Abstract
Purpose:
We report efficacy of a prospective Phase II trial (NCT00450411) of salvage low dose rate (LDR) prostate brachytherapy (BT) for local failure (LF) after prior external beam radiotherapy (EBRT) with minimum 5- years’ follow up.
Materials/Methods:
Eligible patients had low/intermediate risk prostate cancer (PCa) prior to EBRT and biopsy-proven LF > 30 months after EBRT, with PSA < 10 ng/mL and no regional/distant disease. The primary endpoint, late GI/GU Adverse Events (AEs) (CTCAE V3.0 ≥ Grade 3) was 14%. With minimum 5-year follow up after salvage BT, secondary clinical outcomes including disease-free (DFS; includes death from any cause), disease-specific (DSS), and overall survival (OS) were estimated using the Kaplan-Meier method and modelled using Cox proportional hazards regression. Local tumor progression (LF), distant and biochemical failure (DF/BF) were estimated using cumulative incidence. Time to LF, DF and BF were modeled by cause-specific Cox proportional hazards regression.
Results:
From 05/2007 –01/2014, 20 centers registered 100 patients (92 analyzable). Median follow up is 6.7 years (range: 0.3–11.2); median age 70 years (range: 55–82); median prior EBRT dose 74 Gy (IQR: 70–76) at a median of 85 months prior(IQR: 60–119). Androgen deprivation was combined with salvage BT in 16%. 10-year OS is 70% (95% confidence interval [CI]: 58%–83%). 19 patients died (5 PCa, 10 other, 4 unknown). 10-year failure rates are local 5% (95% CI:1–11), distant 19% (95% CI:10–29) and biochemical 46% (95% CI:34–57). DFS is 61% at 5 years; 33% at 10 years. No baseline characteristic was significantly associated with any clinical outcome.
Conclusion:
This is the first prospective multicenter trial reporting outcomes of salvage LDR BT for LF after EBRT. Five-year freedom from BF is 68%, comparable to other salvage modalities. Although further LF is rare (5%), BF climbs to 46% by10-years.
Keywords: Prostate cancer, radiotherapy, local failure, salvage brachytherapy, low dose rate brachytherapy
Introduction
Although local persistence of tumour is not infrequent after external beam radiotherapy (EBRT) for prostate cancer (1) (2) (3) (4) (5), local salvage therapy is rarely employed (6). Several options exist, including salvage prostatectomy, cryotherapy, high intensity focussed ultrasound, or whole gland or partial gland high dose rate (HDR) or low dose rate (LDR) brachytherapy. Most published results are retrospective from single centers(7) (8) (9) (10). Due to advanced patient age, co-morbidities and/or fear of toxicity, the most common approach remains androgen deprivation (11), which may be administered in a delayed or intermittent schedule.
In 2005, the RTOG, now NRG, commenced a phase II trial of salvage whole gland LDR brachytherapy. In 2018 the primary endpoint, the rate of late Grade 3 Gastrointestinal/Genitourinary adverse events (GI/GU AEs) with a minimum 2 years of follow up was reported at 14%. Now, with a minimum 5 years of follow up, we report clinical outcomes for efficacy in terms of survival, and biochemical, local and distant failure.
Materials and Methods
Eligible patients had received EBRT more than 30 months before enrollment (maximum prescribed dose 78 Gy/39 fractions or 81 Gy/45 fractions), had biopsy-proven local recurrence confirmed on central pathology review, prostate specific antigen (PSA) at trial entry < 10 ng/mL, and no evidence of regional or distant metastases on Tc99 bone scan and abdominal/pelvic computed tomography (CT). Original tumour presentation prior to EBRT was favorable or intermediate risk, PSA ≤ 20 ng/mL, Gleason Score ≤ 7 (Grade groups 1,2 and 3) and clinical stage ≤ T2c.
Treatment consisted of transperineal, template-guided LDR brachytherapy using either Iodine-125 (140 Gy minimum target dose) or Paladium-103 seeds (120 Gy minimum target dose). Partial prostate treatment was permitted if the dominant lesion was identified with appropriate biologic imaging (DCE CT, MRI or MRspect). Androgen deprivation therapy (ADT) was permitted in conjunction with salvage brachytherapy at the discretion of the treating physician for a maximum duration of 6 months.
The primary endpoint of late GI/GU AEs was previously reported (12). Secondary endpoints included clinical outcomes of overall survival (OS), disease-specific survival (DSS), disease-free survival (DFS), as well as local, distant and biochemical failure. For all secondary clinical outcome endpoints, all patients were to be followed for a minimum of 5 years. Follow up was mandated every 3 months for the first year post brachytherapy, every 6 months in years 2–5 and then annually. The time to failure was measured from the date of registration to the date of death from any cause for OS, date of death due to prostate cancer (including death in association with clinical tumor progression with or without further salvage therapy, or death from a complication of therapy) for DSS, and date of disease progression or date of death for DFS. DFS and OS were estimated using the Kaplan-Meier method with unadjusted and adjusted hazard ratios (HRs), with corresponding 95% confidence intervals (CIs) calculated from Cox proportional hazards regression models. DSS, local failure (LF), distant failure (DF), and biochemical failure (BF) were estimated using nonparametric estimation of cumulative incidence of the event of interest, accounting for competing risks of death without an event. The time to failure was measured from the date of registration for the trial to the date of LF (determined by clinical exam), BF (PSA ≥ current nadir + 2 ng/mL), or DF (documented lymphatic or hematogenous metastatic disease), respectively. Adjusted and unadjusted HRs for time to death from prostate cancer and LF, DF, and BF were obtained from cause-specific Cox proportional hazards regression models. Adjustment in all models was made for factors such as T-stage, PSA, Gleason score, and age.
Results
From May 2007 to January 2014 100 patients were enrolled from 20 centers. As 8 patients were excluded(12), the subsequent statistical analyses focused on the 92 eligible patients who received protocol treatment (12). Median age at study entry was 70 years. Zubrod performance status was 0 in 92% of eligible patients, and 52% had Gleason score 6 while 48% were Gleason 7. No patient had extracapsular extension clinically at baseline or at the time of salvage. Baseline PSA (prior to EBRT) was ≤10 ng/ml in 84% (median: 7.3 ng/ml). Median PSA prior to salvage LDR brachytherapy was 7.3 ng/ml (IQR: 5.5–9.3 ng/ml). Table 1 provides the clinical characteristics of the patients. Concurrent or prior ADT was prescribed for 16% of patients at the time of salvage. Only 2 patients received a partial prostate implant.
