Abstract
Oxidative stress in the retinal pigment epithelium (RPE) can cause mitochondrial dysfunction and is likely a causative factor in the pathogenesis of age-related macular degeneration (AMD). Under oxidative stress conditions, some of the RPE cells become senescent and a contributory role for RPE senescence in AMD pathology has been proposed. The purpose of this study is to 1) characterize senescence in human RPE; 2) investigate the effect of an αB Crystallin chaperone peptide (mini Cry) in controlling senescence, in particular by regulating mitochondrial function and senescence-associated secretory phenotype (SASP) production and 3) develop mouse models for studying the role of RPE senescence in dry and nAMD. Senescence was induced in human RPE cells in two ways. First, subconfluent cells were treated with 0.2 μg/mL doxorubicin (DOX); second, subconfluent cells were treated with 500 μM H2O2. Senescence biomarkers (senescence-associated beta-galactosidase (SA-βgal), p21, p16) and mitochondrial proteins (Fis1, DRP1, MFN2, PGC1-α, mtTFA) were analyzed in control and experimental groups. The effect of mini Cry on mitochondrial bioenergetics, glycolysis and SASP was determined. In vivo, retinal degeneration was induced by intravenous injection of NaIO3 (20 mg/kg) and subretinal fibrosis by laser-induced choroidal neovascularization. Increased SA-βgal staining and p16 and p21 expression was observed after DOX- or H2O2-induced senescence and mini Cry significantly decreased senescence-positive cells. The expression of mitochondrial biogenesis proteins PGC-1 and mTFA increased with senescence, and mini Cry reduced expression significantly. Senescent RPE cells were metabolically active, as evidenced by significantly enhanced oxidative phosphorylation and anaerobic glycolysis, mini Cry markedly reduced rates of respiration and glycolysis. Senescent RPE cells maintain a proinflammatory phenotype characterized by significantly increased production of cytokines (IFN-ɣ, TNF-α, IL1-α IL1-β, IL-6, IL-8, IL-10), and VEGF-A; mini Cry significantly inhibited their secretion. We identified and localized senescent RPE cells for the first time in NaIO3-induced retinal degeneration and laser-induced subretinal fibrosis mouse models. We conclude that mini Cry significantly impairs stress-induced senescence by modulating mitochondrial biogenesis and fission proteins in RPE cells. Characterization of senescence could provide further understanding of the metabolic changes that accompany the senescent phenotype in ocular disease. Future studies in vivo may better define the role of senescence in AMD and the therapeutic potential of mini Cry as a senotherapeutic.
Keywords: αB crystallin peptide, mitochondrial dysfunction, oxidative stress, retinal pigment epithelium, SASP, senescence, senolytic drugs, subretinal fibrosis
1. Introduction
Age-related macular degeneration (AMD), a degenerative disease that affects the macula area of the neural retina and the monolayer retinal pigment epithelium (RPE), is the most common cause of vision loss in the elderly in the US (Ambati and Fowler, 2012). Advanced AMD exists in two morphological forms: wet (neovascular) and dry (non-neovascular). Dry AMD is characterized by geographic atrophy (GA), accompanying loss of the RPE and overlying photoreceptors (Bowes Rickman et al., 2013). Wet AMD is characterized by choroidal neovascularization (CNV) resulting in the growth of blood vessels from the choroid under the neural retina (Ambati and Fowler, 2012). While there are effective therapies for active CNV, there is limited effectiveness for the complication of subretinal fibrosis in treated CNV (Yang et al., 2016).
Strong evidence exists that oxidative stress is a major contributor to the pathogenesis of AMD. In addition, RPE cells are known to generate reactive oxygen species (ROS) when exposed to certain environmental factors. RPE is a polarized monolayered tissue found at the base of the retina; in adults, these cells lying in the central macula remain in a quiescent state, but peripheral RPE cells can be rapidly activated by a variety of stimuli (Bhutto and Lutty, 2012; Blasiak et al., 2017). Damaged RPE cells in the central retina can be replaced by the proliferating peripheral RPE cells (Blasiak et al., 2017). Others and our lab have shown that oxidative stress can induce senescence in cultured RPE cells (Lee et al., 2021; Marazita et al., 2016; Sreekumar et al., 2016; Sreekumar et al., 2020b; Zhu et al., 2009; Zhuge et al., 2014). As stated above, when most of the macular peripheral RPE cells are senescent, the ability to replace injured or damaged macular RPE cells could be lost, and this can lead to AMD.
Cellular senescence is a state of permanent growth arrest plus phenotypic changes that have a significant role in maintaining physiological homeostasis (Campisi, 2013; Campisi et al., 2019; Coppe et al., 2010). Senescent cells are known to have multiple phenotypes, such as cellular flattening and hypertrophy, a complex senescence-associated secretory phenotype (SASP), resistance to apoptotic cell death, altered mitochondrial regulation, senescence-associated β-galactosidase activity (SAβG), and decreased cell cycle signaling events (Campisi, 2013; Sreekumar et al., 2020a). Although the number of senescent cells is generally very few, they can be a source of pathology because of the sizeable changes to surrounding cells and tissues, due to their pro-inflammatory SASP and paracrine effects (bystander effect). Accumulating evidence indicates that senescent cells can contribute to tissue remodeling and many age-related and other diseases (Baker et al., 2011; Chae et al., 2021; Childs et al., 2015; Kim and Kim, 2019; Lee et al., 2021; Sreekumar et al., 2020a). Furthermore, senescent cells have been detected in the brain and blood vessels in old-age human retinas (Lopez-Luppo et al., 2017), in the RPEs of older (16 and 29-year-old) primates (Mishima et al., 1999) and they accumulate in the neovascular area in patients with proliferative retinopathy (Crespo-Garcia et al., 2021). While recent studies provide evidence for the generation of senescent RPE in culture, data demonstrating the production and regulation of senescence in vivo are lacking owing to the scarcity of suitable mouse models. Thus, developing suitable animal models and targeting senescent RPE cells might provide a new therapeutic approach for treating AMD.
αB-crystallin is a prominent member of the small heat shock protein (sHSP) family and is secreted by RPE cells primarily from the apical domain (Kannan et al., 2012; Sreekumar et al., 2010). sHSPs exert diverse biological activities in both normal and stressed cells. Our laboratory showed that RPE cells lacking αB-crystallin are more susceptible to oxidative and endoplasmic reticulum stress (Dou et al., 2012; Yaung et al., 2007; Zhou et al., 2014), whereas RPE cells that overexpress αB-crystallin show resistance to apoptosis (Sreekumar et al., 2012). We also worked extensively on the role of αB-crystallin in ocular angiogenesis, CNV formation, and subretinal fibrosis (Ishikawa et al., 2016; Kase et al., 2010). In addition, it was demonstrated that a 20-mer peptide (mini Cry) derived from amino acid residues 73–92 of αB crystallin has chaperone properties and protects RPE cells from oxidative stress-induced cell death by inhibiting caspase-3 activation in in vivo AMD models (Sreekumar et al., 2013; Sreekumar et al., 2018). However, the senolytic (drugs that selectively kill senescent cells)/senomorphic (drugs that selectively suppress certain SASP modules) functions of this 20-mer peptide are not known. Given the known antioxidant and anti-inflammatory functions, we hypothesized that the 20-mer peptide could delay RPE senescence by inhibiting SASP production and regulating energy metabolism. Our present work also addresses the effect of aging in the development of senescence in mice.
2. Materials and methods
2.1. Retinal Pigment Epithelial Cell Culture
All procedures adhered to the tenets of the Declaration of Helsinki for research involving human subjects. RPE cells were isolated from human fetal eyes obtained from Advanced Bioscience Resources Inc. (Alameda, CA, USA) and Novogenix Laboratories, LLC (Los Angeles, CA, USA) and cultured as described (Dridi et al., 2012; Ferrington et al., 2017; Ghosh et al., 2018; Sippy et al., 1995; Sonoda et al., 2009a; Sreekumar et al., 2010). In brief, hRPE cells were grown in Dulbecco’s modified Eagle medium (DMEM, #15-013-CV, Corning, NY, USA) with 10% fetal bovine serum (FBS, # 4800-500HI, Laguna Scientific, Laguna Niguel CA, USA). Upon reaching confluency, cells were subcultured and passages 2 to 4 were used in all experiments.
2.2. Induction of Cellular Senescence
Senescence was induced in RPE using two stimuli described below.
2.2.1. Doxorubicin (DOX) induced senescence
Subconfluent (50-60% confluent at the beginning of the study) human fetal RPE cells were treated with 0.2 μg/mL doxorubicin (DOX) (Sigma-Aldrich, # D1515, St. Louis, MO, USA) for 3 h in medium containing 0.5% FBS, washed in PBS and switched to fresh medium containing 10% FBS. Cells were maintained for 6 d, with the culture medium replaced every 48 h. For the peptide-treated group, 75 μg/mL mini Cry (DRFSVNLDVKHFSPEELKVK) or a scrambled (Scr.) (DLPLKKNVEDKFHRSFVESV) peptide was added to the culture medium. The peptides used consisted of a 20-mer of αB-crystallin, and a 20-mer of scrambled sequence that were custom-synthesized with a purity of > 98% as per the manufacturer (NeoPeptide, Cambridge, MA).
2.2.2. H2O2-induced RPE senescence
Sub confluent human RPE cells were treated with 500 μM H2O2 (Sigma-Aldrich # H1009, Saint Louis, MO, USA) alone or 500 μM H2O2 and 75 μg/mL mini Cry for 2 h. This concentration of mini Cry was chosen from our previous studies on dose-dependent antiapoptotic and anti-inflammatory properties of mini Cry in RPE cells (Sreekumar et al., 2013). The H2O2 treatment was repeated the following day. The medium was replaced, cells were washed in PBS and incubated with fresh medium containing 10% FBS. Cells were kept for another 48 h, and medium was replaced every 24 h. Mini Cry (75 μg/ml) was present in one of the wells previously co-treated with H2O2.
2.3. Senescence-associated β-galactosidase (SA-β gal) assay
SA-βgal activity was evaluated in RPE cells at the end of the experiment by using the Senescence Detection Kit (Sigma-Aldrich, #CS0030) per the manufacturer’s instructions. Briefly, cells were seeded in 4-well chamber slides, and at the end of the experiment were washed with PBS, then fixed with 4% PFA for 10 min at room temperature. Cells were washed with PBS and incubated overnight at 37° C without CO2 and with the β-gal substrate. Following washing, cells were examined with a microscope and photographed (Keyence, Itasca, IL, USA). The development of a perinuclear blue color was taken as an indicative of a senescent cell. For quantification, blue-stained cells and total cells were counted microscopically and the percentage of cells expressing β-galactosidase (senescent cells) calculated (Sreekumar et al., 2016).
2.4. Cellular respiration, and extracellular acidification (ECAR) measurements
2.4.1. Cell Mito Stress Test Kit:
This kit measures basal respiration, ATP-linked respiration, maximal and reserve capacities. Mitochondrial respiration was determined by measuring the oxygen consumption rate (OCR) of RPE cells using a Seahorse XFe96 Analyzer (Cell Mito Stress Test Kit #103015-100, Agilent, Santa Clara, CA, USA). Induction of senescence and mini Cry co-treatment of sub confluent cells were performed as described above. Assays were initiated by replacing the growth medium with 180 μL XF assay medium containing glucose (25 mM), sodium pyruvate (1 mM), and glutamine (2 mM) (Agilent). The concentrations of inhibitors were oligomycin (ATP-Synthase inhibitor) at 1.5 μM, carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP, mitochondrial membrane depolarizer) at 1.5 μM and a mixture of 0.5 μM of each rotenone (complex I inhibitor) and antimycin A (complex III inhibitor). Each sample was measured in 8 to 10 wells per condition, and the results were averaged. The OCR data were expressed as pmol/min/μg protein. Seahorse Wave Desktop software was used for data analysis.
2.4.1. Glycolysis stress test:
Seahorse XF Glycolysis Stress Test Kit (Agilent, # 103020-100) was used as per the standard protocol by Agilent Seahorse. The kit measures glycolytic pathway capacity after glucose starvation. As glycolysis occurs, the resulting acidification of the medium surrounding the cells is measured directly by the analyzer and reported as the Extracellular Acidification Rate (ECAR). RPE cells were seeded in 96 well plates and treated with H2O2 (500 μM) as described above (2.2.2). To study the glycolytic potential of cells after the treatment period, the ECAR was measured in 17-20 wells/experimental condition before and after sequential injections of glucose (10 mM), oligomycin (1 μM), and 2-Deoxy-D-glucose (2-DG; 50 mM). The data were expressed as pmol/min/μg protein.
