Abstract
Studies on microalgae interspecific interactions have so far focused either on nutrient competition or allelopathic effects due to excreted substances from Harmful Algal Bloom (HAB) species. Evidence from plants, bacteria and specific microalgae groups, point to a range of responses mediated by sensing or direct chemical impact of exometabolites from foreign species. Such processes remain under-investigated, especially in non-HAB microalgae, despite the importance of such knowledge in ecology and industrial applications. Here, we study the directional effect of exometabolites of 4 “foreign” species Heterosigma akashiwo, Phaeocystis sp., Tetraselmis sp. and Thalassiosira sp. to each of three “target” species across a total of 12 treatments. We disentangle these effects from nutrient competition by adding cell free medium of each “foreign” species into our treatment cultures. We measured the biomass response, to the foreign exometabolites, as cell number and photosynthetic biomass (Chla), whereas nutrient use was measured as residual phosphorus (PO4) and intracellular phosphorus (P). Exometabolites from filtrate of foreign species were putatively annotated by untargeted metabolomics analysis and were discussed in association to observed responses of target species. Among others, these metabolites included L-histidinal, Tiliacorine and dimethylsulfoniopropionate (DMSP). Our findings show that species show a range of responses with the most common being biomass suppression, and less frequent biomass enhancement and intracellular P storage. Filtrate from the green microalgae Tetraselmis caused the most pronounced negative effects suggesting that non-HAB species can also cause negative chemical interference. A candidate metabolite inducing this response is L-histidinal which was measured in high abundance uniquely in Tetraselmis and its L-histidine form derived from bacteria was previously confirmed as a microalgal algicidal. H. akashiwo also induced biomass suppression on other microalgae and a candidate metabolite for this response is Tiliacorine, a plant-derived alkaloid with confirmed cytotoxic activity.
Keywords: Microalgae, Exometabolites, Intracellular phosphorus, Luxurious phosphorus uptake, L-histidinal, Tiliacorine
Graphical abstract
Highlights
-
•
We show that microalgae demonstrate a range of responses to foreign exometabolites.
-
•
Biomass suppression caused by Tetraselmis was linked to L-histidinal.
-
•
Biomass enhancement was only observed in Heterosigma akashiwo.
-
•
Luxurious P uptake can also occur as a response to foreign species exometabolites.
-
•
Tiliacorine is reported for the first time from the mixotroph H. akashiwo.
1. Introduction
Competition for nutrients is the most well-known process intrinsic to microalgae assemblages leading to predictable composition under specific environmental conditions [1]. On the other hand, chemical interference via metabolites excreted from competitor microalgae (exometabolites) is less well understood. Studies on chemical interference between microalgae have focused on effects of Harmful Algal Bloom (HAB) species recording mostly negative but also positive responses on physiology and growth, aka allelopathy [2]. Evidence from other microbial groups such as bacteria have shown that foreign exometabolites can be sensed by target species and lead to more complex responses including both aggressive and defensive strategies [3]. To advance research in the field it is essential to explore a broader range of responses in microalgae, by expanding experimental settings beyond the effects of single species to crossed designs using HAB and non-HAB species of different taxonomic groups. It is also essential that such investigations, tease apart effects of nutrient competition that are inevitably masking chemical interference in co-cultures. Combining such information with comparative metabolomics of focal species, can shed light into processes sustaining microalgae co-existence, biomass dynamics and nutrient use applicable in field settings and industrial production systems.
The bulk of research on microalgal chemical interference has focused on physiological aspects of cell viability such as growth, biomass and respiration [2]. The main assumption is that compounds excreted from toxin producing species (aka allelochemicals) would impede the growth process and cell viability of the target species via direct chemical effects such as algicidal action. This raises the question on whether microalgae are able to respond to foreign species, via processes other than physiological impairment, as a result of sensing the exometabolites of their competitor. Competition sensing has been reported in bacteria, where toxins released induce responses to competitor species [3]. However, more complex interactions (i.e. quorum quenching) have also been reported whereby the release of toxins by competitor species interferes with substances used for communication between conspecifics of other species (known as quorum sensing signals) [4]. Defensive strategies are also known to occur in bacteria as a response to chemical cues, including damage repair or changes in gene regulation [5]. For example, nutrient starvation leads to transcriptional switching from proliferation-related genes to maintenance-related genes in Escherichia coli [6]. In microalgae, a maintenance-related mechanism is luxurious phosphorus (P) uptake, a well-documented process whereby cells adapt to nutrient fluctuations by maximizing their intracellular P storage [7], [8]. Luxurious P uptake might either lead other species, whose P uptake is slower, to starvation, or storing more P than required for growth for supporting other potentially defensive functions [8]. It is thus plausible, that under stress conditions induced by the sensing of competitors, a species shifts its strategy from growth to nutrient storage to gain a competitive advantage over the long term. This process has not yet been explicitly investigated as a response to foreign species exometabolites and could provide the first basis of understanding sensing or direct chemical impacts between microalgae when investigated alongside biomass responses and accompanying information on foreign exometabolites.
Investigations of chemical interference between microalgae species have focused on effects of toxin-producing, Harmful Algal Bloom (HAB) species on other toxic and non-toxic microalgae [2]. The bulk of these studies has reported growth inhibition on target microalgae linked to cell lysis, shifts in respiration, protein synthesis and gene expression [9], [10], [11], [12], [13], [14], [15], [16], [17]. However, positive allelopathy has also been reported by a limited number of studies [15], [18], [19], [20]. This suggests that microalgae do not show a consistent response to exometabolites of foreign microalgae even if the latter are toxin-producing species known to cause adverse effects to higher trophic levels during HABs. Such differences in response directionality are hindering our understanding of community assembly and prediction of phytoplankton assemblage composition while increasing the uncertainty of co-cultivation outcomes when aiming to maximize industrial microalgae production. It is thus critical to understand whether microalgae can respond to exometabolites of non-HAB foreign microalgae and whether such effects extend beyond biomass and physiological changes to defensive strategies such as nutrient use.
In regards to non-HAB species, chemical effects have reported negative allelopathy [21], [22], [31], [32], [23], [24], [25], [26], [27], [28], [29], [30]. However, chemical interference is often tested in experimental designs involving co-cultured species sharing common resources. This poses a challenge in disentangling the effects of chemical interference from competition for nutrients. According to theory, a co-culture of two co-occurring species growing under a constant resource supply in continuous cultures, will end up being exclusively occupied by the species that is more competitive for the limiting resource [1]. It is thus plausible that negative effects to co-cultured target microalgae are not due to chemical interference but rather to the higher competitive ability of the focal species for the limiting resource [32]. Although it is possible to isolate the effect of allelopathy from resource competition by using either culture filtrate or cellular lipophilic extract of the focal species [24], [33], [34], [35], it is impossible to isolate the effect of resource competition from that of chemical interference in co-cultures [32], [36]. This poses the necessity to investigate chemical interference in isolation, while carefully controlling for nutrient concentrations, as an essential step towards understanding how both processes act antagonistically or synergistically to sustain species co-existence and biomass.
To obtain further insights into the mechanisms governing the responses of microalgae to competitor species exometabolites, it is necessary to characterize the exometabolites excreted from a target species. Metabolomics recognizes that alterations in cell function are perhaps more evident at the level of small molecule metabolism and offers a powerful biochemical approach for revealing molecular phenotypes [37], [38]. In the context of this study, secondary metabolites are a key focus, i.e. substances that are not essential for algal growth and that mostly participate in the defensive and protective mechanisms of the cell. Many components in the algal exometabolome can cause interspecific allelopathic effects [39] and the potential of metabolomics to investigate chemical interactions has been already highlighted by different studies [20], [40].
