Abstract
Mast cells, sentinel immune cells, are most abundantly expressed in vascularized tissues that interface the external environment, such as the skin and ocular surface. Our previous reports have shown mast cells reside closely with vascular endothelial cells and mediate the pathogenic angiogenic response. However, the contribution of mast cells and their underlying mechanisms on lymphangiogenesis have not been fully deciphered. Using a murine model of inflammatory corneal angiogenesis, we observed adjacent migration of activated mast cells with new lymph vessel growth. Our in vitro co-culture assays demonstrate that mast cells with high expression of VEGF-D directly promote lymphatic endothelial cell tube formation and proliferation. Moreover, our loss-of-function approach, using mast cell knockout mice and cromolyn-mediated mast cell inhibition, showed mast cell deficiency suppresses the induction of inflammatory lymphangiogenesis and VEGF-D expression at the ocular surface following corneal tissue insult. Our findings suggest blockade of mast cells as a potential therapeutic strategy to inhibit pathological lymphangiogenesis.
Keywords: mast cells, lymphangiogenesis, ocular surface, cKit
Introduction
Mast cells, tissue-resident innate immune cells distributed throughout the body, are particularly abundant in tissues interfacing the external environment, such as the skin and mucosal surfaces [1, 2]. Anatomically localized in close proximity to vessels, mast cells have been identified in nearly all vascularized organs, including the heart, lungs, gastrointestinal tract, and the brain [3–6]. Despite its abundance in various tissues, mast cells have historically been viewed as effectors of allergy, and only recently has it gained attention for their role in physiological and pathological processes that are independent of the IgE-mediated allergic immune responses. Indeed, mast cells have been shown to modulate innate and adaptive immune responses by promoting the infiltration of inflammatory cells during injury and allosensitzation [7–9].
Lymphangiogenesis, characterized by the generation of new lymphatic vessels from pre-existing lymphatics, occurs during various physiological and pathological responses [10]. In particular, lymphangiogenesis has been shown to play a critical role in tumor metastasis and inflammatory diseases by facilitating immune cell trafficking [10, 11]. In fact, clinicopathological studies have shown a significant correlation of lymphangiogenesis and the production of lymphangiogenic factors with cancer progression [12]. Interestingly, studies have observed a similar trend in correlation with mast cell density and tumor severity [13–15]. However, the majority of the studies have been limited to mast cell-mediated angiogenesis or observational clinical findings [16]. Despite the unique characteristics of mast cells as major innate effector cells that reside closely to lymphatic vessels and release preformed and de novo synthesized inflammatory and vasoactive mediators, the functional implication and mechanism of mast cell-mediated lymphangiogenesis has not been fully deciphered.
Our recent report demonstrated that ocular surface mast cells promote corneal angiogenesis by secreting high levels of VEGF-A [17]. In this study, we conducted a series of experiments to determine whether mast cells mediate inflammatory lymphangiogenesis using a well-established model of inflammatory corneal lymphangiogenesis. Cornea, transparent in nature and devoid of blood and lymphatic vessels at a naïve state, serves as an ideal site for angiogenic studies [18]. Lymph vessels and ocular surface mast cells, restricted to the limbal area, grow and infiltrate towards the central cornea following an inflammatory insult allowing close observation of lymphangiogenic in context to lymphatic-oriented mast cells.
In this study, we evaluated the expression of lymphangiogenic factors, particularly VEGF-D, by mast cells and its interaction with endothelial cells to investigate whether mast cells promote lymphangiogenesis. To delineate the direct contribution of mast cells, we utilized mast cell-deficient cKitw-sh mice and pharmacological inhibition of mast cells in the setting of inflammatory lymphangiogenesis at the ocular surface.
Materials and Methods
Animals
Six- to eight-week-old BALB/c mice, fully congenic cKitw-sh mouse strain on a C57BL/6J genetic background (Stock No: 012861), and sex and age-matched C57BL/6J mice as controls were utilized for the described experiments (Jackson Laboratory, Bar Harbor, ME). Littermates were used for each set of experiments. cKitw-sh mice were confirmed for their deficiency in mast cells at the ocular surface and in the peritoneum. cKitw-sh mice, unlike other mast cell-deficient strains (cKitw-v) which exhibit basal neutropenia and macrocytic anemia, have a comparable generation of total CD45+ cells and myeloid cells in the bone marrow compared to wild type mice [9, 19]. The mice were housed in the Schepens Eye Research Institute animal vivarium and treated according to the ARVO guidelines for Use of Animals in Ophthalmic and Vision Research. All procedures were approved by the Animal Care and Use Committee of Schepens Eye Research Institute (2020N000177).
Corneal lymphangiogenesis model
Corneal lymphangiogenesis was induced by placing a single intrastromal suture on anesthetized mice, as previously described [20]. Suture placement induces growth of new lymph and blood vessels and tissue inflammation and has been used as a model of inflammatory corneal angiogenesis [21]. Briefly, a single figure-of-eight suture was intrastromally placed on the nasal side of the cornea, 1.0 mm from the limbal area using 11.0 nylon sutures (MANI, Tochigi, Japan) (Fig. 1a). Following suture placement, a single line of triple antibiotic ointment (Vetropolycin) was applied topically. To subside suture-induced pain, buprenorphine was administered subcutaneously. Ocular surface tear wash (5 μL/wash) was collected at 0, 1, 3, and 6h following suture placement to measure ocular surface mast cell activation. Mice were assessed using a slit lamp biomicroscope on alternating days to monitor angiogenesis. Mice were euthanized on day 7 when new blood vessels reached the suture and their corneas (including the corneal limbus and conjunctiva) were harvested for further analysis.
Fig. 1. Inflammatory model of corneal lymphangiogenesis and activation of mast cells.
