Abstract
Comparison of epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) monotherapy or with bevacizumab in real-world non-small cell lung cancer (NSCLC) patients was lacking. 310 patients of advanced NSCLC with common EGFR mutation receiving first-generation EGFR-TKI monotherapy or with bevacizumab were included and propensity-score matched. Progression-free survival (PFS), overall survival (OS) and secondary T790M mutation were analysed. Patients receiving EGFR-TKI and bevacizumab were significantly younger, had better performance status and with high incidence of brain metastasis (55.8%). In the propensity-score matched cohort, PFS (13.5 vs. 13.7 months; log-rank p = 0.700) was similar between the two groups. The OS (61.3 vs. 34.2 months; log-rank p = 0.010) and risk reduction of death (HR 0.42 [95% CI 0.20–0.85]; p = 0.017) were significantly improved in EGFR-TKI plus bevacizumab group. Analysis of treatment by brain metastasis status demonstrated EGFR-TKI plus bevacizumab in patients with brain metastasis was associated with significant OS benefit compared to other groups (log-rank p = 0.030) and these patients had lower early-CNS and early-systemic progressions. The secondary T790M did not significantly differ between EGFR-TKI plus bevacizumab and EGFR-TKI monotherapy groups (66.7% vs. 75.0%, p = 0.460). Forty-one (31.1%) and 31 (23.5%) patients received subsequent osimertinib and chemotherapy, respectively. The post-progression OS of osimertinib and chemotherapy were 22.1 and 44.9 months in EGFR-TKI plus bevacizumab group and were 10.0 and 14.1 months in EGFR-TKI monotherpay group, respectively. First-generation EGFR-TKI with bevacizumab improved treatment efficacy in real-world patients of NSCLC with EGFR mutation. Patients with brain metastasis received additional OS benefit from this treatment.
Subject terms: Cancer, Lung cancer, Non-small-cell lung cancer
Introduction
Erlotinib and gefitinib are first-generation EGFR-TKIs with non-covalent and reversible binding activity to receptor tyrosine kinase and are both approved treatments for patients of advanced NSCLC harboring sensitizing EGFR mutation1,2. Although patients treated by first-generation EGFR-TKI demonstrated an improved efficacy compared to platinum-based chemotherapy, this treatment usually exhibited variable objective response rates at approximately 50–75% and a median PFS between 9 to14 months3–6.
A number of combination strategies were attempted in the hope to improve the treatment efficacy of single-agent EGFR-TKI. This includes anti-angiogenesis agents that specifically target vascular endothelial growth factor (VEGF) or VEGF receptor 2 (VEGFR2)7. The long-established role of angiogenesis in the progression of lung cancer has supported the rationale to combine anti-angiogenesis agents with active anti-cancer treatments8. Two previous randomized studies, the JO25567 and NEJ026 trials, which mainly involved Japanese patients, have demonstrated PFS benefit of erlotinib plus bevacizumab compared to erlotinib monotherapy in the front-line treatment of advanced NSCLC with sensitizing EGFR mutation9,10. However, the OS benefit from bevacizumab add-on was not observed in these studies which may be partly related to the cross-over design of the trials.
The superior efficacy of erlotinib and anti-angiogenesis combination over single-agent erlotinib did not seem to be consistent across different clinical trials. Stinchcombe et al. conducted a phase II randomized study comparing erlotinib monotherapy and with bevacizumab in a Caucasian cohort of EGFR-mutant NSCLC patients11. In this study, PFS and OS were similar in the two groups. Ichihara et al. reported another phase II single-arm study on gefitinib and bevacizumab combination. The study did not meet the primary endpoint of 1-year PFS though a seemingly longer PFS than historical data of gefitinib monotherapy was observed12.
Previously, administration of bevacizumab has demonstrated clinical benefit for both primary brain tumor and brain metastasis from advanced NSCLC, likely as a result of suppressing tumor angiogenesis and reducing intracranial vasogenic edema13,14. Lately, significantly improved OS of bevacizumab treatment in advanced NSCLC patients with brain metastasis compared to those without was also observed in a real-world US cancer registry and claim database15. Thus, the survival benefit of bevacizumab treatment in EGFR-mutant NSCLC patients with brain metastasis warrants further investigation.
Currently, the third-generation EGFR-TKI osimertinib has arisen as a preferred first-line treatment option for advanced EGFR-mutant NSCLC16,17. Recently, combination of osimertinib and bevacizumab has been investigated in both second-line and first-line setting. Akamatsu et al. demonstrated that efficacy of osimertinib plus bevacizumab is statistically equivalent to, yet numerically lower than, osimertinib monotherapy in patients of acquired EGFR T790M-positive NSCLC18. In contrary, a phase I/II single arm study reported by Yu et al. showcased a positive result in which osimertinib plus bevacizumab in treatment-naïve EGFR-mutant NSCLC patients met the pre-specified effectiveness end point19. On the other side, treatment of osimertinib plus ramucirumab has also been investigated in two phase I studies whereas the efficacy data was premature20,21. Overall, whether combination of third-generation EGFR-TKI and anti-angiogenesis agents will be a new standard of treatment remains unsettled.
In this study, we analysed a real-world, brain metastasis-enriched cohort of NSCLC patients with EGFR-sensitizing mutation who received first-line erlotinib/gefitinib monotherapy or with bevacizumab. The treatment efficacy and the development of secondary T790M were compared between the two groups.