Table 1:
Pre-treatment clinical, tumor and treatment characteristics of the patients.
| Factor | Median | Range | Interquartile Range |
|---|---|---|---|
| Age (years) | 70 | 55–82 | 66–74 |
| TRUS volume (cm3) | 25 | 14–44 | 22–31 |
| Interval EBRT to BT (months) | 85.4 | 39.3–199.4 | 60–119.4 |
| EBRT dose (Gy) | 73.8 | 45–81 | 70.2–76 |
| Baseline PSA | 7.26 | 0.38–19.56 | 5.45–9.25 |
| Proportion | Proportion | ||
| Combined Gleason score | 3–6: 52% | 7: 48% | |
| Gleason 1+2 | 1.1% | ||
| Gleason 2+2 | 2.2% | ||
| Gleason 2+3 | 3.3% | ||
| Gleason 3+2 | 2.2% | ||
| Gleason 3+3 | 43.5% | ||
| Gleason 3+4 | 37.0% | ||
| Gleason 4+3 | 10.9% | ||
| Zubrod Performance Status | 0: 92% | 1: 8% | |
| T-Stage | T1: 48.9% | T2: 51.1% |
TRUS= Transrectal Ultrasound; EBRT=external beam radiation therapy, BT=brachytherapy, PSA=prostate specific antigen.
Median follow up is 6.7 years. The 10-year overall survival is 70% (95% CI: 58–83)(Figure 1). Of the 19 patients who died, 5 died due to the prostate cancer, 10 from other causes and 4 of unknown cause.
Figure 1:
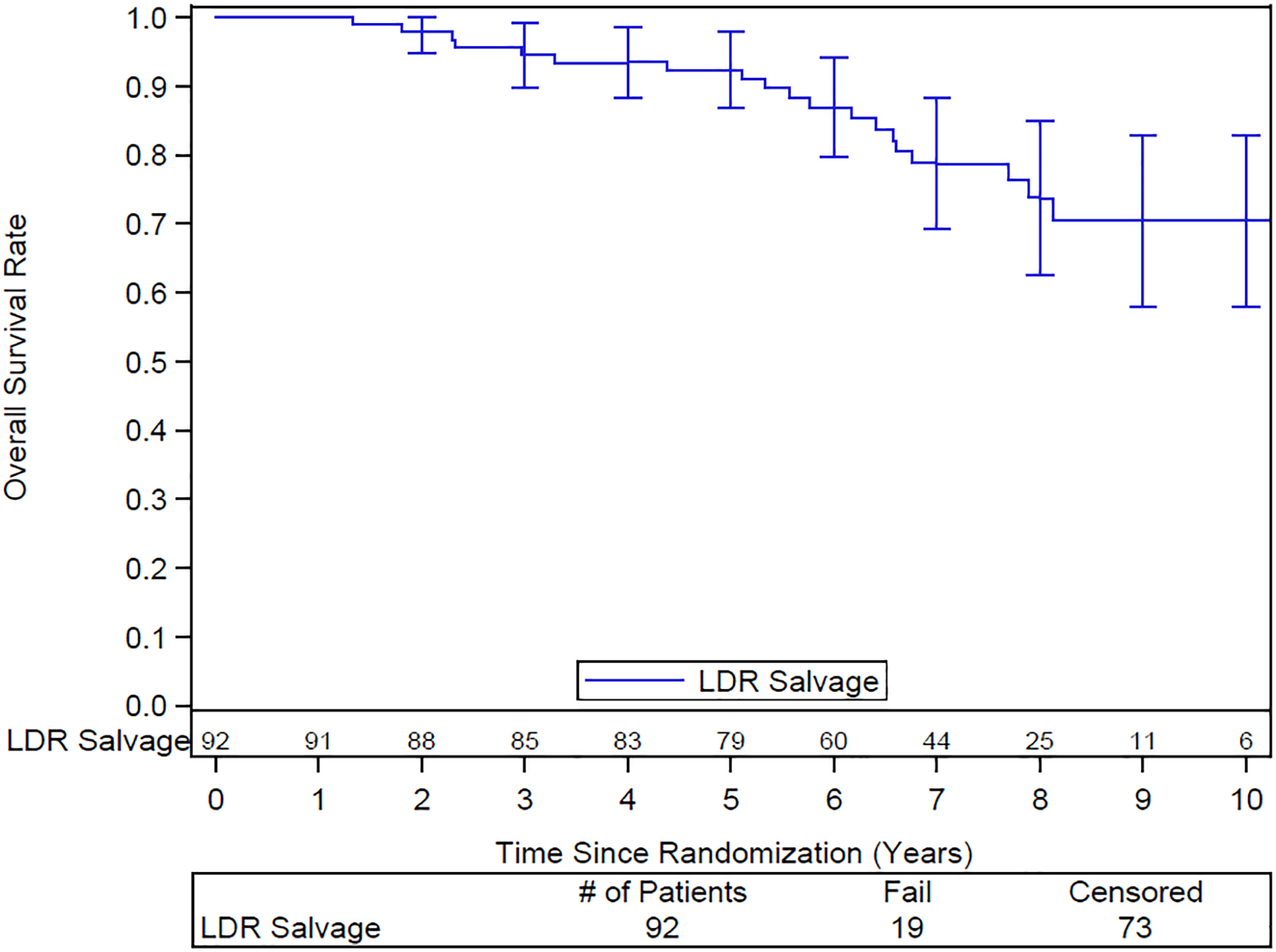
Overall survival. Numbers of patients at risk are shown above the x-axis.
The 10-year DFS rate is 33% (95% CI: 21–45). The 10-year DSS rate is 70% (95% CI: 58–83). 10-year distant failure rate is 19% (95% CI: 10–29) (Figure 2) and local failure 5% (95% CI: 2–11) (Figure 3). Only 2 of the 14 patients with distant failure also had local failure. Distant failures were detected at a median of 3.9 years (IQR 1.5–6.2). The 10-year biochemical failure rate is 46% (95% CI: 34–57) (Figure 4). The median PSA at 6 years for those patients who are failure free (n=51) was 0.1 ng/ml, suggesting a durable ablative effect of the salvage brachytherapy. Of the 37 patients for whom salvage was unsuccessful, 29 were treated with ADT, 1 received pelvic EBRT, 2 had surgery, and 5 had other forms of treatment.
