Abstract
Purpose:
Autophagy is a resistance mechanism to BRAF/MEK inhibition in BRAFV600-mutant melanoma. Here we used hydroxychloroquine (HCQ) to inhibit autophagy in combination with dabrafenib 150 mg twice daily and trametinib 2 mg every day (D+T).
Patients and Methods:
We conducted a phase I/II clinical trial in four centers of HCQ + D+T in patients with advanced BRAFV600-mutant melanoma. The primary objectives were the recommended phase II dose (RP2D) and the one-year progression-free survival (PFS) rate of >53%.
Results:
Thirty-four patients were evaluable for one-year PFS rate. Patient demographics were as follows: elevated lactate dehydrogenase: 47%; stage IV M1c/M1d: 52%; prior immunotherapy: 50%. In phase I, there was no dose-limiting toxicity. HCQ 600 mg orally twice daily with D+T was the RP2D. The one-year PFS rate was 48.2% [95% confidence interval (CI), 31.0%–65.5%], median PFS was 11.2 months (95% CI, 5.4–16.9 months), and response rate (RR) was 85% (95% CI, 64%–95%). The complete RR was 41% and median overall survival (OS) was 26.5 months. In a patient with elevated LDH (n = 16), the RR was 88% and median PFS and OS were 7.3 and 22 months, respectively.
Conclusions:
HCQ + D+T was well tolerated and produced a high RR but did not meet criteria for success for the one-year PFS rate. There was a high proportion of patients with pretreated and elevated LDH, an increasingly common demographic in patients receiving targeted therapy. In this difficult-to-treat population, the RR and PFS were encouraging. A randomized trial of D+T + HCQ or placebo in patients with BRAFV600-mutant melanoma with elevated LDH and previous immunotherapy is being conducted.
Translational Relevance.
Autophagy is a resistance mechanism to MAPK-targeted therapy in MAPK-mutant cancers. Hydroxychloroquine (HCQ) is FDA approved for rheumatoid arthritis and lupus, and has demonstrated the ability to block autophagy in previous cancer clinical trials. This multi-institutional trial of dabrafenib and trametinib and the autophagy inhibitor HCQ established the safety and activity of combining HCQ with MAPK-targeted therapy in patients with BRAFV600-mutant melanoma. This trial had a high proportion of patients with elevated lactate dehydrogenase (LDH) and prior immunotherapy, reflecting the most common patient population that gets treated with targeted therapy in the modern era. The three-drug regimen was safe and produced high response rates. Progression-free survival did not meet the prespecified threshold for the entire cohort, but looked especially promising in patients with elevated LDH and prior treatment. A national randomized study has been launched to study this regimen further in patients with poor prognosis BRAFV600-mutant melanoma.
Introduction
BRAF and MEK inhibitors are the main targeted therapy option for patients with BRAFV600-mutant melanoma and have improved survival when tested in the first-line setting (1). However, resistance to BRAF and MEK inhibition remains a major problem, especially in patients with elevated LDH. With the adoption of immune checkpoint inhibitors as preferred adjuvant and first-line therapies for stage III and stage IV melanoma, respectively (2), it is common for patients to be treated with targeted therapies in the second- or third-line setting where tumors are often larger and more difficult to treat. Autophagy is a cellular pathway where organelles and proteins are sequestered in autophagic vesicles and degraded in the lysosome. Lysosomal degradation of autophagic cargo allows for nutrient recycling, fueling further growth of cancer cells (3). Autophagy is a major adaptive resistance mechanism to BRAF and/or BRAF and MEK inhibition in BRAFV600-mutant cancers (4–9). Chloroquine derivatives, such as hydroxychloroquine (HCQ), are lysosomal inhibitors that effectively block autophagic flux. Chloroquine derivatives are not simply weak bases that deacidify the lysosome. In fact, they are targeted therapies that bind and inhibit palmitoyl protein thioesterase 1 (PPT1) in the lysosome, leading to autophagy inhibition (10, 11). We have previously conducted clinical trials involving combinations of HCQ and cancer drugs (12–16). These trials have demonstrated the safety of the approach in patients with advanced solid tumor, and pharmacokinetic–pharmacodynamic studies have demonstrated at doses of 400 mg twice daily or above, HCQ effectively blocks autophagy in multiple combinations. Collectively, these preclinical and clinical findings provided the rationale for conducting a multi-institution single-arm phase I/II trial of dabrafenib plus trametinib and HCQ in BRAFV600-mutant melanoma.
Patients and Methods
Patient Selection
Eligible patients were adults with stage IV or unresectable stage III BRAF (V600E, V600K, V600R, or V600D)-mutant melanoma, measurable disease by RECIST 1.1 (17), an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, adequate baseline organ function [absolute neutrophil count ≥ 1.2 × 109/L, hemoglobin ≥ 9 g/dL, platelet count ≥ 100×109/L, prothrombin time/international normalized ratio and partial thromboplastin time ≤ 1.3 × upper limit of normal (ULN), total bilirubin ≤1.5× ULN, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) ≤ 2.5 × ULN, serum creatinine ≤ 1.5 mg/dL], and left ventricular ejection fraction (LVEF)≥ lower limit of normal by ECHO. Patients with treated brain metastases were allowed if they had evidence of radiographic stability (3 weeks after gamma knife, and 2 months after whole-brain radiotherapy). Subjects were excluded if they had leptomeningeal disease or spinal cord compression. Any number and type of prior anticancer therapies were allowed except BRAF or MEK inhibitors. Patients with history of malignancy with a RAS mutation were ineligible. Patients receiving cytochrome P450 enzyme–inducing anticonvulsant drugs were ineligible. For full eligibility criteria, see Supplementary Data. The study was approved by the institutional review boards at the University of Pennsylvania (Philadelphia, PA), Rutgers University (New Brunswick, NJ), Washington University (St. Louis, MO), and Northwestern University (Evanston, IL), and was conducted in accordance with U.S. and international standards for Good Clinical Practice (FDA Title 21 part 312 and International Conference on Harmonization guidelines), the Declaration of Helsinki, and the Belmont Report. Written informed consent was obtained from each patient prior to study entry.
