Abstract
Study Design:
Prospective observational study.
Objective:
We present the natural history, including survival and function, among participants in the prospective observational study of spinal metastases treatment (POST) investigation.
Summary of Background Data:
Surgical treatment has been touted as a means to preserve functional independence, quality of life, and survival. Nearly all prior investigations have been limited by retrospective design and relatively short-periods of post-treatment surveillance.
Methods:
This natural history study was conducted using the records of patients who were enrolled in the POST study (2017-2019). Eligible participants were 18 or older and presenting for treatment of spinal metastatic disease. Patients were followed at pre-determined intervals (1, 3, 6, 12 and 24-months) following treatment. We conducted cox proportional hazard regression analysis adjusting for confounders including age, biologic sex, number of co-morbidities, type of metastatic lesion, neurologic symptoms at presentation, number of metastases involving the vertebral body, vertebral body collapse, New England Spinal Metastasis Score (NESMS) at presentation, and treatment strategy.
Results:
We included 202 patients. Twenty-three percent of the population had died by 3-months following treatment initiation, 51% by 1-year, and 70% at 2-years. There was no significant difference in survival between patients treated operatively and non-operatively (p=0.16). No significant difference in HRQL between groups were appreciated beyond 3-months following treatment initiation. NESMS at presentation (scores of 0 [HR 5.61; 95% CI 2.83, 11.13] and 1 [HR 3.00; 95% CI 1.60, 5.63]) was significantly associated with mortality.
Conclusion:
We found that patients treated operatively and non-operatively for spinal metastases benefitted from treatment in terms of HRQL. Two-year mortality for the cohort as a whole was 70%. When prognosticating survival, the NESMS appears to be an effective utility, particularly among patients with scores of 0 or 1.
Level of Evidence:
II
Keywords: spinal metastases, survival, health-related quality of life, surgery, non-operative management, New England Spinal Metastasis Score
Introduction
Despite advances in the treatment of cancer, surgical techniques, radiotherapy, chemotherapy, and immunologics, the effective management of spinal metastatic disease remains challenging. Even in the context of technological advancements and improvements in peri-operative optimization and medical management, spinal metastases are overwhelmingly associated with functional decline, loss of independence, severe pain, and mortality.1–8 Many modern large-scale investigations report 50% survival at 1-year following diagnosis9,10. These realities complicate effective clinical decision making as management strategies must account for the patient’s capacity to benefit from surgery or non-operative care, while minimizing the potential for complications and accelerated functional deterioration.5,11
Over the last 15 years, surgical treatment has been touted as a means to preserve functional independence, quality of life and improve the prospect for survival.4,5,12–15 A balanced assessment of the literature supporting these notions, however, raises concern for both selection bias and confounding by indication. Non-randomized clinical series were limited as only patients with favorable survival characteristics were eligible for surgery, while those who would not tolerate surgical intervention, or who were deemed poor candidates, received palliative non-operative care.7,8,12–15 Those studies that used randomized approaches, or statistical techniques to account for bias in the decision for surgery, often still found advantages for surgery although the benefits tended to be more limited in nature.16,17 Nearly all prior investigations have been restricted to relatively short-periods of post-treatment observation, as well as the prospect of surveillance bias and patients being lost to follow-up. 1–4,7,8,13–15
We recently completed the Prospective Observational study of Spinal metastasis Treatment (POST), which was originally designed to examine aspects of the New England Spinal Metastasis Score (NESMS) and its prognostic capacity in the setting of spinal metastatic disease.18 The objective of this work was to present the natural history, including ultimate survival and functional outcomes, among the patients who participated in the POST study. We believe that these results can be used to help inform patients and families regarding natural history, anticipated outcomes and treatment trade-offs when considering operative and non-operative approaches at the time of presentation.
Materials and Methods
Data Collection
This natural history study was conducted using the complete records of patients who had participated in the POST study, which was initiated in 2017 at three participating centers following IRB approval: Brigham and Women’s Hospital, Massachusetts General Hospital and, Dana Farber Cancer Institute (all in Boston, MA). The inclusion and exclusion criteria, enrollment goals, means of data collection and study aims have all been previously published.18 In brief, eligible participants were 18 years or older and presenting for treatment of spinal metastatic disease at one of the three participating centers. Sixty-four percent of eligible candidates agreed to participate. Decisions regarding operative and non-operative management were made by treating clinicians.9,18 All patients consented to participation in the investigation and completed intake assessment, including abstraction of sociodemographic and clinical characteristics, baseline neurologic function graded according to the American Spinal Injury Association (ASIA) scale, determination of baseline prognostic scores (e.g. NESMS, Tokuhashi, Tomita and Spinal Instability Neoplastic Score [SINS]6,19) and health-related quality of life (HRQL; Euro-Quol-5 dimension [EQ-5D], Short Form [SF]-12 physical component score [PCS] and mental component score [MCS]).