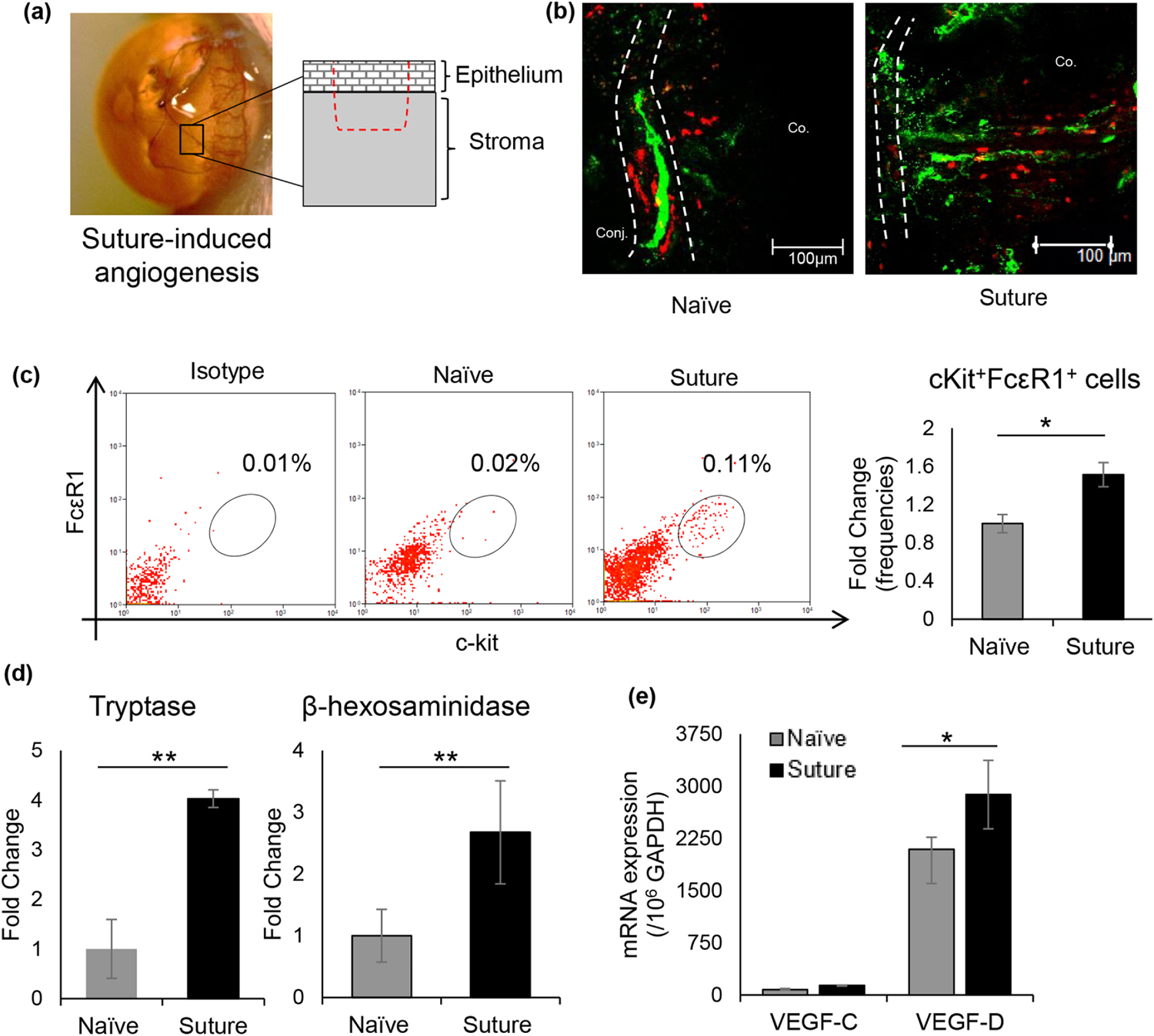
(a) Schematic drawing of the inflammatory model of corneal angiogenesis illustrating the depth of the intrastromal suture placed 1.0 mm from the limbal vessel. (b) Representative immunohistochemistry micrographs of mast cells infiltration (Avidin – Texas red) and lymph vessel growth (LYVE-1 – AlexaFluor488) into the cornea on day 7 post-intrastromal suture placement (Scale bar, 100 μm). (c) Representative flow cytometric dot plots (left) and cumulative bar chart (right) showing frequencies of cKit+FcεR1+ mast cells (gated on CD45+ cells; fold change relative to naïve controls) at the ocular surface following suture placement. Corneas were harvested 6 hours following suture placement, and single-cell suspensions were prepared for analysis. (d) Bar chart showing fold change in levels of mast cell activation markers tryptase (left) and β-hexosaminidase (right) in ocular surface tear wash collected at 0h, 1h, 3h, and 6h following suture placement (5 μl/wash). (e) Bar chart depicting expression of VEGF-C and VEGF-D in the cornea 6 hours post-suture placement compared to naive corneas, as quantified by real-time PCR. Representative data from three independent experiments are shown, and each experiment consisted of 4 animals per group. Co., Cornea; Conj., Conjunctiva. Data are represented as mean ± SD (error bar). t-test; *p<0.05, **p < 0.01.
Mast cell inhibitor administration
Three microliters of 2% sodium cromolyn in PBS (Sigma-Aldrich Corp., St. Louis, MO, USA) or control PBS were administered topically to sutured corneas at seven-time points on the day of suture placement (−3, −1, 0, and 1, 3, 6 and 9h postoperatively). Thereafter, topical treatment was administered 6 times a day (every 2 hours) for seven days.
Corneal tissue isolation and digestion
Single-cell suspensions were prepared from corneas, as previously described [22]. Briefly, corneas were digested in RPMI media (Lonza, Walkersville, MD, USA) containing 4 mg/mL collagenase type IV (Sigma-Aldrich, St. Louis, MO) and 2 mg/mL DNase I (Roche, Basel, Switzerland) for 45 minutes at 37°C. Following digestion, cells were filtered through a 70-μm cell strainer.
Cell culture assays
LAD2 cells, derived from human mat cell leukemia [23], were kindly supplied by Drs. Kirshenbaum and Metcalfe (NIAID, Bethesda, Maryland). LAD2, closely resembling CD34+-derived primary human mast cells, were cultured in the presence of human stem cell factor (SCF; 100 ng/ml) [23]. The culture media was changed once a week for 3 weeks after thawing prior to utilizing them in the experiments. LAD2 cells were primed by incubating the cells in 100 ng/ml of biotinylated human myeloma IgE overnight at 37°C. Thereafter, LAD2 cells were stimulated with 10 ng/ml of IL-33 (Biolegend, CA, USA) for 3 hours at 37°C. The cells were harvested for co-culture assays and to measure VEGF-C and VEGF -D levels using methods described below.
Primary human dermal lymphatic microvascular endothelial cells (LECs) were cultured in endothelial cell basal medium-2 (EGM-2MV media) supplemented with growth factors [5% FBS, VEGF, FGF, EGF, IGF] at 37°C (Lonza, USA).
Tube formation assay
A Matrigel assay was set up in a flat-bottom 96-well plate in triplicates as previously described [17]. In brief, matrigel basement membrane (Millipore, MA, USA) was plated into each well (50 μl/well) and incubated for 1 hour at 37°C until adequate polymerization. Next, 5×103 LECs were cultured in basal media alone (negative control), with supplemented growth factors [5% FBS, VEGF, FGF, EGF, IGF] (positive control) or with 5×103 LAD2 cells (stimulated with 10 ng/ml of IL-33 for 3hrs) (1:1 ratio; 200 μl/well) on the matrigel surface and was incubated at 37°C. Tube formation was observed for 12 hours, and micrographs of co-cultures were captured using an inverted brightfield microscope (Leica DMi1, USA).
Proliferation assay
LEC proliferation was measured using the BrdU proliferation kit. Briefly, in a flat-bottom 96-well plate 5×103 LECs were cultured alone in EBM-2 basal medium (Lonza, USA) (negative control), with growth factors [5% FBS, VEGF, FGF, EGF, IGF] (positive control) or with 5×103 LAD2 cells (stimulated with 10 ng/ml of IL-33 for 3hrs) for 12 hours at 37°C. BrdU label was added after 12 hours and cell cultures were incubated for an extra 12 hours at 37°C. Subsequently, the culture plate was processed according to the manufacturer’s protocol. A SpectraMax Plus 384 Microplate Reader (Molecular Devices, San Jose, CA, USA) was used to measure absorbance at 450/550 nm.