The aim of this study was thus to investigate the response of each of 4 target microalgal species of different taxonomic groups to the presence of exometabolites from each of the other 3 species. The exposure to the exometabolome was performed in the absence of resource competition, by using a nutrient-enriched cell free filtrate, and we quantified responses related to biomass yield (photosynthetic activity and cell number) and phosphorus use (intracellular P storage and residual P in the medium). These responses were linked to specific exometabolites following comparative analysis of the metabolic profile of monoculture filtrate from each species. Evidence that exometabolites of foreign species can lead to multiple responses regarding biomass production and nutrient use could lead to a shift in the way that we predict microalgae dynamics and composition outcomes and can influence decisions on species co-cultivations in industrial biomass production.
2. Material and methods
2.1. Experimental design & procedure
To address the research questions, we used four HAB and non-HAB marine phytoplankton species representing different taxonomic groups: Heterosigma akashiwo (Ochrophyta) and Phaeocystis sp. (Haptophyta), both known to form HABs [41], [42], [43], Tetraselmis sp. (Chlorophyta) and Thalassiosira sp. (Bacillariophyta). A high volume (2 L) mother culture (MC) of each species was used to obtain the cell-free filtrate, by filtration through GF/C glass microfiber filters, which was then added in our treatment cultures. For each species, there were three treatments where MC filtrate of each “foreign” species was added and a control treatment where plain seawater was added (Fig. 1). Each treatment and control comprised of three replicate cultures (200 mL/flask). Thus, the experimental design comprised of 4 species × (3 treatments+1control) × 3 replicates = 48 cultures.
Fig. 1.
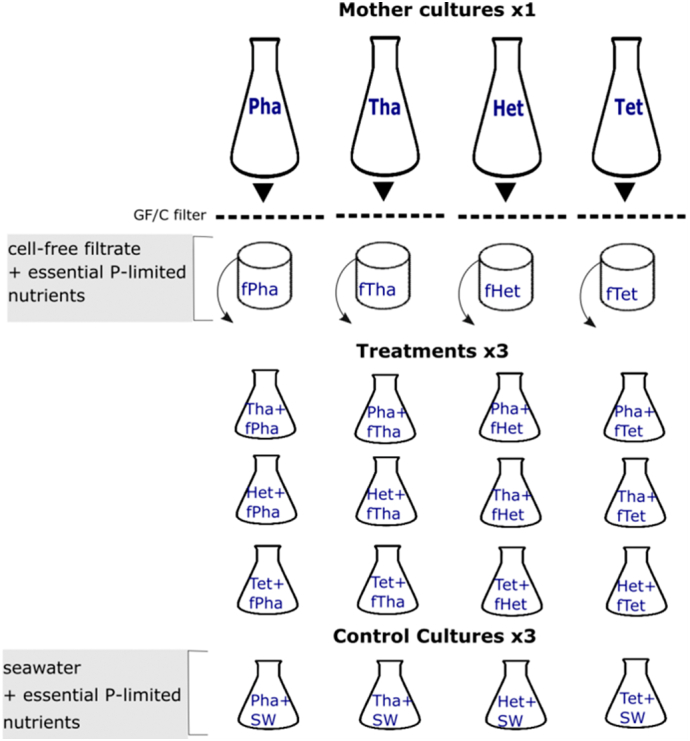
Experimental design testing for the effect of filtrate with exometabolites from four “foreign” microalgae on the biomass and nutrient use by “target” microalgae representing four taxonomic groups: Heterosigma akashiwo (Ochrophyta), Phaeocystis sp. (Haptophyta), Tetraselmis sp. (Chlorophyta) and Thalassiosira sp. (Bacillariophyta). The experiment consisted of the treatments whereby triplicate monocultures of each species were treated with filtrate from each “foreign” species MC (fspecies) and controls whereby we added plain artificial seawater (SW) in triplicate monocultures of each species. The total sample size was thus 48 monocultures: 4 “target” species × (3 “foreign” species treatments + 1 control) × 3 replicates.
We wanted to see the effect of the addition of foreign species' filtrate on the biomass and nutrient use of the target species at stationary phase, thus aiming at testing for effects at the population level. Two filtrate additions were carried out to intensify any effect of allelochemicals on the treatment cultures mimicking conditions also encountered in the field where species are exposed in a more continuous manner to exometabolites of foreign species. The aim was to test the effects and responses of microalgae when different species interact via their exometabolome, rather than to quantify the exometabolite dose concentrations that would cause the observed effects. Additions occurred when MCs of the four species had reached stationary phase because at this stage allelochemicals from microalgae are in higher concentrations [35], [44]. At this stage, susceptibility of target species to allelochemicals was also expected to be higher than the control since they were under higher stress due to nutrient limitation [45]. The first filtrate addition occurred when treatment and control cultures were at the end of the exponential phase and the second occurred two days later, when cultures had reached stationary phase. Based on pilot data, the growth rate of each species was calculated (see supplementary material “methods”) so that all species' cultures would reach stationary phase simultaneously. Samples were collected at two time points. The first sampling occurred prior to the first filtrate addition, to record the initial biomass of the cultures before any effects of exometabolites took place. These measurements were included in our statistical models to account for differences in initial conditions. The second sampling took place two days after the second addition to allow sufficient time for the populations to react to foreign exometabolites.
In each of the two filtrate addition time points we removed 30% of the volume (i.e. 60 mL) from each of the 48 cultures and replaced it with the same volume of MC filtrate for our treatment cultures or plain seawater for our controls. At these time points residual P concentrations were very low and prior to the addition, essential nutrients were added into treatments and control cultures (i.e. F/2 with P-limitation) to minimize any effects of residual nutrients in the MC on the growth of treatment cultures.
2.2. Experimental conditions
Phytoplankton cultures of Phaeocystis sp. and Thalassiosira sp. were obtained from the algal collection of the Hellenic Centre for Marine Research (HCMR) and cultures of Tetraselmis sp. (Florida Aqua Farms - 352-567-0226) and Heterosigma akashiwo (CCAP 934/7, Oban, Scotland) were maintained in the algal collection of aquatic ecology laboratory of University of Glasgow. All cultures were incubated at 21 °C, in a 24-hour continuous photoperiod under fluorescent light and in artificial ultra-pure autoclaved seawater with salinity 35 psu. Mother cultures (2 L) had a continuous ventilation system whereas in treatment and control cultures (200 mL), regular stirring was applied instead.
All cultures were initiated with a concentration of 5000 cells mL−1 apart from Phaeocystis sp. which was inoculated with 10,000 cells mL−1 due to the much higher population carrying capacity of this species. This was to achieve a synchronization among treatment cultures and MCs from which they received filtrate. Synchronization refers to the growth stage of the cultures, i.e. all cultures being at the stationary stage during filtrate additions. MCs and treatments, including controls, were cultured in medium F/2 Guillard (1975) (see supplementary material) but with P-limitation (i.e. 3 μΜ instead of 36μΜ P) because allelopathy is known to be higher under nutrient-limited conditions [17], [27], [35], [46], [47]. In this study we focus on P-limitation, because previous research from coastal ecosystems, where these species are usually encountered, has shown that P limitation can occur on a seasonal basis depending on the intensity of freshwater terrestrial inflows into the coastal environment [48], [49]. All species were acclimated to the experimental conditions for at least a month prior to the initiation of the experiment. These conditions (including light and growth medium) were the same across all species, as we aimed to test for species interactions under conditions when species co-occur in nature.
