Abstract
Mosquitoes and mosquito-borne diseases represent an increasing global challenge. Plant extract and/or oils could serve as alternatives to synthetic insecticides. The larvicidal effects of 32 oils (1000 ppm) were screened against the early 4th larvae of Culex pipiens and the best oils were evaluated against adults and analyzed by gas chromatography-mass spectrometry (GC mass) and HPLC. All oils had larvicidal activity (60.0–100%, 48 h Post-treatment, and their Lethal time 50 (LT50) values ranged from 9.67 (Thymus vulgaris) to 37.64 h (Sesamum indicum). Oils were classified as a highly effective group (95–100% mortalities), including Allium sativum, Anethum graveolens, Camellia sinensis, Foeniculum vulgare, Nigella sativa, Salvia officinalis, T. vulgaris, and Viola odorata. The moderately effective group (81–92% mortalities) included Boswellia serrata, Cuminum cyminum, Curcuma aromatic, Allium sativum, Melaleuca alternifolia, Piper nigrum, and Simmondsia chinensis. The least effective ones were C. sativus and S. indicum. Viola odorata, Anethum graveolens, T. vulgaris, and N. sativa provide 100% adult mortalities PT with 10, 25, 20, and 25%. The mortality percentages of the adults subjected to 10% of oils (H group) were 48.89%, 88.39%, 63.94%, 51.54%, 92.96%, 44.44%, 72.22%, and 100% for A. sativum, An. graveolens, C. sinensis, F. vulgare, N. sativa, S. officinalis, T. vulgaris, and V. odorata, respectively. Camellia sinensis and F. vulgare were the most potent larvicides whereas V. odorata, T. vulgaris, An. graveolens and N. sativa were the best adulticides and they could be used for integrated mosquito control.
Subject terms: Developmental biology, Ecology
Introduction
Mosquitoes are an ancient nuisance pest and mosquito-borne diseases represent an increasing global health challenge, threatening over 40% of the world’s population and it is expected that almost half of the world’s population will be at risk of arbovirus transmission by 20501. Culex pipiens (Diptera: Culicidae) is widely distributed, transmitting dreadful diseases leading to severe morbidity and sometimes mortality to humans and animals2–5.
Vector control is the primary method for reducing public concerns about mosquito-borne diseases6–11. Controlling adults and larvae through repellents and insecticides12,13, are the most effective approach for reducing mosquito bites. Using synthetic insecticides led to insecticide resistance, environmental pollution, and health hazards to human health and non-target organisms.
Searching for eco-friendly alternatives in botanicals such as essential oils (EOs) is a curtail need. EOs are volatile components found in many plant families like Asteraceae, Rutaceae, Myrtaceae, Lauraceae, Lamiaceae, Apiaceae, Piperaceae, Poaceae, Zingiberaceae, and Cupressaceae14. EOs contain complicated mixtures of products as phenols, sesquiterpenes, and monoterpenes15.
EOs have antibacterial, antiviral, and antifungal activities. They also possess insecticidal effect interfering with insects' physiological, metabolic, behavioral, and biochemical functions through inhalation, ingestion, or skin absorption of EOs inducing a neurotoxic action16. EOs act as adulticides, larvicides, deterrents, and repellents. They are less toxic, biodegradable, and overcome insecticidal resistance15,17,18.
EOs have higher popularity with organic growers and environmentally conscious consumers and suitability for urban areas, homes, and other sensitive areas.
The role of EOs in mosquito control has been discussed15,19. This study aimed to screen and evaluate the lethal time values of the larvicidal effects of thirty-two oils and evaluate the adulticidal effect and phytochemical analyses of the most effective ones against Cx. pipiens.
Materials and methods
Plant oils
Thirty- two oils were purchased from EL CAPTAIN Company for extracting natural oils, plants, and cosmetics "Cap Pharm," El Obor, Cairo, Egypt and Harraz for Food Industry & Natrual products, Cairo, Egypt (Table 1).
Table 1.
Plants species screened (oil No = 32) used for larvicidal activity.
| No. | Oil name | Plant oils | ||
|---|---|---|---|---|
| Order | Family | English name | ||
| 1 | Allium sativuma | Asparagales | Amaryllidaceae | Garlic |
| 2 | Anethum graveolensa | Apiales | Apiaceae | Dill |
| 3 | Argania spinosab | Ericales | Sapotaceae | Argan |
| 4 | Boswellia serrata R.a | Sapindales | Burseraceae | Olibanum |
| 5 | Brassica carinataa | Brassicales | Brassicaceae | Mustard |
| 6 | Camellia sinensisa | Ericales | Theaceae | Green Tea |
| 7 | Cedrus libani Aa | Pinales | Pinaceae | Cedar wood |
| 8 | Citrullus colocynthis Lb | Cucurbitales | Cucurbitaceae | Bitter apple |
| 9 | Crocus sativus L.a | Asparagales | Iridaceae | Saffron crocus |
| 10 | Cucurbita maxima D.a | Cucurbitales | Cucurbitaceae | Pumpkin |
| 11 | Cuminum cyminum La | Apiales | Apiaceae | Cumin |
| 12 | Cupressus sempervirensb | Pinales | Cupressaceae | Italian cypress |
| 13 | Curcuma aromatica S.a | Zingiberales | Zingiberaceae | Curcuma |
| 14 | Curcuma longa L.a | Zingiberales | Zingiberaceae | Common turmeric |
| 15 | Foeniculum vulgare M.a | Apiales | Apiaceae | Sweet fennel |
| 16 | Gadus morhuaa | Gadiformes | Gadidae | Cod Liver |
| 17 | Lepidium sativum L.a | Brassicales | Brassicaceae | Garden pepperwort |
| 18 | Linum usitatissimum L.a | Malpighiales | Linaceae | Common flax |
| 19 | Melaleuca alternifoliaa | Myrtales | Myrtaceae | Tea tree |
| 20 | Nigella sativaa | Ranunculales | Ranunculaceae | Black cumin |
| 21 | Panax ginsenga | Apiales | Araliaceae | Chinese ginseng |
| 22 | Piper nigrum L.a | Piperales | Piperaceae | Black pepper |
| 23 | Prunus dulcisb | Rosales | Rosaceae | Almond |
| 24 | Ruta chalepensis L.a | Sapindales | Rutaceae | Rues |
| 25 | Salvia officinalis L.a | Lamiales | Lamiaceae | Sage |
| 26 | Sesamum indicuma | Lamiales | Pedaliaceae | Sesame |
| 27 | Simmondsia chinensisb | Caryophyllales | Simmondsiaceae | Jojoba |
| 28 | Syzygium aromaticum L | Myrtales | Myrtaceae | Clove |
| 29 | Tilia americana L.a | Malvales | Malvales | Tilia |
| 30 | Thymus vulgaris L | Lamiales | Lamiaceae | Garden |
| 31 | Viola odorata L.a | Malpighiales | Violaceae | Sweet violet |
| 32 | Zingiber officinalea | Zingiberales | Zingiberaceae | Ginger |
aPlant oils purchased from EL CAPTAIN company for extracting natural oils, plants and cosmetics “Cap Pharm”.
bPlant oils purchased from Harraz for Food Industry & Natural products.