Real-Time PCR
Corneas were harvested and lysed using the freeze-thaw method. In brief, corneas were digested in Trizol and alternatively placed in dry ice and 37°C water bath. Corneal tissue was mechanically lysed using a pellet pestle (Life Sciences) between every cycle. A total of 7 freeze-thaw cycles were completed before preceding to RNA isolation. RNeasy Micro Kits (Qiagen, Valencia, CA, USA) were used to isolate total RNA. Quantitative real-time PCR was conducted using Taqman Universal PCR Mastermix and preformulated primers for murine and human Vegfc (Mm00437313_m1; Hs00173626_m1), Vegfd (Mm00438965_m1; Hs01128659_m1), Vegfr3 (Mm00433337_m1), and glyceraldehyde-3-phosphate dehydrogenase (Gapdh, Mm99999915_gl; Hs02786624_g1) in a Mastercycler Realplex 2 (Eppendorf, Hamburg, Germany). The comparative threshold cycle method was used to analyze the results, which were normalized to Gapdh as an internal control.
Flow Cytometry
Single-cell suspensions were stained with fluorochrome-conjugated anti-CD45 (30-F11; Rat IgG2b k),), anti-CD11b (M1/70; Rat IgG2b k), anti-cKit (2B8; Rat IgG2b k), and anti-FcεR1 (MAR-1; Armenian Hamster IgG) antibodies or with their respective isotype controls. All antibodies and isotype controls were purchased from Biolegend (San Diego, CA, USA). LSR II flow cytometer (BD Biosciences, San Jose, CA, USA) and Summit software (Dako Colorado, Inc., Fort Collins, CO, USA) were used to acquire and analyze the stained cells.
Tryptase assays
Mast Cell Degranulation Assay Kits (Sigma-Aldrich) were used to quantify levels of tryptase. Ocular surface tear wash were incubated with 0.1 mg/mL tosyl-gly-pro-lys-pNA (substrate) for 3 hours at 37°C. A SpectraMax Plus 384 Microplate Reader (Molecular Devices, San Jose, CA, USA) was used to quantify cleaved chromophore p-nitroaniline (pNA) at 405 nm.
β-hexosaminidase assays
Levels of β-hexosaminidase enzyme were quantified using β-n-acetylglucosaminidase assay kits (Sigma-Aldrich). The kit measures the level of 4-Nitrophenyl N-acetyl-β-d-glucosaminide (NP-GlcNAc) hydrolysis. Ocular surface wash was incubated with 0.1 mg/mL NP-GlcNAc (substrate) for 1 hour at 37°C and was stopped with with 5 mg/mL sodium carbonate. Absorbance at 405 nm was measured using SpectraMax Plus 384 Microplate Reader (Molecular Devices, San Jose, CA, USA). β-hexosaminidase levels were estimated using the formula: U/mL = (A405sample – A405blank) × 0.05 × 0.3 × DF/A405 standard × time × volume of sample in milliliters.
Immunohistochemistry
Corneas with limbus were harvested and immunostained as previously described [24]. Briefly, cornea stroma was separated from the epithelial layer by incubating the cornea in EDTA for 30 minutes at 37°C and thereafter was fixed using 4% paraformaldehyde. Tissues were incubated at 4°C with LYVE-1 (Biolegend) overnight and stained with AlexaFluor488-conjugated or Rhodamine-conjugated secondary antibodies for 2 hours at room temperature. To stain for mast cells, the harvested cornea was fixed using 4% paraformaldehyde and was subsequently incubated at 4°C with Texas red-conjugated Avidin (ThermoFisher), which specifically binds to mast cells, for 6 hours [25]. Stained corneas were whole-mounted on slides using VECTASHIELD mounting medium (Vector Laboratories) and visualized using a confocal microscope (Leica TCS-SP5; Buffalo Grove, IL, USA). The area covered by lymph vessel (LYVE1+) and the number of mast cells (Avidin+) were calculated using ImageJ 1.52v software.
Statistical analysis
Unpaired two-tailed Student t-tests were used to compare means between two groups. The significance level was set at p < 0.05. Data are presented as the mean ± standard deviation. The results shown are representative of at least three independent experiments.
Results
Activation of mast cells and lymphangiogenesis following corneal suture placement
To assess the distribution and activation of mast cells at the ocular surface in conjunction with lymph vessels, we employed a model of inflammatory corneal angiogenesis by placing a single figure-of-eight intrastromal suture 1 mm from the limbus (Fig. 1a). Corneas were harvested from naïve and suture-placed Balb/c mice and immunostained with fluorochrome-conjugated avidin and LYVE-1. Avidin staining, which specifically binds to heparin granules of mast cells [25], showed mast cells juxtapose along the peripheral lymph vessels (Fig. 1b). Furthermore, intrastromal suture placement induced infiltration of mast cells into the central cornea, alongside new lymph vessel growth (Fig. 1b). To confirm the centripetal infiltration of mast cells into the cornea, which is devoid of mast cells in naïve state, corneas were harvested 6 hours post-suture placement, and single-cell suspensions were made for flow cytometry analysis. Corneal cells were stained with fluorochrome-conjugated CD45, CD11b, c-Kit, and FCεR1 monoclonal antibodies. As shown in immunohistochemistry, increased frequencies of cKit+ FCεR1+ mast cells were observed at the ocular surface following suture placement (Fig. 1c). Increased levels of mast cell activation markers, tryptase and β-hexosaminidase [26], in the ocular tear wash collected within 6 hours of suture placement further confirmed upregulated activation of ocular surface mast cells following suture placement (Fig. 1d). Next, to ascertain the underlying pathway of our inflammatory model lymphangiogenesis, we harvested the corneas at 6 hours post-suture placement and evaluated the expression of vascular endothelial growth factor C and D (VEGF-C, VEGF-D) using real-time PCR. A significant increase in VEGF-D expression is observed following suture placement, compared to naïve controls (Fig. 1e). However, no significant difference in expression of VEGF-C is observed following suture placement. (Fig. 1e). Our data demonstrate concurrent infiltration of activated mast cells with lymphangiogenesis, suggesting a potential role of mast cells in inflammatory lymph vessel growth.
Mast cells promote lymphatic endothelial cell proliferation and tube formation
Given the increased frequencies and activation of ocular mast cells jointly with lymphangiogenesis, we sought to determine whether mast cells directly promote lymph vessel growth. Human primary lymphatic endothelial cells (LECs) were cultured in basal media alone, with endothelial growth factors (5% FBS, VEGF, FGF, EGF, IGF), or with LAD2 human mast cells (in basal media only) [23], for 24 hours. LECs were cultured on a gel matrix, and tube formation was observed using brightfield microscopy (Fig. 1a). LECs cultured in basal media alone did not show any tube formation; however, LECs co-cultured with mast cells exhibited significantly higher tube formation, as assessed by the multiple components of tube formation quantified using the ‘Angiogenesis Analyzer’ plugin in ImageJ 1.52s software (Fig. 1b). Moreover, LEC-mast cell co-cultures resulted in a comparable number of branches, branch length and node formation as LECs cultured with known growth factors (Fig 1b). To investigate the mechanism of mast cell-induced lymphatic endothelial tube formation, we evaluated whether mast cells promote proliferation of LECs. Our data demonstrate a significant increase in proliferation of LECs co-cultured with mast cells, comparable to LECs cultured with growth factors (Fig. 2c). Given the known pro-angiogenic function of VEGF-C and -D [27, 28], we specifically assessed the expression of these factors by mast cells purified from the cultures. Of note, mast cells selectively showed high expression of VEGF-D, similar to the observation of increased VEGF-D expression in the inflammatory model of corneal lymphangiogenesis (Fig. 1d). Taken together, these data suggest mast cells selectively secrete high levels of pro-lymphangiogenic factor VEGF-D and directly promote proliferation and tube formation of lymphatic endothelial cells.