Methods
Patients and treatment
Patients who received a first-generation EGFR-TKI (gefitinib or erlotinib) monotherapy or with bevacizumab as the first-line treatment of advanced NSCLC with common EGFR mutation (exon 19 deletion or exon 21 L858R) were retrospectively included from January 2014 to December 2019. The dose of EGFR-TKI administered were gefitinib 250 mg/day and erlotinib 150 mg/day, respectively; and bevacizumab was administered at a dose of 7.5 mg/kg every 3 weeks. The lower dose strength was chosen because bevacizumab is not reimbursed by National Health Insurance of Taiwan for NSCLC treatment and this dosage has been demonstrated to achieve an equivalent efficacy and numerically lower adverse events compared to the 15 mg/kg dose strength22,23 Patients were excluded if the first dose of bevacizumab was given 3 weeks behind the first dose of EGFR-TKI and those who received an EGFR-TKI monotherapy less than 14 days were also excluded. The progression-free survival (PFS) was defined as the interval between the date of starting EGFR-TKI treatment and the date of radiologically or clinically determined progression or death. The treatment response, including complete response (CR), partial response (PR), stable disease, and progressive disease, was evaluated according to the Response Evaluation Criteria in Solid Tumors (version 1.1). The study used data from the Chang Gung Research Database and the study protocol and the waiver of informed consent form were approved by the Ethics Committee of Chang Gung Memorial Hospital.
Statistical analysis
The Mann–Whitney test was used to determine the statistical significance of continuous variables between the two groups and Fisher exact test was used for evaluating the categorical variables. The Kaplan–Meier survival curves were generated using the R package survival, and the hazard ratio (HR) was analysed using the Cox regression model. The propensity-score-matched analysis was used to balance the clinical characteristics between the treatment groups as previously described, in which the distance measure was defined by generalized linear model, the matching method was nearest neighbor matching and the caliper was 0.1 in standard deviation unit6. Briefly, the EGFR-TKI plus bevacizumab and EGFR-TKI monotherapy groups served as the dependent variables and the covariates used included age, ECOG PS, EGFR mutation subtypes, brain metastasis and type of EGFR-TKI administered. Paired patients treated with EGFR-TKI plus bevacizumab or EGFR-TKI alone with equivalent propensity scores were selected in a 1:2 manner using the R package MatchIt. The disease progression patterns (CNS progression alone without death versus systemic progression without death) were treated as competing risk events of which the cumulative incidence functions were calculated. The modified Cox regression model for the subdistribution hazard of the cumulative incidence function was applied to calculate the disease progression hazard from a given pattern in the presence of competing events by using the R package cmprsk. All reported p-values are two sided; p < 0.05 was considered statistically significant. Data were also analysed using SPSS (version 10.1; SPSS, Chicago, IL, USA).
Ethics statement
The study was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki. The Ethics Committee of Chang Gung Memorial Hospital approved the study (No. 201801967B0) and granted permission for access to the Chang Gung Research Database and approved the waiver of the informed consent form.
Results
Baseline patient characteristics
Overall, 310 patients were included in present study in which 267 (86.1%) patients received the treatment of single-agent EGFR-TKI and 43 (13.9%) patients received the treatment of EGFR-TKI plus bevacizumab. Compared to patients who received EGFR-TKI monotherapy, those who received bevacizumab combination in real-world practice were significantly younger (60.4 ± 9.8 vs. 71.2 ± 12.1; p < 0.001), more likely to have erlotinib as treatment partner (90.7% vs. 55.1%; p < 0.001), have a better performance status (ECOG PS 0–1 88.4% vs. 72.7%; p = 0.036) and more likely to present a brain metastasis (55.8% vs. 42.3%; p = 0.140, Table 1). The other patient characteristics including smoking status, sex, EGFR mutation subtype and liver metastasis were similar in the two groups.
Table 1.
Overall patient characteristics.
