Sex differences in metabolic effects of angiotensin-(1–7) treatment in obese mice. Biol Sex Differ. Jul 17 2019;10(1):36. doi: 10.1186/s13293-019-0251-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Medina D, Mehay D, Arnold AC. Sex differences in cardiovascular actions of the renin-angiotensin system. Clin Auton Res. Aug 29 2020; 30(5): 393–408. doi: 10.1007/s10286-020-00720-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Matsukawa T, Sugiyama Y, Watanabe T, Kobayashi F, Mano T. Gender difference in age-related changes in muscle sympathetic nerve activity in healthy subjects. Am J Physiol. Nov 1998;275(5):R1600–4. doi: 10.1152/ajpregu.1998.275.5.R1600 [DOI] [PubMed] [Google Scholar]
- 105.Lima R, Wofford M, Reckelhoff JF. Hypertension in postmenopausal women. Curr Hypertens Rep. Jun 2012;14(3):254–60. doi: 10.1007/s11906-012-0260-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Harvey RE, Ranadive SM, Limberg JK, et al. Forearm vasodilatation to a beta2 -adrenergic receptor agonist in premenopausal and postmenopausal women. Exp Physiol. May 2020;105(5):886–892. doi: 10.1113/EP088452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Williams SF, Nicholas SB, Vaziri ND, Norris KC. African Americans, hypertension and the renin angiotensin system. World J Cardiol. Sep 26 2014;6(9):878–89. doi: 10.4330/wjc.v6.i9.878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sagnella GA. Why is plasma renin activity lower in populations of African origin? J Hum Hypertens. Jan 2001;15(1):17–25. doi: 10.1038/sj.jhh.1001127 [DOI] [PubMed] [Google Scholar]
- 109.Drew RC, Charkoudian N, Park J. Neural control of cardiovascular function in black adults: implications for racial differences in autonomic regulation. Am J Physiol Regul Integr Comp Physiol. Feb 1 2020;318(2):R234–R244. doi: 10.1152/ajpregu.00091.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]