2.5. Western Blot Analysis
Protein was extracted from cells with RIPA buffer (Cell Signaling Tech, # #9806, Danvers, MA) containing protease inhibitor (Sigma-Aldrich # P8340) and the concentration of soluble protein was measured using BSA (Bio-Rad #500-0207, Hercules, CA, USA) as a standard. Equal amounts of protein (30-60 μg) were resolved on Mini-PROTEAN TGX Precast Protein Gels (Bio-Rad) and transferred to PVDF blotting membranes (Millipore #IPVH00010, Billerica, MA, USA). Membranes were incubated with primary antibodies against p16, p21, Fis1, DRP1, Mfn2, PGC1-α, mtTFA, GAPDH or β-actin in 5% milk (Nonfat Dry milk, Bio-Rad # 170-6404) in PBS overnight at 4° C (see Table 1 for a list of antibodies used). After incubation with appropriate secondary antibodies (Vector Laboratories, Burlingame, CA, USA), protein bands were visualized using the SuperSignal West Pico PLUS Chemiluminescence substrate (Thermo Scientific, #34580, IL, USA). Equal protein loading was confirmed with β-actin or GAPDH.
Table 1.
List of antibodies used
| Antibody | Source | Application | Product catalogue |
|---|---|---|---|
| Actin | Mouse | Western blotting (1:5000) | Santa Cruz Biotech (sc-8432) |
| mtTFA | Mouse | Western blotting (1; 1000) | Santa Cruz Biotech (sc-sc-166965) |
| P16INK4a | Rabbit monoclonal | Western blotting (1:2000) Immunofluorescence (1:100) |
Abcam (ab108349) |
| p21 | Mouse | Western blotting (1;1000), Immunofluorescence (1:100) |
Santa Cruz Biotech (sc-6246) |
| Fis-1 | mouse | Western blotting (1;1000) | Santa Cruz Biotech (sc-376447) |
| Mfn2 | Mouse | Western blotting (1:1000) | Santa Cruz Biotech (sc- 100560) |
| DRP1 | Mouse | Western blotting (1:1000) | Santa Cruz Biotech (sc-271583) |
| PGC-1α | Mouse | Western blotting (1:500) | Santa Cruz Biotech (sc-518038) |
| GAPDH | Mouse | Western blotting (1:5000) | EMD Millipore (MAB374) |
| Beta-actin | Mouse | (Western blotting (1:3000) | Santa Cruz Biotech (sc-47778)) |
2.6. Multiplex Proteomic assays in Culture Supernatants
Multiplex fluorescent bead assays for several secreted SASP proteins were performed by Eve Technologies Inc. (Alberta, Canada) using their standard proprietary protocols. The following panel groups were selected for multiplex assays: Human Cytokine Array / Chemokine Array 48-Plex (HD48) and Human MMP and TIMP Array Panel for Cell Culture and non-blood samples (HMMP/TIMP). 24 h conditioned media was collected after senescence induction on day 4, centrifuged to remove dead cells and debris and used in analysis. All results are expressed as pg/ml. The background control consisted of medium alone and control values were subtracted from all sample values. Triplicate samples were analyzed for each experimental condition.
2.7. Animal studies
Six-week-old and 13 month-old C57BL6/J male mice that do not carry the rd8 and Pde6brd1 mutations were purchased from The Jackson Laboratory (The Jackson Laboratory, Bar Harbor, ME, USA). The Animal Research Committee at the University of California, Los Angeles approved the experimental protocols. All procedures used in this study were conducted in accordance with National Institutes of Health guidelines and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic Vision Research.
Two mouse models were used in this study: the NaIO3 model of RPE atrophy and the Laser-induced Subretinal Fibrosis model.
2.7.1. NaIO3 induced retinal degeneration model
Sodium iodate (NaIO3) injection has been extensively used as a preclinical model of RPE degeneration and GA (Bhutto et al., 2018; Enzbrenner et al., 2021; Enzmann et al., 2006; Kannan and Hinton, 2014; Zhou et al., 2014). This model has been well characterized with respect to the loss of RPE cells and the death of photoreceptors, resulting in thinning of the outer nuclear layer (ONL) and a significant reduction in retinal functions (Enzbrenner et al., 2021; Franco et al., 2009; Sreekumar et al., 2018; Zhou et al., 2014). Systemic administration of NaIO3 specifically targets the RPE, resulting in patchy loss, and subsequent death of adjacent photoreceptors (Enzmann et al., 2006; Zhou et al., 2014). Thus, NaIO3 model can be used to delineate the molecular mechanism of cell death associated with GA. Based on our recent studies (Su et al., 2019), we used a NaIO3 (Sodium Iodate # S4007, Sigma-Aldrich) concentration of 20 mg/kg body weight in the current study. Three-month-old or 13-month-old C57BL6/J male mice were divided into two experimental groups. (1) Control group (Control): Mice with tail vein injection of PBS (vehicle) and (2) NaIO3-treated group (NaIO3): Mice with tail vein injection of NaIO3. After 7 days, eyes were enucleated, and frozen sections of retinal tissue were processed for p16 and p21 immunofluorescence. In brief, airdried retinal sections were fixed in 4% PFA, followed by permeabilization in 0.1% triton. Sections were blocked in 5% normal goat serum and incubated overnight with p16 or p21 antibodies. Sections were washed and incubated with corresponding fluorescein labelled secondary antibodies (Vector Lab, # FI-2000, Burlingame, CA). For quantification of immunofluorescence, digital images were analyzed (n = 3–5), five images (20x) were taken from each section and the average total corrected fluorescence (background subtracted) was calculated using ImageJ (US NIH, Bethesda, MD) (Burgess et al., 2010; Sreekumar et al., 2018).
2.7.2. Laser-Induced CNV and subretinal fibrosis
CNV was generated as described in C57BL6/J male mice (Ishikawa et al., 2016). 6-8 weeks old mice were used. Briefly, laser photocoagulation (532 nm, 150 mW, 50 milliseconds, 75 μm) was applied to each fundus using a coverslip as a contact lens on day 0 in four laser spots per eye. The production of a subretinal bubble at the time of laser application indicated a rupture of Bruch’s membrane. We excluded animals that developed burns with bleeding. Animals were euthanized on day 35 after laser photocoagulation. Cryostat sections (6 μm thick) of snap-frozen mouse eyes were obtained from 4 mice and frozen sections were processed for Senescence-associated β-galactosidase (SA-β gal) staining, and p16 and p21 immunofluorescence.
2.8. Statistical Analysis
All data are expressed as mean ± SEM. Data were analyzed using one-way ANOVA followed by Tukey post-test using graphing software (GraphPad Prism, version 5; GraphPad Software, Inc., La Jolla, CA, USA). p<0.05 was considered significant.
3. Results
We used the anthracycline antibiotic doxorubicin (DOX) to model therapy-induced senescence (TIS) based on reports in multiple cell types (Chae et al., 2021; Demaria et al., 2017; Kim et al., 2018). We performed a dose response with various doses of DOX to determine the optimal dose for induction of senescence while not completely reducing cell viability (data not shown). Based on preliminary studies, we selected 0.2 μg/ml, a dose at which we observed a complete growth arrest without significant reduction in viability. At day 6, many cells showed a senescence morphology, which was confirmed by SA-β-gal staining (Fig. 1 A, B). We also demonstrated that cotreatment with mini cry, and not the scr. peptide, significantly (p<0.01 vs scrambled) reduced SA-β-gal positive cells. In addition, we observed DOX at 0.2 μg/ml showed a significant increase in SA-β-gal activity and marked upregulation of the cyclin-dependent kinase inhibitors p16 and p21, as determined by mRNA and protein analysis (Fig.1A, B).
Fig. 1. Inhibition of Doxorubicin (DOX)- and H2O2 induced senescence (β-gal +ve cells) by mini Cry.
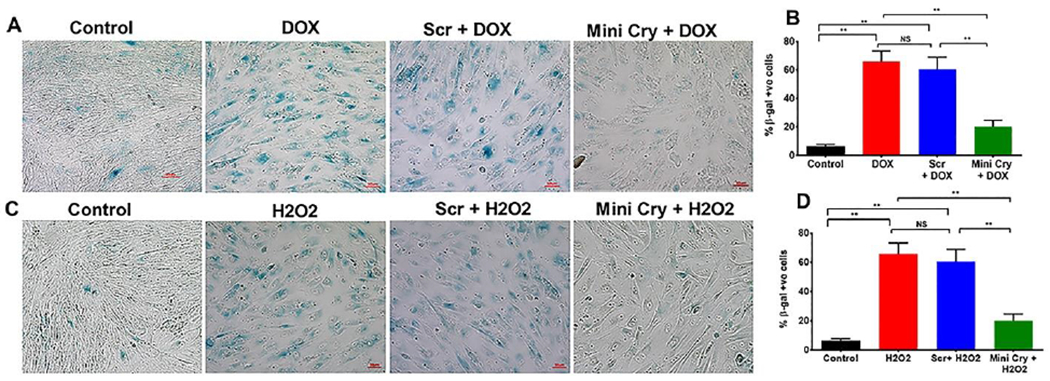
DOX-induced (A, B) and H2O2-induced senescence (C, D) was inhibited by co-treatment with 75 μg/ml mini Cry. Subconfluent hRPE cells were treated with DOX (0.2 μg/ml) for 3h in 0.5% FBS containing medium. The medium was switched to 10% FBS medium for 6 d. RPE cells were treated with H2O2 (500 μM) in serum-free medium for 2h, and medium replaced with 10% FBS containing medium. The H2O2 treatment was repeated the next day, and cells were maintained in 10% FBS for 4 d. Samples were processed for β-gal staining. Oxidative stress significantly increased the number of β-Gal–positive cells and RPE cell senescence was considerably lower with mini Cry co-treatment (B, D). ** p< 0.01. NS- not significant, Scale bar: 50 μm.
To test the hypothesis that mini Cry delayed cellular senescence, we used a model where subconfluent hRPE cells were treated with 500 μM H2O2 (Sreekumar et al., 2016). Oxidative stress showed a significant (p<0.01 versus control cells) increase in SA-β-Gal–positive cells (Fig. 1C, D). Co-treatment with mini Cry showed a significant (p<0.01 versus H2O2-treated or Scr-treated cells) (50%) decrease compared to scr. peptide + H2O2 treated cells (Fig. 1D). We note that the hRPE cells used were non-polarized and do not represent the native RPE monolayer. In addition, the RPE monolayer is resistant to cell death induced by oxidative stress and has higher levels of certain cytoprotective growth factors than nonpolarized RPE (Bailey et al., 2004; Sonoda et al., 2009b; Sreekumar et al., 2008; Zhu et al., 2010).
Mini Cry reduced Senescence in RPE Cells
We next examined the expression of two other senescence markers, p16 and p21 (Demaria et al., 2017; Hernandez-Segura et al., 2018; LaPak and Burd, 2014; Sreekumar et al., 2020a). Expression of these senescence-related genes at day 6 in both DOX-treated RPE cells showed a significant 4-fold upregulation of p16, p21 mRNA levels and over 2-fold increase in protein levels (Fig. 2A–D). Both p16 and p21 are known to the cell cycle arrest in senescent cells. Mini Cry co-treatment significantly (p< 0.01 vs scr. peptide or DOX treated cells) inhibited upregulation of both p16 and p21 at the mRNA and protein levels.
Fig. 2. Attenuation of senescence in DOX–induced and H2O2-induced senescence by mini Cry.
