Abstract
Survival after blood or marrow transplantation (BMT) for inborn errors of metabolism (IEM) is excellent; however, the burden of morbidity in long-term survivors of BMT for IEM remains understudied. This study examined the risk of chronic health conditions (CHC) in ≥2-year survivors of allogeneic BMT for IEM performed between 1974 and 2014 using the BMT Survivor Study. In this retrospective cohort study, participants (or their parents; n = 154) reported demographic data and CHCs (graded using Common Terminology Criteria for Adverse Events version 5), and transplantation characteristics were obtained from institutional databases. Unaffected siblings (n = 494) served as a comparison group. Logistic regression was used to estimated the odds of severe/life-threatening CHCs compared with siblings. Cox proportional hazards regression was used to estimate factors associated with severe/life-threatening/fatal CHCs in survivors of BMT for IEM. Survivors of allogeneic BMT for IEM (leukodystrophies, 43.5%; mucopolysaccharidoses, 41.0%) were at 12.5-fold higher odds of severe/life-threatening CHCs (95% confidence interval [CI], 5.4 to 28.9) compared with their siblings. The mean 10-year post-BMT cumulative incidence of grade 3–5 CHCs was 47.5 ± 4.0%. Reduced-intensity conditioning (RIC) was associated with a 2.7-fold higher risk (95% CI, 1.2 to 6.2; P = .02) of any grade 3–5 CHC, a 6.7-fold higher risk of grade 3–5 cardiopulmonary conditions (95% CI, 1.3 to 35.4), and a 3.0-fold higher risk of severe hearing/vision deficits (95% CI, 1.4 to 6.6). Older (age >26 years) BMT survivors were significantly less likely to graduate from college (odds ratio [OR], 0.3; 95% CI, 0.1 to 0.7) or marry (OR, 0.01; 95% CI, 0.004 to 0.07) compared with their siblings. Survivors of BMT for IEM carry a significant burden of morbidities, which affects their ability to attain adult milestones. Efforts to reduce chronic health conditions in this population are needed.
Keywords: Inborn errors of metabolism, Morbidity, Chronic health conditions, Blood or marrow transplantation
INTRODUCTION
Inborn errors of metabolism (IEM) constitute a heterogeneous group of inherited disorders and can be broadly classified as mucopolysaccharidoses (eg, Hurler syndrome, Hunter syndrome), leukodystrophies (eg, X-linked adrenoleukodystrophy, metachromatic leukodystrophy, globoid leukodystrophy), or other lysosomal disorders (eg, fucosidosis, mannosidosis). Left untreated, IEMs result in progressive decline in organ function and premature death. Novel therapies, such as enzyme replacement for Hurler syndrome [1] and gene therapy for leukodystrophies [2,3], may mitigate the disease burden, but allogeneic blood or marrow transplantation (BMT) remains the standard of care for several IEM. Improvements in transplantation practices (improved graft selection, early BMT when indicated, avoidance of reduced-intensity conditioning [RIC] and T cell depletion) have resulted in improved outcomes, including lower rates of graft failure and improved functional status post-BMT [4,5]. However, mortality rates remain elevated for decades post-BMT compared with the age- and sex-matched general US population [6–8], and progressive organ dysfunction from primary disease remains the leading cause of death [6]. Therefore, it is important to understand the long-term burden of morbidity experienced by BMT recipients to inform follow-up for early detection and mitigation of morbidities.
Compromised functional health in patients with Hurler syndrome [4,9] and progressive neurologic deterioration and premature death in patients with leukodystrophies [10–12] have been reported several years after BMT. However, data are lacking on the long-term cumulative burden of chronic health conditions in patients with IEM treated with BMT compared with unaffected siblings. There is also limited information regarding functional outcomes among survivors. We sought to describe the risk of chronic health conditions among survivors of BMT for IEM using the resources offered by the BMT Survivor Study (BMTSS).
METHODS
The BMTSS is a retrospective cohort study that seeks to describe the long-term outcomes after BMTs performed between 1974 and 2014 at 1 of 3 transplantation centers in the United States (City of Hope, University of Minnesota and University of Alabama at Birmingham). Inclusion criteria for this analysis consisted of allogeneic BMT for IEM and survival of ≥2 years after BMT, irrespective of current vital status. A randomly selected cohort of siblings (not necessarily related to the BMT survivors and never having undergone BMT themselves) constituted a comparison group. The Human Subjects Committee at each participating site approved the study; the University of Alabama Birmingham served as the single Institutional Review Board of record. Informed consent was obtained in accordance with the Declaration of Helsinki.
Data Collection
Details regarding primary IEM, age at BMT, preparative regimens (specific chemotherapeutic agents with or without total body irradiation [TBI]), conditioning intensity (myeloablative/RIC), stem cell source (bone marrow, cord blood, or peripheral blood stem cells [PBSCs]), type of donor (related/unrelated), >1 BMT (yes/no), and history of chronic graft-versus-host disease (GVHD) were collected from institutional transplant databases. IEM diagnoses were grouped into 3 primary categories: leukodystrophies (X-linked adrenoleukodystrophy [X-ALD], metachromatic leukodystrophy [MLD], and globoid leukodystrophy [GLD]), mucopolysaccharidoses (Hurler syndrome, Hunter syndrome, Maroteaux-Lamy syndrome, and Sanfilippo syndrome), and other IEM (all remaining IEM).
Parents of participants (BMT recipients and siblings age <18 years at the study or ≥18 years at the study but unable to complete the survey) or participants (if age ≥18 years at the study and able to complete the survey independently) completed a BMTSS survey by mail, by phone, or online and reported the presence or absence of chronic health conditions as diagnosed by a healthcare provider. An example of a question in the survey addressing chronic health conditions is as follows: “Has a doctor or other healthcare professional told you that you have or have had the following health conditions... deafness in both ears not completely corrected by hearing aid?. If yes, can you provide age of onset?” The survey elicited sociodemographic characteristics: sex, race/ethnicity, highest education, marital status, employment, annual household income, and health insurance). It also assessed the ability of survivors to carry out activities of daily living (ADLs; eg, eating, bathing, dressing) and instrumental activities of daily living (IADLs; eg, shopping). The reliability and validity of the BMTSS questionnaire have been verified, and the responses confirm that survivors are able to report adverse medical conditions with accuracy [13].