| Total (%) N = 310 |
TKI plus bevacizumab (%) N = 43 |
TKI alone (%) N = 267 |
p value | |
|---|---|---|---|---|
| Age (mean ± SD) | 60.4 ± 9.8 | 71.2 ± 12.1 | < 0.001 | |
| Age ≥ 65 | 208 (67.1) | 15 (34.9) | 193 (72.3) | < 0.001 |
| ECOGPS 0–1 | 232 (74.8) | 38 (88.4) | 194 (72.7) | 0.036 |
| Gender | ||||
| Male | 105 (33.9) | 14 (32.6) | 237 (34.1) | 1.000 |
| Current/ex-smoker | 73 (23.5) | 11 (25.6) | 62 (23.2) | 0.703 |
| Histology | ||||
| Adenocarcinoma | 307 (99.0) | 43 (100.0) | 264 (98.9) | 1.000 |
| Others | 3 (1.0) | 0 | 3 (1.1) | |
| EGFR mutation | ||||
| L858R | 185 (59.7) | 23 (53.5) | 162 (60.7) | 0.405 |
| 19deletion | 125 (40.3) | 20 (46.5) | 105 (39.3) | |
| Disease stage | ||||
| III | 18 (5.6) | 1 (2.3) | 17 (6.4) | 0.485 |
| IV | 292 (94.4) | 42 (97.7) | 250 (93.6) | |
| Site of metastasis | ||||
| Brain | 137 (44.2) | 24 (55.8) | 113 (42.3) | 0.140 |
| Liver | 40 (12.6) | 7 (16.3) | 33 (12.4) | 0.469 |
| EGFR TKI administered | ||||
| Gefitinib | 124 (40.0) | 4 (9.3) | 120 (44.9) | < 0.001 |
| Erlotinib | 186 (60.0) | 39 (90.7) | 147 (55.1) | |
Cox regression analyses of overall survival in all patients
Cox regression survival analyses were performed to determine the independent factors that associated with OS in all study subjects. In univariate analyses, ECOG PS 0–1 (HR 0.44 [95% CI 0.31–0.62]; p < 0.001) and bevacizumab treatment (HR 0.41 [95% CI 0.21–0.78]; p = 0.006) were associated with a prolonged OS whereas EGFR L858R mutation (HR 1.49 [95% CI 1.06–2.09]; p = 0.022) and liver metastasis (HR 1.43 [95% CI 0.89–2.31]; p = 0.139, Table 2) were associated with a reduced OS. In multivariate analyses, ECOG PS 0–1 (HR 0.46 [95% CI 0.33–0.66]; p < 0.001) and bevacizumab treatment (HR 0.48 [95% CI 0.25–0.92]; p = 0.027) remained predictive of an improved OS. On the other side, EGFR L858R mutation (HR 1.42 [95% CI 1.01–2.02]; p = 0.047, Table 2) still served as a negative predictor of OS.
Table 2.
Cox regression overall survival analysis.
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age ≥ 65 | 1.22 | 0.86–1.73 | 0.259 | – | – | – |
| ECOG 0, 1 | 0.44 | 0.31–0.62 | < 0.001 | 0.46 | 0.33–0.66 | < 0.001 |
| Male | 1.24 | 0.89–1.73 | 0.196 | – | – | – |
| Current/ex-smoker | 1.30 | 0.91–1.87 | 0.152 | – | – | – |
| EGFR L858R | 1.49 | 1.06–2.09 | 0.022 | 1.42 | 1.01–2.02 | 0.047 |
| Bevacizumab treatment | 0.41 | 0.21– 0.78 | 0.006 | 0.48 | 0.25–0.92 | 0.027 |
| Brain metastasis | 1.15 | 0.83–1.60 | 0.397 | |||
| Liver metastasis | 1.43 | 0.89–2.31 | 0.139 | 1.20 | 0.73–1.96 | 0.464 |
Treatment outcomes in the propensity-score matched patients
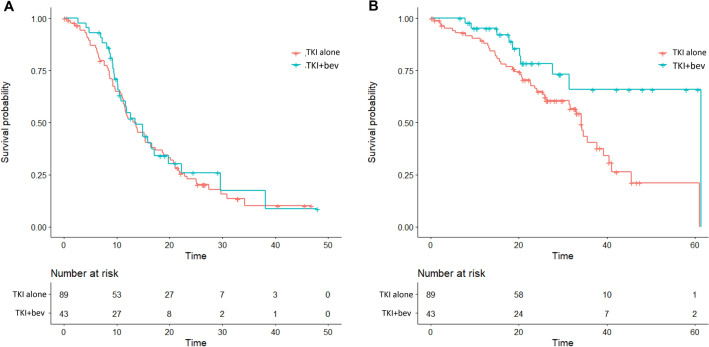
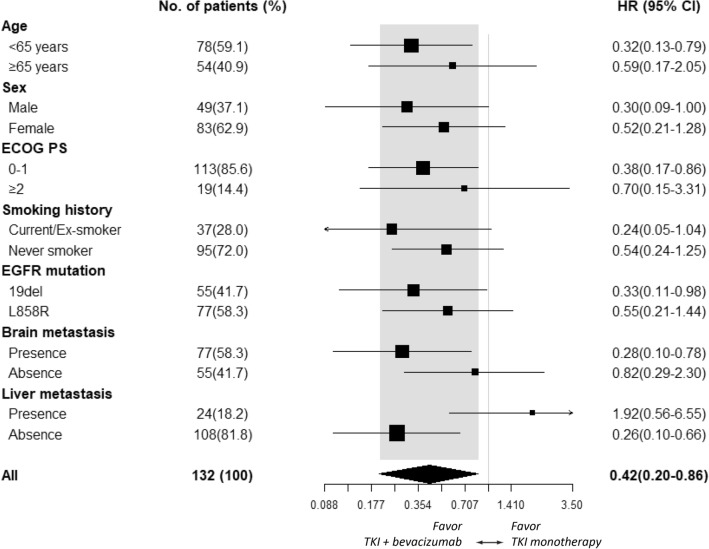
