Abstract
Heterosexual transmission of human immunodeficiency virus type 1 (HIV-1) is the major cause of the ongoing AIDS epidemic. Application of chemical barrier methods is expected to contribute to the worldwide control of this epidemic. Bovine β-lactoglobulin modified by 3-hydroxyphthalic anhydride (3-hydroxyphthaloyl-β-lactoglobulin [3HP-β-LG]) was shown to inhibit HIV-1, HIV-2, simian immunodeficiency virus (SIV), herpes simplex virus type 1 and 2, and Chlamydia trachomatis infection in vitro. Here, we show that 3HP-β-LG not formulated into any vehicle protected three of six rhesus monkeys against vaginal infection by SIV. Incorporation of the compound into an appropriate vehicle is expected to increase the degree of protection. 3HP-β-LG may be effective as a vaginal inhibitor of HIV-1 infection in humans.
A safe, effective strategy to prevent transmission of human immunodeficiency virus type 1 (HIV-1) would be an important advancement in the worldwide control of the AIDS epidemic. Eighty percent of new infections occur through heterosexual transmission in developing countries. Therefore, the selected prophylactic agents must be cost-effective and stable under a variety of storage conditions.
3HP-β-LG, a form of bovine β-lactoglobulin (β-LG) (a protein present in whey and milk) modified by 3-hydroxyphthalic anhydride (3HP), has been shown to inhibit HIV-1, HIV-2, simian immunodeficiency virus (SIV), herpes simplex virus type 1 and 2 (HSV-1 and HSV-2), and Chlamydia trachomatis infection in vitro (3, 4, 14–16). Against HIV-1, 3HP-β-LG has been shown to be a more potent antiviral inhibitor than sulfated polysaccharides; it appears both to be virucidal and to block virus attachment to the CD4 receptor (4, 13, 15). 3HP-β-LG has been demonstrated to have broad-spectrum activity against various HIV-1 clades and against syncytium- and non-syncytium-inducing viruses (14, 15). It does not appear to be affected by exposure to elevated temperatures (13, 14).
In this study, we used the SIV female rhesus monkey model of sexual transmission to evaluate the efficacy of 3HP-β-LG in preventing vaginal infection with cell-free SIV. Although the exact mode of HIV-1 transmission across the vaginal mucosa is not well understood, it is likely that cell-free virus plays a major role. SIV infection of rhesus monkeys causes an acquired immunodeficiency disease with a striking similarity to HIV-1 and HIV-2 infections in humans and is therefore a relevant model for HIV-1 infection of humans (6). Genetically, the virus has the same auxiliary gene structure as HIV-2 and shows similar modes of infection, cellular and tissue tropism, and clinical disease course. The transmission of cell-free virus across the vaginal mucosa has been well described (1, 2, 7, 17), and the rhesus monkey model has been used to test the efficacy of nonoxynol 9 in the prevention of infection. Here, we describe the successful prevention of infection in 50% of the rhesus monkeys that were treated intravaginally with 3HP-β-LG.
The study was conducted with eight cycling female rhesus monkeys enrolled into either a treatment group (six animals) or a control group (two animals). Control animals were inoculated with virus, to demonstrate inoculum viability, and not treated with a vehicle. This study was designed to determine if the formulation (vehicle and active ingredient) was protective, not to evaluate each component. Adult female rhesus monkeys were received from the Oregon Regional Primate Research Center, Beaverton; Laboratory Animal Breeders and Services, Yemassee, S.C.; or Yerkes Regional Primate Research Center, Atlanta, Ga. Prior to the study, all animals were tested and determined to be seronegative for antibodies to SIV, type D retrovirus, and simian T-cell lymphotrophic virus type 1. All animal care and use procedures conformed to the revised Public Health Service Policy on the Humane Care and Use of Laboratory Animals (16a). The animals were anesthetized with ketamine intramuscularly prior to all procedures. The SIV stock used in this study contained 105 50% tissue culture infective doses (TCID50) and ≈4.3 × 109 SIV RNA copies per ml. This stock has been shown to infect 19 of 19 females after a single intravaginal inoculation (6a, 12a). The animals were treated and challenged on day 1 of the study and then were monitored by virus recovery and antibody assays for seroconversion for at least 28 weeks.
3HP-β-LG (60 mg/ml in phosphate-buffered saline [PBS] [13]) and virus were inoculated with a 1.0-ml syringe as previously described (9). 3HP-β-LG (30 mg in 0.5 ml) was administered approximately 10 min prior to and 60 min after a single virus inoculation. The animals were monitored for approximately 6 months postchallenge.