Study endpoints
The primary objective of the phase I portion of the trial was the recommended phase II dose (RP2D) HCQ when administered with D+T in patients with advanced BRAFV600-mutant melanoma. The primary objective of the phase II trial was to determine the proportion of patients achieving one-year progression-free survival (PFS). Secondary objectives were to estimate the toxicity rates, response rate (RR), and overall survival (OS).
Treatment plan and safety assessments
Patients were treated with a run-in of dabrafenib 150 mg orally twice daily and trametinib 2 mg orally daily (D+T) for one week. Patients then started HCQ in combination with D+T in week 2. One cycle was defined as 4 weeks of therapy to coincide with CT scanning performed every 8 weeks (two cycles). The starting phase I dose for HCQ was 400 mg orally twice daily. Three patients were treated at the starting dose and the dose was escalated by predetermined increments. Before accrual to the next dose level began, all patients in each cohort completed the first 4 weeks of combination treatment permitting toxicities to be evaluated. The following events were considered a DLT if they occurred in the first 4 weeks of combined D+T + HCQ therapy: grade 4 neutropenia with fever, grade 4 thrombocytopenia, and any nonhematologic toxicity of grade 3 or higher that was at least possibly treatment-related and that was refractory to supportive measures. Treatment continued until disease progression, and continued treatment beyond progression was allowed when there was an isolated progression that could be locally treated. For full treatment protocol, see Supplementary Data. All subjects received echocardiograms at baseline and every 3 months, electrocardiograms (12-lead ECGs) at every visit, and comprehensive ophthalmologic exams at baseline and at the beginning of the following cycles (+/− one week): cycle 2, cycle 7, and every 6 months. Ophthalmologic exams included multimodal retinal imaging with spectral domain optical coherence tomography (SD-OCT), fundus autofluorescence with short-wavelength (SW), near-infrared (NIR) excitation lights, and visual fields using Humphrey Field Analyzer and a 10–2 protocol (18). This study utilized the Common Terminology Criteria for Adverse Events version 4.03 for toxicity and adverse event reporting. Specific guidelines for dose modification for each of the three medications are detailed in Supplementary Data.
Measurement of effect
Patients were evaluable for response if they received ≥ 4 weeks of D+T + HCQ. Patients were reevaluated for response every two cycles. Objective response and disease progression were evaluated by RECIST 1.1 (17) and responses were required to be confirmed by repeat scans not less than 4 weeks following the initial response scan. Investigator assessment of RECIST response was used to make treatment decisions and to assess the primary endpoint. Central radiology review was also performed by the Radiology RECIST core at the University of Pennsylvania with the goal of assessing the accuracy of the RR. Definitions of clinical outcomes RR, PFS, and OS can be found in Supplementary Data.
RNA sequencing and bioinformatics
RNA sequencing (RNA-seq) data was aligned using bowtie2 (19) algorithm against hg38 human genome version and RSEM v1.2.12 software (20) was used to estimate read counts and RPKM values using gene information from Ensemble transcriptome version GRCh38.p12. Raw counts were used to estimate significance of differential expression difference between any two groups using DESeq2 (21). Overall gene expression changes were considered significant if passed a FDR <5% threshold. Genes that passed nominal significance threshold of P < 0.05 were used for enrichment analysis using QIAGEN's Ingenuity Pathway Analysis software (IPA, QIAGEN Redwood City, www.qiagen.com/ingenuity) using “Upstream Regulators” option. Regulators of interest were those that passed a P < 0.05 threshold and had a significantly predicted activation state (absolute Z-score calculated by IPA of at least 2) with at least 15 target genes.
Statistical analysis
For the phase I study, a traditional 3+3 escalation design was used. The first dose cohort was HCQ 400 mg orally twice daily, and the second dose cohort was HCQ 600 mg orally twice daily. For full details of dose escalation, see protocol. If the MTD was undefined due to lack of DLTs, then HCQ 600 mg orally twice a day would be declared the recommended phase II dose (RP2D). Patients were evaluable for DLT if they completed 1 week of combined HCQ and D+T, but patients treated with any amount of HCQ were evaluable for toxicity reporting. Once the MTD or RP2D for HCQ combined with D+T was determined, a single-arm open-label Simon 2 stage (22) phase II trial was conducted. The null hypothesis that 40% of patients achieve one-year PFS (23) was tested against a one-sided alternative that 60% of patients would achieve one-year PFS with the three-drug combination. In the first stage, if ≤7 of 17 patients reached a one-year PFS, then the study would be stopped. Enrollment continued while the first-stage data matured. If >7 of the first 17 evaluable patients achieved one-year PFS, then 24 additional evaluable patients would be accrued for a total of 41. The null hypothesis would be rejected if >22/41 (>53%) patients were alive and progression-free at one year. This design had a type I error rate of 5% and power of 80% when the true proportion of patients achieving one-year PFS was 60%. For data analyses, any variables requiring transformation because of skewing or other violations of distributional assumptions were appropriately transformed prior to analysis. Overall and complete RRs and 95% exact CIs were computed. Median and one-year PFS and OS rates were estimated by the Kaplan–Meier method. Comparisons between subgroup survival curves were performed by log-rank test. Analyses were performed using either GraphPad Prism or SPSS. All P values were two-sided and P values < 0.05 were considered statistically significant.
Data sharing
Data collected for the study, including individual participant data and a data dictionary defining each field in the set, will be made available to others. This includes deidentified participant clinical data and RNA-seq data. The study protocol and informed consent form will be made available. All of these materials will be available with publication for use by scientific colleagues that contact ravi.amaravadi@pennmedicine.upenn.edu. RNA-seq data has been deposited in the NCBI GEO database with accession number GSE193157. Inquiries will be considered on the basis of their scientific merit of the request and a signed data access agreement.