Fig. 2. Mast cells express high levels of VEGF-D and promote proliferation and tube formation of lymphatic endothelial cells.
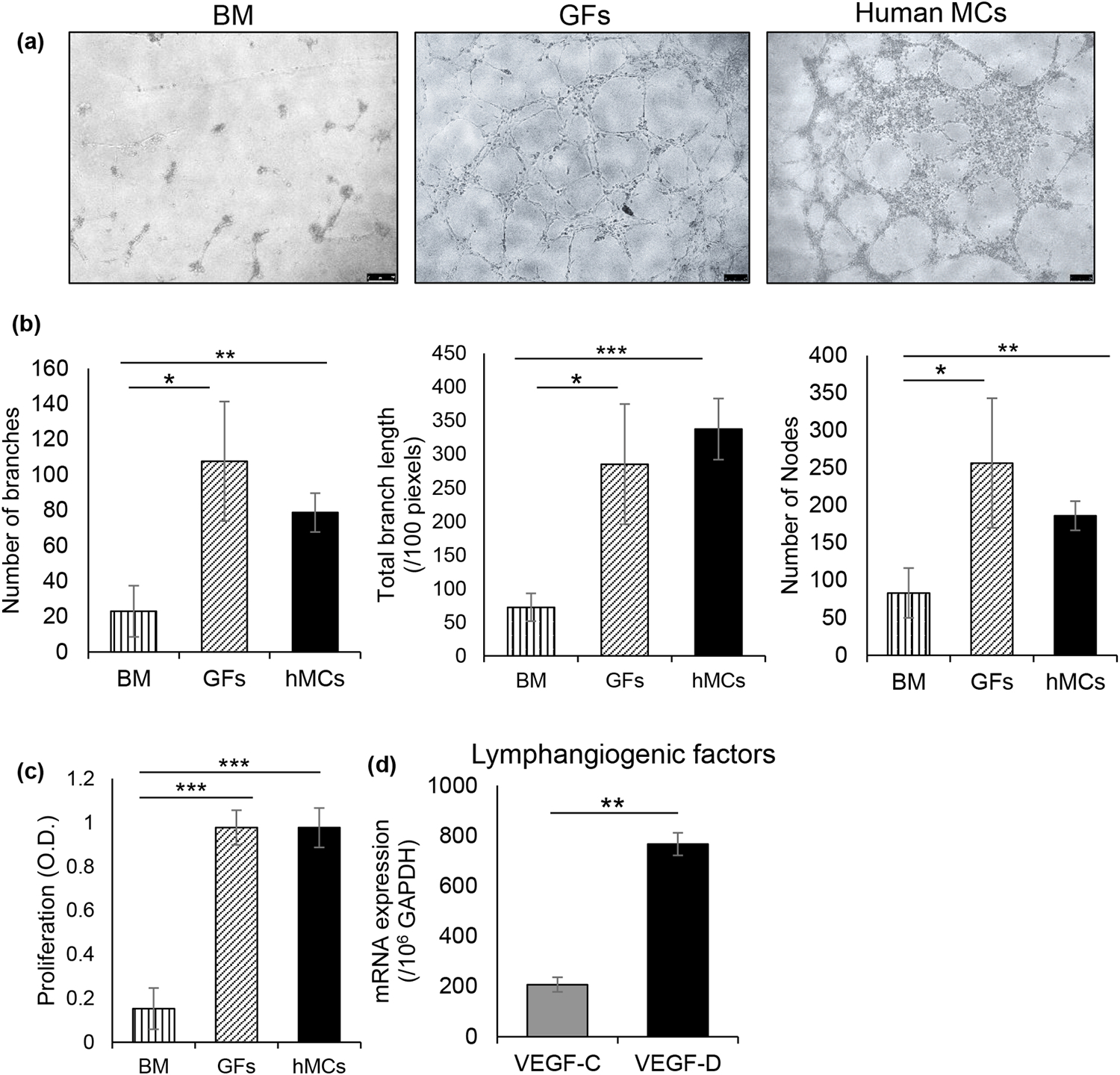
Primary human lymphatic endothelial cells were cultured alone in basal media, with growth factor supplements (GFs) or with IgE coated human mast cells (hMCs) at 1:10 ratio (LECs:hMCs) for 24 hours at 37°C. (a) Representative brightfield micrographs showing tube formation of lymphatic endothelial cells of indicated co-cultures. (b) Bar chart showing measures of tube formation (number of branches, total branch length, number of nodes) in the indicated co-cultures at 8 hours. Tube formation was assessed using the ‘Angiogenesis analyzer’ plugin in ImageJ 1.52s software. (c) Cumulative bar chart showing proliferation of lymphatic endothelial cells in the indicated co-cultures, measured using BrdU incorporation assays. (d) Bar chart depicting expression of VEGF-C and VEGF-D by mast cells, as quantified by real-time PCR. Representative data from four independent experiments are shown. Data are represented as mean ± SD (error bar). t-test; *p<0.05, **p < 0.01, ***p < 0.001. BM, basal media; GFs, growth factors; hMCs, human mast cells.
Mast cell deficiency abridges inflammatory corneal lymphangiogenesis
To confirm the direct role of mast cells in facilitating lymphatic endothelial cell proliferation and tube formation, we employed the model of inflammatory corneal lymphangiogenesis in mast cell-deficient (cKitw-sh) and its respective wild type control (C57BL/6) mice. To attest their deficiency in ocular surface mast cells, naïve corneas of wild-type and cKitw-sh mice were harvested and immunostained with fluorochrome-conjugated avidin. cKitw-sh mice showed no mast cells at the ocular surface (Fig. 3a). Tear wash collected within 6 hours post-suture placement showed significant increase in mast cell activation marker tryptase in wild-type mice, with no significant increase in cKitw-sh, further verifying the absence of ocular surface mast cells in cKitw-sh mice. To assess the underlying pathway of lymphangiogenesis in the absence of mast cell deficiency, expression of VEGF-D in corneas harvested on day 7 post-suture placement was evaluated using real-time PCR. No significant change in VEGF-D was observed in cKitw-sh mice versus the significant upregulation of VEGF-D expression in wild-type mice, suggesting VEGF-D expression at the ocular surface following suture placement is largely induced by mast cells (Fig. 3c). Next, to observe the difference in lymph vessel growth in the absence of mast cells, corneas were harvested and immunostained with fluorochrome-conjugated LYVE-1. Area of lymph vessel growth was quantified using ImageJ 1.52s software. cKitw-sh mice showed significantly less lymphangiogenesis compared to wild-type mice (Fig. 1d). Furthermore, harvested corneas were lysed, and the expression of lymphatic endothelial cell-specific VEGFR3 [29] was evaluated using real-time PCR. In line with the immunohistochemistry observation, significantly less expression of VEGFR3 was observed in cKitw-sh mice compared with wild-type mice. In fact, only a minimal increase in VEGFR3 expression was observed following suture placement, relative to its naïve control, in cKitw-sh mice. Collectively, our data suggest mast cells play a critical role in inflammatory corneal lymphangiogenesis by regulating the VEGF-D-VEGFR3 axis.