Virus load was determined by limiting dilution coculture of isolated peripheral blood mononuclear cells (PBMC) with CEMx174 cells (18). Twelve serial 1:3 dilutions of PBMC, beginning with 106 cells, were cocultured in duplicate with 105 CEMx174 cells per well in 24-well plates. Supernatant samples were collected after 21 days of culture and stored frozen at ≤70°C until analyzed for p27 antigen with a p27 antigen assay kit (Coulter Immunology, Hialeah, Fla.). Serum was analyzed for anti-SIV antibodies by using a whole-virus enzyme-linked immunosorbent assay (ELISA) as described previously (18).
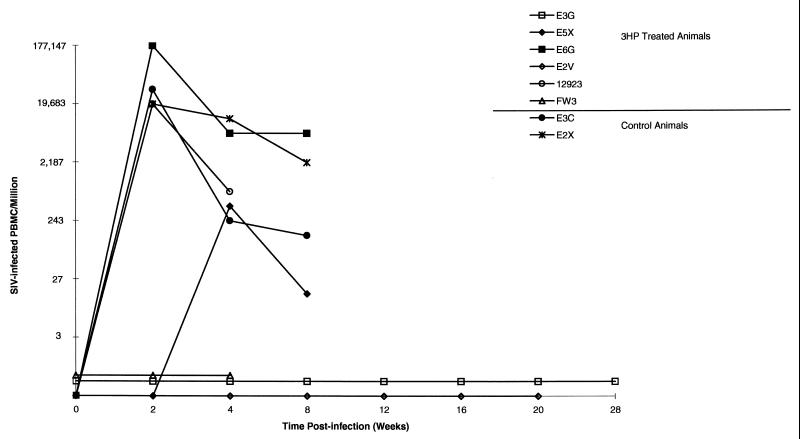
Three of the six treated monkeys (E3G, E2V, and FW3) were negative for virus recovery by limiting dilution coculture (Fig. 1). Virus was recovered from the remaining three treated animals, as well as from two control animals inoculated with a single dose of virus. ELISA for anti-SIV antibodies confirmed the results of the virus recovery assays; the development of antibodies in virus-positive animals was observed by 4 to 8 weeks postchallenge (Table 1), while ELISA values remained below the assay cutoff (optical density, 0.250) in the virus-negative animals. Control monkeys had recoverable virus by 2 weeks postinoculation; viral loads remained high through week 9.
FIG. 1.
Cell-associated viral loads. The numbers of PBMC needed to recover SIV were quantitated by threefold limiting dilution coculture. Control animals and treated animals that were virus positive were not monitored past 4 or 8 weeks. Virus-negative animals were assigned values of 0, 0.5, and 0.7 to facilitate interpreting the graph. 3HP, 3HP-β-LG.
TABLE 1.
Anti-SIV antibody responses of monkeysa
| Animal | Treat-ment | Absorbance at 405 nm at wk:
|
||||||
|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 8 | 12 | 16 | 20 | 28 | ||
| E3G | 3HP | 0.039 | 0.092 | 0.039 | 0.022 | 0.230 | 0.020 | 0.008 |
| E2V | 3HP | 0.114 | 0.106 | 0.167 | 0.186 | 0.074 | 0.062 | 0.082 |
| FW3 | 3HP | 0.036 | 0.024 | 0.019 | 0.022 | 0.028 | 0.021 | ND |
| E5X | 3HP | 0.044 | 0.056 | 2.194 | ND | ND | ND | ND |
| E6G | 3HP | 0.355 | 0.843 | ND | ND | ND | ND | ND |
| 12923 | 3HP | 0.024 | 0.072 | 0.871 | 1.042 | 1.246 | ND | ND |
| E2X | Ctrl | 1.178 | 2.244 | ND | ND | ND | ND | ND |
| E3C | Ctrl | 0.762 | 0.671 | ND | ND | ND | ND | ND |
The virus recovery results were confirmed by testing for seroconversion to SIV with a whole-virus antigen ELISA. The control animals and treated animals that were virus positive were not monitored past 4 or 8 weeks. The historical cutoff for this assay is approximately an optical density of 0.250. Positive responses are in bold type. ND, not done; 3HP, 3HP-β-LG; Ctrl, control.
A similar degree of protection has been obtained with two forms of nonoxynol 9, foam and gel (8), where three of six and two of six animals, respectively, were protected against vaginal transmission of SIV. One difference between the present study and the nonoxynol 9 study is that 3HP-β-LG was not formulated in any vehicle gel or foam but only suspended in PBS. Therefore, it appears that the level of protection reported here must be due to 3HP-β-LG or PBS. The protective efficacy of 3HP-β-LG against vaginal infection of mice by HSV-2 was greatly enhanced by its incorporation into a gel formulation (5). Similar enhancement of the antiviral effect of 3HP-β-LG would be expected in the SIV-monkey model.