Figure 2:
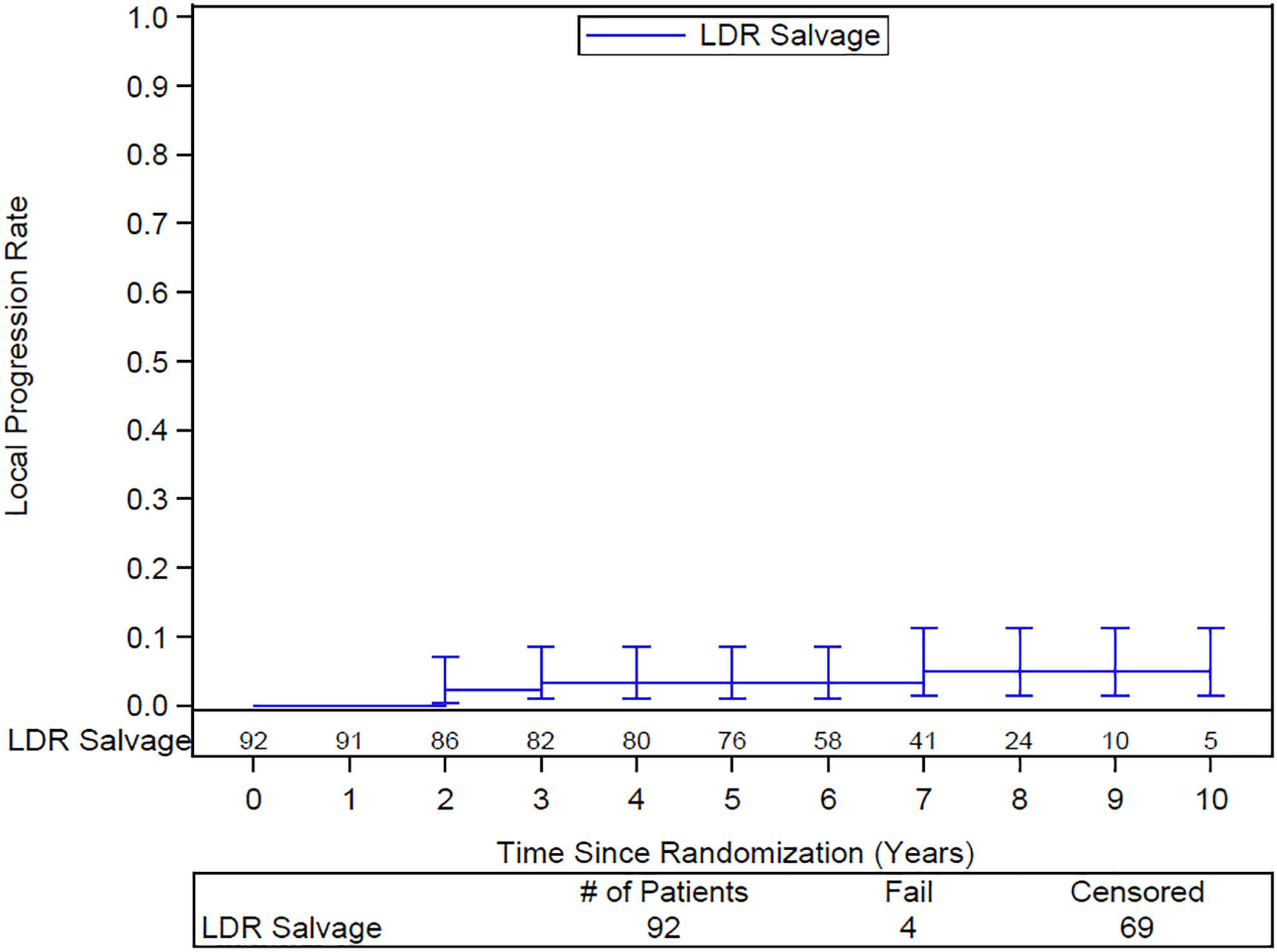
Actuarial local failure following salvage brachytherapy. Numbers of patients at risk are shown above the x-axis
Figure 3:
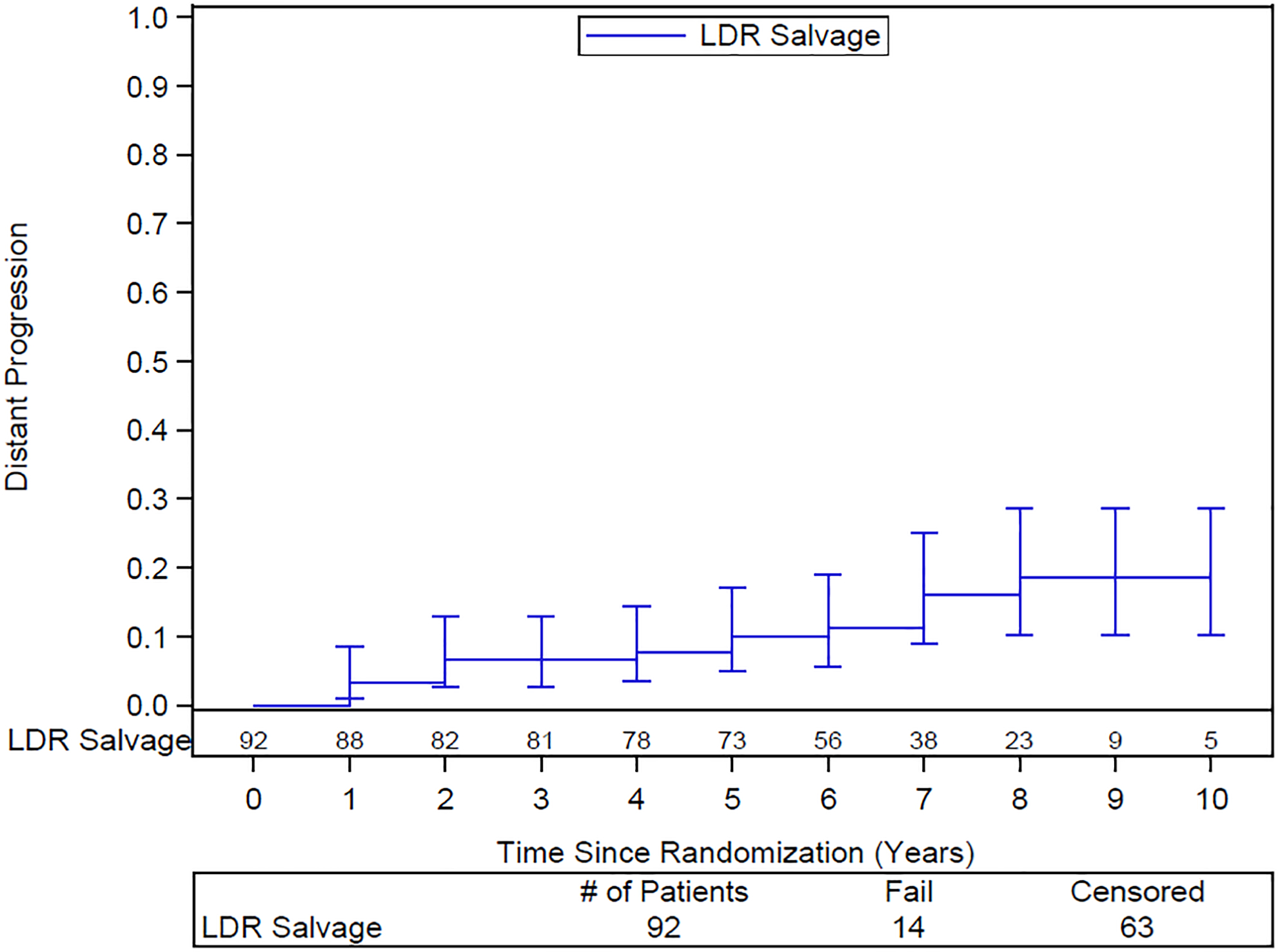
Actuarial distant failure after salvage brachytherapy. Numbers of patients at risk shown above the x-axis
Figure 4:
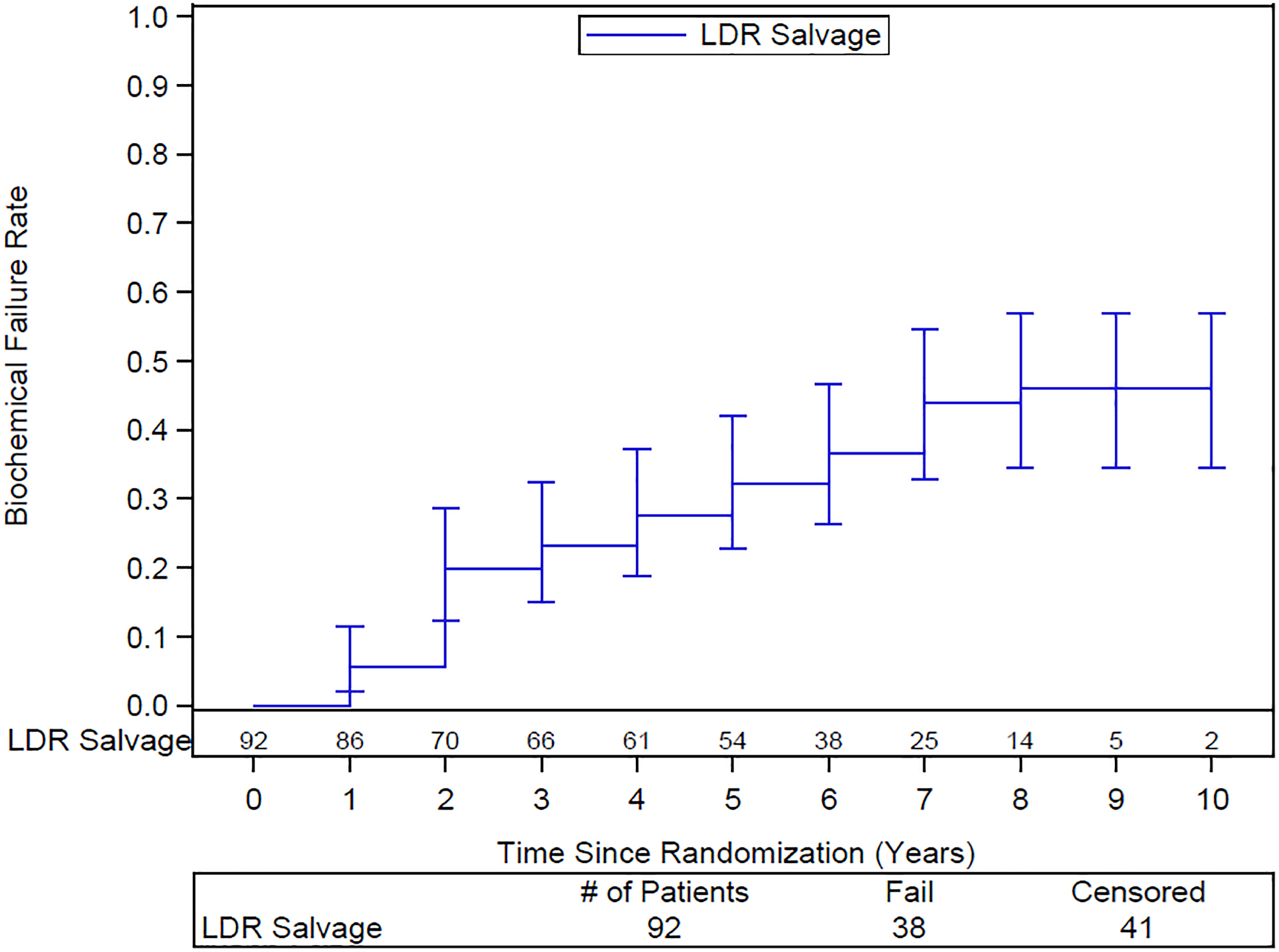
Actuarial rate of biochemical failure after LDR salvage brachytherapy. Numbers of patients at risk shown above the x-axis
None of the baseline characteristics, including proportion of the evaluated target volume covered by 100% of the prescription dose (V100) and time from completion of EBRT to brachytherapy implant were significantly associated with any clinical endpoint. Table 2 shows the Cox proportional hazards model.