Culex pipiens
Culex pipiens (anautogenous strain) was provided from the colony reared at the Department of Entomology, Faculty of Science, Benha University, Egypt, and maintained at 27 ± 2 °C, 75–85% RH and 14: 10 h (L/D) photoperiod.
Larvicidal efficacy
Thirty-two oils were screened for their larvicidal efficacy20 against the early fourth instar larvae, Cx. pipiens. Oils were added to a solvent (emulsifier) consisting of dechlorinated water plus 1.0 mL 0.5% Tween-20, through a shaker plate to yield a homogenous solution. Oils were added to a solvent consisting of dechlorinated water plus 5% tween 20. For each oil, twenty larvae were placed in a 500 mL glass beaker containing 250 mL of 1000 ppm. The experiment and the control group, treated with the solvent only, were replicated three times. Larval mortalities were recorded 0.5, 2, 8, 24, and 48 h post-treatment (PT).
Adulticidal efficacy
Susceptibility tests for adult mosquitoes were performed for the promising larvicidal oils through the CDC bottle bioassays21 with modifications. For each concentration, three bottles were coated. Several concentrations for each oil were prepared using pure ethanol as a solvent. The bottles were coated with the desired concentrations and left overnight at 27 ± 2 °C for solvent evaporation.
Adult mosquitoes (15–10, aged 3–4 days) fed on 10% sucrose solution were released to each bottle using a hand aspirator. The exposure time was set to 30 min. The mosquitoes were removed from the bottles. Mosquito groups were added to separate transparent paper cups (10 × 9 × 6 cm) having 10% sucrose solution and mortalities were checked after 24 h. Three replicates were made for each concentration.
GC/MS analysis
A Thermo Scientific Trace GC Ultra/ISQ Single Quadrupole MS, TG-5MS fused silica capillary column was used for the GC/MS study (0.1 mm, 0.251 mm and 30 m film thickness). An electron ionisation device with a 70 eV ionisation energy was employed for GC/MS detection. At a constant flow rate of 1 mL/min, helium gas was used as the carrier gas. Temperatures were established at 280 °C for the injector and MS transfer line. The oven temperature was set at 50 °C (hold for 2 min), then increased to 150 °C at a rate of 7 °C per minute, then to 270 °C at a rate of 5 °C per minute (hold for 2 min), and finally to 310 °C at a rate of 3.5 °C per minute (hold 10 min). A percent relative peak area was used to explore the quantification of all of the discovered components. The chemicals were tentatively identified by comparing their respective retention times and mass spectra to those of the NIST, WILLY library data from the GC/MS instrument. The identification was done using mass spectra and a computer search of user-generated reference libraries. To check peak homogeneity, single-ion chromatographic reconstruction was used. When identical spectra could not be identified, only the structural type of the relevant component was provided based on its mass spectral fragmentation. When possible, reference compounds were co-chromatographed to confirm GC retention durations22.
Data analysis
Data were analyzed through one-way analysis of variance (ANOVA), Duncan’s multiple range tests, and Probit analysis for calculating the lethal concentration (LC) and lethal time (LT) values using the computer program PASW Statistics 2009 (SPSS version 22). The relative efficacies (RE) were calculated18 according to the following formula:
Non-parametric, Kruskal–Wallis test was performed to compare the mean differences of more than two groups followed by the Mann–Whitney test to compare the mean differences between the effective oil groups.
Results
The larvicidal effect of 32 oils was screened against the early 4th larvae, Cx. pipiens. The results showed that all plant oils had larvicidal activity (60.0–100%, 48 h PT) and their Lethal time 50 (LT50) values ranged from 9.67 (Thymus vulgaris) to 37.64 h (Sesamum indicum), Tables 2 and 3.
Table 2.
Larval mortality (%) of plant oils used at 1000 ppm through different time periods.
| Oils | Mortality % (mean ± SD)/h | Grouping | ||||
|---|---|---|---|---|---|---|
| 0.5 | 2 | 8 | 24 | 48 | ||
