Electronic cigarette (EC) use (vaping) has grown at an alarming rate in recent years. Originally used as a cessation-aid for combustible cigarette smoking, the popularity of vaping has grown exponentially in adolescents and teenagers.1 While there are clear pulmonary consequences of EC use,2 adolescent cardiac health has not been examined despite the well-documented impact of particulate matter (found in EC aerosol) on the heart.3 Therefore, the cardiovascular system may encounter significant detriment following EC exposure throughout the sensitive period of adolescence. To investigate this, we used a mouse model of adolescent EC exposure.
All animal use was approved and conducted in accordance with institutional guidelines. FVB mice (3–5 weeks old) were exposed to EC aerosol (50:50 mixture of propylene glycol and vegetable glycerin) with nicotine (20.2 mg/mL) or vehicle alone at relevant levels (one 70 mL puff per minute for 4 hrs/day, 5 days/week), or to HEPA-filtered air as control (FA) for 3 weeks or 3 months. EC aerosol was produced from a third-generation EC device attached to an exposure chamber (SCIREQ, Montreal, CA). Data supporting the findings from this study are available from the corresponding author.
Heart weight/tibia length ratio was not altered after 3-months of exposure in male or female mice (Figure [A]). Male mice displayed reduced fractional shortening and impaired diastolic function using echocardiography after exposure to EC aerosol with nicotine for 3 months, but not in response to vehicle alone (Figure [B]). These changes were not found in mice exposed at adulthood (3 months old), adolescent female mice (Figure [B]), adolescent mice exposed to a “casual-use” timeframe (1 hr/day, 3 days/week), or adolescent mice exposed for only 3 weeks in duration. Systolic/diastolic blood pressure were unchanged as measured by tail-cuff. Upon sacrifice after 3 months of exposure, pressure-volume (PV) loop recordings of the left ventricle (LV) revealed that the male, adolescent EC and EC(+Nic) groups had reduced contractility (Ees), and the EC(+Nic) group had reduced preload-recruitable stroke work compared to FA (Figure [C]), collectively indicating a reduced contractile capacity as a result of exposure. PV-loop analysis also revealed no change in Tau in the EC(+Nic), as compared to FA, indicating the E/A alteration observed via echocardiography may not accurately depict the diastolic function of these mice (Figure [C]). Trichrome staining of cardiac tissue revealed increased perivascular fibrosis in the male, adolescent vehicle group, relative to control and the nicotine group, implicating a protective effect of nicotine (Figure [D]). Similarly, ELISA targeting type I collagen (Novus Biologicals, Centennial, CO) showed a slight, nonsignificant increase in the vehicle group (p=.14 via ANOVA; Figure [D] bottom right). RT-qPCR demonstrated that adolescent EC exposure with nicotine increased cardiac COL1A1 and COL3A1 expression in males after 3 weeks of exposure, which was reversed by 3 months (Figure [E]). This inverted response may be due to timing of exposure in mouse development, or a protective effect of nicotine after prolonged exposure. We performed RNA-sequencing on the LV of male mice (NCBI GEO accession GSE183614) and found that when comparing FA with EC(+Nic), expression of many genes was altered in adolescent mice (71), and very few altered in adult mice (4) (Figure [F]). Further, gene ontology (GO) analyses revealed that the genes changed included those involved in the inflammatory response, stress response, fibroblast signaling, and endothelial cell signalling.
Figure.