Table 2:
Unadjusted Cox proportional hazards model for influence of clinical factors on efficacy outcomes.
| Factor | Overall Survival | Distant Failure | Local Failure | Biochemical Failure | |||||
|---|---|---|---|---|---|---|---|---|---|
| HR 95% CI | P value | HR 95% CI | P value | HR 95% CI | P value | HR 95% CI | P value | ||
| T stage | T1 vs. T2 | 1.41 (0.51–3.92) | 0.51 | 0.58 (0.24–1.39) | 0.22 | 0.61 (0.24–1.59) | 0.31 | 0.85 (0.39–1.85) | 0.69 |
| PSA | Continuous | 1.07 (0.93–1.23) | 0.33 | 0.93 (0.83–1.05) | 0.25 | 0.91 (0.80–1.03) | 0.14 | 0.91 (0.81–1.01) | 0.08 |
| Gleason Score | 2–6 vs. 7 | 2.20 (0.78–6.22) | 0.14 | 0.53 (0.22–1.28) | 0.16 | 0.4 (0.15–1.06) | 0.07 | 0.57 (0.26–1.26) | 0.16 |
| Age | Continuous | 1.09 (0.99–1.20) | 0.06 | 0.95 (0.89–1.02) | 0.19 | 0.96 (0.88–1.03) | 0.25 | 0.98 (0.92–1.04) | 0.43 |
| V100 | Continuous | 0.98 (0.91–1.06) | 0.59 | 1.04 (0.97–1.11) | 0.26 | 1.00 (0.93–1.08) | 0.89 | 1.03 (0.97–1.10) | 0.35 |
| Days from EBRT | Continuous | 1.00 (0.99–1.02) | 0.90 | 1.00 (0.99–1.02) | 0.64 | 1.00 (0.99–1.02) | 0.47 | 1.01 (0.99–1.02) | 0.33 |
| Dose EBRT (Gy) | Continuous | 1.01 (0.92–1.10) | 0.90 | 0.94 (0.85–1.04) | 0.21 | 0.97 (0.88–1.06) | 0.46 | 0.92 (0.84–1.00) | 0.050 |
HR=hazard ratio; CI=confidence interval; PSA=prostate specific antigen; EBRT=external beam radiation therapy; V100%=percentage of target volume receiving prescription brachytherapy dose
Discussion
EBRT is the mainstay of non-surgical management in all stages of prostate cancer, often combined with ADT for higher grade or more locally advanced disease. Although highly effective treatment, patients with longer follow up may experience a slowly rising PSA associated with local persistence or recurrence of viable tumour. Morris et al reported that in a randomized trial of 398 men with unfavorable intermediate or high-risk prostate cancer treated with 78 Gy plus 12 months of ADT, only 30% maintained a PSA < 0.2ng/ml while this was evident in 80% of those treated with the combination of EBRT plus brachytherapy and 12 months of ADT (13). Although a dose response relationship for EBRT has been established in multiple randomized trials of dose escalation (14)(15)(16)(17)(18), the optimal dose of EBRT has yet to be reached. Ample evidence exists for the benefit of the increased dose provided by the addition of brachytherapy (19) (20)(21).
Post-radiation biopsies demonstrate residual tumour in 21–51% of cases biopsied 2–3 years after radiotherapy (3)(5)(1). The frequency varies depending on tumor grade and stage, radiation dose and use of ADT (5)(22). Positive post treatment biopsies of 25% are reported even with a dose of 81 Gy or greater (2). Pucar et al have demonstrated through serial MRI and step-sectioning of salvage prostatectomy specimens that recurrence or persistence is usually at the site of the original dominant lesion, indicating relative radioresistance that may still be successfully managed if sufficient dose can be delivered(23). Persistent or recurrent local disease not only predicts biochemical failure but is associated with subsequent distant metastases and death from prostate cancer (2)(1)(3). Zelefsky et al reported on 382 patients who were biopsied at a median of 38 months after radiotherapy, 30% of whom had positive biopsies. With 15-year follow-up, the risk of distant metastases was 2.6 times higher in those with positive post treatment biopsies and the risk of death from prostate cancer twice as high (1).
Despite these potential consequences, local salvage modalities are rarely offered. Fewer than 5% of men with biochemical recurrence undergo biopsy for clarification of the site of disease and subsequent definitive treatment. Many options exist, including salvage prostatectomy, re-irradiation with either brachytherapy or stereotactic body radiotherapy (SBRT) or tissue destructive modalities such as cryotherapy or high intensity focussed ultrasound (HIFU). Patient selection is important in all modalities, but no striking differences exist in efficacy. Reported biochemical no evidence of disease rates (BNED) for whole gland cryotherapy are 67% at 3 years (24) and 39% at 10 years (25). For SBRT, BNED rates range similarly from 3-year 55% (n=100) (26) to 5-year 60% (n=50) (27). For HIFU Crouzet et al reported on 418 patients with a mean follow up of 3.5 years and found 5-year BNED rates of 58% for initially low risk disease, 51% for intermediate and 36% for patients with initially high risk disease (28). Salvage radical prostatectomy yields 5-year BNED rates of 47–82%, and at 10 years 28–53% (29). Reports of robot-assisted laparoscopic prostatectomy are still lacking the follow up to assess oncologic outcomes. Side effects are more frequent than in de novo treatments with each modality.
If local recurrence after prior EBRT is due to insufficient dose initially and is not totally radio-resistant, then further radiotherapy may be potentially curative. The highly conformal dose distribution achievable with brachytherapy is desirable for re-treatment in previously irradiated tissue in order to limit the dose to adjacent sensitive organs. Several single center reports confirmed feasibility and efficacy (7) (8) (9) prior to the RTOG/NRG initiating a multi-center Phase II trial in 2007.
Patient selection
The NRG/RTOG 0526 eligibility criteria concentrated on tumor-related and treatment-related factors and did not specify demographic criteria, which often carry more weight in determining the appropriateness for salvage treatment. Advanced patient age, diminished life expectancy and the presence of significant co-morbidities often preclude consideration of definitive salvage treatment.