| Allium sativum | 6.67 ± 0.58aE | 22.33 ± 1.53D | 46.67 ± 0.58efgiC | 81.33 ± 1.53dB | 96.67 ± 0.58eA | H |
| Anethum graveolens | 8.33 ± 0.58aE | 23.33 ± 1.15D | 48.67 ± 1.15jC | 83.67 ± 1.53dB | 98.33 ± 0.58eA | H |
| Argania spinosa | 5.00 ± 1.00aE | 11.67 ± 0.58D | 21.67 ± 1.53bcdC | 43.33 ± 1.53cB | 66.67 ± 1.53dA | L |
| Boswellia serrata | 3.33 ± 0.58aE | 15.00 ± 1.00D | 31.67 ± 1.53bcdeC | 70.00 ± 1.00dB | 90.00 ± 1.00eA | M |
| Brassica carinata | 3.33 ± 0.58aE | 13.33 ± 0.58D | 25.00 ± 1.00bcdC | 45.00 ± 1.53cB | 68.33 ± 2.08dA | L |
| Camellia sinensis | 8.33 ± 0.58aE | 23.33 ± 1.00aC | 61.67 ± 1.531jB | 100.00 ± 1.00dA | 100.00 ± 0.58eA | H |
| Cedrus libani | 5.00 ± 1.00abE | 15.00 ± 0.00aD | 25.00 ± 1.00cC | 56.67 ± 1.00dB | 78.33 ± 1.53eA | L |
| Citrullus colocynthis | 3.33 ± 0.58aE | 11.67 ± 0.58cdeD | 33.33 ± 0.58defgC | 65.00 ± 1.00defB | 75.00 ± 1.00deA | L |
| Crocus sativus | 3.33 ± 0.58aE | 10.00 ± 1.00defD | 21.67 ± 1.15hijC | 39.33 ± 1.00hiB | 62.33 ± 1.00fgA | L |
| Cucurbita maxima | 3.33 ± 0.58aE | 10.00 ± 1.00defD | 21.67 ± 1.53hijC | 48.33 ± 1.53ghB | 65.00 ± 1.35efgA | L |
| Cuminum cyminum | 3.33 ± 0.58aE | 8.33 ± 0.58efD | 33.33 ± 1.53defgC | 63.33 ± 1.53defB | 88.33 ± 1.53bcA | M |
| Cupressus sempervirens | 5.00 ± 1.00aE | 8.33 ± 0.58efD | 16.67 ± 0.58ijC | 41.67 ± 2.08hiB | 63.33 ± 2.00fgA | L |
| Curcuma aromatic | 5.00 ± 1.00aE | 16.67 ± 1.53abcdeD | 35.00 ± 1.73defC | 71.67 ± 1.53cdB | 88.33 ± 1.53bcA | M |
| Curcuma longa | 5.00 ± 1.00aE | 10.00 ± 1.00defD | 20.00 ± 1.00ijC | 40.00 ± 2.08hiB | 61.67 ± 1.53fgA | L |
| Foeniculum vulgare | 8.33 ± 0.58aE | 25.00 ± 1.15aC | 63.33 ± 0.58aB | 100.00 ± 1.00aA | 100.00 ± 0.00aA | H |
| Gadus morhua | 5.00 ± 1.00abE | 13.33 ± 0.58bcdeD | 31.67 ± 1.53defghC | 55.00 ± 1.00fgB | 75.00 ± 1.00deA | L |
| Lepidium sativum | 6.67 ± 0.58aE | 15.00 ± 1.00abcdeD | 36.67 ± 1.15deC | 70.00 ± 1.00cdeB | 90.00 ± 1.00abcA | M |
| Linum usitatissimum | 3.33 ± 0.58aE | 15.00 ± 1.00abcdeD | 40.00 ± 1.00cdC | 55.00 ± 1.00fgB | 75.00 ± 1.00deA | L |
| Melaleuca alternifolia | 6.67 ± 0.58aE | 10.00 ± 1.00defD | 40.00 ± 1.00cdC | 71.67 ± 1.53cdB | 81.67 ± 0.58cdA | M |
| Nigella sativa | 5.00 ± 1.00aE | 20.00 ± 1.00abcdD | 50.00 ± 1.00bcC | 78.67 ± 1.53bcB | 95.00 ± 1.00abA | H |
| Panax ginseng | 5.00 ± .1.00aE | 11.67 ± 0.58cdeD | 30.00 ± 1.73defghC | 48.33 ± 1.53ghB | 71.67 ± 1.15defA | L |
| Piper nigrum | 5.00 ± 1.00aE | 20.00 ± 1.00abcdD | 38.33 ± 0.58dC | 70.00 ± 1.00cdeB | 88.33 ± 1.58bcA | M |
| Prunus dulcis | 3.33 ± 0.57aE | 13.33 ± 0.33bcdeD | 31.67 ± 0.88defghC | 50.00 ± 0.57ghB | 75.00 ± 0.57deA | L |
| Ruta chalepensis | 3.33 ± 0.58aE | 15.00 ± 1.00abcdeD | 33.33 ± 2.08defgC | 60.00 ± 2.00efB | 80.00 ± 1.00cdA | L |
| Salvia officinalis | 6.67 ± 0.58aE | 21.67 ± 1.53abcD | 51.67 ± 1.53bC | 80.00 ± 1.53bcB | 97.33 ± 1.00abA | H |
| Sesamum indicum | 3.33 ± 0.58aE | 8.33 ± 1.15efD | 15.00 ± 1.00jC | 36.67 ± 1.15iB | 60.00 ± 1.15gA | L |
| Simmondsia chinensis | 5.00 ± 1.00aE | 11.67 ± 0.58cdeD | 36.67 ± 1.53deC | 70.00 ± 2.0cdeB | 91.67 ± 0.58abA | M |
| Syzygium aromaticum | 5.00 ± 1.00aE | 13.33 ± 0.58bcdeD | 23.33 ± 1.15ghijC | 50.00 ± 1.00ghB | 76.673 ± 1.53dA | L |
| Tilia americana | 5.00 ± 0.57aE | 15.00 ± 0.0abcdeD | 25.00 ± 0.57fghijC | 56.67 ± 0.88fgB | 88.33 ± 0.88bcA | L |
| Thymus vulgaris | 8.33 ± 0.58aE | 21.67 ± 0.58abcD | 58.33 ± 2.08abC | 85.00 ± 0.58bB | 100.00 ± 1.00aA | H |
| Viola odorata | 8.33 ± 0.58aE | 23.33 ± 1.00abD | 58.67 ± 1.53abC | 89.67 ± 1.53abB | 100.00 ± 0.00aA | H |
| Zingiber officinale | 5.00 ± 1.00aE | 13.33 ± 0.58bcdeD | 26.67 ± 1.53efghiC | 48.33 ± 1.53ghB | 75.00 ± 1.00deA | L |
| Control | 0.33 ± 0.33aA | 0.33 ± 0.33fA | 0.33 ± 0.33kA | 0.33 ± 0.33jA | 0.33 ± 0.33hA | L |
Numbers of the same raw followed by the same small letter are not significantly different (one-way ANOVA, Duncan’s MRT, P > 0.05).
H: The highly effective (95–100% mortalities), 8 oils.
M: The moderately effective group (81–92% mortalities), 7 oils.
L.: The moderately effective group, include the rest of oils, 17 oils.
Table 3.
Lethal time values of applied oils (1000 ppm) against Culex pipiens larvae.