Chronic health conditions after BMT were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5 [14], and were classified as grade 1 (mild), grade 2 (moderate), grade 3 (severe), grade 4 (life-threatening), or grade 5 (death from a chronic health condition). Cause of death was ascertained from the National Death Index Plus program and/or medical records. Chronic health conditions reported by siblings (or their parents) were classified as grade 1 to 4 (all siblings were alive at study participation). For participants with more than 1 chronic health condition, the condition associated with the highest grade was included in the analysis. A detailed description of questions included in the BMTSS survey, the corresponding chronic health condition categories, and the scoring are presented in the Supplementary Material.
Statistical Analyses
The prevalence of chronic health conditions (grade 1–4, grade 1–2, grade 3–4) was compared between BMT survivors who were alive at study (all IEMs taken together, as well as subcategories of IEMs) and siblings. The cumulative incidence of chronic health conditions by attained age was estimated for survivors and siblings. Logistic regression models were used to estimate the odds of grade 3–4 CHCs among survivors (entire cohort as well as IEM subcategories) compared with siblings. Models were adjusted for age at study (continuous variable), sex, race/ethnicity (non-Hispanic white; others), education (less than high school; high school and/or some college; college graduate/postgraduate), annual household income (<$20,000/≥$20,000), and current health insurance (yes/no).
In the analyses restricted to BMT recipients (alive at study, as well as those who had died after surviving ≥2 years after BMT), the cumulative incidence rates of any grade 1–5 chronic health condition and any grade 3–5 condition were estimated using competing-risk methods, with death from other causes treated as a competing risk. Factors associated with grade 3–5 conditions were estimated using Cox proportional hazards models and included demographics, age at BMT, stem cell source (bone marrow, cord blood, or PBSCs), donor type (related/unrelated), conditioning intensity (myeloablative/RIC), use of TBI (yes/no), year of BMT (<2000, 2000 to 2009, ≥2010), >1 BMT (yes/no), chronic GVHD status (yes/no), and GVHD prophylaxis (cyclosporine [yes/no], mycophenolate mofetil [yes/no] and T cell depletion [yes/no]).
In a subgroup analysis restricted to survivors age >26 years at the study (to allow time for graduation from college), we examined functional outcomes including maximum education, employment, and marital status, as well as the need for assistance with ADL and IADLs. We examined the following factors associated with requiring assistance with ADLs and IADLs after adjusting for age at study: sex, race/ethnicity, year of BMT, stem cell source, donor type, conditioning intensity, use of TBI, chronic GVHD status, and grade 3–4 chronic health conditions. Owing to a low number of events for certain outcomes, parsimonious models were created that included factors with P < .10 in multivariable models.
Variables with >10% missing values were excluded (continuous) or included as “missing” (categorical). All statistical tests were 2-sided, and P < .05 was considered to indicate statistical significance. Analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Overall, 269 patients with IEM survived for ≥2 years after allogeneic BMT; of these, 48 (17.8%) died after surviving ≥2 years. Of the 221 alive BMT recipients, 21 (9.5%) were lost to follow-up. Of the 200 patients approached, 106 completed the survey (53%) and 94 (47%) refused participation. As shown in Supplementary Table S1, compared with nonresponders, responders were more likely to be non-Hispanic whites (92.5% versus 80.6%; P = .009) and to have received bone marrow/PBSC transplantation (67% versus 47.6%; P = .003) and less likely to have received an unrelated donor BMT (62.3% versus 81.6%; P = .002).
Table 1 summarizes the demographic and clinical characteristics of the 154 BMT recipients (106 alive; 48 deceased). The median age at BMT was 3.0 years (range, 0.2 to 44.2 years), and the median duration of follow-up was 10.8 years (range, to 38.1 years); 49.3% were at >10 years post-BMT at study participation. The majority of the BMT recipients were non-Hispanic white (90.3%), and the majority received myeloablative conditioning (81.8%). The prevalence of chronic GVHD in the cohort was 9.8%. Leukodystrophies (43.5%) and mucopolysaccharidoses (41.0%) were the most prevalent IEM categories (Supplementary Table S2). Patients with leukodystrophies were older at BMT compared to patients with mucopolysaccharidoses or other IEMs (median age, 8.8 years [range, 0.2 to 44.2 years] versus 1.6 years [range, 0.4 to 20.4 years] versus years [range, 0.2–23.1 years]; P < .0001) (Supplementary Table S3).
Table 1.