2.3. Sampling & metabolomics analysis
At each of the two sampling points (pre-filtrate addition and post-filtrate addition), 5 mL were removed from each culture for cell counting, they were preserved with a drop of Lugol solution and stored in the refrigerator (4 °C). Cell counting was carried out under an optical Leica microscope at 200× magnification using Fast-Read® 102 disposable counting chambers (immune systems). An additional 50 mL aliquot of each culture was collected and from this 25 mL was filtered onto 25 mm Whatman GF/C glass microfiber filters for chlorophyll-a (Chla) analysis and the other 25 mL was filtered for intracellular P analysis, while the 50 mL filtrate was used to evaluate the residual P in the medium. Filters for Chla and intracellular P analysis were placed in aluminum foil and together with samples for nutrients (PO4) were frozen at −20 °C. Chla and residual phosphorus in the medium were quantified according to Parsons et al. (1984) (see supplementary material). Determination of intracellular P was carried out according to Caceres et al. (2019) and details are provided in the supplementary material under “Methods”.
Metabolomics analysis was carried out to detect the unique exometabolites released by each species in the medium. These were determined from 5 mL samples taken from our control cultures of the four species at the second sampling point. Samples for metabolomics analysis (5 mL) were quenched by rapidly cooling cells in ice for 10 min. The cells were removed by centrifugation for 10 min at 3000g at 4 °C and 25 μL of supernatant was taken from all the samples. In each sample 1 mL of chloroform/methanol/water (1:3:1 v/v/v) was added, the samples were vortexed for 1 min and centrifuged again for 3 min at 10,000g at 4 °C. Finally, 300 μL of supernatant was added in cryovials (three times from each sample to create back-up technical replicates) and stored at −80 °C. A sample from the growth medium was also taken (i.e. artificial saltwater with F/2, P/24 nutrients) to control for any substances present therein. For quality analysis purposes, a pooled sample of all the samples at each sampling time point was also used for a metabolomics analysis. An untargeted metabolomics approach was employed to determine the metabolic profiles of the cultures. Liquid chromatography-mass spectrometry (LC-MS) analysis was performed with a Thermo Orbitrap Q-Exactive mass spectrometer interfaced to a Dionex UltiMate 3000 RSLC system. Samples (10 μL) were injected onto a Merck Sequant ZIC-pHILIC column (150 mm × 4.6 mm; 5 μm) maintained at 30 °C. Mobile phase A consisted of water containing 20 mM ammonium carbonate and mobile phase B consisted of acetonitrile. The initial conditions for analysis were 20% mobile phase A–80% mobile phase B and the percentage of mobile phase A was increased to 95% over 15 min with a hold for 2 min before re-equilibration to the starting conditions over 9 min. The flow rate was 0.3 mL/min. Analysis was operated in polarity switching mode over the mass-to-charge ratio (m/z) range of 70 to 1050 at a resolution of 70,000. Data sets were processed with IDEOM [50] which uses the XCMS [51] and mzMatch [52] software in the R environment and PiMP [53]. The levels of reliability of the spectral assignment to metabolites, as defined by the Metabolomics Standard Initiative were as follows: ‘MSI:1 (identified metabolites)’: high resolution mass (3 ppm) and retention time (5%) matched to an authentic standard, ‘MSI:2 (putatively annotated compounds)’: high resolution mass matched to a public library (3 ppm).
2.4. Data analysis
For each of our response variables (cell counts, Chla, medium PO4 and intracellular P) the effect of the addition of exometabolites of foreign species on each target species was tested using the following statistical approach. We first fitted a model for each of our four response variables with which we accounted for any differences -in the response variable- between the cultures of a given species prior to the filtrate additions from the “foreign” species. An example of the Generalized Linear Model (GLM) structure in the case of Chla was:
where, fspecies stands for the filtrate addition level, Cspecies stands for the species' culture and their product is testing for the interaction between the two factor variables. After fitting the models, we extracted the fitted values for each response variable and used these instead of the original data values for further analysis. This approach is helping us isolate differences between cultures prior to filtrate addition from the actual treatment effects. Using the fitted values for each response variable we then carried pairwise comparisons between treatment cultures and their respective controls based on the Tukey method for adjustment of means using the package emmeans. The outcome of this analysis was a “contrast value” indicating the difference between treatment and respective control and a corresponding p-value. This difference between treatment and control (from now on referred to as Δ) indicated a significant positive effect of exometabolites on the tested variable when Δ >0 and p < 0.05, a significant negative effect when Δ < 0 and p < 0.05, and no effect when p > 0.05. To illustrate, when the target species Heterosigma (Het) receives filtrate from Tetraselmis (fTet), then the effect on Chla would be negative if ΔChla<0, p < 0.05, indicating that Chla in the replicates of the Het cultures treated with “foreign” species filtrate was on average significantly lower than the replicates of the control Het which received plain artificial seawater.
To determine exometabolites that were excreted by the species, we first excluded from our analysis all the metabolites that were present in a sample taken from the growth medium. Then we fitted the following model, with the response variable being each metabolite and the explanatory variable being the factor variable “species” with four levels corresponding to the control cultures of each species:
Using the F-ratio and associated p-value from this analysis, we identified exometabolites that showed significant differences between the 4 species. These were further explored with pairwise comparisons using the package emmeans for pairwise comparison of means based on the Tukey method. Metabolites that had significantly higher abundance in specific species were discussed as potential substances driving the observed responses in target species at the physiological level (i.e. biomass production, nutrient use).
Statistics were performed using R programming language version 4.0.5 (13-04-2021) [54] in the software R-Studio Desktop. Packages ggplot2 [55], and ggpubr [56] was used for data visualization and emmeans (i.e. Estimated Marginal Means (Least-Squares Means)) was used for pairwise comparison of means based on the Tukey method [57]. Function glm in package stats [52] was used for the GLM.