Fig. 3. Reduced lymphangiogenesis following suture placement in cKitw-sh/w-sh mice.
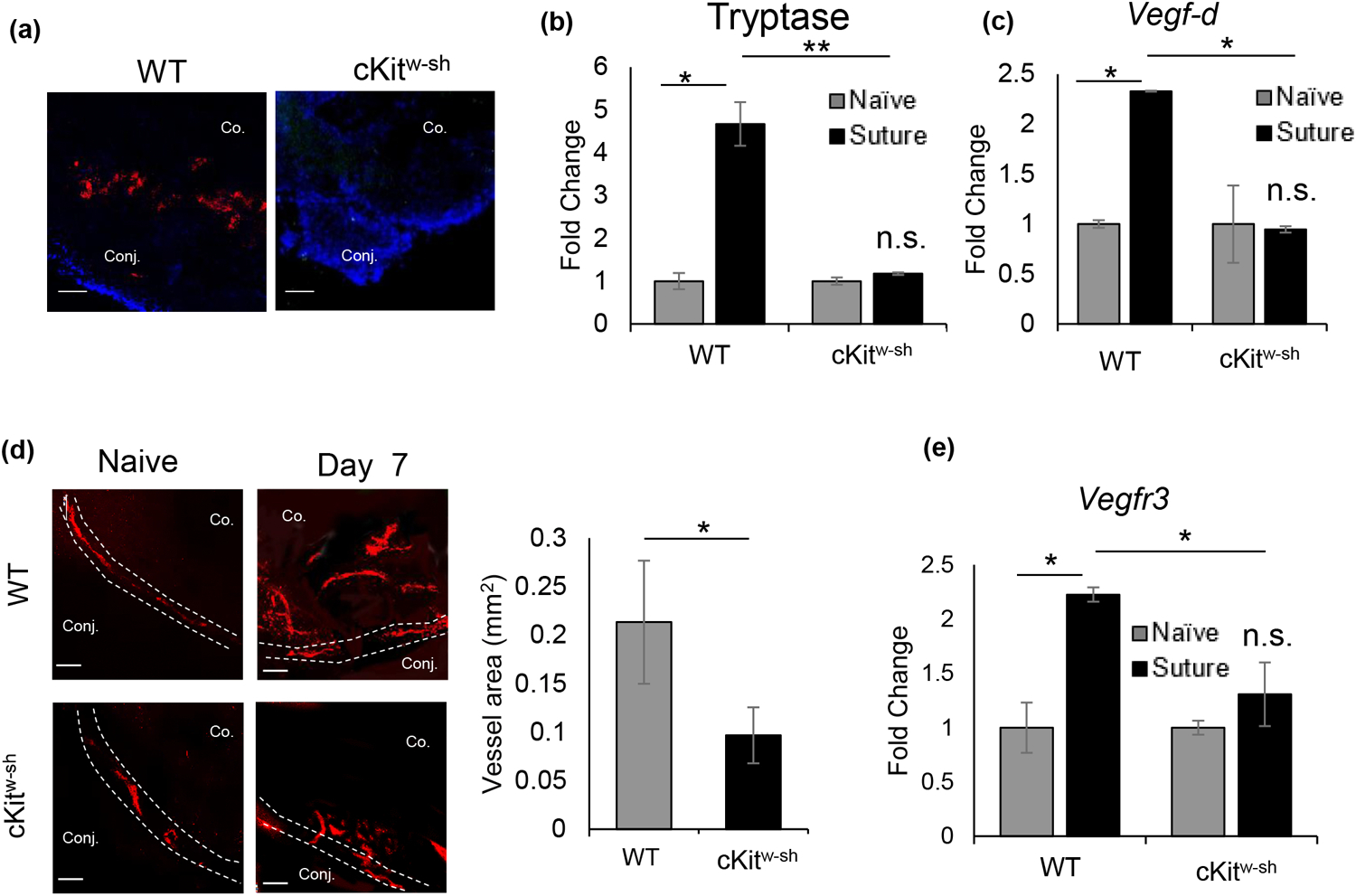
(a) Representative immunohistochemistry micrographs of mast cell expression (avidin-Texas red) at the ocular surface of wild type (C57BL/6) and cKitw-sh mice. (b) Bar chart showing levels of mast cell activation marker tryptase (fold change) at ocular surface tear wash collected at 0h, 1h, 3h, and 6h following suture placement (5 μl/wash) in wild-type and cKitw-sh mice. (c) Bar chart depicting expression of VEGF-D, relative to naïve state, in the cornea harvested on day 7 post-suture placement in wild type and cKitw-sh mice, as quantified by real-time PCR. (d) Representative immunohistochemistry micrographs (left) of naïve corneas and corneas harvested on day 7 post-suture placement and immunostained with LYVE-1 (Rhodamine) (left; Scale bar, 100 μm). Cumulative bar chart (right) showing lymph vessel area in corneas of wild-type and cKitw-sh mice, as quantified using ImageJ 1.52s software. (e) Bar chart showing VEGFR3 expression, relative to its naïve baseline, in corneas harvested on day 7 post-suture placement in wild type and cKitw-sh mice. Representative data from two independent experiments are shown, and each experiment consisted of n = 3 animals/group. Data are represented as mean ± SD (error bar). t-test; *p < 0.05, n.s.; not significant. Co., Cornea; Conj., Conjunctiva.
Pharmacological blockade of mast cell activation suppresses inflammatory corneal lymphangiogenesis
Finally, to assess whether pharmacological intervention to inhibit mast cell activation prevents corneal lymphangiogenesis, sutured corneas were treated with cromolyn, a known mast cell inhibitor [30]. Corneas were treated topically with 2% cromolyn six times a day for seven days, as outlined in the experimental design (Fig. 4a). PBS served as a control. To assess the efficacy of topical cromolyn treatment, tryptase was measured in tear wash collected within 6 hours following intrastromal suture placement. Cromolyn treatment significantly suppressed the upregulation of tryptase at the ocular surface post-suture placement (Fig. 4b). Next, to evaluate the effect of pharmacological inhibition of mast cells on pro-lymphangiogenic factor at the ocular surface, expression of VEGF-D was quantified in corneas harvested post-suture placement using real-time PCR. Significantly less expression of VEGF-D was observed in cromolyn-treated corneas compared to PBS-treated corneas (Fig. 4c). To further confirm the effect of mast cell inhibition in lymphangiogenesis, corneas were harvested on day 7 post-suture for immunohistochemistry analysis. Cromolyn-treated corneas showed significantly smaller area of lymph vessel growth compared to PBS-treated controls as quantified by the ImageJ 1.52s software (Fig. 4d). Moreover, cromolyn treatment resulted in significantly less expression of VEGFR3 in the corneas harvested on day 7 post-suture, further confirming the suppressive effect of cromolyn in corneal lymphangiogenesis. Our data show that pharmacological blockade of mast cell activation sufficiently prevents inflammatory corneal lymphangiogenesis.