A propensity-score matching was subsequently conducted in a 1:2 manner between the EGFR-TKI plus bevacizumab and EGFR-TKI monotherapy groups. After matching, a cohort of 132 patients with balanced characteristic was attained which consisted of 43 and 89 patients in EGFR-TKI plus bevacizumab group and single-agent EGFR-TKI group, respectively (Table 3). The median follow-up duration, estimated by reverse Kaplan–Meier method, was 23.1 months and 32.9 months in the EGFR-TKI plus bevacizumab and EGFR-TKI monotherapy groups, respectively. The median cycle of bevacizumab treatment was 11 (7.0–17.5). At the end of follow up, 29 (67.4%) events of disease progression or death were noted in the EGFR-TKI plus bevacizumab group and 69 (77.5%) events were observed in the EGFR-TKI monotherapy group. The median PFS (13.5 vs. 13.7 months; log-rank test p = 0.700), risk reduction toward disease progression (HR 0.90 [95% CI 0.58–1.40]; p = 0.656) and the 24-month PFS rate (26.0% [95% CI 14.3–47.0%] vs. 22.9% [95% CI 15.3–34.2%], Fig. 1A) were similar between the two groups. However, the median OS (61.3 vs. 34.2 months; log-rank test p = 0.010), risk reduction of death (HR 0.42 [95% CI 0.20–0.85]; p = 0.017) and the 3-year OS rate (65.8% [95% CI 48.3–89.6%] vs. 40.5% [95% CI 28.8–56.8%], Fig. 1B) was significantly improved in the EGFR-TKI plus bevacizumab group compared the single-agent EGFR-TKI group. Subgroup OS analysis indicated additional benefit of bevacizumab in patients with brain metastasis (HR 0.28 [95% CI 0.10–0.78]; p = 0.015), patients who had ECOG PS 0–1 (HR 0.38 [95% CI 0.17–0.86]; p = 0.020), patients with EGFR 19 deletion mutation (HR 0.33 [95% CI 0.11–0.98]; p = 0.046) and patients who had no liver metastasis (HR 0.26 [95% CI 0.10–0.66]; p = 0.005, Fig. 2).
Table 3.
Propensity-score matched cohort.
| Total (%) N = 132 |
TKI plus bevacizumab (%) N = 43 |
TKI alone (%) N = 89 |
p value | |
|---|---|---|---|---|
| Age (mean ± SD) | 60.4 ± 9.8 | 62.4 ± 10.6 | 0.284 | |
| Age ≥ 65 | 54 (40.9) | 15 (34.9) | 39 (43.8) | 0.351 |
| ECOG PS 0–1 | 113 (85.6) | 38 (88.4) | 75 (84.3) | 0.606 |
| Gender | ||||
| Male | 49 (37.1) | 14 (32.6) | 35 (39.3) | 0.565 |
| Current/ex-smoker | 37 (28.0) | 11 (25.6) | 26 (29.2) | 0.836 |
| Histology | ||||
| Adenocarcinoma | 132 (100.0) | 43 (100.0) | 89 (100.0) | 1.000 |
| EGFR mutation | ||||
| L858R | 77 (58.3) | 23 (53.5) | 54 (60.7) | 0.456 |
| 19deletion | 55 (41.7) | 20 (46.5) | 35 (39.3) | |
| Disease stage | ||||
| III | 1 (0.8) | 1 (2.3) | 0 | 0.326 |
| IV | 131 (99.2) | 42 (97.7) | 89 (100.0) | |
| Site of metastasis | ||||
| Brain | 77 (58.3) | 24 (55.8) | 53 (59.6) | 0.710 |
| Liver | 24 (18.2) | 7 (16.3) | 17 (19.1) | 0.812 |
| EGFR TKI administered | ||||
| Gefitinib | 13 (9.8) | 4 (9.3) | 9 (10.1) | 1.000 |
| Erlotinib | 119 (90.2) | 39 (90.7) | 80 (89.9) | |
| Local treatment of brain metastasis | ||||
| WBRT | 38 (28.8) | 10 (23.3) | 28 (31.5) | 0.325 |
| SRS | 1 (0.8) | 1 (2.3) | 0 | |
| Surgical resection | 9 (6.8) | 4 (9.3) | 5 (5.6) | |
WBRT whole brain radiotherapy, SRS stereotactic radiosurgery.
Figure 1.
(A) PFS and (B) OS of EGFR-TKI plus bevacizumab and EGFR-TKI monotherapy groups. bev bevacizumab, TKI tyrosin kinase inhibitor.
Figure 2.
Subgroup analysis of OS of EGFR-TKI plus bevacizumab and EGFR-TKI monotherapy groups.