3. Results
3.1. Response of target microalgae to filtrate addition of “foreign” microalgae
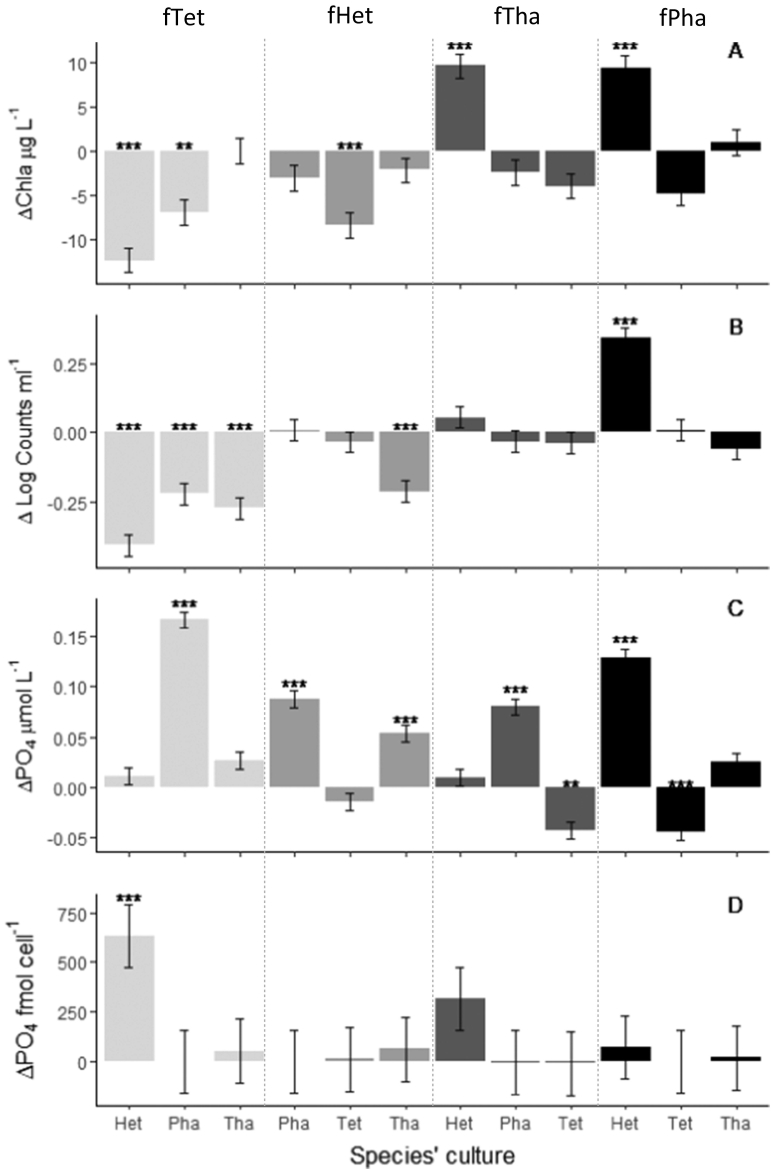
Our results show that filtrate from Heterosigma (fHet) and Tetraselmis (fTet) can cause a significant biomass suppression in other species (expressed either as photosynthetic biomass or counts (ΔChla < 0 & ΔCounts < 0, t-test, p < 0.05) (Fig. 2A & B)). Only exception to this was Phaeocystis which remained unaffected by the filtrate addition of Heterosigma (fHet). On the other hand, cultures treated with filtrate from Thalassiosira (fTha) and Phaeocystis (fPha), showed biomass enhancement in the species Heterosigma compared to the respective controls (ΔChla > 0 & ΔCounts > 0, t-test, p < 0.05) (Fig. 2A&B).
Fig. 2.
Effect of filtrate with exometabolites from foreign microalgae species on biomass production and resource use of target microalgae species: biomass production expressed as Chla (panel A) and log cell number (panel B), and resource use of limiting nutrient P expressed as residual PO4 in the medium (panel C) and intracellular P (panel D). For each of the four response variables examined, Δ indicates the difference between treatment and the respective control which was the culture of that species treated with plain medium. The x-axis presents the 12 cultures across our 4 species (Heterosigma-Het, Phaeocystis-Pha, Thalassiosira-Tha, Tetraselmis-Tet) that were being treated with filtrate from the MCs of the “foreign” species (fTet, fHet, fTha, fPha). Annotations of significance levels based on Tukey adjusted pairwise comparisons: p > 0.05, *: p ≤ 0.05, **: p ≤ 0.01, ***: p ≤ 0.001.
Our results also show that residual PO4 measured in the treated culture medium was increased compared to the controls in some treatments, including all Phaeocystis cultures, suggesting it remained unused (ΔPO4 > 0, t-test, p < 0.05), was negative in two Tetraselmis treatments suggesting that more was used compared to controls (ΔPO4 < 0, t-test, p < 0.05), and remained unaffected in another 5 treatments (ΔPO4 = 0, t-test, p > 0.05) (Fig. 2C). Regarding intracellular P concentrations, no differences were observed between treatments and controls (ΔPO4 = 0, t-test, p > 0.05) with the exception of Heterosigma treated with Tetraselmis filtrate (fTet) whereby although the residual PO4 in the medium remained unchanged, there was a significant increase in intracellular PO4 (Fig. 2D).
3.2. Exometabolites measured in the filtrate of “foreign” microalgae
58 detected ion signals showed statistically significant differences between the four microalgae species (GLM, p < 0.05). From these, 23 were selected as being present in the cultures and not in the growth medium (GM), indicating they were produced endogenously by the algal species. From those, a metabolite putatively identified as L-histidinal was significantly elevated in cultures of Tetraselmis (Fig. 3A). Further, metabolites putatively annotated as tiliacorine and hydrogen iodide were present in higher levels in Heterosigma and Thalassiosira cultures respectively (Fig. 3B & E), together with several unidentified ion signals (SFig. 1A, B & G). The haptophyte Phaeocystis was found to have the largest number of significantly elevated exometabolites. Specifically, cultures from this species showed increased concentrations of a metabolite putatively annotated as S,S-dimethyl-beta-propiothetin also known as dimethylsulfoniopropionate (DMSP), a tetra peptide Asp-Leu-Lys-Gln (Fig. 3C & D) as well as four uncharacterized ion signals (SFig. 1C–F).
Fig. 3.
Putative exometabolites measured from the filtrate of the control monocultures of the four microalgae species (Heterosigma-Het, Phaeocystis-Pha, Thalassiosira-Tha, Tetraselmis-Tet) that were either significantly higher either in specific species or across subgroups of the four microalgae species (GLM, p < 0.05). Annotations of significance levels based on Tukey adjusted pairwise comparisons: p > 0.05, *: p ≤ 0.05, **: p ≤ 0.01, ***: p ≤ 0.001.
4. Discussion
Here we investigate responses of “target” microalgae to exometabolites of “foreign” microalgae by testing for directional effects of all possible combinations of four species belonging to different taxonomic groups and representing HAB and non-HAB species (Fig. 4). Our experiment enabled to disentangle the effect of chemical interference from that of nutrient competition by using cell-fee culture medium from the “foreign” species' cultures. Our findings show that responses of target species strongly depend on which foreign species is affecting them. They also reveal that chemical interference through exometabolites of foreign species can cause a range of responses to target microalgae the most common being biomass suppression (e.g. all species under the influence of Tetraselmis), and less common being biomass enhancement (e.g. Heterosigma under the influence of Phaeocystis) (Fig. 4). The increased intracellular P content in Heterosigma cells under the influence of Tetraselmis also demonstrates the potential of microalgae for growth strategy shifts as a response to sensing competitors. Indeed, although this strategy has been previously documented in Heterosigma which was actively migrating to accumulate P from nutrient-rich deep water layers [8], [58] it has not been previously documented as a response to sensing of competitor species. These findings have obvious implications in the way we understand ecological interactions between microalgae, indicating that interspecific interactions extend beyond nutrient competition and allelopathy and should be carefully factored in species population models predicting assemblage species composition, biodiversity or biomass.
Fig. 4.

Range of responses of “target” microalgae to exometabolites of “foreign” microalgae (Heterosigma-Het, Phaeocystis-Pha, Thalassiosira-Tha, Tetraselmis-Tet). Our crossed experimental design enabled the testing of directional effects of all possible combinations of four species belonging to different taxonomic groups and representing HAB and non-HAB species: Het-Ochrophyta and Pha-Haptophyta (HAB), Tet-Chlorophyta and Tha-Bacillariophyta (non-HAB).