Results
Patients
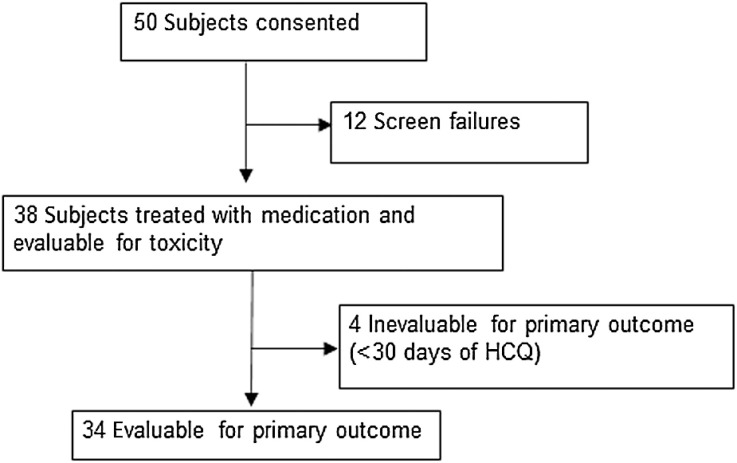
Between December 5, 2014 and January 2, 2020, 50 patients were consented. Twelve patients were excluded during eligibility screening, mainly for occult brain metastases that required treatment. The remaining 38 were treated and evaluable for toxicity. Four patients did not complete 4 weeks of D+T + HCQ due to intolerable fevers and rash typical of D+T and were unevaluable for response. The remaining 34 participants were evaluable for one-year PFS and response (Fig. 1). Patients had more highly advanced stage IV melanoma than most published pivotal studies with BRAF and MEK inhibitors alone (Table 1). M1c or M1d disease was present in 52%, elevated serum LDH in 47%, 50% were previously treated (all with immune checkpoint inhibitors; Supplementary Table S1), and 52% had a baseline tumor size (BTS; sum of target lesions using RECIST criteria) ≥ 5 cm. Of note in patients with elevated serum LDH, 81% also had BTS ≥ 5 cm. BRAFV600 mutations were characterized by next-generation sequencing (NGS) in 50% of patients and single-gene assay in 50%. In tumors that underwent NGS testing, 59% had BRAFV600E as the only pathogenic mutation. BRAFV600K was detected in tumors of 21% of patients (Supplementary Table S2).
Figure 1.
CONSORT diagram for the BAMM trial.
Table 1.
Demographics.
| Age, median (range) | 58 (30–83) |
| Sex | |
| Female | 11/38 (29%) |
| Male | 27/38 (71%) |
| ECOG performance status | |
| ECOG PS 0 | 27/38 (71%) |
| ECOG PS 1 | 11/38 (29%) |
| LDH at study entry | |
| LDH ≤ ULN | 20/38 (53%) |
| LDH > ULN | 18/38 (47%) |
| LDH 1–2X ULN | 15/38 (39%) |
| LDH ≥ 2X ULN | 3/38 (8%) |
| Stage at study entry | |
| Unresectable stage IIIC | 0/38 (0%) |
| Stage IV M1a | 9/38 (24%) |
| Stage IV M1b | 9/38 (24%) |
| Stage IV M1c | 15/38 (39%) |
| Stage IV M1d | 5/38 (13%) |
| No. of prior systemic therapies | |
| 0 | 19/38 (50%) |
| 1 | 8/38 (21%) |
| 2 | 9/38 (24%) |
| 3 | 2/38 (5%) |
| Prior immune checkpoint inhibition | |
| in patients with prior Rx | 19/19 (100%) |
| Baseline tumor size | |
| < 5 cm | 16 (42%) |
| ≥ 5 cm | 20 (52%) |
| N/A | 2 (6%) |
| Brain metastases | 5/38 (13%) |
Abbreviation: Rx, treatment.
Phase I dose escalation and phase II enrollment
Seven patients were enrolled on the phase I dose escalation portion of this study. Three patients were treated at 400 mg orally twice daily with no DLTs. Three patients were then treated at HCQ 600 mg orally twice daily, the highest dose allowed by the FDA without an investigational new drug (IND). One of the patients was noncompliant during the DLT evaluation period but continued on study and became compliant in the second month and was therefore evaluable for response and toxicity. For DLT evaluation at HCQ 600 mg orally twice daily, a fourth patient was added with no DLTs observed. Our previous pharmacokinetic–pharmacodynamic studies of HCQ in patients with cancer have demonstrated that across combinations, HCQ produces a remarkably consistent pharmacokinetic–pharmacodynamic correlation (10, 11). Doses of 400–600 mg twice daily consistently blocked autophagy in peripheral blood mononuclear cells. Therefore, the RP2D of HCQ was 600 mg twice daily combined with dabrafenib 150 mg orally twice daily and trametinib 2 mg orally daily was used for the phase II portion of the study. By August 2018, 20 patients were enrolled and 15 patients were treated with HCQ 600 twice daily and D+T and evaluable for one-year PFS, of which 9 had achieved one-year PFS, so enrollment onto stage II of the phase II study was continued. In March 2020, the study was closed due to slow accrual and the COVID-19 pandemic after accruing a total of 34 response-evaluable patients. Combining patients from both phases of the study, the proportion of patients achieving one-year PFS was 44% (15/34).