Fig. 4. In vivo blockade of mast cell activation suppresses inflammatory corneal lymphangiogenesis.
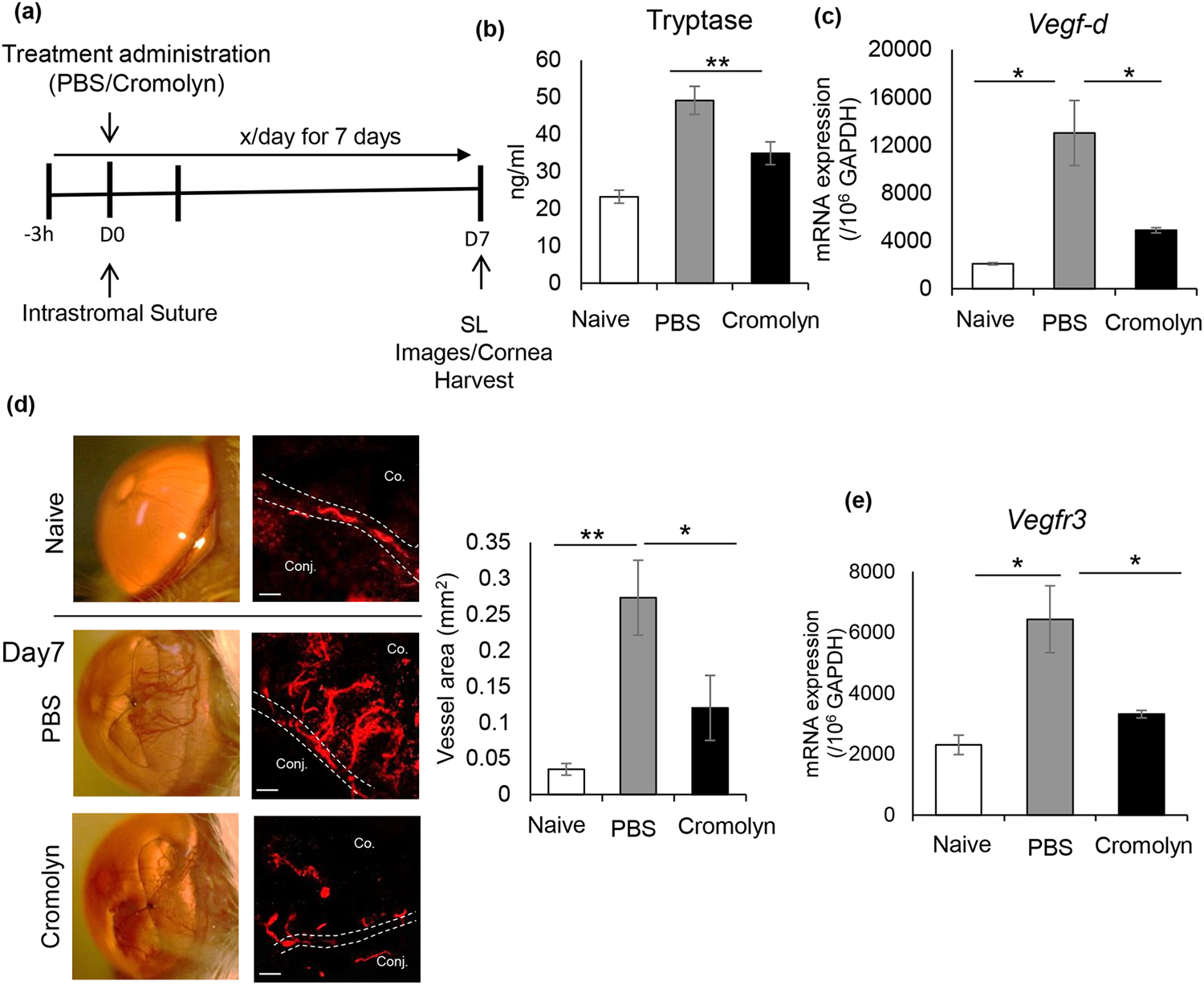
(a) Schematic experimental design showing the time points of topical treatment administration of PBS or 2% cromolyn (3 μl/treatment) to Balb/c mice. Topical treatment was administered 3 h prior to suture placement and subsequently treated 6 times a day for 7 days. Corneas were harvested for immunohistochemistry and mRNA analysis on day 7 post-suture placement. (b) Bar chart showing levels of mast cell activation marker tryptase in ocular surface tear wash collected at 0h, 1h, 3h, and 6h following suture placement (5μl/wash) in PBS and cromolyn treated corneas. (c) Bar chart showing expression levels of VEGF-D in PBS and cromolyn treated-corneas harvested on day 7 post-suture placement, compared to naive controls, as quantified by real-time PCR. (d) Corneas were harvested on day 7 when blood vessels reached the suture, as demonstrated by the slit-lamp micrographs (left). Representative immunohistochemistry micrographs of PBS and cromolyn-treated corneas immunostained with LYVE-1 (Rhodamine), compared to the naïve cornea (Scale bar, 100 μm). Cumulative bar chart (right) showing lymph vessel area in PBS and cromolyn-treated corneas, compared to naïve controls, as quantified using ImageJ 1.52s software. (e) Bar chart depicting expression of VEGFR3 in PBS and cromolyn-treated corneas harvested on day 7 post-suture placement, compared to naïve controls. Representative data from three independent experiments are shown, and each experiment consisted of n = 4 animals/group. Data are represented as mean ± SD (error bar). t-test; *p < 0.05, **p < 0.01. Co., Cornea; Conj., Conjunctiva.
Discussion
Mast cells, as sentinel innate immune cells, play a critical role in early immune response primarily by secreting various pro-inflammatory factors [31]. Previous work by our laboratory and others has demonstrated that mast cells secrete high levels of VEGF-A and promote angiogenesis [17, 32], yet their direct effect on lymphangiogenesis and its underlying pathway have not been explored. Here, using a murine cornea model of inflammatory lymphangiogenesis in genetically modified mast cell knockout mice, and human mast cells, we show that mast cells (i) primarily express pro-lymphangiogenic factor VEGF-D, (ii) directly induce lymphatic endothelial cell proliferation and tube formation, and (iii) promote inflammatory lymphangiogenesis in vivo.
Historically, mast cells have mainly been studied for their function in mediating the allergic response and parasitic infection [33]. Recent research, however, has demonstrated that mast cells exhibit a wide range of immunostimulatory functions that mediate both innate and adaptative immunity, as well as chronic inflammatory conditions, including vascular disease and cancer [34, 35]. Clinical studies have observed a positive correlation between the number of mast cells and the extent of angiogenesis and lymphangiogenesis in various carcinomas [36, 37]. However, research on mast cells in the regulation of lymphatic vessels has largely been limited to its effect on lymphatic permeability and contractility or the tumor microenvironment [38]. In the current study, we demonstrate that mast cells directly promote pathological lymphangiogenesis at the ocular surface, expanding our current understanding of the roles of mast cells in the regulation of lymphatic pathophysiology.