NRG/RTOG 0526 only included patients who were more than 2.5 years from their initial treatment. This makes the interpretation of the biopsy more straightforward with fewer indeterminate results (30) and helps to eliminate those patients whose rising PSA is due to pre-existing metastatic disease (31)(32). All patients had EBRT administered in standard fractionation to a maximum dose of 81 Gy in 1.8 Gy fractions or 78 Gy in 2 Gy fractions. The safety and efficacy of salvage LDR brachytherapy has not been tested following hypofractionated EBRT or SBRT. In addition, to be eligible for NRG/RTOG 0526, the initial presentation of prostate cancer had to be either low or intermediate risk, and the PSA at the time of salvage under 10 ng/ml. Unfortunately, there was no PSA doubling time restriction on eligibility, despite doubling times < 6 months being associated with a component of distant failure (33)(34). The intent was to select a population with a lower risk of pre-existing metastases and a higher chance of ultimate cure. Two-thirds of patients were intermediate risk at initial presentation and despite these precautions, almost 20% of the treated population developed distant metastatic failure by 10 years. As ADT was not mandated in this study and was used for very few patients (16%), its role in combination with salvage brachytherapy cannot be evaluated. Clearly, if the indications for salvage brachytherapy evolve to include the high-risk scenario, ADT will be increasingly important.
Furthermore, the trial antedated availability of prostate specific membrane antigen (PSMA) positron emission tomography (PET) for investigation of patients with a rising PSA following definitive radiotherapy. PSMA-Ga68-PET or other PET-based scanning will play a prominent role in the future for early detection of metastatic disease when selecting patients for definitive salvage treatment. Detection rates with PSA < 0.5 ng/ml are 50–58%, 0.5–2.0 ng/ml 69–93% and > 2.0 ng/ml 86%−97% (35) (36) (37). Given the increased risk of toxicity of salvage treatment compared to the de novo scenario, a negative PSMA PET scan prior to embarking on salvage is recommended. This will be especially important if local salvage is being considered for a patient with initially high-risk disease where co-existent subclinical metastatic disease is more likely.
Partial prostate treatment
Although NRG/RTOG 0526 permitted partial prostate treatment if a focal lesion could be detected by the available metabolic imaging such as SPECT or MR spectroscopy, only 2 of the patients had less than a whole gland implant. The efficacy of focal salvage therapy cannot be evaluated with these data. Because of the available imaging the target for a partial prostate implant was required to be a minimum of 60% of the prostate volume. If focal salvage proves to be effective, toxicity may well be less than whole gland salvage, especially since grade 3 GU toxicity has been shown to be related to higher V100 signifying more complete coverage of the entire gland (12). Focal HDR salvage data is very encouraging with very low rates of grade 3 toxicity reported (38) (39) (40).
Subsequent LF after salvage brachytherapy was observed in only 5% of patients by 10 years. Determination of LF by DRE can be problematic after prostate irradiation such that persistent disease is often unsuspected. This would be especially true after re-irradiation with salvage brachytherapy. Confirmatory biopsies were strongly recommended in the protocol but infrequently collected.
Since LDR brachytherapy provides effective salvage of local recurrence after external beam radiotherapy, could this be preferable use of this modality compared to upfront incorporation as a “boost” in primary management? The cumulative grade III GU toxicity of LDR boost in primary management of high-risk disease reported in the Ascende trial of 18% is a disturbing disincentive to its use(21). Restricting LDR brachytherapy to those patients with LF after EBRT and no DF on PSMA PET might put fewer patients at risk for toxicity. To counter this argument, much of the grade 3 toxicity in Ascende was successfully managed and persistent toxicity at 5 years was only 8%. Furthermore, other multicenter studies of EBRT plus an LDR boost reported much lower rates of late grade 3 GU toxicity (41,42). Further study of LDR brachytherapy to compare upfront boost vs. delayed salvage could address this question with patient reported outcomes playing a key role.
Conclusion
Whole gland salvage LDR brachytherapy is an effective and well-tolerated modality for salvage of local recurrence after definitive radiotherapy in patients selected to have a reasonable chance of cure based on the initial stage and grade at diagnosis, and the timing of the subsequent recurrence. At 10 years, local failure is 4.9%. The toll of distant failure may be reduced by incorporating advanced imaging in the selection criteria. Even without that, the actuarial rate of biochemical failure at 10 years of 46% compares well to other available modalities.
Funding Statement:
This project was supported by grants UG1CA189867 (NCORP), U10CA180868 (NRG Oncology Operations), and U10CA180822 (NRG Oncology SDMC) from the National Cancer Institute (NCI).
Footnotes
Conflict of Interest: Dr.’s Bice, Crook, Helou, Amin, Beyer, Horwitz, Michalski, Murtha, Pisansky, Morton, Raben, Roach III, Rodgers, Trabulsi, and Vigneault have nothing to disclose. Dr. Jani reports personal fees from Blue Earth Diagnostics, Ltd., outside the submitted work; Dr. Pugh reports other from Pfizer, other from Millennium, outside the submitted work; Dr. Sandler reports being a member of the ASTRO Board of Directors.