Response
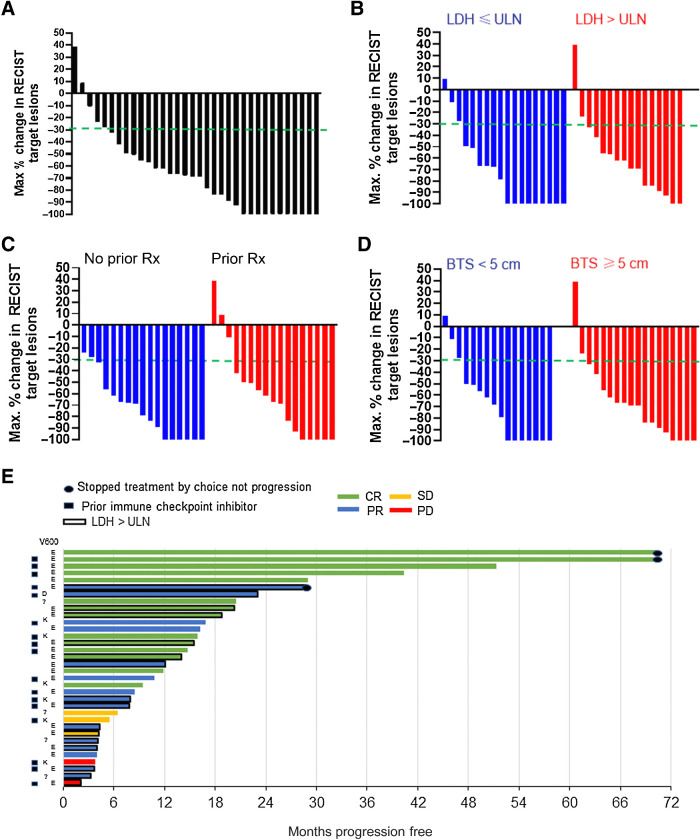
The investigator-assessed overall RR for the combined phase I/II study population was 29/34 [85%; 95% exact confidence interval (CI), 69%–95%; Table 2]. The investigator-assessed complete RR was 14 of 34 (41%; 95% exact CI, 25%–59%). Central review of scans was performed by the University of Pennsylvania Radiology RECIST Core for 31 of 34 evaluable patients, and confirmed the overall RR (Supplementary Fig. S1). For the remaining 3 patients, scans were not retrievable for central review. The objective RR and complete RR for response-evaluable patient subgroups were: 16 patients with elevated serum LDH (88%, 25%), 17 with prior therapy (82%, 35%), and 18 with baseline tumor size ≥ 5 cm (89%, 33%), suggesting that this regimen is effective at eliciting response in the most aggressive stage IV BRAFV600-mutant melanoma cases. Waterfall plots for the entire cohort and these subgroups further demonstrate this finding (Fig. 2A–D). A Swimmer's plot shows that there was a wide variability in durability of response in patients, but patients who were pretreated and had elevated serum LDH were among the patients with the longest PFS (Fig. 2E).
Table 2.
Investigator-assessed RRs.
| Response | Entire study cohort | LDH ≤ ULN | LDH > ULN | No prior therapy | Prior therapy | BTS < 5 cm | BTS ≥ 5 cm |
|---|---|---|---|---|---|---|---|
| CR | 14/34 (41%) | 10/18 (56%) | 4/16 (25%) | 8/17 (47%) | 6/17 (35%) | 8/16 (50%) | 6/18 (33%) |
| PR | 15/34 (46%) | 5/18 (27%) | 10/16 (63%) | 7/17 (41%) | 8/17 (47%) | 5/16 (31%) | 10/18 (56%) |
| SD | 3 (8%) | 2/18 (11%) | 1/16 (6%) | 2/17 (12%) | 1/17 (6%) | 2/16 (13%) | 1/18 (6%) |
| PD | 2 (5%) | 1/18 (6%) | 1/16 (6%) | 0 | 2/17 (11%) | 1/16 (6%) | 1/18 (5%) |
| N | 34 | 18 | 16 | 17 | 17 | 16 | 18 |
| ORR | 29/34 (85%) | 15/18 (83%) | 14/16 (88%) | 15/17 (88%) | 14/17 (82%) | 14/16 (81%) | 16/18 (89%) |
Abbreviations: CR, complete response; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease.
Figure 2.
Waterfall and Swimmer's plots for dabrafenib (D), trametinib (T), and hydroxychloroquine (HCQ). A–D, Waterfall showing maximum change in RECIST target lesions. Dashed green line indicates threshold for partial response. A, Entire study population. B, According to LDH status. C, According to prior therapy. D, According to baseline tumor size (BTS) as determined by the sum of the RECIST target lesions on baseline scans. E, Swimmer's plot showing prior therapy, BRAFV600 mutation, LDH status, response, and PFS in each patient. Rx, treatment.
PFS
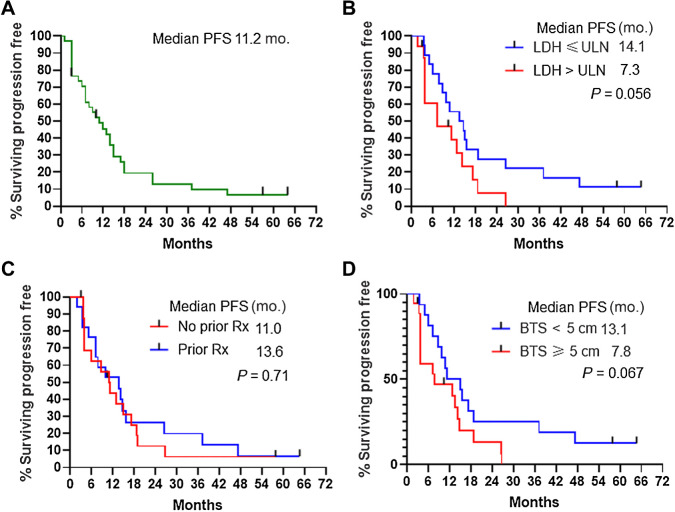
On the basis of 34 response-evaluable patients, the one-year PFS rate from Kaplan–Meier analysis was 48.2% (95% CI, 31%–65.5%) and median was 11.2 months (95% CI, 5.4–16.9 months; Fig. 3A). The median PFS for 16 patients with elevated serum LDH (7.3 months; Fig. 3B), 17 with prior therapy (13.6 months; Fig. 3C), and 18 with baseline tumor size ≥ 5 cm (7.8 months; Fig. 3D) suggests this regimen demonstrates efficacy in previously treated patients and patients with elevated serum LDH. Patients who responded had a median PFS of 14.1 months and there was a nonsignificant trend toward prolonged PFS in patients with BRAFV600E/D compared with BRAFV600K mutation (Supplementary Fig. S2).
Figure 3.
PFS. A, Entire study population. B, According to LDH status. C, According to prior therapy. D, According to baseline tumor size as determined by the sum of the RECIST target lesions on baseline scans. Tick marks indicate censored patients who never progressed by RECIST criteria at the time of last follow-up. Rx, treatment.
Overall survival
With a median follow-up of 43.6 months, the median OS was 26.5 months (95% CI, 9.3–43.7 months; Supplementary Fig. S3). The median OS for patients with elevated serum LDH was 22.2 months; prior therapy was 23 months; and BTS ≥ 5 cm was 22.2 months. The 3-year (47.8%), 4-year (43.3%), and 5-year (37.2%) OS rates for the BAMM trial compare favorably to previously reported OS rates for dabrafenib and trametinib (Supplementary Table S3; ref. 24). In patients with elevated serum LDH in the BAMM trial 2-year (41%), 3-year (32%), 4-year (32%), and 5-year (32%) survival rates were more favorable than previously reported (25).