The significance of this data in predicting the effect of 3HP-β-LG on HIV-1 transmission in humans should be evaluated in the context of the model and the potential epidemiologic impact of this level of protection. The infection of rhesus monkeys with SIV across mucosal surfaces has been extensively studied (1, 2, 7, 17); the localization of virus-infected cells, as well as the reproductive tract pathology in SIV-infected monkeys of both sexes, is similar to that in humans (11, 12). In order to ensure a 100% infection rate in control animals by vaginal inoculation, a dose of 105 TCID50 is utilized in this model, a dose that contains approximately 4.3 × 109 RNA copies per ml. In human semen, RNA copy numbers as high as 1.3 × 107 per ml have been observed. This indicates that our SIV challenge uses a dose at least 100 times that likely to occur during natural exposure. There is no direct correlation between RNA copy number and infectious titer. Thus, direct comparisons of RNA copy number in the SIV stock and semen samples are only crude estimates.
The infectivity of a vaginal viral inoculation is likely to be related to some inherent properties of a particular virus strain; the properties which would favor transmission are not well understood (19) but appear to be related to in vivo replication capacity (12a). In our model, although the exact animal infectious dose of virus is not known, virus stocks with TCID50 of 103 and 104 do not reliably infect rhesus monkeys after one vaginal inoculation (6a). Therefore, it is likely that a best estimation of the virus inoculum used would be between 1 and 10 animal infectious doses.
3HP-β-LG treatment resulted in protection of 50% of the animals under challenge conditions that exceed natural exposure in humans but are within reasonable dosage ranges for other animal protection models. 3HP-β-LG has been shown in vitro to have broad specificity across clades and is an abundant, cost-effective by-product of the dairy industry. In addition, 3HP-β-LG application does not appear to result in any irritation to mucosal tissues (12b), a problem that has been observed with nonoxynol 9 and one that theoretically could promote virus transmission due to vaginal inflammation. The data presented here suggests that 3HP-β-LG may play a significant role as a vaginal inhibitor of HIV-1 infection in humans.
Acknowledgments
This study was supported by Dairy Management, Inc., Rosemont, Ill.
REFERENCES
- 1.Baba T W, Koch J, Mittler E S, Greene M, Wyand M, Pennick D, Ruprecht R. Mucosal infection of neonatal rhesus monkeys with cell-free SIV. AIDS Res Hum Retroviruses. 1994;10:351–357. doi: 10.1089/aid.1994.10.351. [DOI] [PubMed] [Google Scholar]
- 2.Baba T W, Trichel A M, An L, Liska V, Martin L N, Murphey-Corb M, Ruprecht R. Infection and AIDS in adult macaques after nontraumatic oral exposure to cell-free SIV. Science. 1996;272:1486–1489. doi: 10.1126/science.272.5267.1486. [DOI] [PubMed] [Google Scholar]
- 3.Jiang S, Li Y Y, Lin K, Strick N, Neurath A R, George K S, Choudhury S, Esmaeli-Azad B. Vaccines 97. Molecular approaches to the control of infectious diseases. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. Virucidal and antibacterial activities of 3-hydroxyphthaloyl-β-lactoglobulin; pp. 327–330. [Google Scholar]
- 4.Jiang S, Lin K, Li Y Y, Neurath A R. Chemically modified bovine β-lactoglobulin blocks uptake of HIV-1 by colon- and cervix-derived epithelial cell lines. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13:461–470. doi: 10.1097/00042560-199612150-00010. [DOI] [PubMed] [Google Scholar]
- 5.Kokuba H, Aurelian L, Neurath A R. 3-Hydroxyphthaloyl-β-lactoglobulin: IV. Antiviral activity in the mouse model of genital herpesvirus infection. Antivir Chem Chemother. 1998;9:353–357. [PubMed] [Google Scholar]
- 6.Miller C. Use of the SIV rhesus macaque system to test virucides designed to prevent sexual transmission of HIV. In: Mauck C K, et al., editors. Barrier contraceptives: current status and future prospects. Somerset, N.J: Wiley-Liss Inc.; 1994. pp. 213–223. [Google Scholar]
- 6a.Miller, C. Unpublished data.