Data Sharing Statement: Data will be made available per the NCTN Data Archiving Rules-https://nctn-data-archive.nci.nih.gov/
Clinical Trial Registration: NCT00450411
ASTRO Journals: American Society for Radiation Oncology (ASTRO)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (1).Zelefsky MJ, Goldman DA, Reuter V, Kollmeier M, McBride S, Zhang Z, et al. Long-Term Implications of a Positive Posttreatment Biopsy in Patients Treated with External Beam Radiotherapy for Clinically Localized Prostate Cancer. J Urol 2019. Jun;201(6):1127–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Zelefsky MJ, Reuter VE, Fuks Z, Scardino P, Shippy A. Influence of Local Tumor Control on Distant Metastases and Cancer Related Mortality After External Beam Radiotherapy for Prostate Cancer. J Urol 2008. Feb 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Zapatero A, Minguez R, Nieto S, Martin de Vidales C, Garcia-Vicente F. Posttreatment prostate biopsies in the era of three-dimensional conformal radiotherapy: what can they teach us? Eur Urol 2009. Apr;55(4):902–909. [DOI] [PubMed] [Google Scholar]
- (4).Pollack A, Zagars GK, Antolak JA, Kuban DA, II R. Prostate biopsy status and PSA nadir level as early surrogates for treatment failure: analysis of a prostate cancer randomized radiation dose escalation trial. Int J Radiat Oncol Biol Phys 2002. Nov 1;54(3):677–85. [DOI] [PubMed] [Google Scholar]
- (5).Nichol A, Chung P, Lockwood G, Rosewall T, Divanbiegi L, Sweet J, et al. A phase II study of localized prostate cancer treated to 75.6 Gy with 3D conformal radiotherapy. Radiother Oncol 2005. Jul;76(1):11–17. [DOI] [PubMed] [Google Scholar]
- (6).Tran H, Kwok J, Pickles T, Tyldesley S, Black PC. Underutilization of local salvage therapy after radiation therapy for prostate cancer. Urol Oncol 2014. Jul;32(5):701–706. [DOI] [PubMed] [Google Scholar]
- (7).Grado GL, Collins JM, Kriegshauser JS, Balch CS, Grado MM, Swanson GP, et al. Salvage brachytherapy for localized prostate cancer after radiotherapy failure. Urology 1999. Jan;53(1):2–10. [DOI] [PubMed] [Google Scholar]
- (8).Beyer DC. Permanent brachytherapy as salvage treatment for recurrent prostate cancer. Urology 1999. Nov;54(5):880–883. [DOI] [PubMed] [Google Scholar]
- (9).Wong WW, Buskirk SJ, Schild SE, Prussak KA, Davis BJ. Combined prostate brachytherapy and short-term androgen deprivation therapy as salvage therapy for locally recurrent prostate cancer after external beam irradiation. J Urol 2006. Nov;176(5):2020–2024. [DOI] [PubMed] [Google Scholar]
- (10).Nguyen PL, Chen RC, Clark JA, Cormack RA, Loffredo M, McMahon E, et al. Patient-reported quality of life after salvage brachytherapy for radio-recurrent prostate cancer: A prospective Phase II study. Brachytherapy 2009. Oct-Dec;8(4):345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Agarwal PK, Sadetsky N, Konety BR, Resnick MI, Carroll PR, Cancer of the Prostate Strategic Urological Research Endeavor (CaPSURE). Treatment failure after primary and salvage therapy for prostate cancer: likelihood, patterns of care, and outcomes. Cancer 2008. Jan 15;112(2):307–314. [DOI] [PubMed] [Google Scholar]
- (12).Crook J, Zhang P, Pisansky T, et al. A Prospective phase II trial of trans-perineal ultrasound-guided brachytherapy for locally recurrent prostate cancer after external beam radiotherapy. Int J Radiat Oncol Biol Phys 2018;103:335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Morris WJ, Pickles T, Keyes M. Using a surgical prostate-specific antigen threshold of >0.2 ng/mL to define biochemical failure for intermediate- and high-risk prostate cancer patients treated with definitive radiation therapy in the ASCENDE-RT randomized control trial. Brachytherapy 2018. Nov - Dec;17(6):837–844. [DOI] [PubMed] [Google Scholar]
- (14).Peeters ST, Heemsbergen WD, Koper PC, van Putten WL, Slot A, Dielwart MF, et al. Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol 2006. May 1;24(13):1990–1996. [DOI] [PubMed] [Google Scholar]
- (15).Dearnaley DP, Jovic G, Syndikus I, Khoo V, Cowan RA, Graham JD, et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol 2014. Apr;15(4):464–473. [DOI] [PubMed] [Google Scholar]
- (16).Zietman AL, Bae K, Slater JD, Shipley WU, Efstathiou JA, Coen JJ, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from proton radiation oncology group/american college of radiology 95–09. J Clin Oncol 2010. Mar 1;28(7):1106–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Beckendorf V, Guerif S, Le Prise E, Cosset JM, Bougnoux A, Chauvet B, et al. 70 Gy versus 80 Gy in localized prostate cancer: 5-year results of GETUG 06 randomized trial. Int J Radiat Oncol Biol Phys 2011. Jul 15;80(4):1056–1063. [DOI] [PubMed] [Google Scholar]
- (18).Pasalic D, Kuban DA, Allen PK, Tang C, Mesko SM, Grant SR, et al. Dose Escalation for Prostate Adenocarcinoma: A Long-Term Update on the Outcomes of a Phase 3, Single Institution Randomized Clinical Trial. Int J Radiat Oncol Biol Phys 2019. Jul 15;104(4):790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Kishan AU, Cook RR, Ciezki JP, Ross AE, Pomerantz MM, Nguyen PL, et al. Radical Prostatectomy, External Beam Radiotherapy, or External Beam Radiotherapy With Brachytherapy Boost and Disease Progression and Mortality in Patients With Gleason Score 9–10 Prostate Cancer. JAMA 2018. Mar 6;319(9):896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Liss AL, Abu-Isa EI, Jawad MS, Feng FY, Vance SM, Winfield RJ, et al. Combination therapy improves prostate cancer survival for patients with potentially lethal prostate cancer: The impact of Gleason pattern 5. Brachytherapy 2015. Jul-Aug;14(4):502–510. [DOI] [PubMed] [Google Scholar]
- (21).Morris WJ, Tyldesley S, Rodda S, Halperin R, Pai H, McKenzie M, et al. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An Analysis of Survival Endpoints for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost to a Dose-Escalated External Beam Boost for High- and Intermediate-risk Prostate Cancer. Int J Radiat Oncol Biol Phys 2017. Jun 1;98(2):275–285. [DOI] [PubMed] [Google Scholar]