| Oil name | LT50 (lower–upper) | RE (LT50) | LT90 (lower–upper) | RE (LT90) | LT99 (lower–upper) | RE (LT99) | Chi (Sig) | Regrision equation |
|---|---|---|---|---|---|---|---|---|
| Allium sativum | 13.95 (3.16–54.44) | 2.7 | 31.17 (18.49–174.49) | 2.2 | 45.20 (26.92–276.44) | 2.1 | 39.30 (0.000a) | y = 0.86 + 0.06*x |
| Anethum graveolens | 19.90 (11.30–36.52) | 1.9 | 39.41 (27.22–81.32) | 1.8 | 55.31 (37.96–120.10) | 1.8 | 23.13 (0.000a) | y = 1.23 + 0.06*x |
| Argania spinosa | 33.02 (22.75–55.92) | 1.1 | 63.55 (45.59–120.49) | 1.1 | 88.45 (62.33–175.00) | 1.1 | 13.91 (0.008a) | y = 1.31 + 0.04*x |
| Boswellia serrata | 20.78 (12.05–37.26) | 1.8 | 41.01 (28.56–82.20) | 1.7 | 57.50 (39.77–121.10) | 1.7 | 22.42 (0.000a) | y = 1.27 + 0.06*x |
| Brassica carinata | 32.09 (21.04–59.25) | 1.2 | 62.39 (43.53–132.05) | 1.1 | 87.09 (59.69–193.58) | 1.1 | 17.05 (0.002a) | y = 1.33 + 0.04*x |
| Camellia sinensis | 13.02 (3.56–56.12) | 2.9 | 27.65 (16.38–172.03) | 2.5 | 39.58 (23.51–269.84) | 2.4 | 40.31 (0.000a) | y = 0.96 + 0.07*x |
| Cedrus libani A | 26.87 (17.55–44.77) | 1.4 | 52.99 (38.06–98.01) | 1.3 | 74.29 (52.64–143.56) | 1.3 | 16.60 (0.002a) | y = 1.24 + 0.05*x |
| Citrullus colocynthis | 26.08 (12.80–65.61) | 0.0 | 52.72 (34.03–169.10) | 0.0 | 74.44 (47.49–257.33) | 1.3 | 32.23 (0.000a) | y = 1.25 + 0.05*x |
| Crocus sativus | 37.07 (25.39–68.56) | 1.0 | 70.02 (49.05–147.56) | 1.0 | 96.88 (66.53–213.77) | 1.0 | 14.35 (0.006a) | y = 1.41 + 0.04*x |
| Cucurbita maxima | 30.90 (22.00–47.60) | 1.2 | 57.85 (43.01–97.25) | 1.2 | 79.81 (58.44–139.44) | 1.2 | 12.91 (0.012a) | y = 1.44 + 0.05*x |
| Cuminum cyminum | 22.65 (13.54- I40.07) | 1.7 | 43.44 (30.47–86.24) | 1.6 | 60.39 (42.00–126.16) | 1.6 | 22.68 (0.000a) | y = 1.39 + 0.06*x |
| Cupressus sempervirens | 34.67 (26.87–47.96) | 1.1 | 67.29 (52.45–100.54) | 1.0 | 93.88 (71.85–144.86) | 1.0 | 18.16 (0.66a) | y = 1.41 + 0.05*x |
| Curcuma aromatic | 20.49 (10.77–39.97) | 1.8 | 41.98 (28.40–94.24) | 1.7 | 59.51 (40.00–141.25) | 1.6 | 25.53 (0.000a) | y = 1.14 + 0.05*x |
| Curcuma longa | 33.89 (24.46–52.94) | 1.1 | 63.92 (47.28–109.44) | 1.1 | 88.41 (64.29–157.09) | 1.1 | 11.35 (0.023a) | y = 1.37 + 0.04*x |
| Foeniculum vulgare | 10.22 (5.29–21.14) | 3.7 | 20.99 (13.93–49.73) | 3.3 | 29.77 (19.68–74.34) | 3.3 | 21.56 (0.000a) | y = 1.06 = 0.1*x |
| Gadus morhua | 27.64 (16.47–54.29) | 1.4 | 55.69 (37.98–128.11) | 1.3 | 78.56 (52.78–191.03) | 1.2 | 21.54 (0.000a) | y = 1.2 + 0.04*x |
| Lepidium sativum | 20.06 (11.18–36.90) | 1.9 | 41.06 (28.31–84.97) | 1.7 | 58.18 (39.83–126.60) | 1.7 | 22.42 (0.000a) | y = 1.11 + 0.05*x |
| Linum usitatissimum | 26.78 (12.80–77.92) | 1.4 | 55.74 (35.22–213.81) | 1.3 | 79.35 (49.44–328.66) | 1.2 | 31.75 (0.000a) | y = 1.18 + 0.04*x |
| Melaleuca alternifolia | 22.36 (9.11–58.90) | 1.7 | 46.52 (29.47–159.02) | 1.5 | 66.22 (41.73–244.98) | 1.5 | 36.44 (0.000a) | y = 1.12 + 0.05*x |
| Nigella sativa | 15.67 (5.25–46.57) | 2.4 | 33.48 (20.57–130.64) | 2.1 | 48.00 (29.54–202.69) | 2.0 | 36.89 (0.000a) | y = 1.01 + 0.06*x |
| Panax ginseng | 30.16 (19.05–57.39) | 1.2 | 59.66 (41.18–131.40) | 1.2 | 83.70 (56.80–194.15) | 1.2 | 18.86 (0.001a) | y = 1.25 + 0.04*x |
| Piper nigrum | 20.14 (9.84–41.84) | 1.9 | 42.45 (28.17–103.75) | 1.6 | 60.63 (40.01–157.34) | 1.6 | 27.10 (0.000a) | y = 1.07 + 0.05*x |
| Prunus dulcis | 26.75 (19.88–36.78) | 2.6 | 58.25 (45.50–85.63) | 1.4 | 78.56 (64.49–127.36) | 1.2 | 21.11(0.03a) | y = 1.2 + 0.04*x |
| Ruta chalepensis | 25.12 (14.06–50.27) | 1.5 | 50.74 (34.32- 119.52) | 1.4 | 71.63 (47.88- 178.94) | 1.4 | 24.68 (0.000a) | y = 1.24 + 0.05 |
| Salvia officinalis | 15.42 (5.38–41.36) | 2.4 | 34.12 (21.26–116.53) | 2.1 | 49.37 (30.77–181.26) | 2.0 | 32.84 (0.000a) | y = 0.89 + 0.06*x |
| Sesamum indicum | 37.64 (32.87–44.04) | 1.0 | 68.08 (58.97–81.70) | 1.0 | 92.89 (79.68–112.98) | 1.0 | 8.60 (0.720a) | y = 1.54 + 0.04*x |
| Simmondsia chinensis | 19.00 (14.03–25.19) | 1.9 | 40.45 (32.52- 55.17) | 1.8 | 57.95 (46.08- 81.12) | 1.8 | 4.20 (0.241a) | y = 1.23 + 0.06*x |
| Syzygium aromaticum | 32.14 (21.00–44.84) | 1.2 | 63.13 (43.91–102.50) | 1.1 | 88.39 (60.37–19.40) | 1.1 | 16.81 (0.031a) | y = 1.26 + 0.04*x |
| Tilia americana | 26.03 (19.61–35.05) | 1.4 | 52 (43.55–78.29) | 1.3 | 78.62 (61.30–115.31) | 1.2 | 16.6 (0.471a) | y = 1.24 + 0.05*x |
| Thymus vulgaris | 9.67 (3.58–33.79) | 3.9 | 21.89 (13.29–104.01) | 3.2 | 31.86 (19.19–163.28) | 3.0 | 33.04 (0.000a) | y = 0.88 + 0.09*x |
| Viola odorata | 10.31 (3.88–28.58) | 3.6 | 22.15 (13.76–78.00) | 3.2 | 31.81 (19.76–120.35) | 3.0 | 29.95 (0.000a) | y = .96 + 0.09*x |
| Zingiber officinale | 29.27 (19.73–48.49) | 1.3 | 57.30(41.31–105.43) | 1.2 | 80.16 (56.91–153.86) | 1.2 | 14.90 (0.005a) | y = 1.26 + 0.04*x |
| Reference oil | Sesamum indicum | Crocus sativus | ||||||
RE Relative efficacy.