Lymphangiogenesis by facilitating immune cell trafficking plays a critical role in various inflammatory disorders [39]. In the eye, a study has demonstrated that chronic allergic microenvironment results in pathogenic corneal lymphangiogenesis, as evidenced by increased expression of LYVE-1 following ovalbumin inoculation [40]. We and others have observed that mast cells are localized in close proximity to lymph vessels [26]. This concurrence is observed along new lymph vessels as well, as demonstrated by the co-infiltration of mast cells into the central cornea alongside the inflammatory lymph vessel growth. Furthermore, we observed significant upregulation of mast cell activation markers, tryptase, and ß-hexosaminidase, following suture-placement, suggesting a close association with mast cell activation and corneal lymphangiogenesis. We confirmed this association by utilizing a well-established murine model of inflammatory lymphangiogenesis, which demonstrated significantly less lymph vessel growth in mast cell-deficient cKitw-sh mice after induction of inflammatory lymphangiogenesis.
VEGF-C and VEGF-D, members of the VEGF family, are known to promote lymphangiogenesis by binding to their specific receptors VEGFR-2 and VEGFR-3, respectively [40, 41]. Although they exhibit structural homology, VEGF-C and D differ in their regulatory mechanisms, expression pattern, and receptor binding [41]. Several studies have identified various vasoactive and inflammatory mediators known to regulate lymphatic physiology [32, 40]; however, few studies have delineated the differential expression and role of lymphangiogenic factors by mast cells. Interestingly, our data suggest that mast cells specifically secrete higher levels of VEGF-D compared to VEGF-C. Mast cells co-cultured with human lymphatic endothelial cells resulted in more tube formation and proliferation of endothelial cells, similar to a previous finding performed with murine mast cell and endothelial cell lines [44]. Our findings demonstrate that mast cells, secreting high levels of VEGF-D, directly promote proliferation and tube formation of lymphatic endothelial cells. In addition, we observe that mast cell-deficient cKitw-sh mice show no upregulation of VEGF-D expression following suture placement compared to wild-type control mice, denoting mast cells as a primary source of ocular surface VEGF-D during inflammatory lymphangiogenesis. These findings, in conjunction with the significantly less inflammatory lymph vessel growth in cKitw-sh mice, suggest that mast cells directly promote inflammatory corneal lymphangiogenesis.
Finally, to assess whether pharmacological inhibition of ocular surface mast cell activation prevents inflammatory lymphangiogenesis, suture corneas were topically treated with 2% cromolyn. Cromolyn sodium, widely used in the clinic for allergic conjunctivitis [43], inhibits mast cell function by preventing degranulation through stabilizing the cell membrane [44]. The topical treatment effectively curbs mast cell activation, as demonstrated by suppression of tryptase levels at the ocular surface following treatment induction. Moreover, our data show cromolyn treatment reduces upregulation of VEGF-D expression and subsequent inflammatory lymphangiogenesis, replicating our observation in cKitw-sh mice.
Conclusion
In conclusion, our data demonstrate that mast cells, which express higher levels of VEGF-D, directly promote inflammatory lymph vessel growth. Moreover, inhibition of mast cell function via genetic deletion and topical application of cromolyn prevents this inflammatory response. These findings provide novel insights into ocular surface mast cells as a potential therapeutic target for regulating inflammatory lymphangiogenesis.
Highlights.
Activation and infiltration of mast cells into the cornea during inflammatory lymphangiogenesis
Mast cells directly promote lymphatic endothelial cell proliferation and tube formation
Genetic deletion of mast cells abrogates pathological corneal lymphangiogenesis
Pharmacological blockade of mast cell activation suppresses inflammatory lymphangiogenesis
Disclosure/conflicts of interest:
The authors have no financial conflict of interest. This work was supported by the National Institutes of Health grants R01EY029727 (S.K.C) and P30EY003790.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability statement:
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request
References
- 1.Krystel-Whittemore M, Kn D, Wood JG (2016) Mast Cell: A Multi-Functional Master Cell. Mast Cell: A Multi-Functional Master Cell Front Immunol 6:620. 10.3389/fimmu.2015.00620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janssens AS, Heide R, den Hollander JC, et al. (2005) Mast cell distribution in normal adult skin. J Clin Pathol 58:285–289. 10.1136/jcp.2004.017210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta K, Conti P, degli Studi UG, et al. (2019) Mast Cells in Stress, Pain, Blood-Brain Barrier, Neuroinflammation and Alzheimer’s Disease. 10.3389/fncel.2019.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramsay DB, Stephen S, Borum M, et al. (2010) Mast Cells in Gastrointestinal Disease [PMC free article] [PubMed]
- 5.Cruse G, Bradding P Mast cells in airway diseases and interstitial lung disease. 10.1016/j.ejphar.2015.04.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levick SP, Melé Ndez GC, Plante E, et al. Cardiac mast cells: the centrepiece in adverse myocardial remodelling. 10.1093/cvr/cvq272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahu SK, Mittal SK, Foulsham W, et al. (2018) Mast Cells Initiate the Recruitment of Neutrophils Following Ocular Surface Injury. Investigative Ophthalmology & Visual Science 59:1732–1740. 10.1167/IOVS.17-23398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li M, Mittal SK, Foulsham W, et al. (2019) Mast cells contribute to the induction of ocular mucosal alloimmunity. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 19:662–673. 10.1111/AJT.15084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elbasiony E, Mittal SK, Foulsham W, et al. (2020) Epithelium-derived IL-33 activates mast cells to initiate neutrophil recruitment following corneal injury. The Ocular Surface 18:633–640. 10.1016/J.JTOS.2020.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stacker SA, Williams SP, Karnezis T, et al. (2014) Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nature Reviews Cancer 2014 14:3 14:159–172. 10.1038/nrc3677 [DOI] [PubMed] [Google Scholar]
- 11.Tammela T, Alitalo K (2010) Lymphangiogenesis: Molecular Mechanisms and Future Promise. Cell 140:460–476. 10.1016/J.CELL.2010.01.045 [DOI] [PubMed] [Google Scholar]
- 12.Christiansen A, Detmar M (2011) Lymphangiogenesis and Cancer. Genes & Cancer 2:1146. 10.1177/1947601911423028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marichal T, Tsai M, Galli S (2013) Mast cells: potential positive and negative roles in tumor biology. Cancer immunology research 1:269–279. 10.1158/2326-6066.CIR-13-0119 [DOI] [PubMed] [Google Scholar]
- 14.Ullah E, Nagi A, Ashraf M (2013) Angiogenesis and mast cell density as predictors of patient survival in squamous cell carcinoma of lung. Journal of cancer research and therapeutics 9:701–705. 10.4103/0973-1482.126487 [DOI] [PubMed] [Google Scholar]