Our findings have also important implications for industrial production of microalgae as they are challenging the view that co-cultures can be used for maximizing algal biomass in bioreactors [59], [60]. Specifically, here we show that the most common effect of foreign species' filtrate is a decrease in the biomass of the target species and this response was observed for all 3 species under the influence of Tetraselmis and 2 species under the influence of Heterosigma. This is important as Tetraselmis is well known for its commercial potential for biofuel production and high value products [61], [62], [63] as well as fish and shellfish aquaculture feed [64] due to its high-lipid content strain [65]. In co-culture settings within bioreactors, such negative effects due to chemical interference can only be exacerbated due to nutrient competition thus leading to an undesirable underyielding relative to the respective monocultures. Although underyielding was in fact observed in previous experimental studies using species co-cultures [32], [66] the effect of chemical interference was not disentangled from nutrient competition obscuring the mechanisms behind the observed yield patterns.
Interestingly, the species that was shown to produce the most negative responses to target species was Tetraselmis sp., a species not considered as harmful. Indeed, our findings suggest that species traditionally thought of as non-HAB, might be capable of causing biomass inhibition to other microalgae species. The absence of a known toxin in this case, raised the question whether the response of target species is due to sensing and reacting to exometabolites of Tetraselmis via e.g. a growth strategy shift or rather are directly affected by exometabolites that have algicidal action. Further insights to this were obtained by our comparative metabolomics analysis which showed that L-histidinal, a biosynthetic precursor of the amino acid L-histidine, was the only exometabolite present in significantly higher abundance in Tetraselmis. A previous study found that L-histidine produced by bacterial cultures of Bacillus sp. strain B1 could have acted as an algicidal of a Phaeocystis globosa HAB in Zhuhai, China [67], [68]. This suggestion was confirmed by further experimental work testing the effects of commercially purchased L-histidine on Phaeocystis globosa cultures [69]. Our study indicates that L-histidinal can in fact also originate from microalgae species and could adversely affect biomass production of species of different taxonomic groups (Bacillariophyta, Haptophyta, Ochrophyta). Therefore, the potential of this compound as a novel biotoxin used to regulate natural HABs, should be further explored.
Heterosigma akashiwo also caused negative effects on growth of 2 other target species. This HAB raphidophyte species is known to cause extensive fish-killing blooms worldwide [43]. The ichthyotoxicity of H. akashiwo is still under investigation although a number of potential mechanisms have been proposed, such as the production of Reactive Oxygen Species (ROS) compounds, neurotoxins, hemolytic compounds etc. The bloom success of H. akashiwo has been associated with its production of allelochemicals capable of inhibiting the growth of co-occurring microalgae, e.g. high-molecular-weight polysaccharide-protein complexes (Wang et al. [70] and references therein). A putative metabolite uniquely identified in H. akashiwo for the first time in our study is Tiliacorine, an alkaloid originally identified in the edible plant Tiliacora triandra with pharmaceutical potential due to confirmed cytotoxicity of the malarian inducing protozoan Plasmodium falciparum [71] and the tuberculosis inducing bacterium Mycobacterium tuberculosis [72]. Our study indicates the potential of this substance as an algicidal agent and merits further experimentations.
Positive effects on both cell counts and photosynthetic biomass were observed only in the species Heterosigma under the influence of Phaeocystis sp. Phaeocystis showed significantly increased metabolite signals of the putatively annotated as S,S-Dimethyl-beta-propiothetin, also known as dimethylsulfoniopropionate (DMSP). DMSP is actively synthesized by certain microalgae, where it is thought to have osmotic, cryoprotective, predator deterring and antioxidant properties [73] and is produced in very high concentrations during microalgal blooms in the field [74]. Previous studies have found that this might be an important source of carbon and sulfur for bacteria in aquatic systems [30], [75], [76]. The fact that Heterosigma showed increased yield while at the same time leaving the limiting phosphate unused in the medium, suggests that this known mixotroph might have switched the growth strategy from autotrophy to heterotrophy. The latter could have been triggered by boosted bacteria growth due to DMSP in the Phaeocystis treatments. Enhanced microalgal growth due to facilitation effects from bacteria as well as the role of DMSP in these mutualistic associations is being studied [77]. Furthermore, the potential of algae-bacteria interactions, also mediated by DMSP is being increasingly explored in the field of biofuels [78]. The indirect link between a microalgal exometabolite boosting mixotrophic algal yield through bacteria growth merits further research due to the important potential of maximizing algal biomass without nutrient supply, as already highlighted in previous studies for nitrogen [79].
The present study highlights the importance of exometabolites in microalgae interactions and the complexity of responses they invoke. Growth facilitation, growth inhibition, algicidal action, strategies shifts, all seem possible responses to foreign species exometabolites, irrespective of known toxicity effects of the studied species. Furthermore, metabolites that mediate the above interactions could not be assigned to a putative metabolite, highlighting an important gap in metabolite research. Although beyond the scope of the present study, some of our findings suggest the importance of algae-bacteria associations, especially in the case of mixotroph species. Our findings, together with the recognized gaps in our knowledge of microalgae metabolites, have important implications in plankton succession prediction modeling and applied microalgae research, including biofuel industry and water remediation.
CRediT authorship contribution statement
NGA: Investigation, Methodology, Formal analysis, Visualization, Writing - Original Draft.
ES: Conceptualization, Methodology, Formal analysis, Visualization, Funding acquisition, Writing - Review & Editing.
ML: Investigation, Writing - Review & Editing.
IV: Methodology, Resources, Writing - Review & Editing.
PW: Methodology, Investigation, Writing - Review & Editing.
CR: Methodology, Investigation, Writing - Review & Editing.
SS: Conceptualization, Methodology, Formal analysis, Visualization, Supervision, Resources, Funding acquisition, Writing - Review & Editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was conducted as part of the research project “Harnessing environmental metabolomics to understand algal warfare” funded by Wellcome Trust Institutional Strategic Support Fund (ISSF) Excellence and Innovation Catalyst Grant, University of Glasgow- Award number 204820/Z/16/Z. We would like to thank Professor A. Economou-Amilli and Dr. D. Raitsos for their useful suggestions on a preliminary draft.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.algal.2021.102627.
Appendix A. Supplementary data
Supplementary material including the method for growth rate estimation for our cultures, the medium used, the Chla and residual P references, the method for intracellular P estimation and the results of the metabolites that were not reported in the main text.