Significant values are in [bold].
The efficacy of oils could be classified, 48 h post-treatment (PT) as the highly effective group (H group) inducing 95–100% mortalities, including eight oils: Allium sativum, Anethum graveolens, Camellia sinensis, Foeniculum vulgare, Nigella sativa, Salvia officinalis, T. vulgaris, and Viola odorata. Camellia sinensis and F. vulgare provided 100%, 24 h PT (Table 2).
The LT50 values of the H group ranged from 9.67 (T. vulgaris) to 19.91 (An. graveolens) hours and those of LT99 values ranged from 29.97 (Foeniculum vulgare) to 55.32 (An. graveolens). The relative effects (RE) of such oils according to LT50 values were 2.7, 1.9, 2.9, 3.7, 2.4, 2.4, 3.9, and 3.6 times, respectively, times than S. indicum; whereas those of LT99 values were 2.1, 1.8, 2.4, 3.3, 2.0, 2.0, 3.0, and 3.0 times, respectively, than C. sativus. The Chi-square, significance, and regression equations were provided for all teste oils (Table 3).
The moderately effective (M group) group of oils resulted in 81–92% mortalities 48 h PT, including B. serrata, C. cyminum, C. aromatic, L. sativum, M. alternifolia, P. nigrum, and S. chinensis. They provided 63.33–71.67% mortalities, 24 h PT (Table 2).
The LT50 values of M group ranged from 19.00 (S. chinensis) to 22.65 (C. cyminum) hours and those of LT99 values ranged from 57.95 (S. chinensis) to 66.22 (M. alternifolia) (Table 3). Their RE regarding the LT50 values were 1.8, 1.7, 1.8, 1.9, 1.7, 1.9, and 1.9 times than S. indicum, respectively, whereas those of LT99 values were 1.7, 1.6, 1.6, 1.7, 1.5, 1.6, and 1.8 times than C. sativus, respectively (Table 3).
The least effective group (L group) included the other 17 oils, and the least effective ones were C. sativus, and S. indicum, providing 62.33 and 60.00% mortalities, 48 h PT, whereas their LT50 values were 37.07 and 37.64 h and their LT99 values were 96.88 and 92.89 h, respectively (Table 3).
Furthermore, the Kruskal–Wallis test was performed to compare the mean differences of more than two groups, followed by the Mann–Whitney test to compare the mean differences between groups. Whereas Kruskal–Wallis and Friedman's tests showed there are significant indications between the three groups at different times (P = 0.001) (Tables 4 and 5).
Table 4.
Kruskal–Wallis test for larval mosquito mortality (%) of plant oil groups at 1000 ppm.
| Oil groups | Mortality % (mean ± SD)* | ||||
|---|---|---|---|---|---|
| 0.5 h | 2 h | 8 h | 24 h | 48 h | |
| Low | 4.2 ± 0.847 | 12.3 ± 2.278 | 25.980 ± 6.590 | 49.4 ± 7.838 | 71.6 ± 7.39 |
| Medium | 5.0 ± 1.361 | 13.8 ± 4.050 | 35.950 ± 2.864 | 69.5 ± 2.841 | 88.3 ± 3.191 |
| High | 7.5 ± 1.260 | 22.7 ± 1.527 | 54.792 ± 6.389 | 87.1 ± 8.533 | 98.3 ± 1.992 |
| Chi-Square | 16.909** | 18.152** | 23.037** | 25.391** | 25.098** |
| df | 2 | 2 | 2 | 2 | 2 |
| Asymp. Sig | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
*Means produced by non-parametric analysis (Kruskal–Wallis, p 0.05).
**The X2 value is sig. at significant level 1%
H: The highly effective group (95–100% mortalities) are 8 oils (A. sativum, A. graveolens, C. sinensis, F. vulgare, N. sativa, S. officinalis, T. vulgaris, and V. odorata).
M: The moderately effective group (81–92% mortalities) are 7 oils (B. serrata, C. cyminum, C. aromatic, L. sativum, M. alternifolia, P. nigrum,and S. chinensis).
L.: The moderately effective group are included the rest of oils, 17 oils (A. spinosa, B. carinata, C. libani, C. colocynthis, C. sativus, C. maxima, C. sempervirens, C. longa, G. morhua, L. usitatissimum, P. ginseng, P. dulcis, R. chalepensis, S. indicum, S.aromaticum, T. americana, and Z. officinale).
Table 5.
Friedman test for larval mosquito mortality (%) of plant oil groups at 1000 ppm.
| Oil groups | 0.5 h | 2 h | 8 h | 24 h | 48 h | Chi2 Df = 4 |
|---|---|---|---|---|---|---|
| Low | 4.2 ± 0.847 | 12.3 ± 2.278 | 25.980 ± 6.590 | 49.4 ± 7.838 | 71.6 ± 7.39 | 68** |
| Medium | 5.0 ± 1.361 | 13.8 ± 4.050 | 35.950 ± 2.864 | 69.5 ± 2.841 | 88.3 ± 3.191 | 28** |
| High | 7.5 ± 1.260 | 22.7 ± 1.527 | 54.792 ± 6.389 | 87.1 ± 8.533 | 98.3 ± 1.992 | 31.7** |
| total | 5.21 ± 1.733 | 15.21 ± 5.111 | 35.36 ± 13.379 | 63.23 ± 17.613 | 81.93 ± 13.09 | 127.6** |
**The X2 value is sig. at significant level 1%
Viola odorata, A. graveolens, T. vulgaris, and N. sativa provide 100% adult mortalities PT with 10. 25. 20, and 25%. The mortality percentages of the adults subjected to 10% of oils (H group) were 48.89%, 88.39, 63.94, 51.54, 92.96, 44.44, 72.22, and 100.0% for A. sativum, An. graveolens, C. sinensis, F. vulgare, N. sativa, S. officinalis T. vulgaris, and V. odorata, respectively. Their adulticidal LC50 values, 24 h PT, were 15.57, 2.42, 9.01, 15.07, 3.42, 20.46, 3.08, and 1.88%; whereas their LC90 values were 38.86, 9.47, 32.18, 33.34, 5.44, 50.76, 16.08, and 7.37%, respectively. Salvia officinalis followed by A. sativum were the least effective oils against adults. According to LC90, N. sativa, V. odorata and An. graveolens killed mosquitoes 9.3, 6.9, and 5.4 times more than S. officinalis (Table 6).