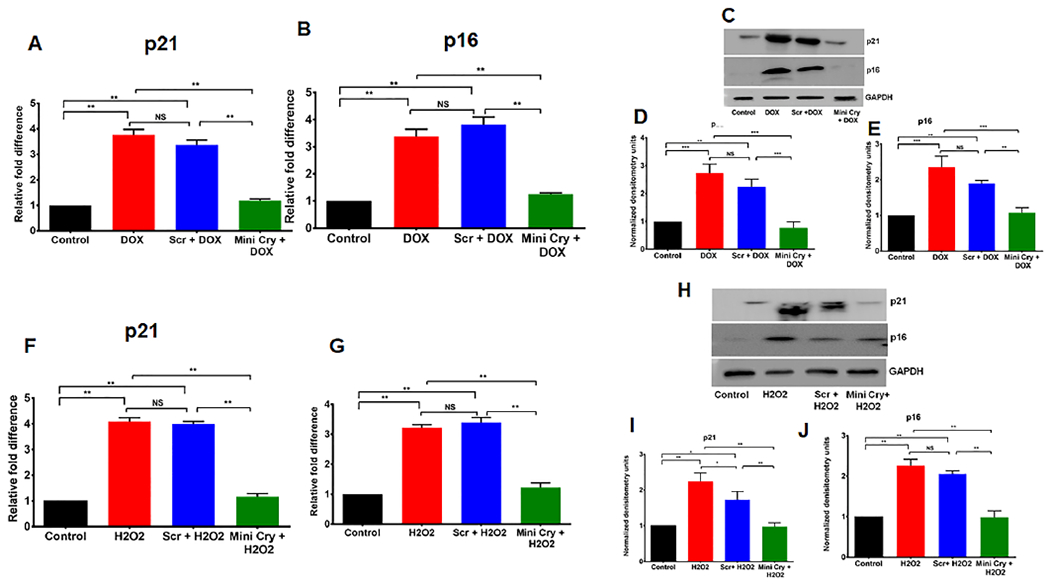
p21 and p16 mRNA (Fig. 2 A, B,F,G) and protein levels (Fig. 2 C,D,E,H, I, J) increased significantly with senescence and mini Cry treatment significantly decreased the levels. The western blot is quantified using densitometry and normalized to GAPDH and presented as fold change from triplicate analysis (D, E, I,J). * p < 0.05,** p < 0.01; *** p < 0.001. NS- No significant. The individual western whole gel bots corresponding to Figure 2 D,E, I & J are shown in the supplementary figure S1.
We also investigated expression of the above senescence markers after treatment with H2O2, which resulted in a significant 4-fold increase in p16 and p21 transcripts (p< 0.01) and was inhibited by mini Cry co-treatment (Fig. 2F,G). We confirmed senescence by significant upregulation of p16 and p21 at the protein level, while mini cry co-treatment decreased expression of p16 and p21 proteins (Fig. 2 H–J).
Mitochondrial biogenesis and energetics are altered by DOX-induced senescence
Mitochondrial fission is mediated by DRP1 (Elgass et al., 2013), which translocates from the cytosol to the outer mitochondrial membrane where it interacts with receptor proteins including fission protein-1 (Fis1) (Hu et al., 2017). We investigated whether DOX-induced cellular senescence maintains mitochondrial dynamics by regulating DRP1. Senescent cells showed a significant (p < 0.01) increase in DRP1 protein, which was blocked by cotreatment with mini Cry (Fig. 3A–a,c). A similar trend was noted for Fis1, where treatment with mini Cry decreased expression (Fig. 3A–a,b). An increasing trend in Mfn2 expression was observed in senescent cells, pointing to alterations in mitochondrial fusion (Fig. 3A & Supplementary Figure S2b).
Fig.3. Regulation of mitochondrial biogenesis and fission-fusion proteins by mini Cry in senescent RPE cells.
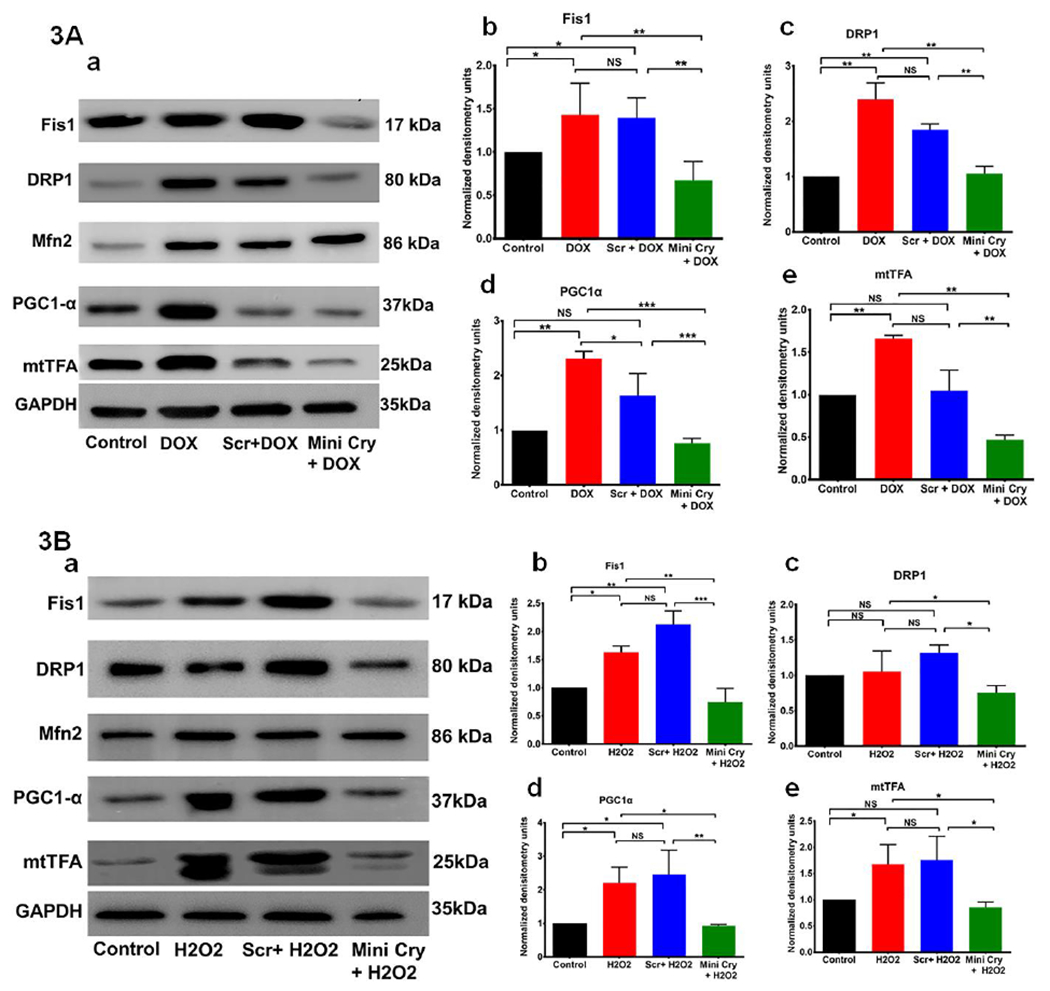
Sub-confluent RPE cells grown were treated with 0.2 μg/mL DOX or 500 μM H2O2 alone or with 75 μg/ml scr. peptide or mini Cry. Protein expression was measured by using Western blot analysis. The quantitative densitometry bar graphs derived from three independent experiments are shown (mean ±SD). Mitochondrial fission proteins (Fis1 & DRP1) increased significantly with senescence and mini cry markedly inhibited their expression and senescence (Fig. 3A–b,c & 3B–b,c). RPE senescence significantly increased biogenesis markers (PGC1 and mtTFA) and mini Cry co-treatment decreased expression (Fig. 3A–d,e & 3B–d,e). However, no major change was noted in the mitochondrial fusion protein Mfn2 (Fig. 3 A,B). DOX: doxorubicin, Scr. Scrambled peptide, mini cry: αB crystallin-chaperone peptide. NS- Not significant, * p< 0.05, ** p< 0.01, *** p< 0.001. The individual western whole gel blots corresponding to Figure 3A&B are shown in the supplementary figure S2a–c.
We also investigated PGC-1α, which is known to play a role in the transcriptional control of mitochondrial biogenesis and respiratory function (Gureev et al., 2019). The mitochondrial transcription factor A (mtTFA) is another important factor regulating mitochondrial biogenesis, and functions in mtDNA transcription initiation and packaging of mtDNA into nucleoids (Kukat and Larsson, 2013). We observed significant increase in protein expression of PGC1α (Fig. 3A–a,d) and a 1.66-fold rise in mtTFA levels in DOX-induced senescent cells (Fig. 3A–a,e).
With respect to the mechanisms of DOX effect, it is possible that the phenomenon observed in our study could be partly due to mitochondrial toxicity (Singh et al., 1999; Wallace et al., 2020). However, it has been reported that low concentrations (100-200 nM) of DOX similar to what we used in our current study (≈ 50 nM) can induce terminal growth arrest with senescence-like alterations in proliferating cells (Bielak-Zmijewska et al., 2014; Maejima et al., 2008; Rebbaa et al., 2003). The effect of DOX on aortic smooth muscle cells follows two mechanisms (Bielak-Zmijewska et al., 2014). DNA damage is followed by activation of the DDR and this signaling pathway was observed in DOX-induced senescence. The level of a protein, not directly involved in DDR pathway but related to senescence, namely p16, increased during DOX-induced senescence. Transient activation of the DDR pathway was also observed during DOX-induced senescence (Bielak-Zmijewska et al., 2014).
A similar trend in these proteins was observed in H2O2-induced senescence (Fig. 3B–a–e). These observations suggest that senescence in RPE cells might be accompanied by an activation of mitochondrial biogenesis and highlight mitochondria as potential pharmacological targets to modulate senescence.
Modulation of energy metabolism and effect of mini Cry in senescent RPE cells
Senescence can be accompanied by changes in energy metabolism that could vary based on the cell type and inducer (Dorr et al., 2013; Kamogashira et al., 2017; Kim et al., 2018; Martinez et al., 2019). We investigated mitochondrial function in H2O2-induced senescence. Senescent cells showed higher basal OCR, greater ATP production and increased maximal respiration compared to non-senescent cells (Fig. 4). These findings demonstrate that senescent cells synthesize more ATP in the mitochondria than non-senescent cells. The maximal respiration rate, obtained after addition of the mitochondrial uncoupler FCCP, also significantly increased in senescent cells, suggesting highly active mitochondria. Co-treatment of RPE cells with mini Cry significantly decreased all mitochondrial bioenergetic parameters to the level of non-senescent cells (Fig. 4).
Fig. 4. Increased respiration and ATP generation in senescence and inhibition by mini Cry.
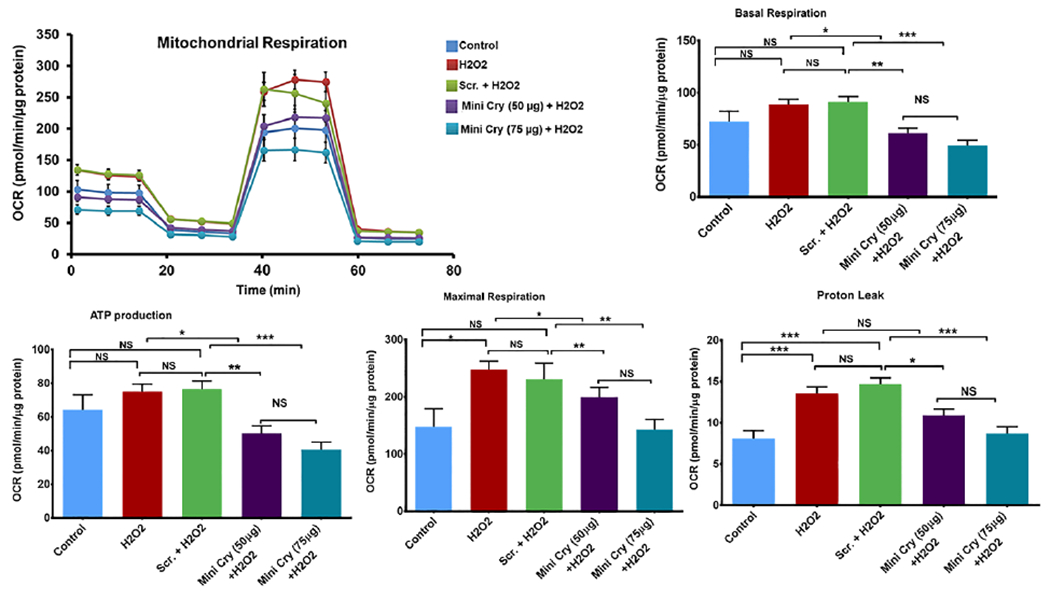
Sub-confluent RPE cells were treated with 500 μM H2O2 in SFM for 2 h, which was replaced with fresh medium containing mini Cry or Scr peptide. H2O2 treatment was repeated the next day. Cells were maintained in 10% FBS for 4 d. Mitochondrial bioenergetics were analyzed by Seahorse XFe96. H2O2-induced RPE senescence significantly increased basal respiration, ATP production and maximal respiration and mini Cry co-treatment dose-dependently reduced mitochondrial bioenergetic parameters. Values are means ± SE (N= 8-10). NS- Not significant; * p< 0.05. ** p< 0.01, *** p< 0.001.