Patients were then followed by study-stuff at pre-determined intervals (1-month, 3-months, 6-months and 12-months) following treatment, with re-evaluation of HRQL at each time-point and surveillance for study specific complications at 1- and 3-months following treatment initiation. Complications that were surveilled included: wound infections, skin/wound breakdown, venous thromboembolic disease (VTED; e.g. deep venous thrombosis and/or pulmonary embolism), sepsis, renal failure, myocardial infarction, pneumonia, urinary tract infection and other, including all other adverse events reported by patients. Observation regarding survival was continued through 24-months following treatment initiation. The study was completed on July 31, 2021.
Statistical Analysis
Comparisons between baseline sociodemographic and clinical characteristics among the operative and non-operative cohorts were made using the chi-square test for categorical variables and the t-test, or Wilcoxon rank-sum test, for parametric and non-parametric continuous data, respectively. Survival in the operative and non-operative groups was evaluated using Kaplan-Meier curves and the log-rank test. We conducted visual inspection of survival plots using KM curves to ensure the proportionality assumption was met in order to support cox proportional hazard regression analysis regarding survival. Factors considered in this analysis were based on conceptual model and included age, biologic sex, number of Deyo-modified Charlson co-morbidities, type of metastatic lesion (e.g. lytic, blastic or mixed), neurologic symptoms at presentation, number of metastases involving the vertebral body, vertebral body collapse, NESMS score at presentation and treatment strategy. The NESMS accounts for primary tumor characteristics and metastatic burden at baseline using the modified Bauer score and also incorporates general health and functional status via serum albumin and ambulatory capacity.18 Analyses using the NESMS relied on a score of 3 (the best possible score in the system18) as the referent. Ambulatory function, performance status and primary tumor type were not included in the cox regression analysis as these parameters are also factored into the NESMS calculation.9
Statistical significance was designated, a-priori, for factors with hazard ratios (HR) and 95% confidence intervals (CI) exclusive of 1.0 and p<0.05. All statistical analyses were conducted using STATA v 15.1 (STATA Corp.; College Station, TX) and all reporting adheres to the STROBE guidelines.
Results
Cohort Characteristics
There were 202 patients in total enrolled in the POST study. Median age at the time of presentation for the entire cohort was 61 (inter-quartile range: 54-69). Forty-five percent of the cohort as a whole was female, with 86% identified as White. The most common primary tumor types overall were lung cancer (20%), breast cancer (18%) and prostate cancer (14%). The most common region involved was the thoracic spine (38%), followed by the lumbar spine (18%), with 31% of the cohort demonstrating involvement in multiple spinal regions. Fifty-eight percent of all patients were independently ambulatory at presentation and 71% were considered neurologically intact (ASIA E).
The most common non-operative treatment modality was combined chemotherapy and radiation (79%). The most common surgical strategy was a fusion-based procedure (79%), of which 30% of the cohort in total also underwent a corpectomy. Fifty-five percent of surgical patients also received chemotherapy and radiation. Surgery was most frequently performed in the thoracic region (70%).
There was no significant difference between the operative and non-operative cohorts in terms of age (p=0.62), biologic sex (p=0.10), racial composition (p=0.66), body mass index (p=0.22), serum albumin at baseline (p=0.76) and the number of vertebral metastases (p=0.10; Table 1). The number of co-morbidities was slightly higher in the non-operative group (2.5 vs 2.3; p=0.02). There were greater degrees of vertebral body collapse in the operative cohort (32% vs 14%; p=0.006), as well as a higher percentage of lytic lesions (67% vs 50%; p=0.01). Patients treated surgically were also more likely to present with neurologic deficits (39% vs 20%; p=0.01).
Table 1.
Demographic and clinical characteristics of the operative and non-operative cohorts as determined at the time of initial presentation*.