Demographic and Clinical Characteristics of IEM Patients Treated with BMT
| Characteristic | Entire Cohort (N = 154) | Alive Participants (N = 106) |
|---|---|---|
| Age at study participation, yr | ||
| Median (range) | 18.2 (2.7–56.5) | 19.8 (4.6–56.5) |
| Mean ± SD | 19.3 ± 11.6 | 21.4 ± 11.1 |
| Sex, n (%) | ||
| Male | 101 (65.6) | 78 (73.6) |
| Race/ethnicity, n (%) | ||
| Non-Hispanic white | 139 (90.3) | 98 (92.5) |
| Hispanic | 8 (5.2) | 4 (3.8) |
| Other | 7 (4.5) | 4 (3.8) |
| Education, n (%) | ||
| Less than high school | - | 37 (44.6) |
| High school and/or some college | - | 36 (43.4) |
| College graduate or higher education | - | 10 (12.0) |
| Annual household income, n (%) | ||
| ≤$20,000 | - | 26 (32.1) |
| >$20,000 | - | 55 (67.9) |
| Current health insurance, n (%) | ||
| Yes | - | 105 (99.0) |
| Age at BMT, yr | ||
| Median (range) | 3.0 (0.2–44.2) | 3.7 (0.2–42.3) |
| Time from BMT to study participation (or death), yr | ||
| Median (range) | 10.8 (2.1–38.1) | 15.2 (3.0–38.1) |
| Primary IEM diagnosis, n (%) | ||
| Leukodystrophies | 67 (43.5) | 50 (47.2) |
| Mucopolysaccharidoses | 63 (41.0) | 48 (45.2) |
| Other IEMs | 24 (15.5) | 8 (7.6) |
| Year of BMT, n (%) | ||
| <2000 | 77 (50.0) | 55 (51.9) |
| 2000–2009 | 51 (33.1) | 28 (26.4) |
| ≥2010 | 26 (16.9) | 23 (21.7) |
| Stem cell source, n (%) | ||
| Bone marrow | 100 (64.9) | 70 (66.0) |
| Cord blood | 52 (33.8) | 36 (33.0) |
| PBSCs | 2 (1.3) | 1 (1.0) |
| Type of donor, n (%) | ||
| Unrelated donor | 99 (64.3) | 66 (62.3) |
| Type of conditioning, n (%) | ||
| Myeloablative conditioning | 126 (81.8) | 89 (84.0) |
| Conditioning agents, n (%) | ||
| Busulfan + cyclophosphamide | 77 (50.0) | 54 (50.9) |
| Cyclophosphamide + TBI4 | 24 (15.6) | 19 (17.9) |
| Fludarabine + melphalan | 28 (18.2) | 17 (16.0) |
| Other conditioning | 25 (16.2) | 16 (15.1) |
| >1 BMT | ||
| Yes | 20 (13.0) | 12 (11.3) |
| Chronic GVHD, n (%) | ||
| Yes | 15 (12.2) | 6 (6.7) |
| No | 108 (87.8) | 84 (93.3) |
| GVHD prophylaxis, n (%) | ||
| Cyclosporine | 138 (93.2) | 99 (96.1) |
| Mycophenolate mofetil | 48 (32.4) | 34 (33.0) |
| T cell depletion | 45 (30.4) | 30 (29.1) |
Percent calculations exclude 23 participants who did not report education status and 25 participants who did not report household income. Chronic GVHD status was not available for 31 patients in the entire cohort and for 16 patients among those alive at study participation (excluded from percent calculations). Six patients were missing information regarding GVHD prophylaxis, among whom 3 were alive at study participation.
Chronic Health Conditions in BMT Survivors and Siblings
Supplementary Table S4 summarizes the demographic characteristics of the 106 BMT survivors who were alive at study and their 494 siblings. Supplementary Figure S1 shows the prevalence of grade 3–4 chronic health conditions among BMT survivors and siblings, and Supplementary Table S5 highlights the most prevalent chronic health conditions among survivors, overall and by disease category. Hearing impairments (27.4%), legal blindness (25.5%), poor balance/vertigo (12.3%), and cataracts (10.4%) were the most prevalent grade 3–4 CHCs in the overall cohort. Grade 3–4 hearing impairment (50.0%) and blindness (28.0%) were the most prevalent CHCs among survivors of mucopolysaccharidoses and leukodystrophies, respectively. Survivors were more likely to have a greater number of grade 1–4 chronic health conditions (≥2: 73.6% versus 28.3%; ≥3: 12.3% versus 0.2%; P < .001) and grade 3–4 conditions (≥2: 28.3% versus 1.6%; ≥3: 12.3% versus 0.2%; P < .001) compared with siblings (Supplementary Table S6). There were no differences in median grade 3–4 CHCs per patient by era of transplantation (<2000: 1 [range, 0 to 4]; 2000–2009: 1 [range, 0 to 5]; ≥2010: 1 [range, 0 to 2]; P = .7).
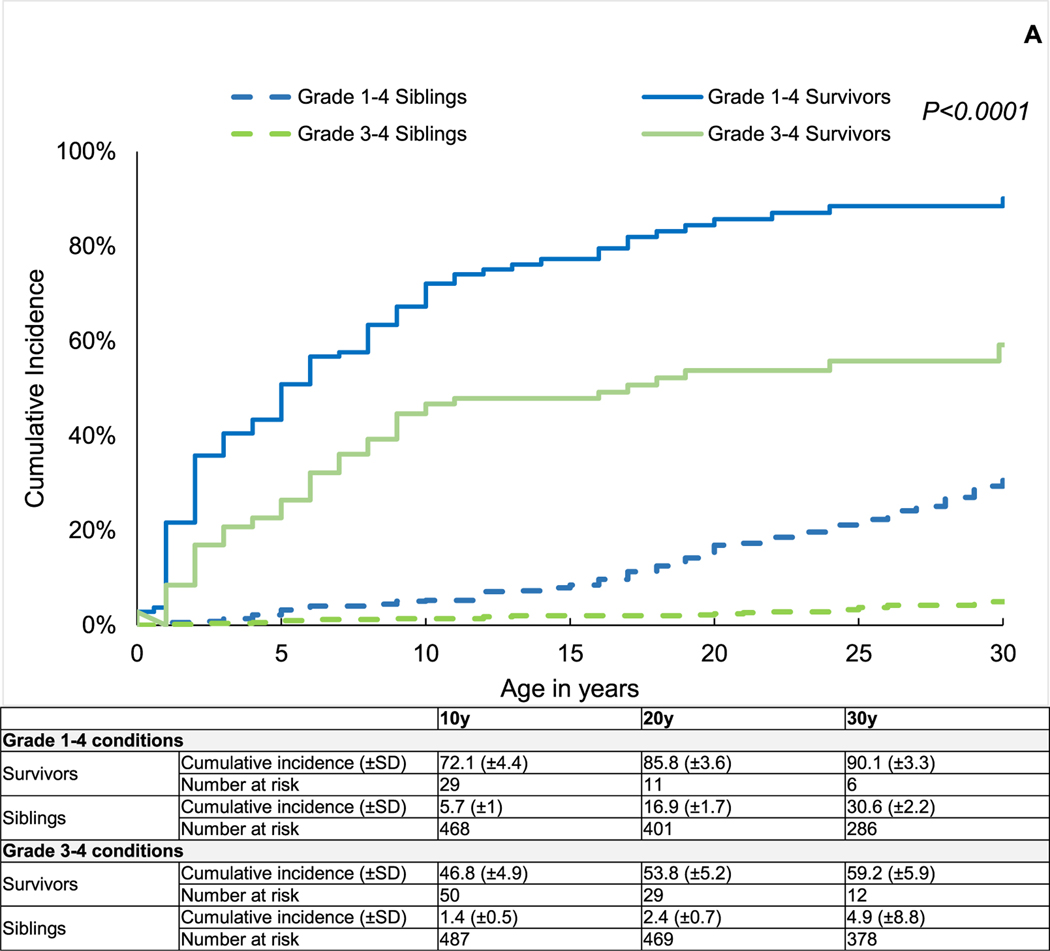
As shown in Figure 1A, by age 10 years, the mean cumulative incidence of chronic health conditions was significantly higher among survivors compared with siblings (grade 1–4: 72.1 ± 4.4% versus 5.7 ± 1.0%, P < .0001; grade 3–4: 46.8 ± 4.9% versus 1.4 ± 0.5%, P < .0001).