- (22).Zelefsky MJ, Yamada Y, Fuks Z, Zhang Z, Hunt M, Cahlon O, et al. Long-term results of conformal radiotherapy for prostate cancer: impact of dose escalation on biochemical tumor control and distant metastases-free survival outcomes. Int J Radiat Oncol Biol Phys 2008. Jul 15;71(4):1028–1033. [DOI] [PubMed] [Google Scholar]
- (23).Pucar D, Hricak H, Shukla-Dave A, Kuroiwa K, Drobnjak M, Eastham J, et al. Clinically significant prostate cancer local recurrence after radiation therapy occurs at the site of primary tumor: magnetic resonance imaging and step-section pathology evidence. Int J Radiat Oncol Biol Phys 2007. Sep 1;69(1):62–69. [DOI] [PubMed] [Google Scholar]
- (24).Williams AK, Martinez CH, Lu C, Ng CK, Pautler SE, Chin JL. Disease-free survival following salvage cryotherapy for biopsy-proven radio-recurrent prostate cancer. Eur Urol 2011. Sep;60(3):405–410. [DOI] [PubMed] [Google Scholar]
- (25).Spiess PE, Levy DA, Pisters LL, Mouraviev V, Jones JS. Outcomes of salvage prostate cryotherapy stratified by pre-treatment PSA: update from the COLD registry. World J Urol 2013. Dec;31(6):1321–1325. [DOI] [PubMed] [Google Scholar]
- (26).Pasquier D, Martinage G, Janoray G, Rojas DP, Zerini D, Goupy F, et al. Salvage Stereotactic Body Radiation Therapy for Local Prostate Cancer Recurrence After Radiation Therapy: A Retrospective Multicenter Study of the GETUG. Int J Radiat Oncol Biol Phys 2019. Nov 15;105(4):727–734. [DOI] [PubMed] [Google Scholar]
- (27).Fuller D, Wurzer J, Shirazi R, Bridge S, Law J, Crabtree T, et al. Retreatment for Local Recurrence of Prostatic Carcinoma After Prior Therapeutic Irradiation: Efficacy and Toxicity of HDR-Like SBRT. Int J Radiat Oncol Biol Phys 2020. Feb 1;106(2):291–299. [DOI] [PubMed] [Google Scholar]
- (28).Crouzet S, Blana A, Murat FJ, Pasticier G, Brown SCW, Conti GN, et al. Salvage high-intensity focused ultrasound (HIFU) for locally recurrent prostate cancer after failed radiation therapy: Multi-institutional analysis of 418 patients. BJU Int 2017. Jun;119(6):896–904. [DOI] [PubMed] [Google Scholar]
- (29).Chade DC, Eastham J, Graefen M, Hu JC, Karnes RJ, Klotz L, et al. Cancer control and functional outcomes of salvage radical prostatectomy for radiation-recurrent prostate cancer: a systematic review of the literature. Eur Urol 2012. May;61(5):961–971. [DOI] [PubMed] [Google Scholar]
- (30).Crook JM, Bahadur YA, Robertson SJ, et al. Evaluation of radiation effect, tumor differentiation, and prostate specific antigen staining in sequential prostate biopsies after external beam radiotherapy for patients with prostate carcinoma. Cancer 1997;79:81–89 [PubMed] [Google Scholar]
- (31).Buyyounouski MK, Hanlon AL, Horwitz EM, Pollack A. Interval to biochemical failure highly prognostic for distant metastasis and prostate cancer-specific mortality after radiotherapy. Int J Radiat Oncol Biol Phys 2008. Jan 1;70(1):59–66. [DOI] [PubMed] [Google Scholar]
- (32).Shilkrut M, McLaughlin PW, Merrick GS, Vainshtein JM, Feng FY, Hamstra DA. Interval to biochemical failure predicts clinical outcomes in patients with high-risk prostate cancer treated by combined-modality radiation therapy. Int J Radiat Oncol Biol Phys 2013. Jul 15;86(4):721–728. [DOI] [PubMed] [Google Scholar]
- (33).Nguyen PL, D’Amico AV, Lee AK, Suh WW. Patient selection, cancer control, and complications after salvage local therapy for postradiation prostate-specific antigen failure: a systematic review of the literature. Cancer 2007. Oct 1;110(7):1417–1428. [DOI] [PubMed] [Google Scholar]
- (34).D’Amico AV, Moul J, Carroll PR, Sun L, Lubeck D, Chen MH. Surrogate end point for prostate cancer specific mortality in patients with nonmetastatic hormone refractory prostate cancer. J Urol 2005. May;173(5):1572–1576. [DOI] [PubMed] [Google Scholar]
- (35).Emmett L, van Leeuwen PJ, Nandurkar R, Scheltema MJ, Cusick T, Hruby G, et al. Treatment Outcomes from (68)Ga-PSMA PET/CT-Informed Salvage Radiation Treatment in Men with Rising PSA After Radical Prostatectomy: Prognostic Value of a Negative PSMA PET. J Nucl Med 2017. Dec;58(12):1972–1976. [DOI] [PubMed] [Google Scholar]
- (36).Afshar-Oromieh A, Holland-Letz T, Giesel FL, Kratochwil C, Mier W, Haufe S, et al. Diagnostic performance of (68)Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging 2017. Aug;44(8):1258–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Morigi JJ, Stricker PD, van Leeuwen PJ, Tang R, Ho B, Nguyen Q, et al. Prospective Comparison of 18F-Fluoromethylcholine Versus 68Ga-PSMA PET/CT in Prostate Cancer Patients Who Have Rising PSA After Curative Treatment and Are Being Considered for Targeted Therapy. J Nucl Med 2015. Aug;56(8):1185–1190. [DOI] [PubMed] [Google Scholar]
- (38).Yamada Y, Kollmeier MA, Pei X, Kan CC, Cohen GN, Donat SM, et al. A Phase II study of salvage high-dose-rate brachytherapy for the treatment of locally recurrent prostate cancer after definitive external beam radiotherapy. Brachytherapy 2014. Mar-Apr;13(2):111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Maenhout M, Peters M, van Vulpen M, Moerland MA, Meijer RP, van den Bosch MAAJ, et al. Focal MRI-Guided Salvage High-Dose-Rate Brachytherapy in Patients With Radiorecurrent Prostate Cancer. Technol Cancer Res Treat 2017. Dec;16(6):1194–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Slevin F, Hodgson S, Rodda SL, Bownes P, Bottomley D, Adiotomre E, et al. Efficacy and toxicity outcomes for patients treated with focal salvage high dose rate brachytherapy for locally recurrent prostate cancer. Clin Transl Radiat Oncol 2020. Mar 27;23:20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Lee WR, Bae K, Lawton C, Gillin M, Morton G, Firat S, et al. Late toxicity and biochemical recurrence after external-beam radiotherapy combined with permanent-source prostate brachytherapy: analysis of Radiation Therapy Oncology Group study 0019. Cancer 2007. Apr 15;109(8):1506–1512. [DOI] [PubMed] [Google Scholar]
- (42).Hurwitz MD, Halabi S, Archer L, McGinnis LS, Kuettel MR, DiBiase SJ, et al. Combination external beam radiation and brachytherapy boost with androgen deprivation for treatment of intermediate-risk prostate cancer: long-term results of CALGB 99809. Cancer 2011. Dec 15;117(24):5579–5588. [DOI] [PMC free article] [PubMed] [Google Scholar]