| Characteristic | Non-Operative | Operative | p-value |
|---|---|---|---|
| Age | - | - | 0.62 |
| 50 or younger | 20 (17) | 14 (16) | - |
| 51-60 | 40 (35) | 26 (30) | - |
| 61-70 | 31 (27) | 31 (36) | - |
| 71 or older | 24 (21) | 16 (18) | - |
| Biologic Sex | - | - | 0.10 |
| Male Sex | 58 (50) | 54 (62) | - |
| Female Sex | 57 (50) | 33 (38) | - |
| White | 98 (85) | 76 (87) | 0.66 |
| Body Mass Index (mean, SD) | 26.9 (6.1) | 27.7 (6.1) | 0.22 |
| Number of Co-morbidities (mean, SD) | 2.5 (0.9) | 2.3 (0.8) | 0.02 |
| Vertebral Body Collapse | - | - | 0.006 |
| No collapse with <50% involvement | 19 (17) | 14 (16) | - |
| No collapse with ≥50% involvement | 38 (33) | 28 (32) | - |
| Collapse with <50% involvement | 42 (37) | 17 (20) | - |
| Collapse with ≥50% involvement | 16 (14) | 28 (32) | - |
| Serum Albumin | - | - | 0.76 |
| Albumin <3.5g/dL | 34 (30) | 24 (28) | - |
| Albumin ≥3.5g/dL | 81 (70) | 63 (72) | - |
| Vertebral Body Metastases | - | - | 0.10 |
| 1 | 21 (19) | 21 (25) | - |
| 2 | 17 (15) | 20 (24) | - |
| 3 or more | 74 (66) | 43 (51) | - |
| Neurologic Status at Presentation | - | - | 0.01 |
| Neurologic Intact | 91 (79) | 52 (60) | - |
| Neurologic Deficits | 23 (20) | 34 (39) | - |
| Type of Lesion | - | - | 0.01 |
| Blastic/Mixed | 58 (50) | 28 (33) | - |
| Lytic | 57 (50) | 57 (67) | - |
- All values are presented as raw number and percentage (rounded to the nearest whole number) except where noted.
The values of prognostic utilities were relatively evenly distributed across both operative and non-operative cohorts (Table 2), with no significant difference identified for the NESMS (p=0.76), Tokuhashi (p=0.62), and Tomita scales (p=0.90) between those who received surgery and those who did not. The SINS score, however, was significantly different between the operative (11) and non-operative (9.7) cohorts (p=0.006).
Table 2.
Prognostic score values among the operative and non-operative cohorts as determined at the time of initial presentation.
| Characteristic | Non-Operative | Operative | p-value |
|---|---|---|---|
| New England Spinal Metastases Score (number/percentage) | - | - | 0.76 |
| 0 | 16 (14) | 14 (16) | - |
| 1 | 26 (23) | 23 (26) | - |
| 2 | 48 (42) | 30 (34) | - |
| 3 | 25 (22) | 20 (23) | - |
| Tokuhashi Score (mean/SD) | 8.5 (2.9) | 8.3 (2.9) | 0.62 |
| Tomita Score (mean/SD) | 5.9 (2.5) | 5.9 (2.8) | 0.90 |
| Spinal Instability Neoplastic (SINS) Score (mean/SD) | 9.7 (3.1) | 11 (3.2) | 0.006 |
Survival
Average survival for the cohort as a whole was 248 days (SD 220.9), with 25% of the population surviving 76 days or less. Twenty-three percent of the population had died by 3-months following treatment initiation, 37% by 6-months, 51% by 1-year and 70% by the time of study completion. There was no significant difference in survival between patients treated surgically and those managed non-operatively (Figure 1; p=0.16). Visual inspection of the survival plots revealed that there was no violation of the proportionality assumption.
Figure 1.
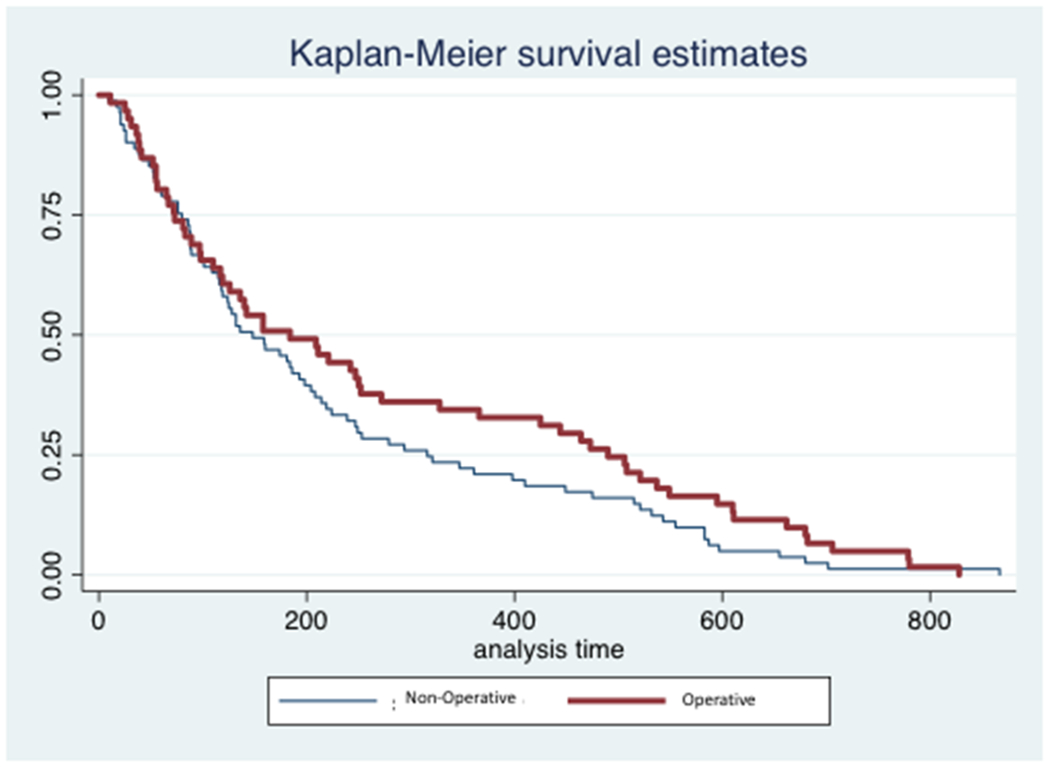
Kaplan-Meier curve displaying survival over the study-period for patients treated operatively and non-operatively for spinal metastatic disease. The x-axis (analysis time) represents time of survival expressed in days.