Figure 1.
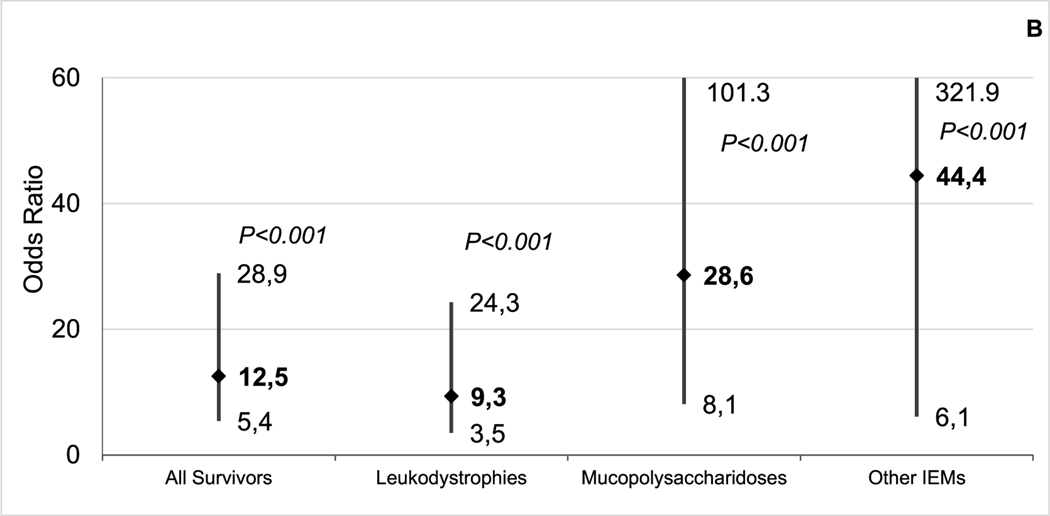
(A) Cumulative incidence of chronic health conditions among 2-year survivors of BMT for IEM and siblings by attained age. (B) Multivariable regression models for grade 3–4 chronic health conditions among 2-year survivors of BMT for IEM compared with siblings. Models adjusted for age at study participation, sex, race/ethnicity (non-Hispanic white versus other), education (less than high school, high school plus some college, college graduate or higher), household income (<$20,000 versus $20,000), and current health insurance (yes/no).
After adjusting for age at study participation, sex, race/ethnicity, education, annual household income, and current health insurance, BMT survivors were significantly more likely than their siblings to have grade 3–4 chronic health conditions (odds ratio [OR], 12.5; 95% CI, 5.4 to 28.9; P < .001) (Figure 1B), and the odds of developing chronic health conditions remained elevated across IEM subcategories (Figure 1B).
Supplementary Table S7 shows the cumulative incidence of specific grade 3–4 chronic health conditions by attained age among BMT survivors and siblings. By age 10 years, the mean cumulative incidence of grade 3–4 hearing/vision deficit was 44.8 ± 4.9% among BMT survivors compared with 1.0 ± 0.5% among siblings (P < .001). The BMT survivors had a significantly higher mean incidence of grade 3–4 cardiovascular and/or respiratory conditions (5.8 ± 2.3% versus 0.2 ± 0.2%; P < .001), grade 3–4 musculoskeletal impairments (2.9 ± 1.7% versus 0 ± 0%; P < .001), and grade 3–4 endocrine conditions (2.9 ± 1.7% versus 0%; P < .001). Adjusting for age at study participation, sex, race/ethnicity, education, annual household income, and current health insurance, survivors were significantly more likely than siblings to report grade 3–4 hearing/vision deficits (OR, 27.7; 95% CI, 9.9 to 77.2; P < .0001).