Adverse events
All adverse events that were possibly, probably, or definitely related to study medication and occurred between December 5, 2014 and December 5, 2020 are reported in Table 3. The addition of HCQ to D+T seems to be very well tolerated, with low rates of grade 3 toxicities. Less than 10% of patients had grade 3 anorexia, diarrhea, nausea, and constipation, symptoms associated with HCQ but also overlapping with D+T. The most common grade 3 adverse event attributed to D+T was fever/pyrexia (5%) and rash (13%). No grade 3 QTc prolongation was observed in this study despite some patients being treated with HCQ 400–600 mg twice daily for years. There were 3 patients with lowered ejection fraction, but all were asymptomatic. One patient discontinued MEK inhibitor and continued on dabrafenib and HCQ after lowered ejection fraction and continued to benefit from therapy. Dose reductions were used in 65% of patients to effectively manage toxicity (Supplementary Table S4). Mild MEK inhibitor–associated retinopathy (MEKAR) was observed in both eyes of one visually asymptomatic patient (01–06), although subclinical distancing of the neurosensory retina from the retinal pigment epithelium was identified in most (17/22) patients soon after treatment initiation, which subsided in most (15/17) despite continued treatment, confirming earlier observations (Supplementary Table S5; Supplementary Fig. S4; ref. 18). One patient (01–10) showed the earliest signs of HCQ toxicity after 34 months of continuous treatment with irreversible loss of the photoreceptor outer segment on SD-OCT imaging in the parafoveal retina bilaterally (Supplementary Fig. S5). This patient was visually asymptomatic and had no change in visual acuity, but showed approximately 10 dB of mean sensitivity loss on perimetry (Supplementary Table S5). There were no changes on follow-up 3 months after discontinuation of HCQ. The rest of the patients did not show signs of retinal toxicity on multimodal imaging or psychophysics, supporting the overall ocular safety of the treatment combination for the duration of the study (Supplementary Table S5). Multimodal imaging of the central retina, particularly SD-OCT, proved a sensitive and practical way of monitoring for subclinical signs of retinal toxicity.
Table 3.
Treatment-related adverse events in 38 patients.
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total | |
|---|---|---|---|---|---|
| Adverse events | n (%) | n (%) | n (%) | n | n (%) |
| Abdominal pain | 6 (16%) | 1 (3%) | 0 (0%) | 0 | 7 (18%) |
| ALT increased | 4 (11%) | 1 (3%) | 2 (5%) | 0 | 7 (18%) |
| Alopecia | 3 (8%) | 0 (0%) | 0 (0%) | 0 | 3 (8%) |
| Anemia | 2 (5%) | 2 (5%) | 0 (0%) | 0 | 4 (10%) |
| Anorexia | 11 (29%) | 4 (11%) | 1 (3%) | 0 | 16 (47%) |
| Arthralgia | 3 (8%) | 4 (11%) | 0 (0%) | 0 | 7 (18%) |
| AST increased | 5 (13%) | 1 (3%) | 1 (3%) | 0 | 7 (18%) |
| Blurred vision | 4 (11%) | 0 (0%) | 0 (0%) | 0 | 4 (12%) |
| Chills | 13 (34%) | 7 (18%) | 1 (3%) | 0 | 21 (55%) |
| Constipation | 4 (11%) | 3 (8%) | 0 (0%) | 0 | 7 (18%) |
| Creatinine increased | 0 (0%) | 4 (11%) | 0 (0%) | 0 | 4 (10%) |
| Dehydration | 1 (3%) | 4 (11%) | 3 (8%) | 0 | 8 (21%) |
| Diarrhea | 14 (37%) | 5 (13%) | 2 (5%) | 0 | 21 (55%) |
| Dizziness | 5 (13%) | 0 (0%) | 0 (0%) | 0 | 5 (13%) |
| Dry mouth | 7 (18%) | 1 (3%) | 0 (0%) | 0 | 8 (21%) |
| Dysgeusia | 6 (16%) | 0 (0%) | 0 (0%) | 0 | 6 (16%) |
| Dyspepsia | 2 (5%) | 2 (5%) | 0 (0%) | 0 | 4 (10%) |
| Edema limbs | 4 (11%) | 2 (5%) | 0 (0%) | 0 | 6 (16%) |
| Ejection fraction decreased | 0 (0%) | 2 (5%) | 1 (3%) | 0 | 3 (8%) |
| Prolonged QTc | 6 (16%) | 2 (5%) | 0 (0%) | 0 | 8 (21%) |
| Encephalopathy | 0 (0%) | 0 (0%) | 1 (3%) | 0 | 1 (3%) |
| Fatigue | 8 (21%) | 12 (32%) | 2 (5%) | 0 | 22 (57%) |
| Fever | 12 (32%) | 12 (32%) | 1 (3%) | 0 | 25 (66%) |
| Headache | 6 (16%) | 4 (11%) | 2 (5%) | 0 | 12 (32%) |
| Lymphocyte count decreased | 0 (0%) | 1 (3%) | 1 (3%) | 0 | 2 (5%) |
| Malaise | 4 (11%) | 2 (5%) | 0 (0%) | 0 | 6 (16%) |
| Mucositis oral | 0 (0%) | 0 (0%) | 1 (3%) | 0 | 1 (3%) |
| Myalgia | 13 (34%) | 1 (3%) | 0 (0%) | 0 | 14 (37%) |
| Nausea | 16 (42%) | 6 (16%) | 2 (5%) | 0 | 24 (63%) |
| Neutrophil count decreased | 0 (0%) | 1 (3%) | 1 (3%) | 0 | 2 (5%) |
| Sigmoid polyp | 0 (0%) | 1 (3%) | 0 (0%) | 0 | 1 (3%) |
| Squamous cell carcinoma | 0 (0%) | 3 (8%) | 0 (0%) | 0 | 3 (8%) |
| Palmar–plantar erythrodysesthesia | 2 (5%) | 2 (5%) | 0 (0%) | 0 | 4 (10%) |
| Pruritus | 7 (18%) | 1 (3%) | 0 (0%) | 0 | 8 (21%) |
| Rash | 9 (24%) | 8 (21%) | 5 (13%) | 0 | 22 (58%) |
| Retinopathy | 2 (5%) | 0 (0%) | 0 (0%) | 0 | 2 (5%) |
| Seizure | 0 (0%) | 0 (0%) | 1 (3%) | 0 | 1 (3%) |
| Sepsis | 0 (0%) | 0 (0%) | 1 (3%) | 0 | 1 (3%) |
| Vomiting | 7 (18%) | 1 (3%) | 0 (0%) | 0 | 8 (21%) |
| Weight loss | 3 (8%) | 3 (8%) | 0 (0%) | 0 | 6 (16%) |
RNA-seq identifies signaling regulators associated with short PFS