Given the significant increase in mitochondrial function with RPE senescence, we reasoned that the main catabolic pathways would likely be altered. Therefore, we studied changes in glycolysis by measuring the extracellular acidification rate (ECAR) under glucose starvation and subsequent addition. Senescent cells showed increased ECAR, glycolytic capacity, and glycolytic reserve compared with controls. This finding suggests that senescent cells contribute more to glycolysis than non-senescent cells. To understand whether senescence causes changes in glycolysis, we inhibited it with mini Cry which delayed senescence. Mini Cry co-treatment significantly reduced glycolytic function in senescent cells. Thus, senescent RPE cells are relatively reliant on both glycolysis and OXPHOS.
Mini cry regulates the SASP in RPE senescence
To determine if human RPE cells secrete SASP proteins upon senescence, we exposed RPE cells to H2O2 alone or H2O2 and mini Cry to test the senolytic properties of the peptide. Senescent cells are metabolically active and display a SASP, consisting of different pro-inflammatory cytokines, chemokines, metalloproteinase and growth factors which act in a paracrine manner to alter and regulate tissues (Basisty et al., 2020; Coppe et al., 2010; Tchkonia et al., 2013). The SASP is considered a potential driver of and therapeutic target for multiple age-related pathologies, including AMD (Blasiak, 2020; Blasiak et al., 2017; Lee et al., 2021). Therefore, we studied the SASP under our experimental conditions.
We collected conditioned medium (CM) from control and senescent cells for 24 h. Exposure to H2O2 significantly increased the production of proinflammatory mediators such as IFN-ɣ, TNF-α, IL1-β, and IL1-β (p<0.01 vs control non-senescent cells) (Fig. 6A). Other proinflammatory cytokines that were differentially regulated are shown in Supplementary Table 1 (Supplementary Table 1). Interleukins such as IL-6, IL-8, IL-9 and IL-10 were also secreted significantly more by senescent than non-senescent cells, with IL-8 being the most highly secreted (Fig. 6B, Supplementary Table 1). The secretion of chemokines by senescent RPE cells are shown in the Table (Supplementary Table 1). Matrix metalloproteases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) play important roles in regulating extracellular matrix (ECM) turnover and structural integrity. The activities of MMPs are inhibited by TIMPs. Among MMPs, only MMP3 secretion increased significantly while other MMPs decreased in senescent cells. The secretion of TIMPs was lower in senescent cells (Fig. 6C). Among the growth factors, VEGF secretion increased significantly (p<0.01 vs non-senescent cells) in senescent RPE cells. As expected, based on the known anti-inflammatory functions of mini Cry, cotreatment with mini Cry significantly reduced secretion of all inflammatory cytokines and interleukins (Fig. 6 A.B). The secretion of all chemokines and growth factors including VEGF was significantly inhibited by mini Cry (Supplementary Table 1). Secretion of MMPs and TIMPs was also significantly inhibited by mini Cry (Fig. 6C). In addition, senescent RPE cells upregulated VEGF and mini cry treatment reduced its secretion significantly (Supplementary Table 1).
Fig. 6. Effect of mini Cry on regulation of SASP members in senescent RPE cells.
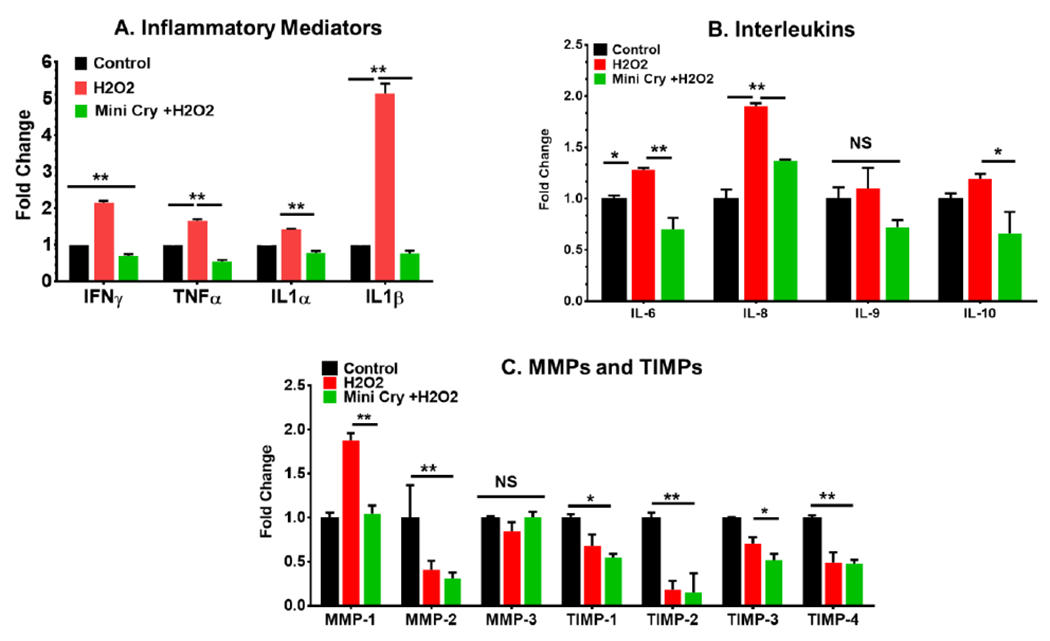
Sub confluent human RPE cells were treated with 500 μM H2O2 alone or with 75 μg/mL mini Cry for 2 h. The H2O2 treatment was repeated the following day. RPE conditioned medium was collected at 24h and assayed by Cytokine/ Chemokine array and MMP and TIMP array (Eve Technologies, Canada). Data are converted to fold change relative to controls and are derived from three samples/experimental condition. N=3, Mean ± SEM. *p<0.05, ** p<0.01. TIMPs- tissue inhibitors of metalloproteinases, MMPs- Matrix metalloproteinases.
Based on the present findings, it is tempting to speculate that SASP regulation may play a contributory role in our recent in vivo demonstration in which nano mini Cry administration showed significant protective effects in the prevention of age-related degeneration (Sreekumar et al., 2018).
Identification of senescent cells in the RPE monolayer in mouse models of AMD.
We used two independent mouse models of AMD to characterize senescence in vivo NaIO3-induced oxidative stress in mice is widely used a dry model of AMD (Kannan and Hinton, 2014; Sreekumaret al., 2018; Su etal., 2019; Zhou etal., 2014) and laser-induced subretinal fibrosis is a model for late neovascular AMD (Ishikawa et al., 2016).
We treated mice with a single systemic injection of NaIO3 and after one-week retinal sections were stained for senescent markers such as p16 and p21. The number of p16 and p21 positive cells increased 7 days after NaIO3 injection, suggesting induction of senescent cells in the RPE layer (Fig.7A). These results suggested that NaIO3-induced retinal degeneration could be a suitable model for studying senescence in a dry form of AMD. Next, we analyzed cellular senescence in mice subjected to laser-induced subretinal fibrosis following the procedure we optimized in previous work (Ishikawa et al., 2016). After day 35 post laser, retinal sections were stained for β-Gal, p16 and p21. RPE cells stained for β-Gal, p16 and p21 providing evidence for senescent cells in vivo during subretinal fibrosis development (Fig. 7B).
Fig.7. Evidence for senescence in RPE cells in dry AMD (A) and subretinal fibrosis (B) mouse models.
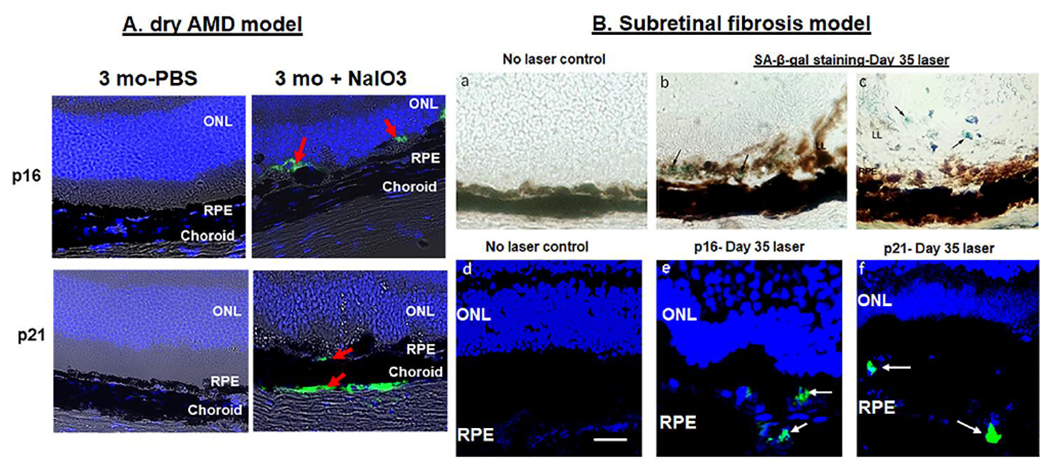
Dry AMD refers to confocal images on day 7 after mice were given NaIO3 (20mg/kg BW) intravenously as a single injection and subretinal fibrosis was produced from laser-irradiation on Day 35. Evidence for the presence of senescent RPE cells by SA-β-gal, p16, and p21 expression in day 35 post-laser retina from 8 wk old mice. Panels (b,c) show SA-β-gal + cells (arrows; blue-green cells). Panels (e,f) show positive immunoreactivity for p16 and p21 (red arrows in A and white arrows in B). No primary antibody control for p21 and p16 was used as a negative control (not shown). Scale bar 5μm. RPE- Retinal pigment epithelium, ONL- Outer nuclear layer.
Thus, our results provide compelling evidence for the presence of senescent cells in the RPE layer in two commonly used animal models of dry and wet AMD. Together, they indicated that these two mouse models could be used to study the mechanistic role of senescence in the progression of dry and wet forms of AMD. Furthermore, demonstration of senescence-associated markers in experimental subretinal fibrosis suggest that the RPE is a potential source of senescent cells in nAMD.
Increased senescence in RPE cells cultured from aged retina and retinas of stressed aged mice
It was reported from many laboratories, including ours, that oxidative stress induces RPE cell senescence. However, information on the role of growth and development in RPE senescence is scarce (Idda et al., 2020). We studied the effect of age on development of senescence using unstressed fetal RPE cells vs RPE cells cultured from old donors. We found that RPE cells from old donors cultured for 4 days showed a significantly higher percentage of SA β-gal positive cells compared to fetal RPE cells cultured under the same conditions (Supplementary Fig. 3), suggesting that aged RPE cells are susceptible to senescence-induced damage than fetal RPE cells under the same culture conditions.
We then investigated whether this phenomenon of age-dependent differences in senescence occurred in vivo in the mouse RPE/retina using young and old mice. Interestingly, we found no appreciable staining for senescent RPE cells in young or old mice, like earlier reports where no senescence markers were detected in 18-week-old WT mice (Yousefzadeh et al., 2020). However, when challenged with NaIO3, there was a marked upregulation of p16 in the RPE layer of old mice compared to young mice challenged with NaIO3 (Fig.8). The expression of p21 showed a trend towards an increase in the RPE but the increase was not statistically significant (Fig. 8). Notably, senescent cells were also observed in other layers of the retina, in the choroid in particular. Our results demonstrate that aging increases susceptibility of retinal tissues, particularly of RPE cells, to the stress of NaIO3-mediated RPE senescence.
Fig.8. Increased senescence with age in stressed mouse retina.

A. Evidence of senescent RPE cells in young (3 month) and old (13-month) old mice after oxidative stress. Mice were treated with NaIO3 (20mg/kg) for 1 wk and expression of p16 and p21 (red arrows) was assessed by immunostaining. B. Quantification of p16 and p21 fluorescence intensity in the RPE monolayer. Mean fluorescence intensity of p16 significantly increased in the RPE layer of old mice treated with NaIO3. The increase in fluorescence intensity of p21 in old mice was not statistically significant. No primary antibody control for p21 and p16 was used as a negative control. Data are mean ± SEM (N=3–5). Scale 50 μm. ** p<0.01. NS- Not significant.