Chronic Health Conditions among BMT Recipients
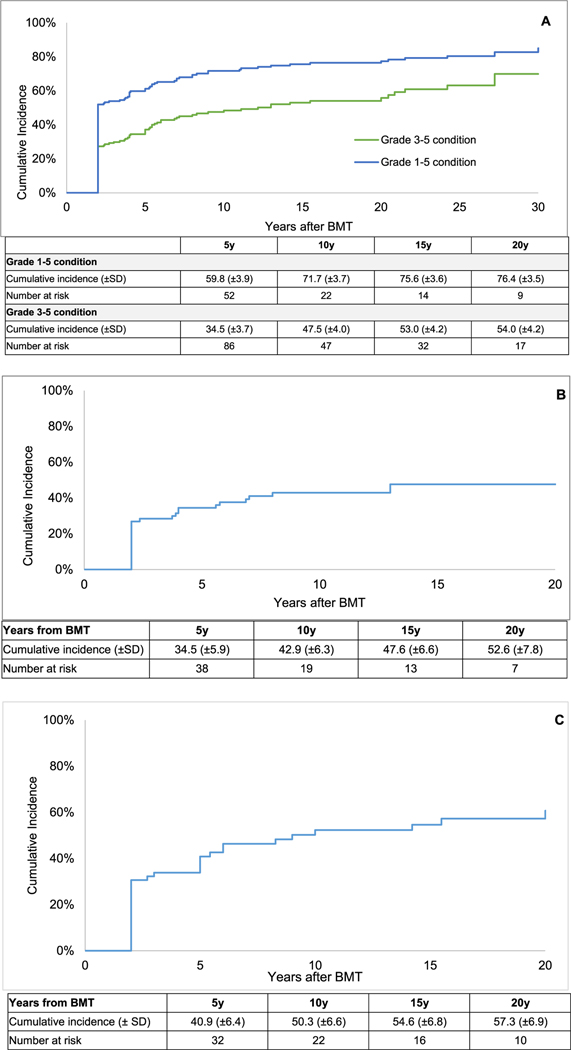
This analysis included all patients who were alive at study participation (n = 106), as well as those who survived for ≥2 years but died after (n = 48). The prevalence of any grade 3–5 chronic health condition was 55.2% overall, 62.9% for patients with mucopolysaccharidoses, and 46.3% for patients with leukodystrophies. Impaired hearing (24.7%), visual impairment (22.1%), and cardiovascular disease (9.7%) were the most prevalent grade 3–5 chronic health conditions reported by survivors (Supplementary Table S8). In addition, 8.4% of survivors in our study died from infectious causes. At 10 years post-BMT, the mean cumulative incidence of any grade 1–5 chronic health condition was 71.7 ± 3.7% and grade 3–5 chronic health conditions was 47.5 ± 4.0% (Figure 2A).
Figure 2.
(A) Cumulative incidence of chronic health conditions among 2-year survivors of BMT for IEM by time from BMT. (B) Cumulative incidence of serious, life-threatening, and fatal chronic health conditions among 2-year survivors of BMT for leukodystrophies by time from BMT. The center diamond represents the odds of grade 3–4 chronic health conditions among 2-year survivors of BMT for IEM. The line represents the upper and lower 95% CI for the OR. (C) The cumulative incidence of serious, life-threatening, and fatal chronic health conditions among 2-year survivors of BMT for mucopolysaccharidoses by time from BMT.
As shown in Supplementary Table S9, the 10-year mean cumulative incidence of grade 3–5 hearing/vision impairment was 48.2 ± 4.8%, and that of grade 3–5 cardiopulmonary disorders was 9.4 ± 2.4%. The 10-year cumulative mortality due to infections was 6.3 ± 2.0%. Figure 2B and C highlights the 10-year cumulative incidence of grade 3–5 chronic health conditions among survivors of BMT for leukodystrophies (42.9 ± 6.3%) and mucopolysaccharidoses (50.3 ± 6.8%).
Multivariable analysis revealed an association between RIC and grade 3–5 chronic health conditions among all BMT recipients (hazard ratio [HR], 2.7; 95% CI, 1.2 to 6.2; P = .02; reference: myeloablative conditioning) and in patients with leukodystrophies (HR, 4.2; 95% CI, 1.2 to 14.9; P = .03) (Table 2).
Table 2.
Risk of any Serious Complications (Grade 3), Life-Threatening Complications (Grade 4), or Death (Grade 5) from Chronic Health Conditions among 2-Year Survivors of BMT for IEM
| Variable | All Survivors | Leukodystrophies | Mucopolysaccharidoses | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Age at BMT | ||||||
| Per year increase | 1.0 (0.97–1.01) | .4 | 1.0 (0.97–1.03) | .9 | 0.9 (0.8–1.1) | .3 |
| Sex (reference: female) | ||||||
| Male | 0.9 (0.5–1.4) | .5 | 0.7 (0.3–1.3) | .2 | 1.1 (0.5–2.3) | .9 |
| Race (reference: non-Hispanic white) | ||||||
| Other | 0.5 (0.2–1.0) | .05 | 0.2 (0.09–0.5) | .001 | 1.5 (0.3–7.7) | .6 |
| Stem cell source (reference: cord blood) | ||||||
| Bone marrow or PBSCs | 1.6 (0.6–3.7) | .3 | 1.7 (0.4–7.5) | .5 | 1.7 (0.6–5.2) | .3 |
| Type of donor (reference: related) | ||||||
| Unrelated | 2.0 (0.9–4.2) | .08 | 1.7 (0.5–6.5) | .4 | 2.0 (0.7–5.6) | .2 |
| Type of conditioning (reference: myeloablative) | ||||||
| RIC | 2.7 (1.2–6.2) | .02 | 4.2 (1.2–14.9) | .03 | 2.2 (0.5–9.6) | .3 |
| Year of BMT (reference: <2000) | ||||||
| 2000—2009 | 0.9 (0.5–1.9) | .8 | 1.1 (0.2–5.1) | .9 | 1.5 (0.5–4.0) | .5 |
| ≥ 2010 | 0.9 (0.4–2.4) | .8 | 0.8 (0.1–5.7) | .9 | 1.9 (0.4–8.2) | .4 |
| >1 BMT | ||||||
| Yes | 1.2 (0.6–2.4) | .6 | 2.6 (0.7–9.5) | .2 | 0.7 (0.3–1.9) | .5 |
| Chronic GVHD (reference: no GvHD) | ||||||
| Yes GVHD | 1.3 (0.6–2.6) | .5 | 1.9 (0.5–7.0) | .3 | 0.9 (0.3–2.9) | .9 |
| TBI (reference: no TBI) | ||||||
| Yes TBI | 0.8 (0.4–1.6) | .5 | 0.5 (0.2–1.6) | .3 | 2.4 (0.6–8.8) | .2 |
| Cyclosporine (reference: no cyclosporine) | ||||||
| Yes cyclosporine | 0.9 (0.4–2.3) | .8 | 0.2 (0.1–0.7) | .008 | 1.0 (0.2–4.6) | .9 |
| (reference: no MMF) | ||||||
| Yes MMF | 1.3 (0.6–2.8) | .5 | 1.8 (0.5–7.1) | .4 | 0.8 (0.4–2.0) | .7 |
| T cell depletion (reference: no T cell depletion) | ||||||
| Yes T cell depletion | 1.4 (0.5–3.6) | .6 | 1.9 (0.4–9.6) | .4 | 0.5 (0.1–1.9) | .3 |
MMF, mycophenolate mofetil.