Seventeen patients had pretreatment tumor tissue suitable for RNA-seq. Ten patients had long PFS (PFS ≥ 1 year) and 7 patients had short PFS (PFS < 1 year). Heat maps of differentially expressed genes based on short versus long PFS identified very few genes that were significantly elevated in patients with PFS < 1 year (Supplementary Fig. S6). A previous study indicated that elevated aldehyde dehydrogenase A1 (ALDH1A1) predicts sensitivity to HCQ (26). ALDH1A1 was significantly decreased in all patients with short PFS (nominal P value 0.04) and in patients with elevated serum LDH and short PFS (P = 0.004; DESe2 method). The single most upregulated gene in patients with short PFS was SLC16A6, the key monocarboxylate transporter for lactate. Looking specifically for autophagy and lysosomal genes, ATG12, BNIP3, and several components of the lysosomal vacuolar ATPase were significantly upregulated in the pretreatment tumors of patients with short PFS (Supplementary Table S6). We next analyzed significantly differentially expressed genes (P < 0.05) for enrichment of upstream regulators known to regulate a significant number of those targets (Supplementary Fig. S7). On the basis of the known regulator effect on the target and the target mRNA change direction, a Z-score was used to classify the regulator as activated or inhibited in patients with short versus long PFS. TGFB1 was the regulator with highest number of genes significantly increased in the tumors of patients with short PFS and in patients with elevated serum LDH and short PFS (Supplementary Fig. S7). TGFB1 has not previously been linked to BRAF inhibitor resistance, but increased TGFB1 signaling has been shown to deplete lysosomes, raising the possibility that tumors with elevated TGFB1 would be resistant to lysosomal inhibition (27). In patients with elevated serum LDH, there was increased expression of many regulators previously linked to BRAF inhibitor resistance including VEGF (28), EGF (29), ERBB2 (30), HGF (31), FOS (32), and ERK (9).
Discussion
There is limited data for the efficacy of BRAF and MEK inhibition in patients treated in the second-line or third-line setting. Patients with advanced melanoma treated in the second- or third-line often have elevated serum LDH, larger tumors at baseline, and therefore a poorer prognosis. Due to early closure, the BAMM trial could not determine whether the proportion of patients achieving one-year PFS was > 53% in 41 evaluable patients which was the prespecified criteria of the Simon two-stage design needed to reject the null hypothesis. The short PFS observed in some patients with complete response in this study suggests there could be resistance mechanisms that could be activated by chronic autophagy inhibition. RNA-seq data in this study found that upregulation of genes in the TGFβ pathway in pretreatment biopsies was associated with short PFS. These findings need to be further validated at the protein and mechanistic level, but could have significant impact on the development of other more potent and specific autophagy inhibitors. Compared with the pivotal study that established D+T as effective therapy for BRAFV600-mutant melanoma (24), the BAMM trial had a higher percentage of patients with elevated serum LDH, large tumor size at baseline, and importantly previous treatment. Given the difference in demographics in this study, the 48.2% one-year PFS rate could be viewed as signal of durable activity in the poorer prognosis patients who are currently being treated with targeted therapy, but this activity needs to be confirmed in a randomized study. While D+T + HCQ produced a strikingly high response rate, in many cases, responses were transient. However, in patients with elevated serum LDH, a population of patients that historically has poor outcomes with targeted therapy, this regimen appears to be safe and active, producing an 88% response rate and median PFS of 7.3 months. A previous pooled analysis of the COMBI-D and COMBI-V studies demonstrated a median PFS of <6 months BRAFV600 patients with elevated serum LDH (25). In this pooled analysis, only 10% of patients had adjuvant non-ipilimumab–based immunotherapy, and none of the patients were previously treated with programmed cell death protein 1 antibody. The difference in median PFS for elevated LDH patients between BAMM and COMBI-D/V studies may not be striking, but more recent data from EA6134 DREAMseq study shows the outcomes found in BAMM deserve attention. The DREAMseq study (NCT02224781), a study conducted through the National Clinical Trials Network in hundreds of community and academic centers in the United States, randomized treatment-naïve stage IV patients with BRAFV600-mutant melanoma to either immunotherapy or targeted therapy with D+T (2). At the time of progression, patients could then crossover the other treatment. The study found that sequencing immunotherapy before targeted therapy produced a significantly higher rate of 2-year survival than sequencing targeted therapy before immunotherapy. In patients randomized to start with D+T, 40% of patients had elevated serum LDH, which is more similar to BAMM than pivotal studies that led to approval of D+T. In the total population who started with D+T (regardless of LDH), the 43% response rate and median PFS of 8.8 months were both surprisingly low and suggest the BAMM regimen which produced an 85% response rate and 11.2-month median PFS is active in BRAFV600-mutant patients most likely to be seen in practice. No information about the outcomes in patients with elevated LDH are available, but regardless, in this more modern context, D+T produced a lower RR and median PFS than previous pivotal studies that were used as a historical reference to design the BAMM trial.