References
- 1.Tilman D. Vol. 17. Princeton University Press; 1982. Resource Competition and Community Structure. (MPB-17) [DOI] [PubMed] [Google Scholar]
- 2.Tan K., Huang Z., Ji R., Qiu Y., Wang Z., Liu J. A review of allelopathy on microalgae. Microbiol. (United Kingdom) 2019;165:587–592. doi: 10.1099/mic.0.000776. [DOI] [PubMed] [Google Scholar]
- 3.Cornforth D.M., Foster K.R. Competition sensing: the social side of bacterial stress responses. Nat. Rev. Microbiol. 2013;11:285–293. doi: 10.1038/nrmicro2977. [DOI] [PubMed] [Google Scholar]
- 4.Grandclément C., Tannières M., Moréra S., Dessaux Y., Faure D. Quorum quenching: role in nature and applied developments. FEMS Microbiol. Rev. 2015;40:86–116. doi: 10.1093/femsre/fuv038. [DOI] [PubMed] [Google Scholar]
- 5.Poole K. Stress responses as determinants of antimicrobial resistance in gram-negative bacteria. Trends Microbiol. 2012;20:227–234. doi: 10.1016/j.tim.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Sharma U.K., Chatterji D. Transcriptional switching in Escherichia coli during stress and starvation by modulation of σ70 activity. FEMS Microbiol. Rev. 2010;34:646–657. doi: 10.1111/j.1574-6976.2010.00223.x. [DOI] [PubMed] [Google Scholar]
- 7.Cáceres C., Spatharis S., Kaiserli E., Smeti E., Flowers H., Bonachela J.A. Temporal phosphate gradients reveal diverse acclimation responses in phytoplankton phosphate uptake. ISME J. 2019;13 doi: 10.1038/s41396-019-0473-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solovchenko A.E., Ismagulova T.T., Lukyanov A.A., Vasilieva S.G., Konyukhov I.V., Pogosyan S.I., Lobakova E.S., Gorelova O.A. Luxury phosphorus uptake in microalgae. J. Appl. Phycol. 2019;31:2755–2770. doi: 10.1007/s10811-019-01831-8. [DOI] [Google Scholar]
- 9.Poulson K.L., Sieg R.D., Prince E.K., Kubanek J. Allelopathic compounds of a red tide dinoflagellate have species-specific and context-dependent impacts on phytoplankton. Mar. Ecol. Prog. Ser. 2010;416:69–78. doi: 10.3354/meps08788. [DOI] [Google Scholar]
- 10.Adolf J.E., Bachvaroff T.R., Krupatkina D.N., Nonogaki H., Brown P.J.P., Lewitus A.J., Harvey H.R., Place A.R. Species specificity and potential roles of karlodinium micrum toxin. africanJ. Mar. Sci. 2006;28:415–419. doi: 10.2989/18142320609504189. [DOI] [Google Scholar]
- 11.Tillmann U., Hansen P.J. Allelopathic effects of Alexandrium tamarense on other algae: evidence from mixed growth experiments. Aquat. Microb. Ecol. 2009;57:101–112. doi: 10.3354/ame01329. [DOI] [Google Scholar]
- 12.Uchida T., Toda S., Matsuyama Y., Yamaguchi M., Kotani Y., Honjo T. Interactions between the red tide dinoflagellates heterocapsa circularisquama and Gymnodinium mikimotoi in laboratory culture. J. Exp. Mar. Biol. Ecol. 1999;241:285–299. doi: 10.1016/S0022-0981(99)00088-X. [DOI] [Google Scholar]
- 13.Gastineau R., Turcotte F., Pouvreau J.B., Morançais M., Fleurence J., Windarto E., Prasetiya F.S., Arsad S., Jaouen P., Babin M., Coiffard L., Couteau C., Bardeau J.F., Jacquette B., Leignel V., Hardivillier Y., Marcotte I., Bourgougnon N., Tremblay R., Deschênes J.S., Badawy H., Pasetto P., Davidovich N., Hansen G., Dittmer J., Mouget J.L. Marennine, promising blue pigments from a widespread haslea diatom species complex. Mar. Drugs. 2014;12:3161–3189. doi: 10.3390/md12063161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poulin R.X., Hogan S., Poulson-Ellestad K.L., Brown E., Fernández F.M., Kubanek J. Karenia brevis allelopathy compromises the lipidome, membrane integrity, and photosynthesis of competitors. Sci. Rep. 2018;8:1–10. doi: 10.1038/s41598-018-27845-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubanek J., Hicks M.K., Naar J., Villareal T.A. Does the red tide dinoflagellate karenia brevis use allelopathy to outcompete other phytoplankton? Limnol. Oceanogr. 2005;50:883–895. doi: 10.4319/lo.2005.50.3.0883. [DOI] [Google Scholar]
- 16.Prince E.K., Myers T.L., Kubanek J. Effects of harmful algal blooms on competitors: allelopathic mechanisms of the red tide dinoflagellate karenia brevis. Limnol. Oceanogr. 2008;53:531–541. doi: 10.4319/lo.2008.53.2.0531. [DOI] [Google Scholar]
- 17.Fistarol G.O., Legrand C., Selander E., Hummert C., Stolte W., Granéli E. Allelopathy in alexandrium spp.: effect on a natural plankton community and on algal monocultures. Aquat. Microb. Ecol. 2004;35:45–56. doi: 10.3354/ame035045. [DOI] [Google Scholar]
- 18.Tillmann U., Alpermann T., John U., Cembella A. Allelochemical interactions and short-term effects of the dinoflagellate alexandrium on selected photoautotrophic and heterotrophic protists. Harmful Algae. 2008;7:52–64. doi: 10.1016/j.hal.2007.05.009. [DOI] [Google Scholar]
- 19.Vanelslander B., Paul C., Grueneberg J., Prince E.K., Gillard J., Sabbe K., Pohnert G., Vyverman W. Daily bursts of biogenic cyanogen bromide (BrCN) control biofilm formation around a marine benthic diatom. Proc. Natl. Acad. Sci. U. S. A. 2012;109:2412–2417. doi: 10.1073/pnas.1108062109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poulin R.X., Poulson-Ellestad K.L., Roy J.S., Kubanek J. Variable allelopathy among phytoplankton reflected in red tide metabolome. Harmful Algae. 2018;71:50–56. doi: 10.1016/j.hal.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Allen J.L., Ten-Hage L., Leflaive J. Impairment of benthic diatom adhesion and photosynthetic activity by allelopathic compounds from a green alga: involvement of free fatty acids? Environ. Sci. Pollut. Res. 2015;22:13669–13680. doi: 10.1007/s11356-014-3873-9. [DOI] [PubMed] [Google Scholar]
- 22.Thanh Doan N., Rickards R.W., Rothschild J.M., Smith G.D. Allelopathic actions of the alkaloid 12-epi-hapalindole E isonitrile and calothrixin a from cyanobacteria of the genera fischerella and calothrix. J. Appl. Phycol. 2000;12:409–416. doi: 10.1023/a:1008170007044. [DOI] [Google Scholar]
- 23.Volk R.B., Girreser U., Al-Refai M., Laatsch H. Bromoanaindolone, a novel antimicrobial exometabolite from the cyanobacterium Anabaena constricta. Nat. Prod. Res. 2009;23:607–612. doi: 10.1080/14786410802114068. [DOI] [PubMed] [Google Scholar]
- 24.Sun Y., Xu S., Li W., Zhang J., Wang C. Antialgal substances from Isochrysis galbana and its effects on the growth of Isochrysis galbana and six species of feed microalgae. Adv. Intell. Soft Comput. 2012;134 AISC:211–223. doi: 10.1007/978-3-642-27537-1_27. [DOI] [Google Scholar]
- 25.Ikawa M., Haney J.F., Sasner J.J. Inhibition of chlorella growth by the lipids of cyanobacterium Microcystis aeruginosa. Hydrobiologia. 1996;331:167–170. doi: 10.1007/BF00025418. [DOI] [Google Scholar]