Table 6.
The adulticidal effects of selected plant oils against Culex pipiens after 24 h post-treatments.
| Oil name | Conc. % | Mortality% (mean ± SD) | LC50 (lower–upper limit) | RE (LC50) | LC90 (lower–upper limit) | RE (LC90) | LC95 (lower–upper limit) | RE (LC95) | Chi (Sig) | Equation |
|---|---|---|---|---|---|---|---|---|---|---|
| Allium sativum | 0 | 0 ± 0e | 15.57 (8.49–28.46) | 2.4 | 38.86 (26.79–81.87) | 1.9 | 45.47 (31.19–97.80) | 1.9 | 24.40 (0.000a) | Y = 0.051 + 0.008*x |
| 0.5 | 20.00 ± 6.67d | |||||||||
| 2.0 | 24.44 ± 5.88d | |||||||||
| 5.0 | 42.22 ± 2.22c | |||||||||
| 10 | 48.89 ± 4.44c | |||||||||
| 20 | 62.22 ± 8.01b | |||||||||
| 40 | 86.67 ± 3.85a | |||||||||
| Anethum graveolens | 0 | 6.37 ± 18.75d | 2.42 (0.08–4.22) | 8.05 | 9.47 (4.66–17.80) | 5.4 | 23.25 (7.17–129.13) | 2.6 | 33.254 (.000a) | Y = 0.242 + 0.130*x |
| 0.1 | 36.86 ± 15.46bc | |||||||||
| 0.5 | 41.66 ± 27.57b | |||||||||
| 2 | 46.12 ± 11.77b | |||||||||
| 5 | 75.96 ± 18.84a | |||||||||
| 10 | 88.39 ± 7.27a | |||||||||
| 20 | 91.85 ± 9.24a | |||||||||
| 25 | 100.00 ± 0.00a | |||||||||
| Camellia sinensis | 0 | 3.57 ± 20.00c | 9.01 (− 17.75 to 23.09) | 2.3 | 32.18 (19.96–170.57) | 1.6 | 38.754 (24.052–218.98) | 1.5 | 26.52 (0.000a) | Y = 0.644 + 0.106*x |
| 2 | 51.51 ± 2.62b | |||||||||
| 5 | 61.21 ± 6.30ab | |||||||||
| 10 | 63.94 ± 10.22ab | |||||||||
| 15 | 75.35 ± 29.22ab | |||||||||
| 20 | 78.78 ± 16.87ab | |||||||||
| 25 | 91.99 ± 0.45a | |||||||||
| Foeniculum vulgare | 0 | 10.50 ± 25.00d | 15.07 (0.10–104.60) | 1.4 | 33.34 (21.67–789.17) | 1.5 | 38.53 (24.63–986.39) | 1.5 | 22.19 (0.000a) | Y = 0.331 + 0.03*x |
| 5 | 36.73 ± 16.93bc | |||||||||
| 10 | 51.54 ± 11.47ab | |||||||||
| 15 | 51.70 ± 2.27ab | |||||||||
| 20 | 59.00 ± 16.87ab | |||||||||
| 25 | 75.96 ± 1.36a | |||||||||
| Nigella sativa | 0 | 4.95 ± 20.61e | 3.42 (− 53.96 to 30.15) | 6.0 | 5.44 (− 14.41 to 84.13) | 9.3 | 29.95 (15.87-1184.48) | 2.0 | 57.88 (0.000a) | Y = 0.261 + 0.06*x |
| 0.05 | 41.87 ± 12.75 cd | |||||||||
| 0.1 | 60.68 ± 3.73bc | |||||||||
| 0.5 | 72.91 ± 6.45ab | |||||||||
| 1 | 74.54 ± 19.78ab | |||||||||
| 2 | 78.09 ± 18.28ab | |||||||||
| 10 | 92.96 ± 9.44ab | |||||||||
| 25 | 100.00 ± 6.11ab | |||||||||
| Salvia officinalis | 0 | 0 ± 0e | 20.46 (11.34–45.85) | 1.0 | 50.76 (33.24–140.52) | 1.0 | 59.35 (38.59–168.23) | 1.0 | 25.35 (0.000a) | Y = 0.8022 + 0.091*x |
| 0.5 | 17.78 ± 2.22d | |||||||||
| 2.0 | 22.22 ± 2.22d | |||||||||
| 5.0 | 37.78 ± 4.45c | |||||||||
| 10 | 44.44 ± 4.44bc | |||||||||
| 20 | 53.33 ± 3.85b | |||||||||
| 40 | 73.33 ± 7.70a | |||||||||
| Thymus vulgaris | 0 | 3.57 ± 7.15c | 3.08 (− 3.29 to 7.48) | 6.6 | 16.08 (10.43–41.60) | 3.2 | 19.76 (12.83–52.76) | 3.0 | 34.12 (0.000a) | Y = 0.350 + 0.091*x |
| 0.1 | 38.74 ± 4.28b | |||||||||
| 0.5 | 61.66 ± 7.26ab | |||||||||
| 2 | 69.82 ± 9.85ab | |||||||||
| 10 | 72.22 ± 14.69ab | |||||||||
| 20 | 100.00 ± 0.00a | |||||||||
| Viola odorata | 0 | 3.57 ± 7.15d | 1.88 (− 1.80 to 5.29) | 10.8 | 7.37 (4.46–29.82) | 6.9 | 8.92 (5.43–37.58) | 6.6 | 21.99 (0.001a) | Y = 0.190 + 0.112*x |
| 0.1 | 50.00 ± 10.00c | |||||||||
| 0.5 | 54.95 ± 15.61c | |||||||||
| 1 | 57.50 ± 19.20c | |||||||||
| 2 | 65.83 ± 13.21bc | |||||||||
| 6 | 85.05 ± 13.62ab | |||||||||
| 10 | 100.00 ± 0.00a | |||||||||
| Reference oils | Salvia officinalis | |||||||||
Oil phytochemical analysis
Phytochemical analysis of oils of F. vulgare Mill., An. graveolens L., V. odorata L., T. vulgaris L., A. sativum, S. officinalis and C. sinensis by GC/MS and HPLC analysis revealed their major compounds. F. vulgare oil contains Estragole (70.36%); Limonene (8.96%) and 1,3,3-trimethyl Bicyclo [2.2.1]heptan-2-one (2.81%) (Table 7 and Fig. 1).
Table 7.