Complications
The complication rate at 3-months was 21% for the cohort as a whole, with 28% of those managed non-operatively sustaining a complication compared to 13% of patients treated surgically (p=0.009). The most common specific complications in both cohorts were VTED (n=4 in non-operative and 2 in operative) and pneumonia (n=4 in non-operative and 1 in operative). There was one instance of wound breakdown in the surgical cohort.
HRQL
Baseline HRQL was significantly lower in the cohort treated surgically than in the non-operative group, with EQ-5D ratings of 0.51 (SD 0.3) for operative patients compared to 0.63 (SD 0.2) among those receiving non-operative treatment (p=0.002). While improvements in EQ-5D were encountered across both groups, the surgical cohort achieved comparable ratings to the non-operative group starting 3-months after treatment initiation. No significant difference in scores between groups were appreciated beyond 3-months following treatment initiation. Among those who survived to 12-months, EQ-5D scores were above 0.7 on average for patients who received surgery and approximated 0.8 for those treated non-operatively. Similar patterns were encountered for the SF-12 PCS and MCS (Figure 2). Robust comparisons of HRQL measures over time across both cohorts were limited by the high mortality rate within the first year.
Figure 2.
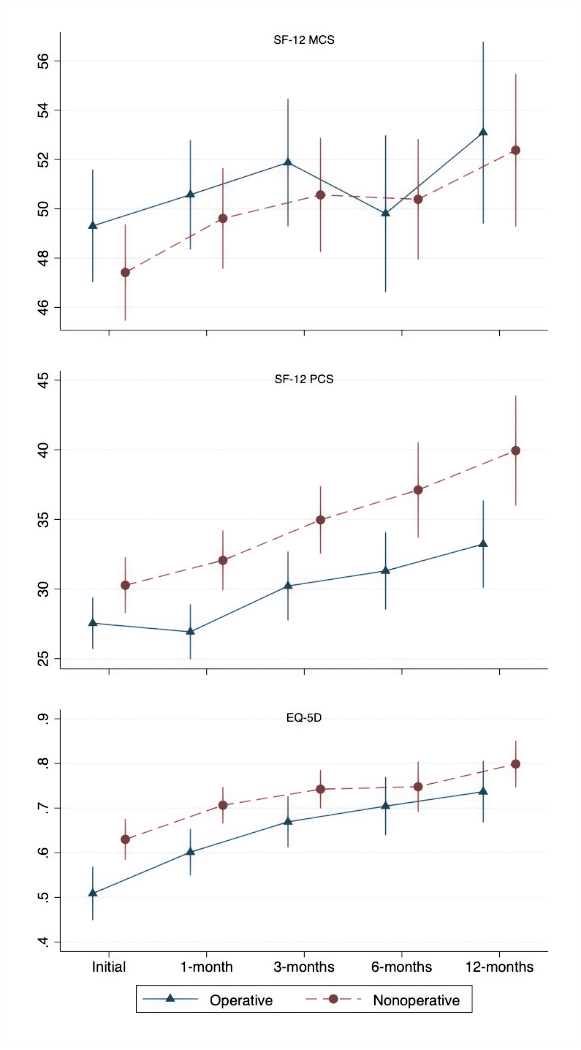
Health-related quality of life outcomes by treatment type over 1 year following treatment initiation. EQ-5D: EuorQol-5-Dimension; SF-12: Short Form survey-12; MCS: mental component score; PCS: physical component score. Data points are intentionally offset between groups to facilitate visibility but apply to the same study time-points.