One of the most surprising findings of the BAMM trial is that the median overall survival for D+T + HCQ was 22.2 months. This is notable as previous reports of median PFS of 13 months in immune checkpoint inhibitor–naïve patients with elevated serum LDH patients treated with D+T (24, 25). The COLUMBUS trial of encorafenib + binimetinib compared with encorafenib or vemurafenib (33) enrolled patients of whom 29% had elevated serum LDH and 33% pretreated with immunotherapy. There was no significant benefit in PFS for this combination compared with single-agent vemurafenib in BRAFV600 patients with elevated serum LDH. The median overall survival for high-dose encorafenib and binimetinib was 11.4 months (34).
The limitations of this study are that it is nonrandomized, and terminated early in part due to poor accrual. However, given the response rate, median PFS, and especially median OS in the elevated LDH population looked promising compared with pivotal BRAF and MEK inhibitor trials, there remains enthusiasm to conduct a randomized study to definitively test the efficacy of adding HCQ to BRAF and MEK inhibition in patients with elevated serum LDH. On the basis of these findings, we have launched a double-blind placebo-controlled randomized phase II study of dabrafenib, trametinib with either HCQ, or placebo in patients with elevated serum LDH (EA6191/BAMM2; NCT04527549).
Supplementary Material
Acknowledgments
This study was an investigator-initiated study partially funded by Novartis. The study was originally funded by GlaxoSmithKline in 2014; dabrafenib and trametinib became assets of Novartis as of March 2, 2015. Support for management of the trial was provided by the HHS/NIH/NCI P30CA016520 (Abramson Cancer Center Support Grant), and HHS/NIH/NCI P50 CA174523 (Skin Cancer SPORE). J. Mehnert was funded by the NYU Melanoma SPORE grant P50CA225450. We thank the patients and their families for their participation.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This article is featured in Highlights of This Issue, p. 1053
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
J.M. Mehnert reports grants and personal fees from Novartis during the conduct of the study, as well as grants from Incyte and personal fees from Merck, Eisai, Seagen, Regeneron, and BMS outside the submitted work. T.C. Mitchell reports personal fees from Merck, BMS, OncoSec, and GigaGen outside the submitted work. R. Groisberg reports employment with Merck & Co. between the time of submitting the manuscript and publication; during the entirety of the submitted work R. Groisberg was an employee of Rutgers University. In addition, R. Groisberg reports research funding/grant support for clinical trials (to his institution) from Regeneron, BMS, Merck/EMD Serono, Amgen, Roche/Genentech, and Philogen; consulting/advisory board from Regeneron; and speaker for Deciphera. L.F. Hernandez-Aya reports other support from Novartis during the conduct of the study; L.F. Hernandez-Aya also reports personal fees and other support from BMS, as well as other support from Merck, Immunocore, Regeneron, Novartis, Genentech, and Foghorn Therapeutics outside the submitted work. S. Chandra reports other support from Novartis outside the submitted work, as well as Advisory Board Member for Novartis, Pfizer, Bristol Myers Squibb, EMD Serono, and Regeneron. P.A. Gimotty reports grants from NIH during the conduct of the study. R.K. Amaravadi reports grants from Novartis during the conduct of the study; R.K. Amaravadi also reports other support from Pinpont Therapeutics, Immunaccel, and Allomek, as well as personal fees from Deciphera and Merck outside the submitted work. In addition, R.K. Amaravadi has a patent for dimeric chloroquines licensed to Pinpoint Therapeutics. No disclosures were reported by the other authors.
Authors' Contributions
J.M. Mehnert: Resources, data curation, formal analysis, supervision, validation, investigation, project administration, writing–review and editing. T.C. Mitchell: Supervision, investigation, writing–review and editing. A.C. Huang: Conceptualization, methodology, writing–review and editing. T.S. Aleman: Conceptualization, resources, data curation, formal analysis, supervision, validation, investigation, methodology, writing–original draft, project administration, writing–review and editing. B.J. Kim: Conceptualization, investigation, writing–review and editing. L.M. Schuchter: Resources, investigation, writing–review and editing. G.P. Linette: Investigation, writing–review and editing. G.C. Karakousis: Investigation, writing–review and editing. S. Mitnick: Conceptualization, resources, data curation, formal analysis, supervision, methodology, project administration, writing–review and editing. L. Giles: Conceptualization, data curation, supervision, investigation, methodology, writing–review and editing. M. Carberry: Conceptualization, data curation, formal analysis, methodology, writing–review and editing. N. Frey: Formal analysis, supervision, project administration, writing–review and editing. A. Kossenkov: Conceptualization, data curation, software, formal analysis, investigation, methodology, writing–original draft, writing–review and editing. R. Groisberg: Investigation, writing–review and editing. L.F. Hernandez-Aya: Investigation, writing–review and editing. G. Ansstas: Investigation, writing–review and editing. A.W. Silk: Investigation, writing–review and editing. S. Chandra: Resources, supervision, investigation, writing–review and editing. J.A. Sosman: Investigation, writing–review and editing. P.A. Gimotty: Conceptualization, data curation, software, formal analysis, supervision, validation, visualization, methodology, writing–original draft, writing–review and editing. R. Mick: Data curation, software, formal analysis, supervision, validation, visualization, methodology, writing–original draft, writing–review and editing. R.K. Amaravadi: Conceptualization, resources, data curation, formal analysis, supervision, funding acquisition, validation, investigation, visualization, methodology, writing–original draft, project administration, writing–review and editing.