Discussion
Cellular senescence is a major risk factor for degenerative retinal disorders (Fu and Smith, 2021; Sreekumar et al., 2020a; Yu et al., 2020); and is implicated in age-dependent eye diseases, such as AMD (Chae et al., 2021; Kozlowski, 2012; Mishima et al., 1999), glaucoma (Rocha et al., 2020; Skowronska-Krawczyk et al., 2015) and diabetic retinopathy (Crespo-Garcia et al., 2021). Our study examined the senolytic potential of mini Cry in two models of RPE senescence. Our key findings are (i) Oxidative stress and DOX induced senescence in RPE cells as evidenced by multiple senescence markers. (Figs. 1 & 2); (ii) Mitochondrial biogenesis (PGC-1α and mTFA) and fission (FIS-1, DRP-1) increased significantly in senescent RPE, and mini Cry inhibited the dysregulated mitochondrial events (Fig. 3); (iii) Senescent RPE cells are metabolically active as shown by enhanced energy production (increased glycolysis and OXPHOS), which was inhibited by mini Cry (Figs. 4 & 5); (iv) Senescent RPE cells are proinflammatory and secrete several SASP factors, and mini Cry inhibited their secretion (Fig.6). We provide evidence for in vivo RPE senescence in dry (NaIO3 model) and late nAMD (laser-induced model) and report that the number of senescent RPE cells increases with age (Fig.7 & 8). Together, our study shows a link between RPE mitochondrial function and senescence. Thus, mitochondria may be a suitable target for interventions to reduce the deleterious impact of senescence in age-related ocular diseases. Our data also suggest the potential use of mouse models to study the role of RPE senescence in the development of AMD.
Fig. 5. Enhanced glycolysis in senescent RPE cells.
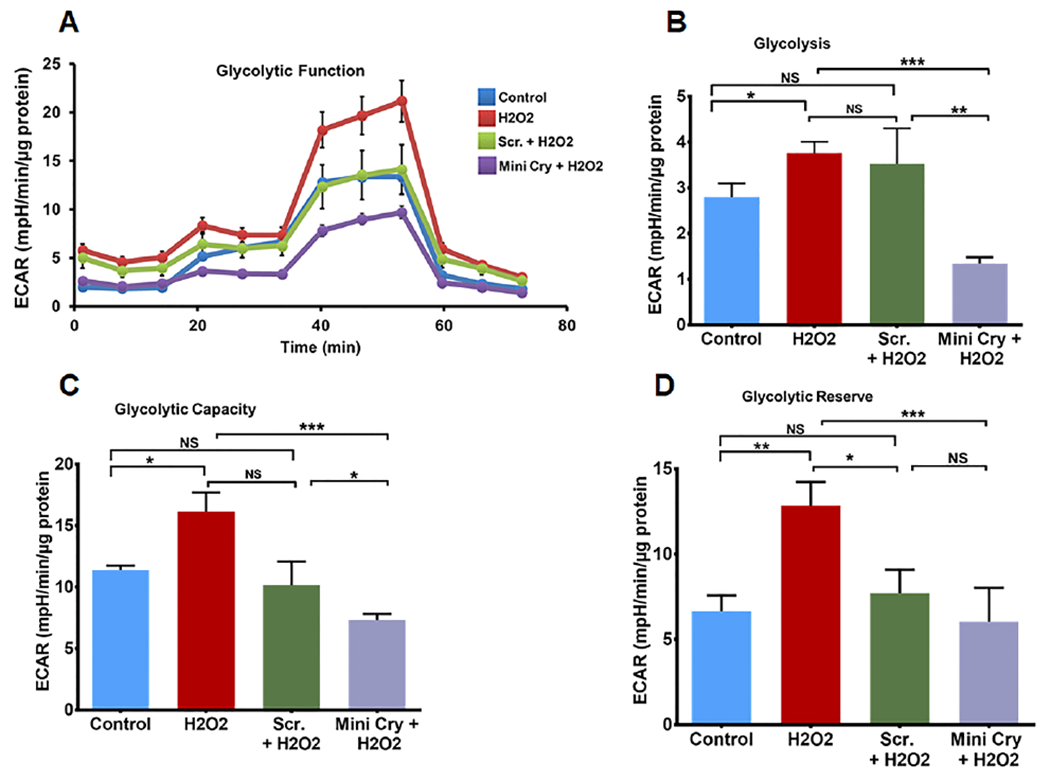
A. Real-time monitoring of glycolysis using the glycolysis stress test kit of key parameters of glycolytic function using Seahorses XFe96. Sequential compound injections (glucose 10 mM, Oligomycin 1μM, 2-deoxy-D-glucose 50 mM) measure glycolysis (B), glycolytic capacity (C), and allow calculation of glycolytic reserve (D). Stress-induced RPE senescence significantly increased all these parameters while co-incubation with mini Cry (75 μg/ml) significantly inhibited all the glycolytic functions. Values are means ± SE (N=6-10) experimental condition. Data normalized to total cellular protein; * p<0.05; ** p<0.01, *** p<0.01, (N=6-10).
As a multifactorial disease, AMD is associated with both genetic and environmental factors that include, but are not limited to, oxidative stress, ageing, smoking, diet etc. (Corso-Diaz et al., 2018; Datta et al., 2017; Kannan et al., 2016). One remarkable impact of oxidative stress is the initiation of RPE senescence, which has been postulated as a key pathophysiological mediator of RPE cell atrophy in geographic atrophy (Kozlowski, 2012; Lee etal., 2021; Sreekumar et al., 2020a).
Previous work showed the presence of senescent RPE cells adjacent to cuticular drusen in aged monkeys (Mishima et al., 1999). A recent study demonstrated the presence of senescent cells in retinas from post-mortem globes of patients diagnosed with proliferative diabetic retinopathy, with senescent cells confined to the outer ganglion cell layer and cells of the RPE (Crespo-Garcia et al., 2021). Further, senescent cells, such as retinal vascular endothelial cells and retinal ganglion cells, occur in the ischemic zones of the retina in a mouse model of oxygen-induced retinopathy (Oubaha et al., 2016). Aging upregulates the expression of several proteins involved in senescence and inflammation in primary human RPE from young (age 29 or 40) and old (ages 84 and 86) donors (Chaum et al., 2015). We found that in both of our cell culture senescence models treatments significantly increased the percentage of SA-β-Gal-positive cells, and p16 and p21 transcripts and protein expression. We showed, for the first time, that pre-treatment with the mini Cry is highly effective at preventing senescence markers in RPE cells.
It is well established that not only cell size but also mitochondrial mass increases in senescent cells and the increase generally occurs before a robust SASP is established (Passos et al., 2010). Enhanced mitochondrial biogenesis, as evidenced by increased expression of the PGC1-α, a master regulator of biogenesis, and mtTFA, a mitochondrial transcription initiator, was observed in our study. Increased mRNA levels of PGC-1α, PGC-1β and TFAM was observed during oncogene-induced senescence (Correia-Melo and Passos, 2015; Vasileiou et al., 2019) and overexpression of PGC1-α induced senescence in lung epithelial cells (Summer et al., 2019). suggesting a role for mitochondrial biogenesis in promoting the mitochondrial mass increase and induction of senescence. Mitochondrial dynamics is regulated by a balance of expression levels between mitochondrial fission (Fis1 and DRP1) and fusion (MFN1, MFN2, and OPA1) proteins (Otera and Mihara, 2011). We observed increased expression of DRP1 and Fis1 in both models of senescence and mini cry treatment significantly reduced expression of both proteins. In human bronchial epithelial cells, cigarette smoke extract-induced senescence increased expression levels of DRP1 and Fis1 in the mitochondrial fraction, suggesting that translocation of DRP1 and Fis1 to the mitochondria is involved in mitochondrial fragmentation (Hara et al., 2013). However, further studies are needed to elucidate more detailed mechanisms of mitochondrial fission in RPE cell senescence.
Cellular energy is produced in the form of adenosine triphosphate (ATP) in the mitochondria via oxidative phosphorylation (OXPHOS) in presence of oxygen, and in the cytosol in the absence of anaerobic glycolysis (Saraste, 1999). Notably, despite their growth arrest, senescent cells are metabolically active (Wiley and Campisi, 2016). However, whether this is a cause or effect of the inflammatory phenotype and changed mitotic status of senescent cells need further studies (Wiley and Campisi, 2016). Our data suggest increased OXPHOS activity and glycolytic rate in senescent RPE cells. An increase in mitochondrial pyruvate oxidation, TCA cycle, and respiration was observed in senescence induced through oncogene activation (Kaplon et al., 2013; Nacarelli et al., 2018; Quijano et al., 2012; Takebayashi et al., 2015), genotoxic stress (Fan and Schmitt, 2019), mitochondrial stress (Nacarelli et al., 2016), and replicative exhaustion (Nacarelli et al., 2016; Takebayashi et al., 2015). The RPE preferably transports glucose to photoreceptors (Kanow et al., 2017), and use of excessive glucose by the RPE leads to photoreceptor glucose shortage and cell death (Zhao et al., 2011). Thus, senescent RPE cells with dysregulated cellular metabolism could result in unmet metabolic needs in photoreceptors and induce angiogenesis (Fu and Smith, 2021). Pyruvate is the key intermediate linking glycolysis and mitochondrial function, and, in this context, it is worthwhile studying the role of the mitochondrial pyruvate carrier (MPC1) given that retina specific deletion of MPC1 results in progressive retinal degeneration and a decline in visual function (Grenell et al., 2019). Even though it is still debatable whether metabolic remodeling is a cause or effect of cellular reprogramming, our study indicates that metabolic pathways can change depending on the state of the cell. Further, our studies demonstrate that mini Cry inhibits mitochondrial biogenesis and glycolysis and can delay or prevent senescence. However, in-depth understanding of the metabolic reprogramming of the senescent cells and potential impact of mini Cry to delay senescence are required in order to devise targeted interventions.
The SASP produces a unique proinflammatory medium and exacerbates senescence through autocrine/paracrine pathways (Coppe et al., 2010). Aging can increase the expression of genes involved in inflammation and regulation of the immune system, including the retina (Chen et al., 2019). An increased production of inflammatory cytokines and activation of immunocompetent cells in the retina could break the blood-retinal barrier and lead to development of neovascular AMD (Parmeggiani et al., 2012). In this context, our findings that senescent RPE cells secreted significantly more pro-inflammatory cytokines such as IFN-ɣ, IL-β, and TNF-α than non-senescent cells, and treatment with mini Cry significantly inhibited this secretion, is significant.
Long-term exposure to H2O2 increased expression of inflammatory cytokines such as IL-1β in ARPE-19 cells (Macchioni et al., 2020) and rat astrocytes (Shang et al., 2020). However, no appreciable change was noticed in TNFα secretion, which could stem either from differences in treatment or use of different cells (primary vs transformed) (Macchioni et al., 2020). Concordant with our study, the role of TNFα in senescence was reported in endothelial cells where TNF-α treatment-induced senescence upregulated several other cytokines, including IL-1β (Kandhaya-Pillai et al., 2017). We hypothesized that oxidative stress induces TNF secretion, which in turn activates other cytokines.
Our results also identified increased production of other cytokines. Among them, IL-6 and IL-8 are most prominent in agreement with other studies demonstrating increased production of IL-6 and IL-8 by senescent RPE cells (Jadeja et al., 2018; Marazita et al., 2016; Wan et al., 2021), astrocytes (Shang et al., 2020) and retinal endothelial cells (Gericke et al., 2021). Increased levels of IL-6 and IL-8 have been reported in the vitreous of AMD patients (Knickelbein et al., 2015; Tan et al., 2020) and may stimulate retinal angiogenesis, induce innate immune responses (Ghasemi, 2018; Ghasemi et al., 2011) and reinforce senescent growth arrests (Ortiz-Montero et al., 2017). These two cytokines are key effectors in inflammasome activation, as reported in degenerative retina, including AMD (Tan et al., 2020).
VEGF is one of the main components of the SASP in senescent RPE cells in our study and is markedly increased in the vitreous of neovascular AMD. Inflammatory cytokines can enhance the secretion of VEGF, which can initiate and cause the pathological CNV and retinal neovascularization of AMD (Nagineni et al., 2012). Failure of current anti-VEGF treatment for nAMD, including resistance to anti-VEGF therapy and development of geographic atrophy or subretinal fibrosis, could at least in part be associated with the occurrence of senescent RPE cells. We also analyzed the secretion by senescent cells of key ECM-modifying proteins such as MMPs and TIMPs. Cellular senescence is associated with changes in both ECM components and remodeling enzymes (Campisi, 1998). TIMPs inhibit many activated MMPs, but in some cases can form complexes with latent form of MMPs, such as MMP2 and MMP9 (Brew et al., 2000). Lower expression of TIMPs was observed in senescent human fibroblasts (Dimozi et al., 2015; Millis et al., 1992; West et al., 1989). H2O2-induced senescent intervertebral disc cells show a reduced mRNA levels of TIMP1, TIMP2 and TIMP3 (Dimozi et al., 2015). Studies have linked cellular senescence with increased (Coppe et al., 2010; Dimozi et al., 2015; Rashid et al., 2018) and decreased (Dimozi et al., 2015; Gutierrez-Fernandez et al., 2015; Vasko et al., 2014) expression of MMP family members. Altogether, expression of MMPs is highly regulated in senescent cells and it is worthwhile to study the activity of MMPs under our experimental conditions.