Regression Model
In the multivariable cox proportional hazards regression analysis that accounted for potential confounders, relatively few parameters in our conceptual model were found to be significantly associated with mortality (Table 3). These included NESMS scores of 0 (HR 5.61; 95% CI 2.83, 11.13) and 1 (HR 3.00; 95% CI 1.60, 5.63). Relative to the referent score of 3, there was a trend toward increased mortality for a NESMS score of 2 (HR 1.67; 95% CI 0.94, 2.97), but this factor did not reach significance with the number of patients available (p=0.08).
Table 3.
Results of the multivariable cox proportional hazard regression model evaluating the adjusted association between clinical/sociodemographic characteristics at presentation with mortality over the course of the study. HR – hazard ratio; CI – confidence interval; Ref – referent
| Characteristics | HR | 95% CI | p-value |
|---|---|---|---|
| New England Spinal Metastases Score | |||
| 0 | 5.61 | 2.83, 11.13 | <0.001 |
| 1 | 3.00 | 1.60, 5.63 | 0.001 |
| 2 | 1.67 | 0.94, 2.97 | 0.08 |
| 3 | Ref | Ref | Ref |
| Male Sex | 1.18 | 0.80, 1.73 | 0.40 |
| Neurologic Deficits at Presentation | 1.39 | 0.87, 2.21 | 0.16 |
| Number of Co-morbidities | 1.01 | 0.80, 1.27 | 0.91 |
| Treatment Strategy | |||
| Non-operative | Ref | Ref | Ref |
| Operative | 0.68 | 0.44, 1.06 | 0.09 |
| Lytic Metastases | 0.92 | 0.62, 1.37 | 0.68 |
| Age | |||
| 50 or younger | Ref | Ref | Ref |
| 51-60 | 0.73 | 0.41, 1.30 | 0.29 |
| 61-70 | 0.52 | 0.29, 0.93 | 0.03 |
| 71 or older | 0.85 | 0.44, 1.63 | 0.63 |
| Vertebral Body Collapse | |||
| No collapse with <50% involvement | Ref | Ref | Ref |
| No collapse with ≥50% involvement | 1.07 | 0.57, 2.00 | 0.84 |
| Collapse with <50% involvement | 1.01 | 0.54, 1.86 | 0.99 |
| Collapse with ≥50% involvement | 0.66 | 0.34, 1.26 | 0.21 |
| Vertebral Body Metastases | |||
| 1 | Ref | Ref | Ref |
| 2 | 0.97 | 0.49, 1.93 | 0.93 |
| 3 or more | 0.89 | 0.49, 1.64 | 0.72 |
There was also a significant finding for patients aged 61-70 (HR 0.52; 95% CI 0.29, 0.93; p=0.03), but there was no evidence of a dose-response effect within the age category overall. Point estimates for HR varied across the age groups and no statistically significant findings were present for ages 51-60 (p=0.29) and 71 or older (p=0.63).
Discussion
As an end-stage manifestation of the cancer disease process, spinal metastases are known to be associated with limited life-expectancy.1–5,11,17 This complicates clinical decision-making, as the risks and benefits of interventions must be balanced against the potential for complications, innate functional declination, and the ability to tolerate treatment in an already frail patient population with multiple other co-morbidities.5,11 Patients with singular spinal metastatic processes and high levels of function at baseline are rarely encountered.10,17 More often, patients presenting for treatment with spinal metastases already have disease involvement in other visceral and osseous locations and are dealing with severe pain, neurologic symptoms, and ambulatory impairment.5,11,19 In this more common scenario, patients and families are most interested in preserving quality of life and independence.11 There is, however, limited research capable of informing discussions regarding treatment, with most available literature retrospective in nature with shorter term followup and prone to confounding by indication.1–4,7,8,13–15 Furthermore, few studies are capable of presenting balanced assessments of the natural history of patients with spinal metastases treated operatively and non-operatively in the same clinical setting.
In this context, we have presented a natural history study that leveraged prospectively collected data from the POST investigation, to delineate the clinical course over a 2-year period of more than 200 patients treated for spinal metastatic disease between 2017-2019. This study is advantaged over prior work given its prospective multi-center design, standardized observation at pre-determined study time-points, inclusion of patients treated operatively and non-operatively by the same clinical teams, and collection of a cohort of patients that encompasses the depth and breadth of clinical variation in spinal metastatic disease. This is exemplified by the normal distribution of prognostic scores across the NESMS, Tokuhashi, Tomita, and SINS utilities encountered within the POST study population.9 The clinical characteristics, including neurologic symptoms, ambulatory function, and baseline HRQL of our population are well aligned with other previously published works1–4,8,10,14,15,19, which we believe supports the translational capacity and generalizability of the findings presented here.