Patients of brain metastasis and pattern of disease progression
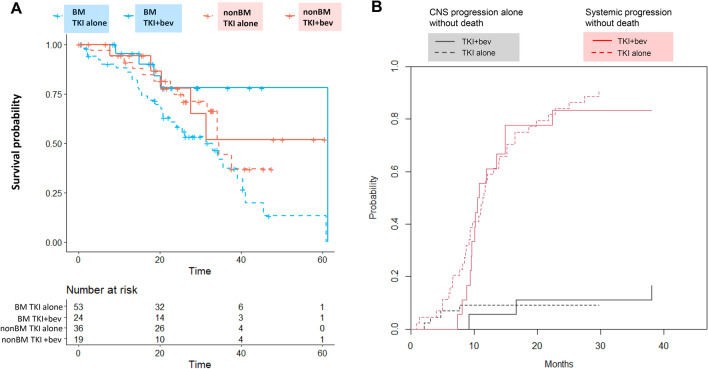
The OS of EGFR-TKI plus bevacizumab and EGFR-TKI monotherapy by the status of baseline brain metastasis was analysed. Patients with brain metastasis who received EGFR-TKI plus bevacizumab and EGFR-TKI monotherapy demonstrated the median OS (61.3 vs. 33.0 months) and the 3-year OS rate (78.1% [95% CI 61.1–99.9%] vs. 37.2% [95% CI 23.6–58.6%]), respectively. In patients without brain metastasis, the median OS (not reach vs. 34.6 months) and the 3-year OS rate (51.9% [95% CI 27.5–98.1%] vs. 44.3% [95% CI 26.0–75.3%], Fig. 3A) were noted in EGFR-TKI plus bevacizumab and single-agent EGFR-TKI groups. A significant OS difference was observed among the four treatment groups (Fig. 3A; log-rank test p = 0.030). The pattern of disease progression in patients with baseline brain metastasis was analysed in terms of the cumulative incidence of systemic or CNS progression. The rates of CNS progression (cause-specific HR, 1.77; 95% CI 0.41–7.56; p = 0.858) and of systemic progression over time (cause-specific HR, 0.88; 95% CI 0.49–1.60; p = 0.692, Fig. 3B) did not differ significantly between EGFR-TKI plus bevacizumab and single-agent EGFR-TKI groups whereas treatment of EGFR-TKI plus bevacizumab was associated with lower emergence of early-CNS and early-systemic progressions which were both delayed to occur beyond 8 to 9 months of treatment (Fig. 3B).
Figure 3.
(A) OS of EGFR-TKI plus bevacizumab and EGFR-TKI monotherapy by the baseline status of brain metastasis (B) Cumulative incidence of systemic progression without death (red) and CNS progression alone without death (black) between EGFR-TKI plus bevacizumab and EGFR-TKI monotherapy groups in patients with baseline brain metastasis. bev bevacizumab, BM brain metastasis, TKI tyrosin kinase inhibitor.
Acquired T790M mutation and efficacy of subsequent post-progression treatment
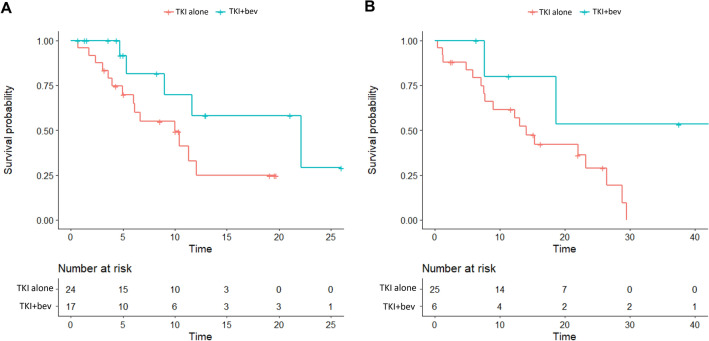
In the original cohort of 310 patients, 119 (38.4%) patients underwent a tissue and/or liquid biopsy for the diagnosis of EGFR T790M mutation where 87 (73.2%) patients were T790M-positive. The T790M-positive rate did not significantly differ between the EGFR-TKI plus bevacizumab and EGFR-TKI monotherapy groups (66.7% vs. 75.0%, p = 0.460). Forty-one (31.1%) and 31 (23.5%) patients of the propensity-score matched cohort, upon disease progression, received subsequent osimertinib treatment or chemotherapy, respectively. Patients of EGFR-TKI plus bevacizumab and EGFR-TKI monotherpay groups demonstrated post-progression osimertinib OS of 22.1 and 10.0 months and post-progression osimertinib 1-year OS rates of 58.2% (95% CI 33.6–100.0%) and 33.0% (95% CI 16.1–67.3%; Fig. 4A), respectively. The post-progression chemotherapy OS were 44.9 and 14.1 months and post-progression chemotherapy 1-year OS rates were 80.0% (95% CI 51.6–100.0%) and 61.6% (95% CI 44.7–84.9%; Fig. 4B) in EGFR-TKI plus bevacizumab and EGFR-TKI monotherpay groups, respectively.
Figure 4.
OS of post-progression (A) osimertinib treatment and (B) chemotherapy between EGFR-TKI plus bevacizumab and EGFR-TKI monotherapy groups. bev bevacizumab, TKI tyrosin kinase inhibitor.
Discussion
The present study demonstrated a superior OS of first-generation EGFR-TKI plus bevacizumab compared to first-generation EGFR-TKI monotherapy in a real-world cohort of EGFR-mutant NSCLC patients with sensitizing mutation. Patients with brain metastasis received additional OS benefit from EGFR-TKI plus bevacizumab. The development of secondary T790M was similar between the two groups. The OS of post-progression treatment, in terms of osimertinib or chemotherapy, in EGFR-TKI plus bevacizumab group were significantly improved compared to single-agent EGFR-TKI group.