- 26.Kearns K.D., Hunter M.D. Toxin-producing Anabaena flos-aquae induces settling of Chlamydomonas reinhardtii, a competing motile alga. Microb. Ecol. 2001;42:80–86. doi: 10.1007/s002480000086. [DOI] [PubMed] [Google Scholar]
- 27.Rengefors K., Legrand C. Broad allelopathic activity in Peridinium aciculiferum (Dinophyceae) Eur. J. Phycol. 2007;42:341–349. doi: 10.1080/09670260701529604. [DOI] [Google Scholar]
- 28.Schmidt L.E., Hansen P.J., Schmidt Hansen 01. Chrysochromulina allelopathy.pdf. 2001;216:67–81. [Google Scholar]
- 29.Wang R., Xue Q., Wang J., Tan L., Zhang Q., Zhao Y., Anderson D.M. and growth effects. 2019:527–534. doi: 10.1016/j.chemosphere.2017.08.024.Effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simó R., Archer S.D., Pedrós-Alió C., Gilpin L., Stelfox-Widdicombe C.E. Coupled dynamics of dimethylsulfoniopropionate and dimethylsulfide cycling and the microbial food web in surface waters of the North Atlantic. Limnol. Oceanogr. 2002;47:53–61. doi: 10.4319/lo.2002.47.1.0053. [DOI] [Google Scholar]
- 31.Vanelslander B., De Wever A., Van Oostende N., Kaewnuratchadasorn P., Vanormelingen P., Hendrickx F., Sabbe K., Vyverman W. Complementarity effects drive positive diversity effects on biomass production in experimental benthic diatom biofilms. J. Ecol. 2009;97:1075–1082. doi: 10.1111/j.1365-2745.2009.01535.x. [DOI] [Google Scholar]
- 32.Papanikolopoulou L.A., Smeti E., Roelke D.L., Dimitrakopoulos P.G., Kokkoris G.D., Danielidis D.B., Spatharis S. Interplay between r- and K-strategists leads to phytoplankton underyielding under pulsed resource supply. Oecologia. 2018;186 doi: 10.1007/s00442-017-4050-x. [DOI] [PubMed] [Google Scholar]
- 33.Wu J.T., Kuo-Huang L.L., Lee J. Algicidal effect of Peridinium bipes on Microcystis aeruginosa. Curr. Microbiol. 1998;37:257–261. doi: 10.1007/s002849900375. [DOI] [PubMed] [Google Scholar]
- 34.Wang R., Xue Q., Wang J., Tan L., Zhang Q., Zhao Y., Anderson D.M. Effects of an allelochemical in Phaeodactylum tricornutum filtrate on heterosigma akashiwo: morphological, physiological and growth effects. Chemosphere. 2017;186:527–534. doi: 10.1016/j.chemosphere.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rengefors K., Legrand C. Toxicity in Peridinium aciculiferum - an adaptive strategy to outcompete other winter phytoplankton? Limnol. Oceanogr. 2001;46:1990–1997. doi: 10.4319/lo.2001.46.8.1990. [DOI] [Google Scholar]
- 36.Smeti E., Roelke D.L., Gremion G., Linhart J.M., Danielidis D.B., Spatharis S. Potential mechanisms of coexistence between two globally important pseudo-nitzschia (Bacillariophyta) species. Hydrobiologia. 2015;762 doi: 10.1007/s10750-015-2340-z. [DOI] [Google Scholar]
- 37.Bujak R., Struck-Lewicka W., Markuszewski M.J., Kaliszan R. Metabolomics for laboratory diagnostics. J. Pharm. Biomed. Anal. 2015;113:108–120. doi: 10.1016/j.jpba.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 38.Sardans J., Peñuelas J., Rivas-Ubach A. Ecological metabolomics: overview of current developments and future challenges. Chemoecology. 2011;21:191–225. doi: 10.1007/s00049-011-0083-5. [DOI] [Google Scholar]
- 39.Kirpenko N.I., Kurashov Y.A., Krylova Y.V. Component composition of exometabolites in cultures of some algae. Hydrobiol. J. 2012;48:59–70. doi: 10.1615/HydrobJ.v48.i3.60. [DOI] [Google Scholar]
- 40.Kuhlisch C., Pohnert G. Metabolomics in chemical ecology. Nat. Prod. Rep. 2015;32:937–955. doi: 10.1039/c5np00003c. [DOI] [PubMed] [Google Scholar]
- 41.Verity P.G., Brussaard C.P., Nejstgaard J.C., Van Leeuwe M.A., Lancelot C., Medlin L.K. Current understanding of phaeocystis ecology and biogeochemistry, and perspectives for future research. Biogeochemistry. 2007;83:311–330. doi: 10.1007/s10533-007-9090-6. [DOI] [Google Scholar]
- 42.Veldhuis M.J.W., Wassmann P. Bloom dynamics and biological control of a high biomass HAB species in European coastal waters: a phaeocystis case study. Harmful Algae. 2005;4:805–809. doi: 10.1016/j.hal.2004.12.004. [DOI] [Google Scholar]
- 43.Edvardsen B., Imai I. In: Ecol. Harmful Algae. Ecol. Stud. Granéli E., Turner J., editors. Vol. 189. Springer; Berlin, Heidelberg: 2006. The ecology of harmful flagellates within Prymnesiophyceae and Raphidophyceae; pp. 67–79. (Analysis Synth). [Google Scholar]
- 44.Romano S., Dittmar T., Bondarev V., Weber R.J.M., Viant M.R., Schulz-Vogt H.N. Exo-metabolome of pseudovibrio sp. FO-BEG1 analyzed by ultra-high resolution mass spectrometry and the effect of phosphate limitation. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reigosa M.J., Sánchez-Moreiras A., González L. 1999. Ecophysiological Approach in Allelopathy. [DOI] [Google Scholar]
- 46.Granéli E., Johansson N. Increase in the production of allelopathic substances by Prymnesium parvum cells grown under N- or P-deficient conditions. Harmful Algae. 2003;2:135–145. doi: 10.1016/S1568-9883(03)00006-4. [DOI] [Google Scholar]
- 47.Granéli E., Hansen P.J. Allelopathy in harmful algae: a mechanism to compete for Resources? Ecol. Harmful Algae. 2006:189–201. doi: 10.1007/978-3-540-32210-8_15. [DOI] [Google Scholar]
- 48.Tamvakis A., Miritzis J., Tsirtsis G., Spyropoulou A., Spatharis S. Effects of meteorological forcing on coastal eutrophication: modeling with model trees. Estuar. Coast. Shelf Sci. 2012;115 doi: 10.1016/j.ecss.2012.09.003. [DOI] [Google Scholar]
- 49.Roelke D.L., Spatharis S. Phytoplankton assemblage characteristics in recurrently fluctuating environments. PLoS One. 2015;10 doi: 10.1371/journal.pone.0120673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Creek D.J., Jankevics A., Burgess K.E.V., Breitling R., Barrett M.P. IDEOM: an excel interface for analysis of LC-MS-based metabolomics data. Bioinformatics. 2012;28:1048–1049. doi: 10.1093/bioinformatics/bts069. [DOI] [PubMed] [Google Scholar]
- 51.Smith C.A., Want E.J., O’Maille G., Abagyan R., Siuzdak G. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006;78:779–787. doi: 10.1021/ac051437y. [DOI] [PubMed] [Google Scholar]
- 52.Scheltema R.A., Jankevics A., Jansen R.C., Swertz M.A., Breitling R. PeakML/mzMatch: a file format, Java library, R library, and tool-chain for mass spectrometry data analysis. Anal. Chem. 2011;83:2786–2793. doi: 10.1021/ac2000994. [DOI] [PubMed] [Google Scholar]
- 53.Gloaguen Y., Morton F., Daly R., Gurden R., Rogers S., Wandy J., Wilson D., Barrett M., Burgess K. PiMP my metabolome: an integrated, web-based tool for LC-MS metabolomics data. Bioinformatics. 2017;33:4007–4009. doi: 10.1093/bioinformatics/btx499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Team R.Core. R Foundation for Statistical Computing; Vienna, Austria: 2021. R: A Language and Environment for Statistical Computing.https://www.r-project.org/ [Google Scholar]