We found that, while patients treated operatively and non-operatively both benefitted from treatment in terms of improvement in HRQL measures, the mortality rate was sobering across both cohorts. We encountered close to 50% mortality at 1-year and 70% mortality by two-years, with no significant differences in mortality rates between the operative and non-operative groups at any time-point. In our cox regression analyses, the most influential factors were low NESMS at presentation (scores of 0 [HR 5.61; 95% CI 2.83, 11.13] and 1 [HR 3.00; 95% CI 1.60, 5.63]). There was a trend toward increased mortality for a NESMS score of 2 (HR 1.67; 95% CI 0.94, 2.97), but this factor did not reach significance (p=0.08). The point estimate, however, would suggest that in an even larger cohort of patients, individuals with a NESMS of 2 at presentation could be at elevated risk of mortality relative to those with a score of 3.
The NESMS accounts for primary tumor characteristics, metastatic burden, general health (via albumin) and functional capacity (via ambulatory ability).9,10,18 The NESMS was originally designed to prognosticate survival at 1-year and the POST study was powered to detect differences in mortality at that time-point.9,18 The NESMS has previously been validated as a prognostic tool with significant differences in survival at 1-year for all iterations relative to the idealized score of 3.9 Our results support the fact that this utility could also inform overall survival, although at time-points beyond 1-year the sensitivity between scores of 2 and 3 may be diminished given the limited life expectancy in patients with spinal metastases as a whole.
While prior studies with a high proclivity for confounding by indication maintained an association between surgical intervention and survival13–15, such a link was not observed in our cohort. The overall mortality rate for our cohort at both 1- and 2-years is aligned with other work, such as that of Ghori et al10, Turner et al14 and the prospective series of Fehlings et al20. In studies that have accounted for selection bias for surgery using causal inference techniques, or other statistical approaches, the survival benefit associated with surgery has been marginal at most and usually only realized over the course of the first 6-months following treatment.5,17 Surgery also impacts short-term quality of life while the patient recovers from the procedure and is more expensive than non-operative care. Surgery also theoretically has a higher complication profile5,11, although this is not always born out in all retrospective series17; nor was it encountered in the current investigation. Schoenfeld et al previously maintained that surgery is the most effective approach for patients with progressive neurologic deterioration, or acute loss of function, whether this be due to spinal instability or compression of neurologic structures from epidural spread.5 In light of these prior determinations and the results of our natural history study, we would maintain that the ideal treatment approach should be contextualized to each patient and there is unlikely to be a single management strategy suitable to all cases of spinal metastases. Patients presenting with acute neurologic deterioration, severe pain and reduced functional independence are likely to benefit from surgery, if practicable.
Limitations
Foremost, while this series of more than 200 subjects represents one of the largest prospective observational studies of patients treated operatively and non-operatively for spinal metastases, participants were enrolled from tertiary medical centers in a single city with the potential for clustering at the provider and institutional levels. Although we attempted to control for confounders through our adjusted statistical models, there is still the potential for residual confounding. We were unable to control for all factors due to the size of the sample and heterogeneity, which restricted the number of patients available for analysis based on histologic grade as well as the region of spinal involvement, among others. While we were able to demonstrate prognostic utility for several NESMS scores, we were underpowered for comparisons between scores of 2 and 3. This is attributable to the high associated mortality rate among patients with spinal metastases and we believe that it does not detract from the clinical utility of the score. We emphasize that our intent was to present a longitudinal, prospectively gathered, experience of patients with spinal metastases over the course of 2-year following treatment initiation. The findings should prove useful in informing patients and families regarding natural history, anticipated tradeoffs between various treatment approaches and the potential utility of the NESMS at informing longitudinal survival, even at time-points beyond 1-year. The results should not be seen as prescriptive and we maintain our findings should not be used to deny surgical intervention to patients who would otherwise be felt to benefit from an operative procedure. The NESMS was designed to prognosticate survival and previous work9 has demonstrated that the NESMS outperforms other scoring utilities in this parameter, including the Tokuhashi, Tomita and SINS scores. The SINS score, however, may be a more useful tool for determining spinal instability and anticipated need for surgical stabilization.
Conclusions
We found that patients treated operatively and non-operatively for spinal metastases benefitted from the selected treatment in terms of HRQL. One-year mortality for the cohort as a whole approximated 50%, with 70% mortality observed by 2-years. There was no appreciable survival advantage, overall, for those patients who received surgery. This supports the notion that surgical intervention is best reserved for those individuals who otherwise can derive demonstrable benefits in terms of quality of life, maintenance of ambulatory, or neurologic function and pain reduction. When prognosticating longitudinal survival, the NESMS appears to be an effective utility, particularly among patients with scores of 0 or 1.