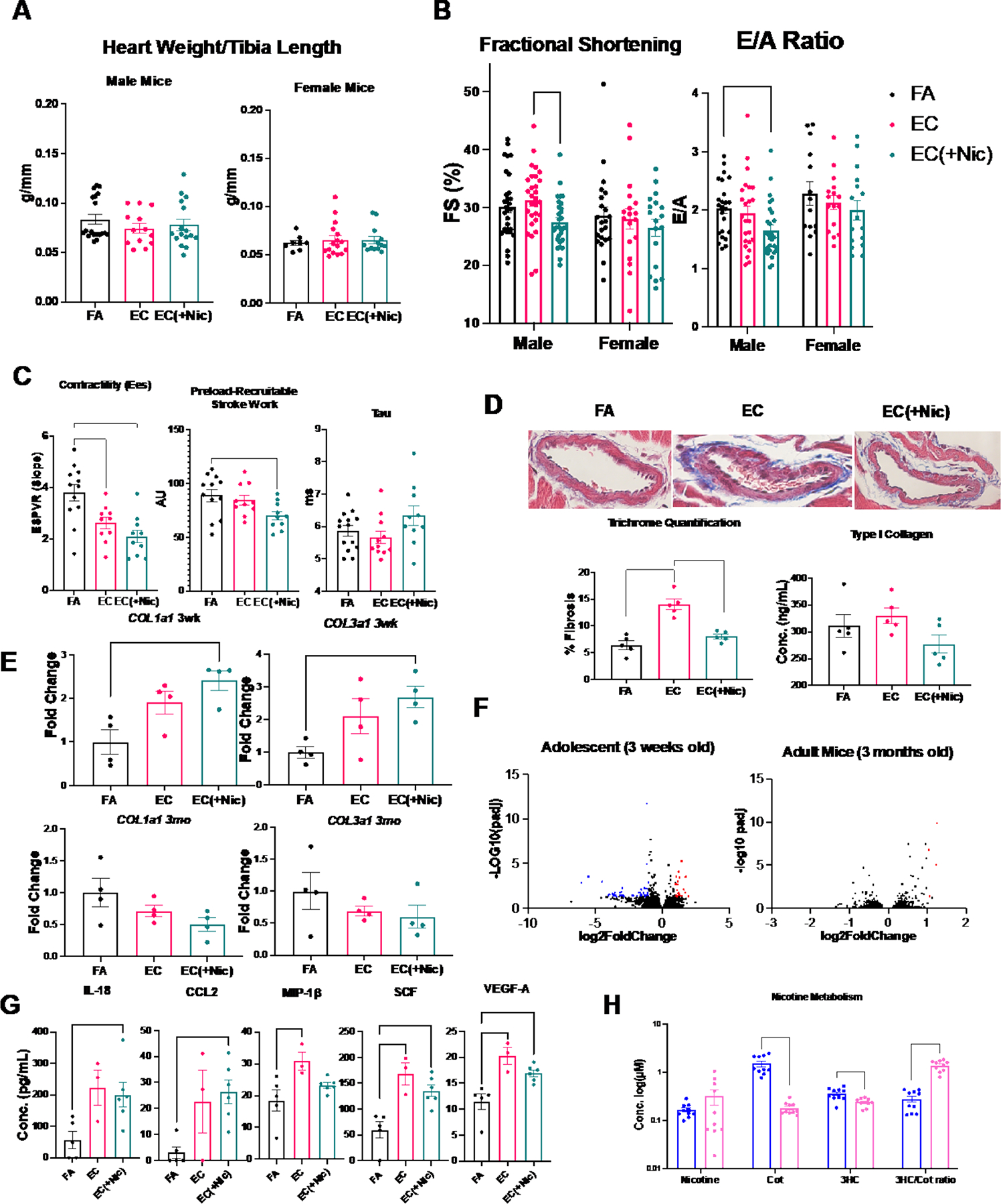
Male and female mice were exposed to either filtered air (FA), electronic cigarette aerosol without nicotine (EC), or with nicotine (EC(+Nic)). Data are expressed as mean ± SEM. *: p<0.05 via unpaired Student’s t-test, or one- or two-way ANOVA with post-hoc Tukey’s multiple comparison test. Statistical tests for RT-qPCR data were conducted on ΔCT values. Panel A: Heart weight/tibia length ratio of male and female mice (n = 8–19) after 3 months exposure. Panel B: Echocardiographic analysis of the left ventricle (LV) of male (n = 24–30) and female (n = 14–22) mice after 3 months of exposure. Male EC(+Nic) mice had reduced fractional shortening (%FS) and reduced transmitral early (E) to late (A) ventricular filling peak velocity ratio (E/A ratio) (*=P<0.05 via ANOVA). Panel C: PV loops from male mice exposed for 3 months: slope of the end-systolic pressure-volume relationship curve is lowered in EC and EC(+Nic) mice as indicated by reduced Ees, preload recruitable stroke work is also reduced in EC(+Nic) mice compared to FA, and Tau is unchanged (*=P<0.05 via ANOVA) Panel D: Representative perivascular images from male mice exposed for 3 months. Sections were subject to Masson’s trichrome staining and imaged using a bright-field light microscope at 60x magnification (top). Percent fibrosis was quantified as percent area of blue staining region averaged across at least 3 images per mouse (n = 5) using ImageJ software (bottom left). ELISA targeting type I collagen in the LV of male mice exposed for 3 months (n = 5, *=P<0.05, via ANOVA) (bottom right). Panel E: Gene expression of COL1A1 and COL3A1 in the LV of male mice exposed for either 3 weeks (3wk, top) or 3 months (3mo, bottom) (n = 4, *=P<0.05 via ANOVA). Panel F: Volcano plots from deseq2 analysis of RNA-sequencing of LV from hearts of adolescent and adult male mice; each dot represents a gene-blue or red color indicates the gene was down-regulated or up-regulated, respectively, with a cutoff value of 2-fold change and adjusted P<0.05. Panel G: Concentration of biomarkers in the serum of mice exposed to FA, EC, and EC(+Nic) (n = 3–6) for 3 months collected via cardiac puncture within 24 hours of exposure (*=P<0.05 via ANOVA). Panel H: Concentration of nicotine and metabolites in serum of male (blue) and female (red) EC(+Nic) mice exposed for 3 months (n = 10) collected via submandibular bleed within 4 hours of exposure (top) (*=P<0.05 via ANOVA).