Although the present study failed to observe PFS benefit of EGFR-TKI plus bevacizumab in EGFR-mutant NSCLC, the OS outcome was significantly improved in patients receiving the regimen. This result was mainly associated with improved efficacies of post-progression treatments after the exposure of EGFR-TKI plus bevacizumab in patients with brain metastasis. Previously, a number of studies have demonstrated satisfactory efficacies of bevacizumab in selected patients with heavily pre-treated symptomatic brain metastasis from advanced solid tumors24–26. Studies which primarily focused on NSCLC-related brain metastasis also revealed bevacizumab as a viable option that improved intracranial response, stabilized neurological symptoms and reduced systemic corticosteroid use27–29. Recently, a retrospective study analysed a cohort of EGFR-mutant NSCLC patients with multiple brain metastasis who received either EGFR-TKI plus bevacizumab or single-agent EGFR-TKI where significant PFS and OS benefits were both observed in the EGFR-TKI plus bevacizumab arm30. In line with these findings, the present study also demonstrated EGFR-TKI plus bevacizumab provided significant OS benefit in brain metastasis patients who have consisted of approximately 60% of subjects of the propensity-score matched cohort. Overall, patients with brain metastasis represented the major source of OS benefit generated by EGFR-TKI plus bevacizumab in present study. In contrary, these patients were excluded in the JO25567 study and consisted of 32% of the overall population in the NEJ 026 study which may be partly associated with the negative OS results in these studies.
Interestingly, the benefit of bevacizumab treatment in patients with brain metastasis did not seem to be CNS-restricted. Tao et al. specifically investigated a cohort of EGFR-mutant NSCLC patients with multiple brain metastasis and thereby demonstrated that EGFR-TKI plus bevacizumab, compared to EGFR-TKI monotherapy, showed improvement of both intracranial and systemic tumor responses30. In present analysis, although the cumulative incidence of CNS and systemic progression over time did not statistically differ between the EGFR-TKI plus bevacizumab and EGFR-TKI monotherapy groups, the early emergence of CNS and systemic progressions were both suppressed in EGFR-TKI plus bevacizumab group and thereby the both types of progression were delayed to occur beyond 8 to 9 months of the treatment. This finding, in association with previous study, likely suggested an improved extracranial tumor control in bevacizumab-treated patients who had intracranial metastasis. Altogether, the bevacizumab treatment-associated CNS and CNS-sparing benefits in patients of brain metastasis may jointly foster a condition that favors the efficacy of subsequent treatments. This was demonstrated in the present analysis that the OS of second-line osimertinib and chemotherapy were both improved in EGFR-TKI plus bevacizumab group compared to single-agent EGFR-TKI.
In addition, other factors related to post-progression treatment may also have impact on OS outcome. Because a cross-over design was allowed in the NEJ 026 study, 28.6% patients of the single-agent erlotnib arm have received subsequent bevacizumab treatment upon disease progression whereas, in present study, only 3.4% of patients who received monotherapy EGFR-TKI have undergone post-progression bevacizumab treatment. Of note, in this analysis, 18.6% of patients who received EGFR-TKI plus bevacizumab continued bevacizumab treatment beyond progression whereas the rate was only 4.5% of the erlotinib plus bevacizumab arm in the NEJ 026 study. The differential rate of bevacizumab treatment beyond progression may also have some impact on OS as previous AvaALL trial has demonstrated a minor benefit of bevacizumab treatment beyond progression compared to standard-of-care alone31.
As a new recommended first-line treatment for advanced EGFR-mutant NSCLC, third-generation EGFR-TKI osimertinib provided an improved OS compared to gefitinib/erlotinib. In the post-hoc analysis, cohort of EGFR exon 19 deletion patients was the major source that contributed to the OS benefit whereas cohort of EGFR L858R patients generally received minimal treatment benefit from osimertinib. In contrary, the post-hoc analysis of NEJ 026 study revealed that bevacizumab likely offered additional benefit to the cohort of EGFR L858R patients and this finding has also been reported in real-world patients32. The present study also observed a trend of OS benefit in EGFR L858R patients who received combination of EGFR-TKI and bevacizumab. Hence, these findings may warrant further investigation of bevacizumab treatment in patients of EGFR L858R genotype.
Previously, pre-clinical study demonstrated activity of bevacizumab to EGFR T790M mutant and a randomized phase II trial also showcased an improved PFS in patients with de novo T790M mutation treated by erlotinib plus bevacizumab33,34. However, the potential activity of anti-angiogenesis agent to EGFR T790M mutant observed previously did not seem to suppress the development of secondary T790M mutation when it was administered simultaneously with EGFR-TKI. This has been observed in the NEJ 026 and RELAY studies in which the rates of secondary T790M were similar between the anti-angiogenesis agent plus erlotinib and monotherapy erlotinib groups35,36. Similarly, only a numerically lower secondary T790M rate by 8.3% was observed in EGFR-TKI plus bevacizumab group in the present study. Nevertheless, more real-world data is still required to clarify the impact regarding treatment of anti-angiogenesis agent and the development of secondary T790M. The limitation of the present study, firstly, is the potential bias due to retrospective nature per se. Therefore, the treatment-related complications of bevacizumab such as hypertension, peripheral edema and bleeding which may be associated with poor prognosis could not be recorded as comprehensive as a prospective study. Secondly, a small portion (9.8%) of patients of the propensity-score matched cohort received gefitinib, instead of erlotinib, as the first-generation EGFR-TKI and thus increased the data heterogeneity. However, an earlier large scale meta-analysis has suggested similar efficacy of the two drugs37 and thus the heterogeneity is likely acceptable.
In conclusion, this study showcased an improved OS of EGFR-TKI plus bevacizumab in a real-world cohort of patients with advanced EGFR-mutant NSCLC in which patients with brain metastasis received most benefit from the regimen. Further study of bevacizumab treatment in brain metastasis-specific cohort of advanced NSCLC patients is warranted.