New SASP members are increasingly discovered. Some are similar among several tissues and cell types but vary quantitively based on the nature of induction of senescence (Lee et al., 2021; Nagineni et al., 2021; Sreekumar et al., 2020a). A recent review proposed that some of these factors are common among photoreceptors, ganglion cells and RPE cells (Lee et al., 2021). Further work will be needed to identify the SASP factors in multiple retinal cell types and their correlation to senescence-induced disease.
Senomorphics, which inhibit modules of the SASP, or senolytics, which specifically kill senescent cells (Romashkan et al., 2021), could be effective to treat AMD. Several senomorphic and senolytic agents have been identified (Lagoumtzi and Chondrogianni, 2021). Our study suggests that mini Cry can inhibit VEGF secretion from senescent RPE cells. We and others presented strong evidence for the neuroprotective, antiinflammatory proprieties of this peptide in different animal models (Kurnellas et al., 2012; Sreekumar et al., 2013; Sreekumar et al., 2018; Wang et al., 2014), Thus, mini Cry is a potential therapeutic molecule that can offer protection as well as inhibit release of SASP factors and is a candidate to be added to the list of potential senomorpic and senolytic agents.
One challenging problem to study the role of RPE senescence in AMD progression is the lack of reproducible models. In this study, we developed two mouse models of RPE senescence that exhibited general features of cellular senescence, including increased SA-β-gal expression and elevated expression of p16 and p21. Although anti-VEGF therapy is a promising therapeutic option for neovascular AMD, poor treatment response or development of subretinal scar formation is also reported. Senescent RPE cells might be responsible for the initiation and progression of neovascularization. The laser-induced subretinal fibrosis model we propose here could be an ideal model to study the effect of anti-VEGF therapy and RPE senescence. The application of senotherapeutics in combination with anti-VEGF is a potentially promising treatment for neovascular AMD and dry AMD. In this context, we recently found an elastin-like polypeptide of mini Cry that inhibited subretinal fibrosis in the laser-induced mouse model (Sreekumar et al. unpublished data), supporting its utility as a potential therapeutic molecule.
Some limitations of the cell culture work in the current study need to be addressed. The rationale for using non-polarized cells in our studies was that in late atrophic AMD non-polarized RPE cells were reported at the edge of GA lesions and in subretinal fibrosis (Roberts et al., 2016; Vogt et al., 2011). hRPE cells cultured in the present study do not fully resemble an intact RPE monolayer. Thus, the extent of senescence with our protocol may not represent what will be observed with polarized preparations of RPE. The use of polarized monolayers will require methodological modifications of isolating intact RPE monolayers from transwell inserts and using viable preparations for induction of senescence (Sonoda et al., 2009a). However, in our investigations using in vivo models we could characterize senescent cells in the RPE layer with multiple senescence biomarkers for the first time in subretinal fibrosis (Ishikawa et al., 2016; Sreekumar et al., 2020a).
In conclusion, we provide evidence of senescence in RPE cells. Mitochondrial biogenesis increased in senescent cells, accompanied by increased glycolysis and OXPHOS for energy production. Our studies provide strong evidence that senescent RPE cells secrete significantly larger amounts of proinflammatory cytokines, chemokines and growth factors. Likewise, our study provides the first evidence for the use of a senotherapeutic peptide (mini Cry) that significantly reduced the number of senescent RPE cells and SASP factors. RPE cells are trophic to the photoreceptors of the neural retina and are one of the first influenced cells in the retina (Fisher and Ferrington, 2018). Dysfunctional senescent RPE cells secrete abnormally high levels of factors (SASP). Our data indicate that mini Cry can protect the RPE and restore its functions. However, it remains crucial to study retinal cell type-specific patterns of senescence and associated SASPs and identify an AMD-related SASP profile. Since SASP factors are linked to regulation of tissue homeostasis and immune cells, the question remains whether senomorphics or senolytics act specifically on senescent cells, or whether they are non-specific. Additional studies are required to delineate the action of mini Cry as a senolytic or sonomorphic peptide.
Supplementary Material
Highlights.
Hydrogen peroxide and doxorubicin induce senescence in hRPE cells
Mitochondrial metabolism was altered in senescent RPE cells
Senescent RPE secretes an array of SASP factors and mini Cry impairs SASP production
RPE senescence plays a role in dry AMD and late nAMD animal models
Age-dependent increase in senescence is observed in retinal degeneration
Acknowledgments:
We sincerely thank Dr. Judith Campisi, Buck Institute for Research on Aging, Novato, CA, USA for helpful discussions. We acknowledge Christine Spee for culturing human RPE and Dr. Chandra Nagineni (NCI) for providing aged human donor RPE. Ernesto Barron is thanked for help with the confocal images. This work was supported by NEI R01 EY30141(RK), William Keck Foundation (RK) and funds from the Ryan Initiative for Macular Research, DEI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Part of this work was presented in the ARVO meeting: “αB Crystallin Chaperone Peptide (mini Cry) Inhibits Senescence in RPE cells by Modulating Mitochondrial Biogenesis and Fission Proteins. Invest. Ophthalmol. Vis. Sci. 2019; 60(9): 1950”.
References
- Ambati J, Fowler BJ, 2012. Mechanisms of age-related macular degeneration. Neuron 75, 26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TA, Kanuga N, Romero IA, Greenwood J, Luthert PJ, Cheetham ME, 2004. Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 45, 675–684. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM, 2011. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basisty N, Kale A, Jeon OH, Kuehnemann C, Payne T, Rao C, Holtz A, Shah S, Sharma V, Ferrucci L, Campisi J, Schilling B, 2020. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol 18, e3000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutto I, Lutty G, 2012. Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol Aspects Med 33, 295–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutto IA, Ogura S, Baldeosingh R, McLeod DS, Lutty GA, Edwards MM, 2018. An Acute Injury Model for the Phenotypic Characteristics of Geographic Atrophy. Invest Ophthalmol Vis Sci 59, AMD143–AMD151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielak-Zmijewska A, Wnuk M, Przybylska D, Grabowska W, Lewinska A, Alster O, Korwek Z, Cmoch A, Myszka A, Pikula S, Mosieniak G, Sikora E, 2014. A comparison of replicative senescence and doxorubicin-induced premature senescence of vascular smooth muscle cells isolated from human aorta. Biogerontology 15, 47–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasiak J, 2020. Senescence in the pathogenesis of age-related macular degeneration. Cell Mol Life Sci 77, 789–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasiak J, Piechota M, Pawlowska E, Szatkowska M, Sikora E, Kaarniranta K, 2017. Cellular Senescence in Age-Related Macular Degeneration: Can Autophagy and DNA Damage Response Play a Role? Oxid Med Cell Longev 2017, 5293258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes Rickman C, Farsiu S, Toth CA, Klingeborn M, 2013. Dry age-related macular degeneration: mechanisms, therapeutic targets, and imaging. Invest Ophthalmol Vis Sci 54, ORSF68–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew K, Dinakarpandian D, Nagase H, 2000. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta 1477, 267–283. [DOI] [PubMed] [Google Scholar]
- Burgess A, Vigneron S, Brioudes E, Labbe JC, Lorca T, Castro A, 2010. Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc Natl Acad Sci U S A 107, 12564–12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, 1998. The role of cellular senescence in skin aging. J Investig Dermatol Symp Proc 3, 1–5. [PubMed] [Google Scholar]
- Campisi J, 2013. Aging, cellular senescence, and cancer. Annu Rev Physiol 75, 685–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, Kapahi P, Lithgow GJ, Melov S, Newman JC, Verdin E, 2019. From discoveries in ageing research to therapeutics for healthy ageing. Nature 571, 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae JB, Jang H, Son C, Park CW, Choi H, Jin S, Lee HY, Lee H, Ryu JH, Kim N, Kim C, Chung H, 2021. Targeting senescent retinal pigment epithelial cells facilitates retinal regeneration in mouse models of age-related macular degeneration. Geroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaum E, Winborn CS, Bhattacharya S, 2015. Genomic regulation of senescence and innate immunity signaling in the retinal pigment epithelium. Mamm Genome 26, 210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Luo C, Zhao J, Devarajan G, Xu H, 2019. Immune regulation in the aging retina. Prog Retin Eye Res 69, 159–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs BG, Durik M, Baker DJ, van Deursen JM, 2015. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med 21, 1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe JP, Desprez PY, Krtolica A, Campisi J, 2010. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5, 99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia-Melo C, Passos JF, 2015. Mitochondria: Are they causal players in cellular senescence? Biochim Biophys Acta 1847, 1373–1379. [DOI] [PubMed] [Google Scholar]
- Corso-Diaz X, Jaeger C, Chaitankar V, Swaroop A, 2018. Epigenetic control of gene regulation during development and disease: A view from the retina. Prog Retin Eye Res 65, 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo-Garcia S, Tsuruda PR, Dejda A, Ryan RD, Fournier F, Chaney SY, Pilon F, Dogan T, Cagnone G, Patel P, Buscarlet M, Dasgupta S, Girouard G, Rao SR, Wilson AM, O’Brien R, Juneau R, Guber V, Dubrac A, Beausejour C, Armstrong S, Mallette FA, Yohn CB, Joyal JS, Marquess D, Beltran PJ, Sapieha P, 2021. Pathological angiogenesis in retinopathy engages cellular senescence and is amenable to therapeutic elimination via BCL-xL inhibition. Cell Metab 33, 818–832 e817. [DOI] [PubMed] [Google Scholar]
- Datta S, Cano M, Ebrahimi K, Wang L, Handa JT, 2017. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog Retin Eye Res 60, 201–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria M, O’Leary MN, Chang J, Shao L, Liu S, Alimirah F, Koenig K, Le C, Mitin N, Deal AM, Alston S, Academia EC, Kilmarx S, Valdovinos A, Wang B, de Bruin A, Kennedy BK, Melov S, Zhou D, Sharpless NE, Muss H, Campisi J, 2017. Cellular Senescence Promotes Adverse Effects of Chemotherapy and Cancer Relapse. Cancer Discov 7, 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimozi A, Mavrogonatou E, Sklirou A, Kletsas D, 2015. Oxidative stress inhibits the proliferation, induces premature senescence and promotes a catabolic phenotype in human nucleus pulposus intervertebral disc cells. Eur Cell Mater 30, 89–102; discussion 103. [DOI] [PubMed] [Google Scholar]
- Dorr JR, Yu Y, Milanovic M, Beuster G, Zasada C, Dabritz JH, Lisec J, Lenze D, Gerhardt A, Schleicher K, Kratzat S, Purfurst B, Walenta S, Mueller-Klieser W, Graler M, Hummel M, Keller U, Buck AK, Dorken B, Willmitzer L, Reimann M, Kempa S, Lee S, Schmitt CA, 2013. Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature 501, 421–425. [DOI] [PubMed] [Google Scholar]
- Dou G, Sreekumar PG, Spee C, He S, Ryan SJ, Kannan R, Hinton DR, 2012. Deficiency of alphaB crystallin augments ER stress-induced apoptosis by enhancing mitochondrial dysfunction. Free Radic Biol Med 53, 1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dridi S, Hirano Y, Tarallo V, Kim Y, Fowler BJ, Ambati BK, Bogdanovich S, Chiodo VA, Hauswirth WW, Kugel JF, Goodrich JA, Ponicsan SL, Hinton DR, Kleinman ME, Baffi JZ, Gelfand BD, Ambati J, 2012. ERK1/2 activation is a therapeutic target in age-related macular degeneration. Proc Natl Acad Sci U S A 109, 13781–13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgass K, Pakay J, Ryan MT, Palmer CS, 2013. Recent advances into the understanding of mitochondrial fission. Biochim Biophys Acta 1833, 150–161. [DOI] [PubMed] [Google Scholar]