References
- 1. Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol 2017;14:463–82. [DOI] [PubMed] [Google Scholar]
- 2. Atkins MB LS, Chmielowski B, Ribas A, Tarhini AA, Truong T, Davar D, et al. DREAMseq (Doublet, Randomized Evaluation in Advanced Melanoma Sequencing): A phase III trial—ECOG-ACRIN EA6134. J Clin Oncol 39, 2021(suppl 36; abstr 356154). [Google Scholar]
- 3. Amaravadi RK, Kimmelman AC, Debnath J. Targeting autophagy in cancer: recent advances and future directions. Cancer Discov 2019;9:1167–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ma XH, Piao SF, Dey S, McAfee Q, Karakousis G, Villanueva J, et al. Targeting ER stress-induced autophagy overcomes BRAF inhibitor resistance in melanoma. J Clin Invest 2014;124:1406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xie X, Koh JY, Price S, White E, Mehnert JM. Atg7 overcomes senescence and promotes growth of BrafV600E-driven melanoma. Cancer Discov 2015;5:410–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sueda T, Sakai D, Kawamoto K, Konno M, Nishida N, Koseki J, et al. BRAF V600E inhibition stimulates AMP-activated protein kinase-mediated autophagy in colorectal cancer cells. Sci Rep 2016;6:18949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goodall ML, Wang T, Martin KR, Kortus MG, Kauffman AL, Trent JM, et al. Development of potent autophagy inhibitors that sensitize oncogenic BRAF V600E mutant melanoma tumor cells to vemurafenib. Autophagy 2014;10:1120–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levy JM, Thompson JC, Griesinger AM, Amani V, Donson AM, Birks DK, et al. Autophagy inhibition improves chemosensitivity in BRAF(V600E) brain tumors. Cancer Discov 2014;4:773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ojha R, Leli NM, Onorati A, Piao S, Verginadis II, Tameire F, et al. ER translocation of the MAPK pathway drives therapy resistance in BRAF-mutant melanoma. Cancer Discov 2019;9:396–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rebecca VW, Nicastri MC, McLaughlin N, Fennelly C, McAfee Q, Ronghe A, et al. A unified approach to targeting the Lysosome's degradative and growth signaling roles. Cancer Discov 2017;7:1266–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rebecca VW, Nicastri MC, Fennelly C, Chude CI, Barber-Rotenberg JS, Ronghe A, et al. PPT1 promotes tumor growth and is the molecular target of chloroquine derivatives in cancer. Cancer Discov 2019;9:220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rangwala R, Chang YC, Hu J, Algazy KM, Evans TL, Fecher LA, et al. Combined MTOR and autophagy inhibition: phase I trial of hydroxychloroquine and temsirolimus in patients with advanced solid tumors and melanoma. Autophagy; 10:1391–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rangwala R, Leone R, Chang YC, Fecher LA, Schuchter LM, Kramer A, et al. Phase I trial of hydroxychloroquine with dose-intense temozolomide in patients with advanced solid tumors and melanoma. Autophagy 2014;10:1369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haas NB, Appleman LJ, Stein M, Redlinger M, Wilks M, Xu X, et al. Autophagy inhibition to augment mTOR inhibition: a phase I/II trial of everolimus and hydroxychloroquine in patients with previously treated renal cell carcinoma. Clin Cancer Res 2019;25:2080–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karasic TB, O'Hara MH, Loaiza-Bonilla A, Reiss KA, Teitelbaum UR, Borazanci E, et al. Effect of gemcitabine and nab-paclitaxel with or without hydroxychloroquine on patients with advanced pancreatic cancer: a phase 2 randomized clinical trial. JAMA Oncol 2019;5:993–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zeh HJ, Bahary N, Boone BA, Singhi AD, Miller-Ocuin JL, Normolle DP, et al. A randomized phase II preoperative study of autophagy inhibition with high-dose hydroxychloroquine and gemcitabine/nab-paclitaxel in pancreatic cancer patients. Clin Cancer Res 2020;26:3126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 18. Nti AA, Serrano LW, Sandhu HS, Uyhazi KE, Edelstein ID, Zhou EJ, et al. Frequent subclinical macular changes in combined BRAF/MEKinhibition with high-dose hydroxychloroquine as treatment for advanced BRAF mutant melanoma: Preliminary results from a phase I/II clinical treatment trial. Retina 2019;39:502–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods 2012;9:357–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-seq data with or without a reference genome. BMC Bioinformatics 2011;12:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014;15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989;10:1–10. [DOI] [PubMed] [Google Scholar]
- 23. Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2012;367:1694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Robert C, Grob JJ, Stroyakovskiy D, Karaszewska B, Hauschild A, Levchenko E, et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med 2019;381:626–36. [DOI] [PubMed] [Google Scholar]
- 25. Schadendorf D, Long GV, Stroiakovski D, Karaszewska B, Hauschild A, Levchenko E, et al. Three-year pooled analysis of factors associated with clinical outcomes across dabrafenib and trametinib combination therapy phase 3 randomised trials. Eur J Cancer 2017;82:45–55. [DOI] [PubMed] [Google Scholar]
- 26. Piao S, Ojha R, Rebecca VW, Samanta A, Ma XH, McAfee Q, et al. ALDH1A1 and HLTF modulate the activity of lysosomal autophagy inhibitors in cancer cells. Autophagy 2017;13:2056–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang C, Chen XC, Li ZH, Wu HL, Jing KP, Huang XR, et al. SMAD3 promotes autophagy dysregulation by triggering lysosome depletion in tubular epithelial cells in diabetic nephropathy. Autophagy 2020;17:2325–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Comunanza V, Cora D, Orso F, Consonni FM, Middonti E, Di Nicolantonio F, et al. VEGF blockade enhances the antitumor effect of BRAFV600E inhibition. EMBO Mol Med 2017;9:219–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun C, Wang L, Huang S, Heynen GJ, Prahallad A, Robert C, et al. Reversible and adaptive resistance to BRAF(V600E) inhibition in melanoma. Nature 2014;508:118–22. [DOI] [PubMed] [Google Scholar]
- 30. Abel EV, Basile KJ, Kugel CH 3rd, Witkiewicz AK, Le K, Amaravadi RK, et al. Melanoma adapts to RAF/MEK inhibitors through FOXD3-mediated upregulation of ERBB3. J Clin Invest 2013;123:2155–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature 2012;487:500–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baron M, Tagore M, Hunter MV, Kim IS, Moncada R, Yan Y, et al. The stress-like cancer cell state is a consistent component of tumorigenesis. Cell Syst 2020;11:536–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dummer R, Ascierto PA, Gogas HJ, Arance A, Mandala M, Liszkay G, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2018;19:603–15. [DOI] [PubMed] [Google Scholar]
- 34. Ascierto PA, Dummer R, Gogas HJ, Flaherty KT, Arance A, Mandala M, et al. Update on tolerability and overall survival in COLUMBUS: landmark analysis of a randomised phase 3 trial of encorafenib plus binimetinib vs vemurafenib or encorafenib in patients with BRAF V600-mutant melanoma. Eur J Cancer 2020;126:33–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.