Acknowledgement:
Contributors to the POST Study group also include: Drs. Michael Groff, Yi Lu, John Chi, Hasan Zaidi, Mai Anh Huynh, Alexander Spektor, Ayal Aizer, Karen Marcus and Larissa Lee. The authors thank Lauren Barton and Justin Blucher for their contributions to the data collection used in this investigation.
Funding Statement:
This research was supported in part by a grant from the Orthopaedic Research and Education Foundation (OREF). The OREF was not involved in the conduct of the study or the preparation of the manuscript. The findings and views expressed here are those of the authors and should not be viewed as reflective of the opinions of the OREF.
This research was supported in part by National Institutes of Health (NIH-NIAMS) grant K23-AR071464 to Dr. Schoenfeld. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the NIH or the Federal government.
Footnotes
Conflicts of Interest: None
Contributor Information
Grace X. Xiong, Harvard Combined Orthopaedic Residency Program, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115.
Miles W.A. Fisher, Department of Orthopaedic Surgery, San Antonio Military Medical Center, 3551 Roger Brooke Drive, Fort Sam Houston, TX 78234.
Joseph H. Schwab, Department of Orthopaedic Surgery, Massachusetts General Hospital, Harvard Medical School, 55 Fruit Street, Boston, MA 02114.
Andrew K. Simpson, Department of Orthopaedic Surgery, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115.
Lananh Nguyen, Department of Orthopaedic Surgery, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115.
Daniel G. Tobert, Department of Orthopaedic Surgery, Massachusetts General Hospital, Harvard Medical School, 55 Fruit Street, Boston, MA 02114.
Tracy A. Balboni, Department of Radiation Oncology, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115.
John H. Shin, Department of Neurosurgery, Massachusetts General Hospital, Harvard Medical School, 55 Fruit Street, Boston, MA 02114.
Marco L. Ferrone, Department of Orthopaedic Surgery, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115.
Andrew J. Schoenfeld, Department of Orthopaedic Surgery, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115.
References
- 1.Karhade AV, Thio QCBS, Ogink PT, Bono CM, Ferrone, Oh KS, Saylor PJ, Schoenfeld AJ, Shin JH, Harris MB, Schwab JH. Predicting 90-Day and 1-Year Mortality in Spinal Metastatic Disease: Development and Internal Validation. Neurosurgery. 2019;85: E671–E681. [DOI] [PubMed] [Google Scholar]
- 2.Paulino Pereira NR, Janssen SJ, van Dijk E, Harris MB, Hornicek FJ, Ferrone ML, Schwab JH. Development of a Prognostic Survival Algorithm for Patients with Metastatic Spine Disease. J Bone Joint Surg Am 2016;98: 1767–1776. [DOI] [PubMed] [Google Scholar]
- 3.Tang Y, Jintao Q, Qu J, Liu H, Chu T, Xiao J, Zhou Y. Effect of surgery on quality of life of patients with spinal metastasis from non-small-cell lung cancer. J Bone Joint Surg Am 2016;98: 396–402. [DOI] [PubMed] [Google Scholar]
- 4.Lo WY, Yang SH. Metastatic spinal cord compression (MSCC) treated with palliative decompression: Surgical timing and survival rate. PLoS One 2017;12: e0190342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schoenfeld AJ, Bensen GP, Blucher JA, Ferrone ML, Balboni TA, Schwab JH, Harris MB, Katz JN, Losina E. The cost-effectiveness of surgical intervention for spinal metastases: A model-based evaluation. J Bone Joint Surg Am 2021. Jul 21. doi: 10.2106/JBJS.21.00023. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smeijers S, Depreitere B. Prognostic scores for survival as decisional support for surgery in spinal metastases: a performance assessment systematic review. Eur Spine J. 2021;30(10):2800–2824. [DOI] [PubMed] [Google Scholar]
- 7.Alamanda VK, Robinson MM, Kneisl JS, Patt JC. Functional and survival outcomes in patients undergoing surgical treatment for metastatic disease of the spine. J Spine Surg. 2018;4(1):28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itshayek E, Candanedo C, Fraifeld S, Hasharoni A, Kaplan L, Schroeder JE, Cohen JE. Ambulation and survival following surgery in elderly patients with metastatic epidural spinal cord compression. Spine J. 2018. Jul;18(7):1211–1221. [DOI] [PubMed] [Google Scholar]