- Enzbrenner A, Zulliger R, Biber J, Pousa AMQ, Schafer N, Stucki C, Giroud N, Berrera M, Kortvely E, Schmucki R, Badi L, Grosche A, Pauly D, Enzmann V, 2021. Sodium Iodate-Induced Degeneration Results in Local Complement Changes and Inflammatory Processes in Murine Retina. Int J Mol Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzmann V, Row BW, Yamauchi Y, Kheirandish L, Gozal D, Kaplan HJ, McCall MA, 2006. Behavioral and anatomical abnormalities in a sodium iodate-induced model of retinal pigment epithelium degeneration. Exp Eye Res 82, 441–448. [DOI] [PubMed] [Google Scholar]
- Fan DNY, Schmitt CA, 2019. Genotoxic Stress-Induced Senescence. Methods Mol Biol 1896, 93–105. [DOI] [PubMed] [Google Scholar]
- Ferrington DA, Ebeling MC, Kapphahn RJ, Terluk MR, Fisher CR, Polanco JR, Roehrich H, Leary MM, Geng Z, Dutton JR, Montezuma SR, 2017. Altered bioenergetics and enhanced resistance to oxidative stress in human retinal pigment epithelial cells from donors with age-related macular degeneration. Redox Biol 13, 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CR, Ferrington DA, 2018. Perspective on AMD Pathobiology: A Bioenergetic Crisis in the RPE. Invest Ophthalmol Vis Sci 59, AMD41–AMD47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco LM, Zulliger R, Wolf-Schnurrbusch UE, Katagiri Y, Kaplan HJ, Wolf S, Enzmann V, 2009. Decreased visual function after patchy loss of retinal pigment epithelium induced by low-dose sodium iodate. Invest Ophthalmol Vis Sci 50, 4004–4010. [DOI] [PubMed] [Google Scholar]
- Fu Z, Smith LEH, 2021. Cellular senescence in pathologic retinal angiogenesis. Trends Endocrinol Metab 32, 415–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gericke A, Suminska-Jasinska K, Breborowicz A, 2021. Sulodexide reduces glucose induced senescence in human retinal endothelial cells. Sci Rep 11, 11532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi H, 2018. Roles of IL-6 in Ocular Inflammation: A Review. Ocul Immunol Inflamm 26, 37–50. [DOI] [PubMed] [Google Scholar]
- Ghasemi H, Ghazanfari T, Yaraee R, Faghihzadeh S, Hassan ZM, 2011. Roles of IL-8 in ocular inflammations: a review. Ocul Immunol Inflamm 19, 401–412. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Shang P, Terasaki H, Stepicheva N, Hose S, Yazdankhah M, Weiss J, Sakamoto T, Bhutto IA, Xia S, Zigler JS Jr., Kannan R, Qian J, Handa JT, Sinha D, 2018. A Role for betaA3/A1-Crystallin in Type 2 EMT of RPE Cells Occurring in Dry Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci 59, AMD104–AMD113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenell A, Wang Y, Yam M, Swarup A, Dilan TL, Hauer A, Linton JD, Philp NJ, Gregor E, Zhu S, Shi Q, Murphy J, Guan T, Lohner D, Kolandaivelu S, Ramamurthy V, Goldberg AFX, Hurley JB, Du J, 2019. Loss of MPC1 reprograms retinal metabolism to impair visual function. Proc Natl Acad Sci U S A 116, 3530–3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gureev AP, Shaforostova EA, Popov VN, 2019. Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction Between the Nrf2 and PGC-1alpha Signaling Pathways. Front Genet 10, 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Fernandez A, Soria-Valles C, Osorio FG, Gutierrez-Abril J, Garabaya C, Aguirre A, Fueyo A, Fernandez-Garcia MS, Puente XS, Lopez-Otin C, 2015. Loss of MT1-MMP causes cell senescence and nuclear defects which can be reversed by retinoic acid. EMBO J 34, 1875–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara H, Araya J, Ito S, Kobayashi K, Takasaka N, Yoshii Y, Wakui H, Kojima J, Shimizu K, Numata T, Kawaishi M, Kamiya N, Odaka M, Morikawa T, Kaneko Y, Nakayama K, Kuwano K, 2013. Mitochondrial fragmentation in cigarette smoke-induced bronchial epithelial cell senescence. Am J Physiol Lung Cell Mol Physiol 305, L737–746. [DOI] [PubMed] [Google Scholar]
- Hernandez-Segura A, Nehme J, Demaria M, 2018. Hallmarks of Cellular Senescence. Trends Cell Biol 28, 436–453. [DOI] [PubMed] [Google Scholar]
- Hu C, Huang Y, Li L, 2017. Drp1-Dependent Mitochondrial Fission Plays Critical Roles in Physiological and Pathological Progresses in Mammals. Int J Mol Sci 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idda ML, McClusky WG, Lodde V, Munk R, Abdelmohsen K, Rossi M, Gorospe M, 2020. Survey of senescent cell markers with age in human tissues. Aging (Albany NY) 12, 4052–4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K, Sreekumar PG, Spee C, Nazari H, Zhu D, Kannan R, Hinton DR, 2016. alphaB-Crystallin Regulates Subretinal Fibrosis by Modulation of Epithelial-Mesenchymal Transition. Am J Pathol 186, 859–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadeja RN, Powell FL, Jones MA, Fuller J, Joseph E, Thounaojam MC, Bartoli M, Martin PM, 2018. Loss of NAMPT in aging retinal pigment epithelium reduces NAD(+) availability and promotes cellular senescence. Aging (Albany NY) 10, 1306–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamogashira T, Hayashi K, Fujimoto C, Iwasaki S, Yamasoba T, 2017. Functionally and morphologically damaged mitochondria observed in auditory cells under senescence-inducing stress. NPJ Aging Mech Dis 3, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandhaya-Pillai R, Miro-Mur F, Alijotas-Reig J, Tchkonia T, Kirkland JL, Schwartz S, 2017. TNFalpha-senescence initiates a STAT-dependent positive feedback loop, leading to a sustained interferon signature, DNA damage, and cytokine secretion. Aging (Albany NY) 9, 2411–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan R, Hinton DR, 2014. Sodium iodate induced retinal degeneration: new insights from an old model. Neural Regen Res 9, 2044–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan R, Sreekumar PG, Hinton DR, 2012. Novel roles for alpha-crystallins in retinal function and disease. Prog Retin Eye Res 31, 576–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan R, Sreekumar PG, Hinton DR, 2016. Alpha crystallins in the retinal pigment epithelium and implications for the pathogenesis and treatment of age-related macular degeneration. Biochim Biophys Acta 1860, 258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanow MA, Giarmarco MM, Jankowski CS, Tsantilas K, Engel AL, Du J, Linton JD, Farnsworth CC, Sloat SR, Rountree A, Sweet IR, Lindsay KJ, Parker ED, Brockerhoff SE, Sadilek M, Chao JR, Hurley JB, 2017. Biochemical adaptations of the retina and retinal pigment epithelium support a metabolic ecosystem in the vertebrate eye. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplon J, Zheng L, Meissl K, Chaneton B, Selivanov VA, Mackay G, van der Burg SH, Verdegaal EM, Cascante M, Shlomi T, Gottlieb E, Peeper DS, 2013. A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature 498, 109–112. [DOI] [PubMed] [Google Scholar]
- Kase S, He S, Sonoda S, Kitamura M, Spee C, Wawrousek E, Ryan SJ, Kannan R, Hinton DR, 2010. alphaB-crystallin regulation of angiogenesis by modulation of VEGF. Blood 115, 3398–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EC, Kim JR, 2019. Senotherapeutics: emerging strategy for healthy aging and age-related disease. BMB Rep 52, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Mehta HH, Wan J, Kuehnemann C, Chen J, Hu JF, Hoffman AR, Cohen P, 2018. Mitochondrial peptides modulate mitochondrial function during cellular senescence. Aging (Albany NY) 10, 1239–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickelbein JE, Chan CC, Sen HN, Ferris FL, Nussenblatt RB, 2015. Inflammatory Mechanisms of Age-related Macular Degeneration. Int Ophthalmol Clin 55, 63–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski MR, 2012. RPE cell senescence: a key contributor to age-related macular degeneration. Med Hypotheses 78, 505–510. [DOI] [PubMed] [Google Scholar]
- Kukat C, Larsson NG, 2013. mtDNA makes a U-turn for the mitochondrial nucleoid. Trends Cell Biol 23, 457–463. [DOI] [PubMed] [Google Scholar]
- Kurnellas MP, Brownell SE, Su L, Malkovskiy AV, Rajadas J, Dolganov G, Chopra S, Schoolnik GK, Sobel RA, Webster J, Ousman SS, Becker RA, Steinman L, Rothbard JB, 2012. Chaperone activity of small heat shock proteins underlies therapeutic efficacy in experimental autoimmune encephalomyelitis. J Biol Chem 287, 36423–36434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagoumtzi SM, Chondrogianni N, 2021. Senolytics and senomorphics: Natural and synthetic therapeutics in the treatment of aging and chronic diseases. Free Radic Biol Med 171, 169–190. [DOI] [PubMed] [Google Scholar]
- LaPak KM, Burd CE, 2014. The molecular balancing act of p16(INK4a) in cancer and aging. Mol Cancer Res 12, 167–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Lin S, Copland DA, Dick AD, Liu J, 2021. Cellular senescence in the aging retina and developments of senotherapies for age-related macular degeneration. J Neuroinflammation 18, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Luppo M, Catita J, Ramos D, Navarro M, Carretero A, Mendes-Jorge L, Munoz-Canoves P, Rodriguez-Baeza A, Nacher V, Ruberte J, 2017. Cellular Senescence Is Associated With Human Retinal Microaneurysm Formation During Aging. Invest Ophthalmol Vis Sci 58, 2832–2842. [DOI] [PubMed] [Google Scholar]
- Macchioni L, Chiasserini D, Mezzasoma L, Davidescu M, Orvietani PL, Fettucciari K, Salviati L, Cellini B, Bellezza I, 2020. Crosstalk between Long-Term Sublethal Oxidative Stress and Detrimental Inflammation as Potential Drivers for Age-Related Retinal Degeneration. Antioxidants (Basel) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima Y, Adachi S, Ito H, Hirao K, Isobe M, 2008. Induction of premature senescence in cardiomyocytes by doxorubicin as a novel mechanism of myocardial damage. Aging Cell 7, 125–136. [DOI] [PubMed] [Google Scholar]
- Marazita MC, Dugour A, Marquioni-Ramella MD, Figueroa JM, Suburo AM, 2016. Oxidative stress-induced premature senescence dysregulates VEGF and CFH expression in retinal pigment epithelial cells: Implications for Age-related Macular Degeneration. Redox Biol 7, 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J, Tarallo D, Martinez-Palma L, Victoria S, Bresque M, Rodriguez-Bottero S, Marmisolle I, Escande C, Cassina P, Casanova G, Bollati-Fogolin M, Agorio C, Moreno M, Quijano C, 2019. Mitofusins modulate the increase in mitochondrial length, bioenergetics and secretory phenotype in therapy-induced senescent melanoma cells. Biochem J 476, 2463–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millis AJ, McCue HM, Kumar S, Baglioni C, 1992. Metalloproteinase and TIMP-1 gene expression during replicative senescence. Exp Gerontol 27, 425–428. [DOI] [PubMed] [Google Scholar]
- Mishima K, Handa JT, Aotaki-Keen A, Lutty GA, Morse LS, Hjelmeland LM, 1999. Senescence-associated beta-galactosidase histochemistry for the primate eye. Invest Ophthalmol Vis Sci 40, 1590–1593. [PubMed] [Google Scholar]
- Nacarelli T, Azar A, Altinok O, Orynbayeva Z, Sell C, 2018. Rapamycin increases oxidative metabolism and enhances metabolic flexibility in human cardiac fibroblasts. Geroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacarelli T, Azar A, Sell C, 2016. Mitochondrial stress induces cellular senescence in an mTORC1-dependent manner. Free Radic Biol Med 95, 133–154. [DOI] [PubMed] [Google Scholar]
- Nagineni CN, Kommineni VK, William A, Detrick B, Hooks JJ, 2012. Regulation of VEGF expression in human retinal cells by cytokines: implications for the role of inflammation in age-related macular degeneration. J Cell Physiol 227, 116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagineni CN, Naz S, Choudhuri R, Chandramouli GVR, Krishna MC, Brender JR, Cook JA, Mitchell JB, 2021. Radiation-Induced Senescence Reprograms Secretory and Metabolic Pathways in Colon Cancer HCT-116 Cells. Int J Mol Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Montero P, Londono-Vallejo A, Vernot JP, 2017. Senescence-associated IL-6 and IL-8 cytokines induce a self- and cross-reinforced senescence/inflammatory milieu strengthening tumorigenic capabilities in the MCF-7 breast cancer cell line. Cell Commun Signal 15, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otera H, Mihara K, 2011. Molecular mechanisms and physiologic functions of mitochondrial dynamics. J Biochem 149, 241–251. [DOI] [PubMed] [Google Scholar]