- 7.Miller C J, Alexander N J, Gettie A, Hendrickx A G, Marx P A. The effect of contraceptives containing nonoxynol-9 on the genital transmission of simian immunodeficiency virus in rhesus macaques. Fertil Steril. 1992;57:1126–1128. [PubMed] [Google Scholar]
- 8.Miller C J, Alexander N J, Sutjipto S, Hendrickx A G, Jennings M, Marx P A. Effect of virus dose and nonoxynol-9 on the genital transmission of SIV in rhesus macaques. J Med Primatol. 1990;19:401–409. [PubMed] [Google Scholar]
- 9.Miller C J, Alexander N J, Sutjipto S, Lackner A A, Gettie A, Hendrickx A G, Lowenstine L J, Jennings M, Marx P A. Genital mucosal transmission of simian immunodeficiency virus: animal model for heterosexual transmission of human immunodeficiency virus. J Virol. 1989;63:4277–4284. doi: 10.1128/jvi.63.10.4277-4284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller C J, Marthas M, Torten J, Alexander N J, Moore J P, Doncel G F, Hendrickx A G. Intravaginal inoculation of rhesus macaques with cell-free simian immunodeficiency virus results in persistent or transient viremia. J Virol. 1994;68:6391–6400. doi: 10.1128/jvi.68.10.6391-6400.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller C J, Vogel P, Alexander N J, Dandekar S, Hendrickx A G, Marx P A. Pathology and localization of simian immunodeficiency virus in the reproductive tract of chronically infected male rhesus macaques. Lab Investig. 1994;70:255–262. [PubMed] [Google Scholar]
- 12.Miller C J, Vogel P, Alexander N J, Sutjipto S, Hendrickx A G, Marx P A. Localization of SIV in the genital tract of chronically infected female rhesus macaques. Am J Pathol. 1992;141:655–660. [PMC free article] [PubMed] [Google Scholar]
- 12a.Miller C J, Marthas M, Greenier J, Lu D, Dailey P J, Lu Y. In vivo replication capacity rather than in vitro macrophage tropism predicts efficiency of vaginal transmission of simian immunodeficiency virus or simian/human immunodeficiency virus in rhesus macaques. J Virol. 1998;72:3248–3258. doi: 10.1128/jvi.72.4.3248-3258.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12b.Neurath, A. R. Unpublished data.
- 13.Neurath A R, Debnath A K, Strick N, Li Y Y, Lin K, Jiang S. 3-Hydroxyphthaloyl-β-lactoglobulin: I. Optimization of production and comparison with other compounds considered for chemoprophylaxis of mucosally transmitted human immunodeficiency virus type 1. Antivir Chem Chemother. 1997;8:131–139. [Google Scholar]
- 14.Neurath A R, Debnath A K, Strick N, Li Y Y, Lin K, Jiang S. 3-Hydroxyphthaloyl-β-lactoglobulin. II. Anti-human immunodeficiency virus type 1 activity in in vitro environments relevant to prevention of sexual transmission of the virus. Antivir Chem Chemother. 1997;8:141–148. [Google Scholar]
- 15.Neurath A R, Jiang S, Strick N, Lin K, Li Y-Y, Debnath A K. Bovine β-lactoglobulin modified by 3-hydroxyphthalic anhydride blocks the CD4 cell receptor for HIV: implications for AIDS prophylaxis. Nat Med. 1996;2:230–234. doi: 10.1038/nm0296-230. [DOI] [PubMed] [Google Scholar]
- 16.Neurath A R, Strick N, Li Y-Y. 3-Hydroxyphthaloyl-β-lactoglobulin: III. Antiviral activity against herpesviruses. Antivir Chem Chemother. 1998;9:177–184. doi: 10.1177/095632029800900209. [DOI] [PubMed] [Google Scholar]
- 16a.Public Health Service. Public health service policy on the humane care and use of laboratory animals. Washington, D.C: U.S. Department of Health and Human Services; 1996. . [Reprint of 1986 edition.] [Google Scholar]
- 17.Trichel A M, Roberts E D, Wilson L A, Martin L N, Ruprecht R M, Murphey-Corb M. SIV/DeltaB670 transmission across oral, colonic, and vaginal mucosae in the macaque. J Med Primatol. 1997;26:3–10. doi: 10.1111/j.1600-0684.1997.tb00313.x. [DOI] [PubMed] [Google Scholar]
- 18.Wyand M S, Manson K H, Garcia-Moll M, Montefiori D, Desrosiers R C. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J Virol. 1996;70:3724–3733. doi: 10.1128/jvi.70.6.3724-3733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu T, Wang N, Carr A, Nam D S, Moor-Jankowski R, Cooper D A, Ho D D. Genetic characterization of human immunodeficiency virus type 1 in blood and genital secretions: evidence for viral compartmentalization and selection during sexual transmission. J Virol. 1996;70:3098–3107. doi: 10.1128/jvi.70.5.3098-3107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]