- 15.Marinaccio C, Ingravallo G, Gaudio F, et al. (2014) Microvascular density, CD68 and tryptase expression in human diffuse large B-cell lymphoma. Leukemia research 38:1374–1377. 10.1016/J.LEUKRES.2014.09.007 [DOI] [PubMed] [Google Scholar]
- 16.Ribatti D, Crivellato E (2012) Mast cells, angiogenesis, and tumour growth. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1822:2–8. 10.1016/J.BBADIS.2010.11.010 [DOI] [PubMed] [Google Scholar]
- 17.Cho WK, Mittal SK, Elbasiony E, Chauhan SK (2020) Activation of ocular surface mast cells promotes corneal neovascularization. The Ocular Surface 18:857–864. 10.1016/J.JTOS.2020.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corneal Lymphatics: Role in Ocular Inflammation as Inducer and Responder of Adaptive Immunity - PubMed. https://pubmed.ncbi.nlm.nih.gov/25580370/. Accessed 5 Aug 2021 [DOI] [PMC free article] [PubMed]
- 19.Grimbaldeston MA, Chen C-C, Piliponsky AM, et al. (2005) Mast Cell-Deficient W-sash c-kit Mutant KitW-sh/W-sh Mice as a Model for Investigating Mast Cell Biology in Vivo. The American Journal of Pathology 167:835. 10.1016/S0002-9440(10)62055-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho WK, Mittal SK, Elbasiony E, Chauhan SK (2021) Spatial Distribution of Mast Cells Regulates Asymmetrical Angiogenesis at the Ocular Surface. American Journal of Pathology 191:1108–1117. 10.1016/J.AJPATH.2021.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giacomini C, Ferrari G, Bignami F, Rama P (2014) Alkali burn versus suture-induced corneal neovascularization in C57BL/6 mice: an overview of two common animal models of corneal neovascularization. Experimental eye research 121:1–4. 10.1016/J.EXER.2014.02.005 [DOI] [PubMed] [Google Scholar]
- 22.Saban DR, Bock F, Chauhan SK, et al. (2010) Thrombospondin-1 Derived from APCs Regulates Their Capacity for Allosensitization. The Journal of Immunology 185:4691–4697. 10.4049/JIMMUNOL.1001133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.K AS, A C, W Y, et al. (2003) Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcepsilonRI or FcgammaRI. Leukemia research 27:677–682. 10.1016/S0145-2126(02)00343-0 [DOI] [PubMed] [Google Scholar]
- 24.Chauhan SK, Jin Y, Goyal S, et al. (2011) A novel pro-lymphangiogenic function for Th17/IL-17. Blood 118:4630. 10.1182/BLOOD-2011-01-332049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tharp MD, Seelig LL, Tigelaar RE, Bergstresser PR (1985) Conjugated Avidin Binds to Mast Cell Granules1. The Journal of Histochemistry and Cytochemistry Inc 33:22–32 [DOI] [PubMed] [Google Scholar]
- 26.Moon TC, Befus AD, Kulka M (2014) Mast Cell Mediators: Their Differential Release and the Secretory Pathways Involved. Frontiers in Immunology 5:. 10.3389/FIMMU.2014.00569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heloterä H, Alitalo K (2007) The VEGF family, the inside story. Cell 130:591–592. 10.1016/J.CELL.2007.08.012 [DOI] [PubMed] [Google Scholar]
- 28.Mäkinen T, Alitalo K (2007) Lymphangiogenesis in development and disease. Novartis Foundation symposium 283:87–98. 10.1002/9780470319413.CH8 [DOI] [PubMed] [Google Scholar]
- 29.Alitalo K (2011) The lymphatic vasculature in disease. Nature Medicine 2011 17:11 17:1371–1380. 10.1038/nm.2545 [DOI] [PubMed] [Google Scholar]
- 30.Orr T, Cox J (1969) Disodium cromoglycate, an inhibitor of mas cell degranulation and histamine release induced by phospholipase A. Nature 223:197–198. 10.1038/223197B0 [DOI] [PubMed] [Google Scholar]
- 31.Gri G, Frossi B, D’Incà F, et al. (2012) Mast Cell: An Emerging Partner in Immune Interaction. Frontiers in Immunology 0:120. 10.3389/FIMMU.2012.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Detoraki A, Staiano RI, Granata F, et al. (2009) Vascular endothelial growth factors synthesized by human lung mast cells exert angiogenic effects. Journal of Allergy and Clinical Immunology 123:1142–1149.e5. 10.1016/J.JACI.2009.01.044 [DOI] [PubMed] [Google Scholar]
- 33.Galli SJ, Tsai M (2010) Mast cells in allergy and infection: Versatile effector and regulatory cells in innate and acquired immunity. European journal of immunology 40:1843. 10.1002/EJI.201040559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.K K, B NR, K MW, et al. (2011) The significant role of mast cells in cancer. Cancer metastasis reviews 30:45–60. 10.1007/S10555-011-9286-Z [DOI] [PubMed] [Google Scholar]
- 35.Bot I, Shi G-P, Kovanen PT (2015) Mast Cells as Effectors in Atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology 35:265–271. 10.1161/ATVBAHA.114.303570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Utrera-Barillas D, Castro-Manrreza M, Castellanos E, et al. (2010) The role of macrophages and mast cells in lymphangiogenesis and angiogenesis in cervical carcinogenesis. Experimental and molecular pathology 89:190–196. 10.1016/J.YEXMP.2010.06.002 [DOI] [PubMed] [Google Scholar]
- 37.Raica M, Cimpean A, Ceausu R, et al. (2013) Interplay between mast cells and lymphatic vessels in different molecular types of breast cancer. Anticancer Research 33: [PubMed] [Google Scholar]
- 38.Pal S, Nath S, Meininger CJ, Gashev AA (2020) Emerging Roles of Mast Cells in the Regulation of Lymphatic Immuno-Physiology. Frontiers in Immunology 0:1234. 10.3389/FIMMU.2020.01234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hampton HR, Chtanova T (2019) Lymphatic Migration of Immune Cells. Frontiers in Immunology 0:1168. 10.3389/FIMMU.2019.01168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee HS, Hos D, Blanco T, et al. (2015) Involvement of corneal lymphangiogenesis in a mouse model of allergic eye disease. Investigative ophthalmology & visual science 56:3140–3148. 10.1167/IOVS.14-16186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davydova N, Harris NC, Roufail S, et al. (2016) Differential Receptor Binding and Regulatory Mechanisms for the Lymphangiogenic Growth Factors Vascular Endothelial Growth Factor (VEGF)-C and -D. Journal of Biological Chemistry 291:27265–27278. 10.1074/JBC.M116.736801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng W, Aspelund A, Alitalo K (2014) Lymphangiogenic factors, mechanisms, and applications. The Journal of clinical investigation 124:878–887. 10.1172/JCI71603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galli SJ, Maurer M, Lantz CS (1999) Mast cells as sentinels of innate immunity. Current Opinion in Immunology 11:53–59. 10.1016/S0952-7915(99)80010-7 [DOI] [PubMed] [Google Scholar]
- 44.de Souza Junior DA, Mazucato VM, Santana AC, et al. (2017) Mast Cells Interact with Endothelial Cells to Accelerate In Vitro Angiogenesis. International Journal of Molecular Sciences 2017, Vol 18, Page 2674 18:2674. 10.3390/IJMS18122674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kray K, Squire E, Tipton W, et al. (1985) Cromolyn sodium in seasonal allergic conjunctivitis. The Journal of allergy and clinical immunology 76:623–627. 10.1016/0091-6749(85)90785-7 [DOI] [PubMed] [Google Scholar]
- 46.Paivandy A, Pejler G (2021) Novel Strategies to Target Mast Cells in Disease. Journal of Innate Immunity 13:131–147. 10.1159/000513582 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request