Other race includes patients of Hispanic, African-American, Asian, and other mixed race/ethnicity. Category of other IEMs could not be analyzed in these models owing to a low number of events.
The use of cyclosporine for GVHD prophylaxis was protective against grade 3–5 chronic health conditions in patients with leukodystrophies (HR, 0.2; 95% CI, 0.1 to 0.7; P = .008). As shown in Table 3, use of RIC also was associated with an elevated risk of grade 3–5 cardiopulmonary conditions (HR, 6.7; 95% CI, 1.3 to 35.4; P = .03) and hearing/vision impairment (HR, 3.0; 95% CI, 1.4 to 6.6; P = .006) compared with myeloablative conditioning. Use of an unrelated donor (HR, 9.3; 95% CI, 1.7 to 50.8; P = .01; reference group: related donor) and bone marrow/PBSCs as the stem cell source were associated with greater risk of grade 3–5 cardiopulmonary conditions (HR, 14.5; 95% CI, 1.7 to 120.2; P = .01; reference group: cord blood). An elevated risk of death due to infections was seen in BMT recipients treated with a T cell-depleted graft (HR, 41.7; 95% CI, 5.8 to 298.9; P = .0002) or with the use of mycophenolate mofetil for GVHD prophylaxis (HR, 11.0; 95% CI, 2.9 to 48.1; P = .0006).
Table 3.
Risk of Serious (Grade 3), Life-Threatening (Grade 4) or Death (Grade 5) from Chronic Health Conditions among 2y Survivors of BMT for IEM by Organ Systems
| Variable | Cardiovascular and/or Pulmonary | Hearing and/or Vision | Infection | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95%CI) | P | |
| Age at BMT | ||||||
| Per year increase | 0.9 (0.8–1.1) | .2 | 0.95 (0.9–1.0) | .05 | 1.0 (0.98–1.1) | .5 |
| Sex (reference: female) | ||||||
| Male | 1.5 (0.5–4.6) | .5 | 0.8 (0.5–1.4) | .5 | 0.1 (0.02–1.1) | .06 |
| Race (reference: non-Hispanic white) | ||||||
| Other | 1.3 (0.2–7.6) | .8 | 0.2 (0.02–1.3) | .09 | 0.3 (0.04–2.3) | .3 |
| Stem cell source (reference: cord blood) | ||||||
| Bone marrow or PBSCs | 14.5 (1.7–120.2) | .01 | 1.5 (0.6–3.5) | .4 | 0.1 (0.01–1.3) | .07 |
| Type of donor (reference: related) | ||||||
| Unrelated | 9.3 (1.7–50.8) | .01 | 1.8 (0.7–4.4) | .2 | 0.08 (0.1–1.6) | .1 |
| Type of conditioning (reference: myeloablative) | ||||||
| Reduced intensity | 6.7 (1.3–35.4) | .03 | 3.0 (1.4–6.6) | .006 | 4.8 (0.5–44.8) | .2 |
| Year of BMT (reference: <2000) | ||||||
| 2000–2009 | 3.9 (1.3–12.1) | .02 | 1.0 (0.5–1.8) | .9 | 2.3 (0.3–14.9) | .4 |
| ≥ 2010 | 3.0 (0.4–21.4) | .3 | 0.9 (0.4–2.3) | .8 | — | — |
| >1 BMT | ||||||
| Yes | 0.8 (0.2–3.1) | .7 | 1.6 (0.9–2.8) | .1 | 3.1 (0.2–5.5) | .9 |
| Chronic GVHD (reference: no GVHD) | ||||||
| Yes GVHD | 1.0 (0.1–10.5) | .9 | 0.6 (0.3–1.3) | .2 | 10.6 (0.6–190.7) | .1 |
| Total body irradiation (reference: no TBI) | ||||||
| Yes TBI | 2.2 (0.5–9.5) | .3 | 1.0 (0.6–1.8) | .9 | 0.1 (0.02–0.8) | .02 |
| Cyclosporine (reference: no cyclosporine) | ||||||
| Yes cyclosporine | 0.3 (0.03–2.5) | .2 | 0.6 (0.2–2.7) | .5 | 14.9 (0.2–1039.3) | .2 |
| MMF (reference: no MMF) | ||||||
| Yes MMF | 0.2 (0.03–1.1) | .06 | 1.2 (0.6–2.2) | .6 | 11.0 (2.9–48.1) | .0006 |
| T cell depletion (reference: no T cell depletion) | ||||||
| Yes T cell depletion | 0.1 (0.01–0.8) | .03 | 0.9 (0.4–2.4) | .8 | 41.7 (5.8–298.9) | .0002 |
Other race/ethnicity includes patients of Hispanic, African-American, Asian, and other mixed race/ethnicity.
Functional Outcomes
Compared with siblings, BMT survivors were less likely to graduate from college (25.9% versus 56.5%; OR, 0.3; 95% CI, 0.1 to 0.7; P = .004) and less likely to marry (2.4% versus 60%; OR, 0.01; 95% CI, 0.004 to 0.07; P < .0001). Employment status could not be examined owing to a low response rate. The BMT survivors were more likely to report assistance with ADLs (52.0% versus 1.2%; OR, 87.5; 95% CI, 35.7 to 214.7; P < .0001) and IADLs (70.2% versus 1.6%; OR, 142.4; 95% CI, 57.9 to 350.2; P < .001). There were no significant differences by disease category between those who completed college (40% for leukodystrophy versus 4% for mucopolysaccharidoses versus 10% for others), got married (4.4% for leukodystrophy versus 0% for mucopolysaccharidoses versus 0% for others), and required assistance with ADLS (55.3% for leukodystrophy versus 52.3% for mucopolysaccharidoses versus 28.6% for others) or IADLs (69.7% for leukodystrophy versus 79.0% for mucopolysaccharidoses versus 40.0% for others).