GC/MS analysis of the Foeniculum vulgare Mill.
| Peak no. | Rt (min.) | MW | MF | Area % | Probabilities of the detected compounds |
|---|---|---|---|---|---|
| 1 | 5.03 | 40 | C3H4 | 0.14 | 1-Propyne |
| 2 | 5.22 | 138 | C7H10N2O | 0.26 | 2,3,3a,4,7,7a-Hexahydro-1H-benzimidazol-2-one |
| 3 | 5.28 | 348 | C19H22ClFN2O | 1.06 | 1-Chloro-3-(3-fluorobenzoyl)-4-(2-(diethylamino)ethylamino)benzene |
| 4 | 6.38 | 136 | C10H16 | 0.41 | Sabinene |
| 5 | 6.49 | 262 | C12H23O4P | 1.01 | Dimethyl{[2,2-dimethyl-3-(2′-methylprop-1′-cyclopropyl]methyl}phosphate |
| 6 | 7.57 | 670 | C44H27DN4Ni | 0.15 | (5,10,15,20-tetraphenyl[2-(2)H1]prophyrin-ato)zinx(II) |
| 7 | 9.17 | 136 | C10H16 | 8.96 | Limonene |
| 8 | 10.90 | 152 | C10H16O | 2.81 | 1,3,3-trimethyl Bicyclo[2.2.1]heptan-2-one |
| 10 | 14.26 | 148 | C10H12O | 70.36 | Estragole |
| 11 | 14.72 | 818 | C44H28Br2N4Ti | 0.11 | Tetraphenylporphyrinatodibromotitanium (IV) |
| 12 | 16.70 | 166 | C11H18O | 0.47 | 3,7-Dimethyl-2,6-Nonadienal |
| 13 | 17.28 | 152 | C10H16O | 1.41 | 2,4-Decadienal |
| 14 | 18.07 | 194 | C14H26 | 0.17 | 1,1′-Bicycloheptyl |
| 15 | 29.40 | 300 | C17H36O2Si | 0.20 | Tetradecanoic acid, trimethylsilyl ester |
| 16 | 32.19 | 160 | C10H21F | 0.15 | Fluoro decane |
| 17 | 32.36 | 244 | C13H24O4 | 0.11 | Oxalic acid isohexylpentyl ester |
| 18 | 33.14 | 328 | C19H40O2Si | 1.74 | Hexadecanoic acid, trimethylsilyl ester |
| 19 | 33.78 | 282 | C18H34O2 | 0.15 | (Z) 9-Octadecenoic acid |
| 20 | 34.03 | 138 | C10H18 | 0.25 | 7-Methyl-1-nonyne |
| 21 | 34.12 | 282 | C18H34O2 | 0.30 | (Z) 9-Octadecenoic acid |
| 22 | 34.58 | 256 | C16H32O2 | 0.12 | Hexadecanoic acid |
| 23 | 35.57 | 280 | C18H32O2 | 1.44 | (Z,Z) 9,12-Octadecadienoic acid |
| 24 | 35.64 | 280 | C18H32O2 | 1.03 | (Z,Z) 9,12-Octadecadienoic acid |
| 25 | 35.70 | 356 | C21H40O4 | 0.53 | 2,3-Dihydroxypropylelaidate |
| 26 | 35.76 | 238 | C16H30O | 1.67 | Z-7-Hexadecenal |
| 27 | 36.25 | 280 | C18H32O2 | 0.23 | (Z,Z )9,12-Octadecadienoic acid |
| 28 | 36.38 | 266 | C18H34O | 0.43 | 12-Octadecenal |
| 29 | 42.83 | 142 | C9H18O | 0.13 | Nonanal |
| 31 | 46.93 | 660 | C20Cl12 | 0.13 | Dodecachloroperylene |
| 32 | 48.70 | 295 | C20H25NO | 0.61 | (R)-1-[N-1-cyclopentylpropionylamino-1-ethyl]naphthalene |
| 33 | 50.05 | 354 | C20H18O6 | 0.38 | Isosesamin |
Figure 1.
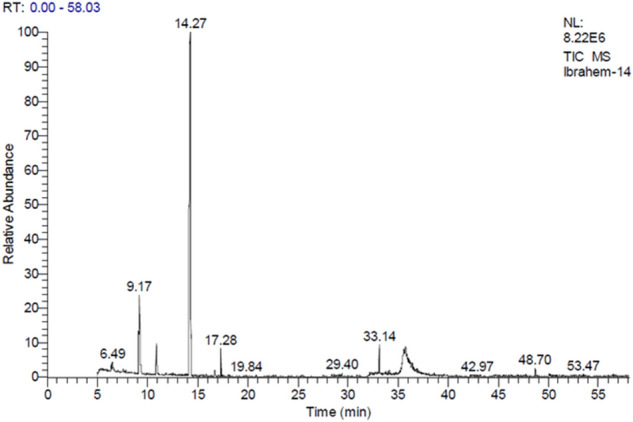
GC/MS analysis of the Foeniculum vulgare Mill.
Anethum graveolens showed abundance of 4-Pyridinecarbaldehyde-4-propyl-3-thiosemicarbazone (32.13%); 1,5-dimethyl-1,5-Cyclooctadiene (17.19%); Dihydrocarvone (5.98%); 3a(1H)-Azulenol,2,3,4,5,8,8a-hexahydro-6,8-adimethyl-3-(1-methylethyl),[3R-(3à,3aà,8aà)] (Carotol) (21.26%); and tricyclic compound Daucol (2.39%) (Table 8 and Fig. 2).
Table 8.
GC/MS analysis of the Anethum graveolens L.