- Oubaha M, Miloudi K, Dejda A, Guber V, Mawambo G, Germain MA, Bourdel G, Popovic N, Rezende FA, Kaufman RJ, Mallette FA, Sapieha P, 2016. Senescence-associated secretory phenotype contributes to pathological angiogenesis in retinopathy. Sci Transl Med 8, 362ra144. [DOI] [PubMed] [Google Scholar]
- Parmeggiani F, Romano MR, Costagliola C, Semeraro F, Incorvaia C, D’Angelo S, Perri P, De Palma P, De Nadai K, Sebastiani A, 2012. Mechanism of inflammation in age-related macular degeneration. Mediators Inflamm 2012, 546786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos JF, Nelson G, Wang C, Richter T, Simillion C, Proctor CJ, Miwa S, Olijslagers S, Hallinan J, Wipat A, Saretzki G, Rudolph KL, Kirkwood TB, von Zglinicki T, 2010. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol 6, 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quijano C, Cao L, Fergusson MM, Romero H, Liu J, Gutkind S, Rovira II, Mohney RP, Karoly ED, Finkel T, 2012. Oncogene-induced senescence results in marked metabolic and bioenergetic alterations. Cell Cycle 11, 1383–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid K, Sundar IK, Gerloff J, Li D, Rahman I, 2018. Lung cellular senescence is independent of aging in a mouse model of COPD/emphysema. Sci Rep 8, 9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebbaa A, Zheng X, Chou PM, Mirkin BL, 2003. Caspase inhibition switches doxorubicin-induced apoptosis to senescence. Oncogene 22, 2805–2811. [DOI] [PubMed] [Google Scholar]
- Roberts P, Sugita M, Deak G, Baumann B, Zotter S, Pircher M, Sacu S, Hitzenberger CK, Schmidt-Erfurth U, 2016. Automated Identification and Quantification of Subretinal Fibrosis in Neovascular Age-Related Macular Degeneration Using Polarization-Sensitive OCT. Invest Ophthalmol Vis Sci 57, 1699–1705. [DOI] [PubMed] [Google Scholar]
- Rocha LR, Nguyen Huu VA, Palomino La Torre C, Xu Q, Jabari M, Krawczyk M, Weinreb RN, Skowronska-Krawczyk D, 2020. Early removal of senescent cells protects retinal ganglion cells loss in experimental ocular hypertension. Aging Cell 19, e13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romashkan S, Chang H, Hadley EC, 2021. National Institute on Aging Workshop: Repurposing Drugs or Dietary Supplements for Their Senolytic or Senomorphic Effects: Considerations for Clinical Trials. J Gerontol A Biol Sci Med Sci 76, 1144–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M, 1999. Oxidative phosphorylation at the fin de siecle. Science 283, 1488–1493. [DOI] [PubMed] [Google Scholar]
- Shang D, Hong Y, Xie W, Tu Z, Xu J, 2020. Interleukin-1beta Drives Cellular Senescence of Rat Astrocytes Induced by Oligomerized Amyloid beta Peptide and Oxidative Stress. Front Neurol 11, 929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KK, Russell J, Sigala B, Zhang Y, Williams J, Keshav KF, 1999. Mitochondrial DNA determines the cellular response to cancer therapeutic agents. Oncogene 18, 6641–6646. [DOI] [PubMed] [Google Scholar]
- Sippy BD, Hofman FM, He S, Osusky R, Sheu SJ, Walker SM, Ryan SJ, Hinton DR, 1995. SV40-immortalized and primary cultured human retinal pigment epithelial cells share similar patterns of cytokine-receptor expression and cytokine responsiveness. Curr Eye Res 14, 495–503. [DOI] [PubMed] [Google Scholar]
- Skowronska-Krawczyk D, Zhao L, Zhu J, Weinreb RN, Cao G, Luo J, Flagg K, Patel S, Wen C, Krupa M, Luo H, Ouyang H, Lin D, Wang W, Li G, Xu Y, Li O, Chung C, Yeh E, Jafari M, Ai M, Zhong Z, Shi W, Zheng L, Krawczyk M, Chen D, Shi C, Zin C, Zhu J, Mellon PL, Gao W, Abagyan R, Zhang L, Sun X, Zhong S, Zhuo Y, Rosenfeld MG, Liu Y, Zhang K, 2015. P16INK4a Upregulation Mediated by SIX6 Defines Retinal Ganglion Cell Pathogenesis in Glaucoma. Mol Cell 59, 931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda S, Spee C, Barron E, Ryan SJ, Kannan R, Hinton DR, 2009a. A protocol for the culture and differentiation of highly polarized human retinal pigment epithelial cells. Nat Protoc 4, 662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda S, Sreekumar PG, Kase S, Spee C, Ryan SJ, Kannan R, Hinton DR, 2009b. Attainment of polarity promotes growth factor secretion by retinal pigment epithelial cells: relevance to age-related macular degeneration. Aging (Albany NY) 2, 28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreekumar PG, Chothe P, Sharma KK, Baid R, Kompella U, Spee C, Kannan N, Manh C, Ryan SJ, Ganapathy V, Kannan R, Hinton DR, 2013. Antiapoptotic properties of alpha-crystallin-derived peptide chaperones and characterization of their uptake transporters in human RPE cells. Invest Ophthalmol Vis Sci 54, 2787–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreekumar PG, Hinton DR, Kannan R, 2020a. The Emerging Role of Senescence in Ocular Disease. Oxid Med Cell Longev 2020, 2583601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreekumar PG, Ishikawa K, Spee C, Mehta HH, Wan J, Yen K, Cohen P, Kannan R, Hinton DR, 2016. The Mitochondrial-Derived Peptide Humanin Protects RPE Cells From Oxidative Stress, Senescence, and Mitochondrial Dysfunction. Invest Ophthalmol Vis Sci 57, 1238–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreekumar PG, Kannan R, Kitamura M, Spee C, Barron E, Ryan SJ, Hinton DR, 2010. alphaB crystallin is apically secreted within exosomes by polarized human retinal pigment epithelium and provides neuroprotection to adjacent cells. PLoS One 5, e12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreekumar PG, Li Z, Wang W, Spee C, Hinton DR, Kannan R, MacKay JA, 2018. Intra-vitreal alphaB crystallin fused to elastin-like polypeptide provides neuroprotection in a mouse model of age-related macular degeneration. J Control Release 283, 94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreekumar PG, Spee C, Ryan SJ, Cole SP, Kannan R, Hinton DR, 2012. Mechanism of RPE cell death in alpha-crystallin deficient mice: a novel and critical role for MRP1-mediated GSH efflux. PLoS One 7, e33420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreekumar PG, Wang M, Spee C, Sadda SR, Kannan R, 2020b. Transporter-Mediated Mitochondrial GSH Depletion Leading to Mitochondrial Dysfunction and Rescue with alphaB Crystallin Peptide in RPE Cells. Antioxidants (Basel);9(5):411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreekumar PG, Zhou J, Sohn J, Spee C, Ryan SJ, Maurer BJ, Kannan R, Hinton DR, 2008. N-(4-hydroxyphenyl) retinamide augments laser-induced choroidal neovascularization in mice. Invest Ophthalmol Vis Sci 49, 1210–1220. [DOI] [PubMed] [Google Scholar]
- Su F, Spee C, Araujo E, Barron E, Wang M, Ghione C, Hinton DR, Nusinowitz S, Kannan R, Reddy ST, Farias-Eisner R, 2019. A Novel HDL-Mimetic Peptide HM-10/10 Protects RPE and Photoreceptors in Murine Models of Retinal Degeneration. Int J Mol Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summer R, Shaghaghi H, Schriner D, Roque W, Sales D, Cuevas-Mora K, Desai V, Bhushan A, Ramirez MI, Romero F, 2019. Activation of the mTORC1/PGC-1 axis promotes mitochondrial biogenesis and induces cellular senescence in the lung epithelium. Am J Physiol Lung Cell Mol Physiol 316, L1049–L1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi S, Tanaka H, Hino S, Nakatsu Y, Igata T, Sakamoto A, Narita M, Nakao M, 2015. Retinoblastoma protein promotes oxidative phosphorylation through upregulation of glycolytic genes in oncogene-induced senescent cells. Aging Cell 14, 689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Zou J, Yoshida S, Jiang B, Zhou Y, 2020. The Role of Inflammation in Age-Related Macular Degeneration. Int J Biol Sci 16, 2989–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL, 2013. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 123, 966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasileiou PVS, Evangelou K, Vlasis K, Fildisis G, Panayiotidis MI, Chronopoulos E, Passias PG, Kouloukoussa M, Gorgoulis VG, Havaki S, 2019. Mitochondrial Homeostasis and Cellular Senescence. Cells 8(7):686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasko R, Xavier S, Chen J, Lin CH, Ratliff B, Rabadi M, Maizel J, Tanokuchi R, Zhang F, Cao J, Goligorsky MS, 2014. Endothelial sirtuin 1 deficiency perpetrates nephrosclerosis through downregulation of matrix metalloproteinase-14: relevance to fibrosis of vascular senescence. J Am Soc Nephrol 25, 276–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt SD, Curcio CA, Wang L, Li CM, McGwin G Jr., Medeiros NE, Philp NJ, Kimble JA, Read RW, 2011. Retinal pigment epithelial expression of complement regulator CD46 is altered early in the course of geographic atrophy. Exp Eye Res 93, 413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace KB, Sardao VA, Oliveira PJ, 2020. Mitochondrial Determinants of Doxorubicin-Induced Cardiomyopathy. Circ Res 126, 926–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan W, Zhu W, Wu Y, Long Y, Liu H, Wan W, Wan G, Yu J, 2021. Grape Seed Proanthocyanidin Extract Moderated Retinal Pigment Epithelium Cellular Senescence Through NAMPT/SIRT1/NLRP3 Pathway. J Inflamm Res 14, 3129–3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Sreekumar PG, Valluripalli V, Shi P, Wang J, Lin YA, Cui H, Kannan R, Hinton DR, MacKay JA, 2014. Protein polymer nanoparticles engineered as chaperones protect against apoptosis in human retinal pigment epithelial cells. J Control Release 191, 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MD, Pereira-Smith OM, Smith JR, 1989. Replicative senescence of human skin fibroblasts correlates with a loss of regulation and overexpression of collagenase activity. Exp Cell Res 184, 138–147. [DOI] [PubMed] [Google Scholar]
- Wiley CD, Campisi J, 2016. From Ancient Pathways to Aging Cells-Connecting Metabolism and Cellular Senescence. Cell Metab 23, 1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Zhao J, Sun X, 2016. Resistance to anti-VEGF therapy in neovascular age-related macular degeneration: a comprehensive review. Drug Des Devel Ther 10, 1857–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaung J, Jin M, Barron E, Spee C, Wawrousek EF, Kannan R, Hinton DR, 2007. alpha-Crystallin distribution in retinal pigment epithelium and effect of gene knockouts on sensitivity to oxidative stress. Mol Vis 13, 566–577. [PMC free article] [PubMed] [Google Scholar]
- Yousefzadeh MJ, Zhao J, Bukata C, Wade EA, McGowan SJ, Angelini LA, Bank MP, Gurkar AU, McGuckian CA, Calubag MF, Kato JI, Burd CE, Robbins PD, Niedernhofer LJ, 2020. Tissue specificity of senescent cell accumulation during physiologic and accelerated aging of mice. Aging Cell 19, e13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Ma J, Li J, Wang D, Wang Z, Wang S, 2020. Mitochondrial phosphatase PGAM5 modulates cellular senescence by regulating mitochondrial dynamics. Nat Commun 11, 2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Yasumura D, Li X, Matthes M, Lloyd M, Nielsen G, Ahern K, Snyder M, Bok D, Dunaief JL, LaVail MM, Vollrath D, 2011. mTOR-mediated dedifferentiation of the retinal pigment epithelium initiates photoreceptor degeneration in mice. J Clin Invest 121, 369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Kannan R, Spee C, Sreekumar PG, Dou G, Hinton DR, 2014. Protection of retina by alphaB crystallin in sodium iodate induced retinal degeneration. PLoS One 9, e98275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Sreekumar PG, Hinton DR, Kannan R, 2010. Expression and regulation of enzymes in the ceramide metabolic pathway in human retinal pigment epithelial cells and their relevance to retinal degeneration. Vision Res 50, 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Wu J, Spee C, Ryan SJ, Hinton DR, 2009. BMP4 mediates oxidative stress-induced retinal pigment epithelial cell senescence and is overexpressed in age-related macular degeneration. J Biol Chem 284, 9529–9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuge CC, Xu JY, Zhang J, Li W, Li P, Li Z, Chen L, Liu X, Shang P, Xu H, Lu Y, Wang F, Lu L, Xu GT, 2014. Fullerenol protects retinal pigment epithelial cells from oxidative stress-induced premature senescence via activating SIRT1. Invest Ophthalmol Vis Sci 55, 4628–4638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