Among BMT survivors, older age at BMT (HRper_year_increase, 1.1; 95% CI. 1.01 to 1.2; P = .02), RIC (HR, 5.2; 95% CI, 1.3 to; P = .02) and unrelated donor BMT (HR, 3.9; 95% CI, 1.5 to 10.5; P = .005) were associated with a greater likelihood of requiring assistance with ADLs. The presence of any grade 3–4 chronic health condition was associated with greater risk of requiring assistance with IADLs (HR, 6.5; 95% CI, 1.1 to 37.8; P = .04) among BMT survivors (Supplementary Table S10). The prevalence of grade 3–4 hearing impairment (55% versus 17.7%), vision deficits (45% versus 35.3%), cardiopulmonary conditions (17.5% versus 11.8%), musculoskeletal disorders (10% versus 5.9%), and endocrine disorders (5.0% versus 0%) was higher among survivors requiring assistance with IADLs compared with those not requiring assistance.
DISCUSSION
Patients with IEM are now surviving longer owing to earlier transplantation and improved transplant strategies [6,7], but survivors continue to experience morbidity secondary to their primary disease [4,9–12]. We show that IEM patients are at a substantially greater risk of developing chronic health conditions compared with their siblings; the cumulative incidence of a serious/life-threatening chronic health condition approaches 50% at 10 years after BMT. Hearing/vision impairments, musculoskeletal and endocrine disorders, cardiopulmonary conditions, and infections are the most common categories of serious/life-threatening/fatal chronic health conditions. We also show that these survivors are significantly less likely to achieve the milestones that are often important for integration into society as adults.
Previous studies have examined cognition, growth, cardiovascular disease, and musculoskeletal problems in selected IEMs [4,15–19]; nevertheless, information on the cumulative burden of morbidity several years after BMT is limited. We show that survivors of BMT for IEM are collectively at a 12.5-fold greater risk of developing serious/life-threatening conditions compared with their siblings. Despite the intended goal of BMT in IEM to slow end-organ dysfunction, a substantial proportion of survivors experienced serious/life-threatening impairment in critical organs, such as the heart, lungs, vision, and hearing. We show that the burden of chronic health conditions increased with time; the cumulative incidence of a serious/life-threatening/fatal chronic health conditions increased from 35% at 5 year post-BMT to 48% at 10 y post-BMT. While normal enzyme levels post-BMT result in improved organ function in patients with Hurler syndrome [4], restoration of enzyme levels is not uniform across all patients or even between various tissues in the same patient. These findings underscore the need for mitigation strategies following transplantation, such as augmentation of gene expression in patients with Hurler syndrome [20], as well as the need for life-long follow-up for early detection of declining organ function. Of particular importance is the need for aggressive management of infections several years after BMT, given the 6.3% incidence of grade 5 infection at 15 years post-BMT. We found that use of T cell–depleted grafts in patients with IEM, previously shown to be a risk factor for graft failure [5] and late mortality [6], was associated with an elevated risk of fatal infections, suggesting the need for infection prevention strategies.
Similar to a previous report [4], in the present study the most prevalent chronic health condition was hearing/vision impairment. By age 20 years, the cumulative incidence of a serious/disabling hearing/vision deficit exceeded 50%. In addition, survivors reported cardiovascular and musculoskeletal complications, which together with hearing/vision impairments are likely to impact their ability to carry out ADLs. Adult survivors reported greater difficulty accomplishing ADLs and IADLs compared with their siblings, and the presence of chronic health conditions was associated with an increased risk of needing assistance with such activities. Aldenhoven et al. [9] used normative data to demonstrate lower functional health post-BMT in patients with Hurler syndrome. We show that patients with IEM are significantly less likely to achieve key milestones during adulthood, such as college graduation or getting married, compared with unaffected siblings. An inability to graduate from college among adult survivors of childhood cancer with serious/life-threatening chronic health conditions has been reported previously [21]. This high burden of morbidity and the associated healthcare needs likely interferes with the ability to achieve educational goals.
RIC emerged as a risk factor for serious/life-threatening/fatal CHCs among survivors, especially those of leukodystrophies, as well as serious/life-threatening hearing/vision impairment and cardiopulmonary compromise in our study. Reduced intensity conditioning is a risk factor for graft failure among patients with IEM [5]. Higher post-BMT enzyme levels lead to improved outcomes among survivors [4]. Although we do not have data on donor carrier status or on engraftment and post-transplantation donor chimerism, RIC likely was associated with inferior engraftment compared with myeloablative conditioning, possibly resulting in lower enzyme levels in tissues, greater accumulation of toxic substrates, and overall decline in organ function. BMT survivors of mucopolysaccharidoses treated with RIC had a 2.2-fold greater hazard of serious/life-threatening/fatal chronic health conditions compared with those treated with myeloablative conditioning, but the difference did not approach statistical significance. This is likely because only 9.7% of mucopolysaccharidoses patients received an RIC preparative regimen, as RIC has been identified as a risk factor for graft failure in patients with Hurler syndrome, and also because of the younger age of these patients at BMT, which may allow for myeloablative conditioning. Because we also do not have data on pretransplantation functional status or comorbidities, it is possible that patients with older age at BMT, lower functional status, or greater comorbidities were offered RIC. RIC is increasingly not recommended as a preparative regimen in the management of IEM, and our study further highlights the downstream sequelae of RIC.
We were unable to measure functional status (especially in mucopolysaccharidoses patients) or other prognostic characteristics (such as Loes score in X-ALD patients) at BMT. Further, we were unable to measure postengraftment enzyme levels or to determine whether patients received enzyme infusions. As such, we were unable to capture criteria used to select patients for BMT or transplantation characteristics such as conditioning intensity. We might not have accurately captured selected health conditions that affect patients with specific IEMs, possibly leading to underestimation of the burden of their healthcare needs. In addition, patients eligible for this study underwent BMT at a single site (University of Minnesota), and these findings might not be generalizable. Finally, patients included in this study span a large time period, and changes in transplantation practices (eg, prompt BMT on diagnosis, improved supportive care) over time may improve the outcomes for these patients. However, our findings do not show a difference in serious/life-threatening chronic health conditions among survivors by era of transplantation. Future studies could focus on identifying whether specific changes (such as enzyme infusion pre-BMT and post-BMT) influence the risk of long-term chronic health conditions.