- 9.Schoenfeld AJ, Ferrone ML, Blucher JA, Agaronnik N, Nguyen L, Tobert DG, Balboni TA, Schwab JH, Shin JH, Sciubba DM, Harris MB. Prospective comparison of the accuracy of the New England Spinal Metastasis Score (NESMS) to legacy scoring systems in prognosticating outcomes following treatment of spinal metastases. Spine J 2021. Mar 16;S1529-9430(21)00118-2. doi: 10.1016/j.spinee.2021.03.007. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghori AK, Leonard DA, Schoenfeld AJ, Saadat E, Scott N, Ferrone ML, et al. Modeling one-year survival after surgery on the metastatic spine. Spine J 2015;15:2345–2350. [DOI] [PubMed] [Google Scholar]
- 11.Lape EC, Katz JN, Blucher JA, Chen AT, Silva GS, Schwab JH, et al. Patient experiences in decision-making in the treatment of spinal metastases: A qualitative study. Spine J 2020;20(6):905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi D, Fox Z, Albert T, Arts M, Balabaud L, Bunger C, Buchowski JM, Coppes MH, Depreitere B, Fehlings MG, Harrop J, Kawahara N, Martin-Benlloch JA, Massicotte EM, Mazel C, Oner FC, Peul W, Quraishi N, Tokuhashi Y, Tomita K, Verlaan JJ, Wang M, Wang M, Crockard HA. Rapid improvements in pain and quality of life are sustained after surgery for spinal metastases in a large prospective cohort. Br J Neurosurg 2016;30: 337–44. [DOI] [PubMed] [Google Scholar]
- 13.Depreitere B, Turner I, Vandoren C, Choi D. Cost-Utility Analysis of Surgery and Radiotherapy for Symptomatic Spinal Metastases in a Belgian Specialist Center. World Neurosurg. 2019;125: e537–e543. [DOI] [PubMed] [Google Scholar]
- 14.Turner I, Kennedy J, Morris S, Crockard A, Choi D. Surgery and Radiotherapy for Symptomatic Spinal Metastases Is More Cost Effective Than Radiotherapy Alone: A Cost Utility Analysis in a U.K. Spinal Center. World Neurosurg 2018;109: e389–e397. [DOI] [PubMed] [Google Scholar]
- 15.Miyazaki S, Kakutani K, Sakai Y, Ejima Y, Maeno K, Takada T, Yurube T, Terashima Y, Ito M, Kakiuchi Y, Takeoka Y, Hara H, Kawamoto T, Sakashita A, Okada T, Kiyota N, Kizawa Y, Sasaki R, Akisue T, Minami H, Kuroda R, Nishida K. Quality of life and cost-utility of surgical treatment for patients with spinal metastases: prospective cohort study. Int Orthop 2017;41: 1265–1271. [DOI] [PubMed] [Google Scholar]
- 16.Patchell RA, Tibbs PA, Regine WF, Payne R, Saris S, Kryscio RJ, Moiuddin M, Young B. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 2005;366: 643–648. [DOI] [PubMed] [Google Scholar]
- 17.Schoenfeld AJ, Losina E, Ferrone ML, Schwab JH, Chi JH, Blucher JA, Silva GS, Chen AT, Harris MB, Kang JD, Katz JN. Ambulatory status after surgical and non-surgical treatment for spinal metastasis. Cancer 2019;125: 2631–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schoenfeld AJ, Blucher JA, Barton LB, Schwab JH, Balboni TA, Chi JH, Shin JH, Kang JD, Harris MB, Ferrone ML. Design of the Prospective Observational study of Spinal metastasis Treatment (POST). Spine J 2020;20: 572–579. [DOI] [PubMed] [Google Scholar]
- 19.Versteeg AL, Sahgal A, Laufer I, Rhines LD, Sciubba DM, Schuster JM, Weber MH, Lazary A, Boriani S, Bettegowda C, Fehlings MG, Clarke MJ, Arnold PM, Gokaslan ZL, Fisher CG; AO Spine Knowledge Forum Tumor. Correlation Between the Spinal Instability Neoplastic Score (SINS) and Patient Reported Outcomes. Global Spine J. 2021. Jul 26:21925682211033591. doi: 10.1177/21925682211033591. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fehlings MG, Nater A, Tetreault L, Kopjar B, Arnold P, Dekutoski M, Finkelstein J, Fisher C, France J, Gokaslan Z, Massicotte E, Rhines L, Rose P, Sahgal A, Schuster J, Vaccaro A. Survival and Clinical Outcomes in Surgically Treated Patients With Metastatic Epidural Spinal Cord Compression: Results of the Prospective Multicenter AOSpine Study. J Clin Oncol. 2016. 20;34: 268–276. [DOI] [PubMed] [Google Scholar]