- 55.Wickham H., Chang W., Henry L., Pedersen T.L., Takahashι K., Wilke C., Woo K., Yutani H., Dunnington D. 2020. Package ‘ggplot2’ : Create Elegant Data Visualisations Using the Grammar of Graphics, R Packag. Version 3.3.0. [Google Scholar]
- 56.Kassambara A., ‘ggpubr’ “ggplot2” based publication ready plots, R packag. Version 0.2.5. 2020 [Google Scholar]
- 57.Lenth R.V., emmeans Estimated marginal means, aka least-squares means. R package version 1.1. Https://CRAN.R-Project.Org/Package=emmeans. 2018 [Google Scholar]
- 58.Watanabe M., Kohata K., Kunugi M. Phosphate accumulation and metabolism by Heterosigma akashiwo (Raphidophyceae) during diel vertical migration i n a stratified microcosm. 1988. pp. 22–28. [Google Scholar]
- 59.Newby D.T., Mathews T.J., Pate R.C., Huesemann M.H., Lane T.W., Wahlen B.D., Mandal S., Engler R.K., Feris K.P., Shurin J.B. Assessing the potential of polyculture to accelerate algal biofuel production. Algal Res. 2016;19:264–277. doi: 10.1016/j.algal.2016.09.004. [DOI] [Google Scholar]
- 60.Bacellar Mendes L.B., Vermelho A.B. Allelopathy as a potential strategy to improve microalgae cultivation. Biotechnol. Biofuels. 2013;6:1. doi: 10.1186/1754-6834-6-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim Z.H., Park H., Lee C.G. Seasonal assessment of biomass and fatty acid productivity by tetraselmis sp. In the ocean using semi-permeable membrane photobioreactors. J. Microbiol. Biotechnol. 2016;26:1098–1102. doi: 10.4014/jmb.1601.01031. [DOI] [PubMed] [Google Scholar]
- 62.Pereira H., Gangadhar K.N., Schulze P.S.C., Santos T., De Sousa C.B., Schueler L.M., Custódio L., Malcata F.X., Gouveia L., Varela J.C.S., Barreira L. Isolation of a euryhaline microalgal strain, tetraselmis sp. CTP4, as a robust feedstock for biodiesel production. Sci. Rep. 2016;6:1–11. doi: 10.1038/srep35663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Skjånes K., Rebours C., Lindblad P. Potential for green microalgae to produce hydrogen, pharmaceuticals and other high value products in a combined process. Crit. Rev. Biotechnol. 2013;33:172–215. doi: 10.3109/07388551.2012.681625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muller-Feuga A. Handb. Microalgal Cult. Appl. Phycol. Biotechnol. Second Ed. 2013. Microalgae for aquaculture: the current global situation and future trends; pp. 615–627. [DOI] [Google Scholar]
- 65.Montero M.F., Aristizábal M., García Reina G. Isolation of high-lipid content strains of the marine microalga Tetraselmis suecica for biodiesel production by flow cytometry and single-cell sorting. J. Appl. Phycol. 2011;23:1053–1057. doi: 10.1007/s10811-010-9623-6. [DOI] [Google Scholar]
- 66.Schmidtke A., Gaedke U., Weithoff G. A mechanistic basis for underyielding in phytoplankton communities. Ecology. 2010;91:212–221. doi: 10.1890/08-2370.1. [DOI] [PubMed] [Google Scholar]
- 67.Zhao L., Chen L., Yin P. Algicidal metabolites produced by bacillus sp. Strain B1 against Phaeocystis globosa. J. Ind. Microbiol. Biotechnol. 2014;41:593–599. doi: 10.1007/s10295-013-1393-0. [DOI] [PubMed] [Google Scholar]
- 68.Hu X., Yin P., Zhao L., Yu Q. Characterization of cell viability in Phaeocystis globosa cultures exposed to marine algicidal bacteria. biotechnolBioprocess Eng. 2015;20:58–66. doi: 10.1007/s12257-014-0437-2. [DOI] [Google Scholar]
- 69.Zhuang L., Zhao L., Yin P. Combined algicidal effect of urocanic acid,: N -acetylhistamine and l-histidine to harmful alga Phaeocystis globosa. RSC Adv. 2018;8:12760–12766. doi: 10.1039/c8ra00749g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang R., Xue Q., Wang J., Tan L. Competitive interactions between two allelopathic algal species: heterosigma akashiwo and Phaeodactylum tricornutum. Mar. Biol. Res. 2020;16:32–43. doi: 10.1080/17451000.2019.1702213. [DOI] [Google Scholar]
- 71.Pavanand K., Webster H.K., Yongvanitchit K., Dechatiwongse T. Antimalarial activity of tiliacora triandra diels against plasmodium falciparum in vitro. Phyther. Res. 1989;3:215–217. doi: 10.1002/ptr.2650030514. [DOI] [Google Scholar]
- 72.Sureram S., Senadeera S.P.D., Hongmanee P., Mahidol C., Ruchirawat S., Kittakoop P. Antimycobacterial activity of bisbenzylisoquinoline alkaloids from tiliacora triandra against multidrug-resistant isolates of mycobacterium tuberculosis, bioorganic med. Chem. Lett. 2012;22:2902–2905. doi: 10.1016/j.bmcl.2012.02.053. [DOI] [PubMed] [Google Scholar]
- 73.Zheng Y., Wang J., Zhou S., et al. Bacteria are important dimethylsulfoniopropionate producers in marine aphotic and high-pressure environments. Nat. Commun. 2020;11:4658. doi: 10.1038/s41467-020-18434-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spielmeyer A., Gebser B., Pohnert G. Investigations of the uptake of dimethylsulfoniopropionate by phytoplankton. ChemBioChem. 2011;12:2276–2279. doi: 10.1002/cbic.201100416. [DOI] [PubMed] [Google Scholar]
- 75.Kiene R.P., Linn L.J. The fate of dissolved dimethylsulfoniopropionate (DMSP) in seawater: tracer studies using 35S-DMSP. Geochim. Cosmochim. Acta. 2000;64:2797–2810. doi: 10.1016/S0016-7037(00)00399-9. [DOI] [Google Scholar]
- 76.Zubkov M.V., Fuchs B.M., Archer S.D., Kiene R.P., Amann R., Burkill P.H. Linking the composition of bacterioplankton to rapid turnover of dissolved dimethylsulphoniopropionate in an algal bloom in the North Sea. Environ. Microbiol. 2001;3:304–311. doi: 10.1046/j.1462-2920.2001.00196.x. [DOI] [PubMed] [Google Scholar]
- 77.Cirri E., Pohnert G. Algae−bacteria interactions that balance the planktonic microbiome. New Phytol. 2019;223:100–106. doi: 10.1111/nph.15765. [DOI] [PubMed] [Google Scholar]
- 78.Patidar S.K., Kim S.H., Kim J.H., Park J., Park B.S., Han M.S. Pelagibaca bermudensis promotes biofuel competence of Tetraselmis striata in a broad range of abiotic stressors: dynamics of quorum-sensing precursors and strategic improvement in lipid productivity. Biotechnol. Biofuels. 2018;11:1–16. doi: 10.1186/s13068-018-1097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stewart J.J., Bianco C.M., Miller K.R., Coyne K.J. The marine microalga, heterosigma akashiwo, converts industrial waste gases into valuable biomass. Front. Energy Res. 2015;3:1–8. doi: 10.3389/fenrg.2015.00012. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material including the method for growth rate estimation for our cultures, the medium used, the Chla and residual P references, the method for intracellular P estimation and the results of the metabolites that were not reported in the main text.