In this study, we describe the cumulative burden of morbidity, as well as the magnitude of risk of specific chronic health conditions several years after BMT for IEM. We show that long-term survivors of BMT for IEM carry a high burden of morbidity, and that this burden continues to increase for several years after BMT. We have identified RIC as associated with increased risk of several chronic health conditions. Future work should focus on developing measures that reduce the burden of morbidity in this population, as well as developing strategies that facilitate a more independent and functional life as an adult.
Supplementary Material
ACKNOWLEDGMENTS
Financial disclosure: This work was supported by grants from National Cancer Institute (U01CA213140) and Leukemia Lymphoma Society (6256–13), both to S.B.
Footnotes
SUPPLEMENTARY MATERIALS
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jtct.2021.11.023.
Conflict of interest statement: There are no conflicts of interest to disclose.
REFERENCES
- 1.Kakkis ED, Muenzer J, Tiller GE, et al. Enzyme-replacement therapy in mucopolysaccharidosis I. N Engl J Med. 2001;344:182–188. [DOI] [PubMed] [Google Scholar]
- 2.Eichler F, Duncan C, Musolino PL, et al. Hematopoietic stem-cell gene therapy for cerebral adrenoleukodystrophy. N Engl J Med. 2017;377:1630–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sessa M, Lorioli L, Fumagalli F, et al. Lentiviral haemopoietic stem-cell gene therapy in early-onset metachromatic leukodystrophy: an ad-hoc analysis of a non-randomised, open-label, phase 1/2 trial. Lancet. 2016;388:476–487. [DOI] [PubMed] [Google Scholar]
- 4.Aldenhoven M, Wynn RF, Orchard PJ, et al. Long-term outcome of Hurler syndrome patients after hematopoietic cell transplantation: an international multicenter study. Blood. 2015;125:2164–2172. [DOI] [PubMed] [Google Scholar]
- 5.Boelens JJ, Wynn RF, O’Meara A, et al. Outcomes of hematopoietic stem cell transplantation for Hurler’s syndrome in Europe: a risk factor analysis for graft failure. Bone Marrow Transplant. 2007;40:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wadhwa A, Chen Y, Holmqvist A, et al. Late mortality after allogeneic blood or marrow transplantation for inborn errors of metabolism: a report from the Blood or Marrow Transplant Survivor Study-2 (BMTSS-2). Biol Blood Marrow Transplant. 2019;25:328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eapen M, Ahn KW, Orchard PJ, et al. Long-term survival and late deaths after hematopoietic cell transplantation for primary immunodeficiency diseases and inborn errors of metabolism. Biol Blood Marrow Transplant. 2012;18:1438–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahn JM, Brazauskas R, Tecca HR, et al. Subsequent neoplasms and late mortality in children undergoing allogeneic transplantation for nonmalignant diseases. Blood Adv. 2020;4:2084–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aldenhoven M, van den Broek BTA, Wynn RF, et al. Quality of life of Hurler syndrome patients after successful hematopoietic stem cell transplantation. Blood Adv. 2017;1:2236–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groeschel S, Kühl JS, Bley AE, et al. Long-term outcome of allogeneic hematopoietic stem cell transplantation in patients with juvenile metachromatic leukodystrophy compared with nontransplanted control patients. JAMA Neurol. 2016;73:1133–1140. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro E, Krivit W, Lockman L, et al. Long-term effect of bone-marrow transplantation for childhood-onset cerebral X-linked adrenoleukodystrophy. Lancet. 2000;356:713–718. [DOI] [PubMed] [Google Scholar]
- 12.Miller WP, Rothman SM, Nascene D, et al. Outcomes after allogeneic hematopoietic cell transplantation for childhood cerebral adrenoleukodystrophy: the largest single-institution cohort report. Blood. 2011;118:1971–1978. [DOI] [PubMed] [Google Scholar]
- 13.Louie AD, Robison LL, Bogue M, Hyde S, Forman SJ, Bhatia S. Validation of self-reported complications by bone marrow transplantation survivors. Bone Marrow Transplant. 2000;25:1191–1196. [DOI] [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE), version 5.0. Available at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5×7.pdf. Accessed 10/22/2020.
- 15.Lum SH, Stepien KM, Ghosh A, et al. Long-term survival and cardiopulmonary outcome in children with Hurler syndrome after haematopoietic stem cell transplantation. J Inherit Metab Dis. 2017;40:455–460. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt M, Breyer S, Lӧbel U, et al. Musculoskeletal manifestations in mucopolysaccharidosis type I (Hurler syndrome) following hematopoietic stem cell transplantation. Orphanet J Rare Dis. 2016;11:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van den Broek BTA, Page K, Paviglianiti A, et al. Early and late outcomes after cord blood transplantation for pediatric patients with inherited leukodystrophies. Blood Adv. 2018;2:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coletti HY, Aldenhoven M, Yelin K, Poe MD, Kurtzberg J, Escolar ML. Long-term functional outcomes of children with Hurler syndrome treated with unrelated umbilical cord blood transplantation. JIMD Rep. 2015;20:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guffon N, Pettazzoni M, Pangaud N, et al. Long-term disease burden post-transplantation: three decades of observations in 25 Hurler patients successfully treated with hematopoietic stem cell transplantation (HSCT). Orphanet J Rare Dis. 2021;16:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisengart JB, Rudser KD, Tolar J, et al. Enzyme replacement is associated with better cognitive outcomes after transplant in Hurler syndrome. J Pediatr. 2013;162:375–380. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bashore L, Merchant Z, Lupo P, et al. Educational attainment in long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study (CCSS). J Clin Oncol. 2019;37(15_suppl):10063. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.