To examine systemic changes manifested by EC, serum was analyzed for biomarkers using immunoassay (Ampersand Biosciences, Saranac Lake, NY). Adolescent male mice exposed to EC aerosol had significantly elevated serum interleukin (IL)-18, chemokine C-C Motif Chemokine Ligand (CCL)2, macrophage inflammatory protein (MIP)-1β, stem cell factor (SCF), and vascular endothelial growth factor (VEGF)-A (Figure [G]). While several of these alterations were nicotine dependent, vehicle alone was sufficient to cause some alterations, including MIP-1β, SCF and VEGF-A. Females exhibited a different serum biomarker profile, as only interferon (IFN)γ was increased compared to FA and vehicle. Thus, EC promoted systemic inflammation, which may cause further downstream changes to organ systems including the heart.
To investigate for sex-dependent differences, we examined nicotine metabolism using liquid chromatography-mass spectrometry. While nicotine concentrations in the serum of male and female mice exposed to EC with nicotine were similar, concentrations of metabolites including cotinine (cot) and 3-hydroxycotinine (3HC) were significantly lower in female compared to male mice. Further, the 3HC/cotinine ratio, a measure of cytochrome P450 2A6 (Cyp2A5 in mice) activity, a critical enzyme in nicotine metabolism,4 was significantly and substantially increased in female mice compared to male mice (Figure [H]). RT-qPCR of liver tissue targeting CYP2A5 also showed increased expression in females as compared to males, further supporting a more rapid nicotine metabolism in female mice (3-fold increase, P<0.05 via t-test). Since reductions in cardiac function and body weight were only observed in male mice exposed to EC aerosol with nicotine, this finding could suggest that females exhibit some degree of protection against EC aerosol from enhanced nicotine metabolism.
To our knowledge, this is the first study to evaluate cardiac function in adolescent mice exposed to EC aerosol. Our results heighten the concern for the dangers of EC use, specifically in youth, and call for further work detailing the mechanistic contributions to the observed cardiac dysfunction. While EC aerosol alone appeared to have a more mild effect on cardiac function, a synergistic effect of nicotine and its metabolites with reactive by-products of combustion, such as various aldehydes,5 could account for the more pronounced consequences following nicotine exposure. Regardless, the novel results of this study demonstrate that EC aerosol exposure can reduce cardiac function in developing male mice.
Acknowledgements:
We thank Drew Miller, Jacob Grimmer, and Michael Muffler for their assistance with mouse exposures and Drs. Gang Chen and Philip Lazarus for their nicotine metabolite analysis.
Sources of Funding:
This work was supported by salary support for Dr. Yael-Natalie H. Escobar (T32HL149637), the National Institutes of Health (R01 HL139348, R01 AG057046) and the American Heart Association (20YVNR35490079) to LEW.
Abbreviations:
- HEPA
high efficiency particulate air
- ELISA
enzyme-linked immunosorbent assay
- RT-qPCR
quantitative reverse transcription polymerase chain reaction
Footnotes
Disclosures: None
References
- 1.Miech R, Johnston L, O’Malley PM, Bachman JG, Patrick ME. Trends in Adolescent Vaping, 2017–2019. N Engl J Med. 2019;381:1490–1491. doi: 10.1056/NEJMc1910739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chun LF, Moazed F, Calfee CS, Matthay MA, Gotts JE. Pulmonary toxicity of e-cigarettes. Am J Physiol Lung Cell Mol Physiol. 2017;313:L193–L206. doi: 10.1152/ajplung.00428.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelin TD, Joseph AM, Gorr MW, Wold LE. Direct and indirect effects of particulate matter on the cardiovascular system. Toxicol Lett. 2012;208:293–299. doi: 10.1016/j.toxlet.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanner J-A, Novalen M, Jatlow P, Huestis MA, Murphy SE, Kaprio J, Kankaanpää A, Galanti L, Stefan C, George TP, et al. Nicotine metabolite ratio (3-hydroxycotinine/cotinine) in plasma and urine by different analytical methods and laboratories: implications for clinical implementation. Cancer Epidemiol Biomarkers Prev. 2015;24:1239–1246. doi: 10.1158/1055-9965.EPI-14-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee YO, Morgan-Lopez AA, Nonnemaker JM, Pepper JK, Hensel EC, Robinson RJ. Latent Class Analysis of E-cigarette Use Sessions in Their Natural Environments. Nicotine Tob Res. 2019;21:1408–1413. doi: 10.1093/ntr/nty164. [DOI] [PubMed] [Google Scholar]