Acknowledgements
We thank to the contributions of research assistants Ms. Yen-Wen Wang and Ms. Yu-Chi Chiang to this study.
Author contributions
T.H.C. wrote and C.S.K. revised the manuscript; C.S.K., C.C.W. and C.T.Y. were responsible for study conception and design; A.C.H., J.S.J., T.H.C., P.H.T. and C.H.H. collected the data; C.S.K., H.W.K., P.C.H., Y.F.F. and C.T.Y. provided study materials and patients; C.S.K. and Y.K.G. analyzed and interpreted the data; all authors read and approved the final manuscript.
Funding
The study received funding support from Chang Gung Medical Foundation CORPG3J0332; CORPG3J0333.
Competing interests
CS-K received speaker honoraria from AstraZeneca, Boehringer Ingelheim, Roche, Pfizer, Eli Lilliy, Novartis, OnO Pharma, Chugai, Merck and Guardant Health. CS-K provided consultation for AstraZeneca, Boehringer Ingelheim, Eli Lilliy, Merck, Chugai, Takeda and Novartis. None of the other authors have any conflict of interest to disclose.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cohen MH, Johnson JR, Chen YF, et al. FDA drug approval summary: Erlotinib (Tarceva) tablets. Oncologist. 2005;10(7):461–466. doi: 10.1634/theoncologist.10-7-461. [DOI] [PubMed] [Google Scholar]
- 2.Kazandjian D, Blumenthal GM, Yuan W, et al. FDA approval of gefitinib for the treatment of patients with metastatic EGFR mutation-positive non-small cell lung cancer. Clin. Cancer Res. 2016;22(6):1307–1312. doi: 10.1158/1078-0432.CCR-15-2266. [DOI] [PubMed] [Google Scholar]
- 3.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 4.Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label. ENSURE study. Ann. Oncol. 2015;26(9):1883–1889. doi: 10.1093/annonc/mdv270. [DOI] [PubMed] [Google Scholar]
- 5.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 6.Huang AC, Huang CH, Ju JS, et al. First- or second-generation epidermal growth factor receptor tyrosine kinase inhibitors in a large, real-world cohort of patients with non-small cell lung cancer. Ther. Adv. Med. Oncol. 2021;13:17588359211035710. doi: 10.1177/17588359211035710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Z, Tam KY. Combination strategies using EGFR-TKi in NSCLC therapy: Learning from the gap between pre-clinical results and clinical outcomes. Int. J. Biol. Sci. 2018;14(2):204–216. doi: 10.7150/ijbs.22955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 2005;23(5):1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 9.Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): Interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20(5):625–635. doi: 10.1016/S1470-2045(19)30035-X. [DOI] [PubMed] [Google Scholar]
- 10.Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014;15(11):1236–1244. doi: 10.1016/S1470-2045(14)70381-X. [DOI] [PubMed] [Google Scholar]
- 11.Stinchcombe TE, Janne PA, Wang X, et al. Effect of erlotinib plus bevacizumab vs erlotinib alone on progression-free survival in patients with advanced EGFR-mutant non-small cell lung cancer: A phase 2 randomized clinical trial. JAMA Oncol. 2019;5(10):1448–1455. doi: 10.1001/jamaoncol.2019.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ichihara E, Hotta K, Nogami N, et al. Phase II trial of gefitinib in combination with bevacizumab as first-line therapy for advanced non-small cell lung cancer with activating EGFR gene mutations: the Okayama Lung Cancer Study Group Trial 1001. J. Thorac. Oncol. 2015;10(3):486–491. doi: 10.1097/JTO.0000000000000434. [DOI] [PubMed] [Google Scholar]
- 13.Kim MM, Umemura Y, Leung D. Bevacizumab and glioblastoma: Past, present, and future directions. Cancer J. 2018;24(4):180–186. doi: 10.1097/PPO.0000000000000326. [DOI] [PubMed] [Google Scholar]
- 14.Besse B, Le Moulec S, Mazieres J, et al. Bevacizumab in patients with nonsquamous non-small cell lung cancer and asymptomatic, untreated brain metastases (BRAIN): A nonrandomized, phase II study. Clin. Cancer Res. 2015;21(8):1896–1903. doi: 10.1158/1078-0432.CCR-14-2082. [DOI] [PubMed] [Google Scholar]
- 15.Ascha MS, Wang JF, Kumthekar P, et al. Bevacizumab for the treatment of non-small cell lung cancer patients with synchronous brain metastases. Sci. Rep. 2019;9(1):17792. doi: 10.1038/s41598-019-54513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 2018;378(2):113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 17.Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N. Engl. J. Med. 2020;382(1):41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 18.Akamatsu H, Toi Y, Hayashi H, et al. Efficacy of osimertinib plus bevacizumab vs osimertinib in patients with EGFR T790M-mutated non-small cell lung cancer previously treated with epidermal growth factor receptor-tyrosine kinase inhibitor: West Japan Oncology Group 8715L phase 2 randomized clinical trial. JAMA Oncol. 2021;7(3):386–394. doi: 10.1001/jamaoncol.2020.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu HA, Schoenfeld AJ, Makhnin A, et al. Effect of osimertinib and bevacizumab on progression-free survival for patients with metastatic EGFR-mutant lung cancers: A phase 1/2 single-group open-label trial. JAMA Oncol. 2020;6(7):1048–1054. doi: 10.1001/jamaoncol.2020.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akamatsu H, Ozawa Y, Oyanagi J, et al. Phase Ib study of osimertinib plus ramucirumab in japanese lung cancer patients with EGFR mutation. Anticancer Res. 2021;41(2):911–917. doi: 10.21873/anticanres.14844. [DOI] [PubMed] [Google Scholar]
- 21.Yu HA, Paz-Ares LG, Yang JC, et al. Phase I study of the efficacy and safety of ramucirumab in combination with osimertinib in advanced T790M-positive EGFR-mutant non-small cell lung cancer. Clin. Cancer Res. 2021;27(4):992–1002. doi: 10.1158/1078-0432.CCR-20-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J. Clin. Oncol. 2009;27(8):1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 23.Huang YC, Shen SM, Liu CY, et al. Impact of prolonged and early bevacizumab treatment on the overall survival of EGFR-mutant and EGFR-wild type nonsquamous non-small cell lung cancer. Thorac. Cancer. 2018;9(12):1648–1655. doi: 10.1111/1759-7714.12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zustovich F, Ferro A, Lombardi G, et al. Bevacizumab as front-line treatment of brain metastases from solid tumors: a case series. Anticancer Res. 2013;33(9):4061–4065. [PubMed] [Google Scholar]
- 25.Berghoff AS, Breckwoldt MO, Riedemann L, et al. Bevacizumab-based treatment as salvage therapy in patients with recurrent symptomatic brain metastases. Neurooncol. Adv. 2020;2(1):38. doi: 10.1093/noajnl/vdaa038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumthekar P, Dixit K, Grimm SA, et al. A phase II trial of bevacizumab in patients with recurrent solid tumor brain metastases who have failed whole brain radiation therapy (WBRT) J. Clin. Oncol. 2019;37(15):2070. doi: 10.1200/JCO.2019.37.15_suppl.2070. [DOI] [Google Scholar]
- 27.De Braganca KC, Janjigian YY, Azzoli CG, et al. Efficacy and safety of bevacizumab in active brain metastases from non-small cell lung cancer. J. Neurooncol. 2010;100(3):443–447. doi: 10.1007/s11060-010-0200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Yang JJ, Tu HY, et al. Retrospective study on bevacizumab in the treatment of non-small cell lung cancer with brain metastases. Int. J. Clin. Oncol. 2020;25(2):267–273. doi: 10.1007/s10147-019-01552-5. [DOI] [PubMed] [Google Scholar]
- 29.Chikaishi Y, Kanayama M, Taira A, et al. Effect of erlotinib plus bevacizumab on brain metastases in patients with non-small cell lung cancer. Ann. Transl. Med. 2018;6(20):401. doi: 10.21037/atm.2018.09.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang T, Zhang Y, Li X, et al. EGFR-TKIs plus bevacizumab demonstrated survival benefit than EGFR-TKIs alone in patients with EGFR-mutant NSCLC and multiple brain metastases. Eur. J. Cancer. 2019;121:98–108. doi: 10.1016/j.ejca.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 31.Gridelli, C., J. Castro Carpeno, A.C. Dingemans, et al., Safety and efficacy of bevacizumab plus standard-of-care treatment beyond disease progression in patients with advanced non-small cell lung cancer: The AvaALL Randomized Clinical Trial. JAMA Oncol. 4(12), 3486 (2018). [DOI] [PMC free article] [PubMed]
- 32.Tsai JS, Su PL, Yang SC, et al. EGFR-TKI plus bevacizumab versus EGFR-TKI monotherapy for patients with EGFR mutation-positive advanced non-small cell lung cancer-A propensity score matching analysis. J. Formos. Med. Assoc. 2021;1:1. doi: 10.1016/j.jfma.2021.03.023. [DOI] [PubMed] [Google Scholar]
- 33.Naumov GN, Nilsson MB, Cascone T, et al. Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin. Cancer Res. 2009;15(10):3484–3494. doi: 10.1158/1078-0432.CCR-08-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosell R, Dafni U, Felip E, et al. Erlotinib and bevacizumab in patients with advanced non-small-cell lung cancer and activating EGFR mutations (BELIEF): An international, multicentre, single-arm, phase 2 trial. Lancet Respir. Med. 2017;5(5):435–444. doi: 10.1016/S2213-2600(17)30129-7. [DOI] [PubMed] [Google Scholar]
- 35.Fukuhara T, Saito H, Furuya N, et al. Evaluation of plasma EGFR mutation as an early predictor of response of erlotinib plus bevacizumab treatment in the NEJ026 study. EBioMedicine. 2020;57:2861. doi: 10.1016/j.ebiom.2020.102861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakagawa K, Garon EB, Seto T, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(12):1655–1669. doi: 10.1016/S1470-2045(19)30634-5. [DOI] [PubMed] [Google Scholar]
- 37.Yang Z, Hackshaw A, Feng Q, et al. Comparison of gefitinib, erlotinib and afatinib in non-small cell lung cancer: A meta-analysis. Int. J. Cancer. 2017;140(12):2805–2819. doi: 10.1002/ijc.30691. [DOI] [PubMed] [Google Scholar]