| Peak no. | Rt (min.) | MW | MF | Area % | Probabilities of the detected compounds |
|---|---|---|---|---|---|
| 1 | 5.14 | 238 | C13H18O4 | 0.49 | Diethyl 3,4-bis(methylene)cyclopentane-1,1-dicarboxylate |
| 2 | 5.21 | 600 | C33H28O11 | 0.69 | (2′S,3S,3′S,P)-hydroxyanhydrophlegmacin-9,10-quinone 8′-O-methylether |
| 3 | 7.65 | 290 | C19H30O2 | 0.06 | 2-(2′-Isopropenyldec-2′-enyl)methylcyclopentane-1,3-dione |
| 4 | 9.18 | 136 | C10H16 | 17.19 | 1,5-Dimethyl-1,5-Cyclooctadiene |
| 5 | 9.35 | 136 | C10H16 | 0.23 | dl-Limonene |
| 6 | 14.05 | 152 | C10H16O | 5.98 | Dihydrocarvone |
| 7 | 14.25 | 152 | C10H16O | 0.86 | CIS-DIHYDROCARVONE |
| 8 | 15.44 | 150 | C10H14O | 14.62 | 2-Methyl-5-(1-methylethenyl)2-Cyclohexen-1-one |
| 9 | 15.80 | 733 | C44H28Cl2N4V | 0.07 | Dichloro(5,10,15,20-tetra phenylporphyrinato)vanadium |
| 10 | 16.71 | 692 | C41H33FeO5P | 0.13 | Dicarbonyl(1,3-5-ü-6-phenyl-2-(phenylethynyl)cyclohept-4-ene-1,3-diyl) triphenoxyphosphaneiron |
| 11 | 17.29 | 110 | C8H14 | 0.47 | octahydro Pentalene |
| 12 | 18.89 | 675 | C44H28CuN4 | 0.09 | (5,10,15,20-tetraphenyl[2-(2)H1]prophyrinato)copper(II) |
| 13 | 20.82 | 204 | C15H24 | 0.10 | à-Humulene |
| 14 | 21.36 | 686 | C37H24Cl2N6O4 | 0.08 | 2,2-Bis[4[[4-chloro-6-(3-ethynylphenoxy)-1,3,5-triazin-2-yl]oxy]phenyl]propane |
| 15 | 21.92 | 134 | C10H14 | 0.14 | 1,2,3,4-Tetramethyl-5-methylenecyclopenta-1,3-diene |
| 16 | 22.07 | 204 | C15H24 | 0.38 | á –Bisabolene |
| 17 | 22.16 | 648 | C35H38Cl2N4O4 | 0.11 | 2,4-bis(á-chloroethyl)-6,7-bis[á-methoxycarbonylethyl]-1,3,5-trimethylporphyrin |
| 18 | 22.36 | 640 | C32H64O5Si4 | 0.23 | OTETRAKIS(TRIMETHYLSILYL)3,5-DIHYDROXY-2-(3-HYDROXY-1-OCTENYL)CYCLOPENTANEHEPTANOATE |
| 19 | 23.34 | 208 | C14H24O | 0.18 | 3-Oxabicyclo[3.3.1]non-6-ene |
| 20 | 24.23 | 222 | C15H26O | 21.26 | 3a(1H)-Azulenol,2,3,4,5,8,8a-hexahydro-6,8-adimethyl-3-(1-methylethyl),[3R-(3à,3aà,8aà)] |
| 21 | 24.57 | 572 | C23H26Br2O7 | 0.10 | Dibromogomisin A |
| 22 | 25.05 | 222 | C10H14N4S | 32.13 | 4-Pyridinecarbaldehyde-4-propyl-3-thiosemicarbazone |
| 23 | 25.28 | 238 | C15H26O2 | 2.39 | Daucol |
| 24 | 26.01 | 194 | C12H18O2 | 0.06 | 3-(1-Hydroxyhexyl)phenol |
| 25 | 27.54 | 220 | C15H24O | 0.06 | Trans-Z-à-Bisaboleneepoxide |
| 26 | 33.01 | 2598 | N/A | 0.07 | YGRKKRRQRRRGPVKRRLDL/5 |
| 27 | 34.16 | 691 | C51H33NO2 | 0.07 | 2,6-Bis(2,3,5-triphenyl-4-oxocyclopentadienyl)pyridine |
| 28 | 35.47 | 733 | C44H28Cl2N4V | 0.08 | Dichloro(5,10,15,20-tetraphenylporphyrinato)vanadium |
| 29 | 40.31 | 739 | C39H81NO4Si4 | 0.13 | (3S,4R,1′E,2″R,3″R)-1-tertButyldimethylsilyl-4-(3′-tertbutyldimethylsilyloxy-2′-methylprop-1′-enyl)-3-(1″,3″ di(tertbutyldimethylsilyloxy)-2″-methylhex-5″-yl]-3-methylazetidin-2-one |
| 31 | 43.48 | 114 | C6H10O2 | 0.13 | 3,4-Hexanedione |
| 32 | 50.56 | 680 | C35H40O5Si5 | 0.06 | Pentamethylpentaphenylcyclopentasiloxane |
| 33 | 51.11 | 733 | C44H28Cl2N4V | 0.09 | Dichloro(5,10,15,20-tetraphenylporphyrinato)vanadium |
Figure 2.

GC/MS analysis of the Anethum graveolens L.
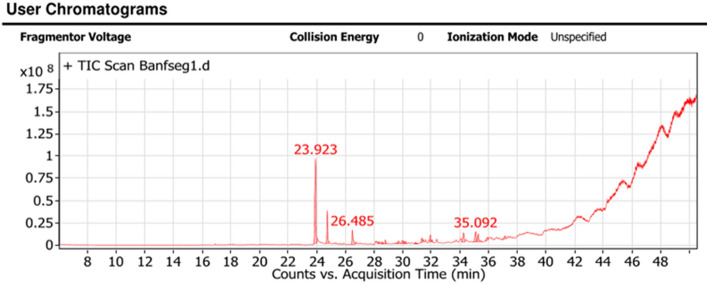
Viola odorata L. oil contains Diphenyl ether (42.04%); alpha.-Ionone(11.87%); (Z)-5-(4-tert-Butyl-1-hydroxycyclohexyl)-3-methylpent-2-en-4-yne (7.22%); 2,3,3a,4,5,5a,6,7,9a,9b-decahydro-3,5a,9-trimethyl-7,9a-peroxy Naphtho-[1,2-b]furan-2-one (6.6%); 2-hexyl-1-Decanol (4.15%); and hexadecahydro-Pyrene (2.79%) (Table 9 and Fig. 3).
Table 9.
GC/MS analysis of the Viola odorata L.
| Peak no. | Rt (min.) | MW | MF | Area % | Probabilities of the detected compounds |
|---|---|---|---|---|---|
| 1 | 23.923 | 170 | C12H10O | 42.04 | Diphenyl ether |
| 2 | 24.735 | 192 | C13H20O | 11.87 | .alpha.-Ionone |
| 3 | 26.485 | 192 | C13H20O | 7.73 | 3-Buten-2-one, 4-(2,6,6-trimethyl-1-cyclohexen-1-yl) |
| 4 | 28.317 | 236 | C15H24O2 | 0.61 | Limonen-6-ol, pivalate |
| 5 | 28.58 | 226 | C13H22O3 | 0.9 | 2-Hydroxy-1,1,10-trimethyl-6,9-epidioxydecalin |
| 6 | 28.786 | 238 | C16H30O | 1.26 | 7-Hexadecenal, (Z)- |
| 7 | 29.599 | 236 | C16H28O | 0.83 | 7,11-Hexadecadienal |
| 8 | 29.713 | 296 | C20H40O | 1.48 | Phytol |
| 9 | 29.959 | 242 | C16H34O | 2.15 | 2-Hexyl-1-Decanol |
| 10 | 30.074 | 378 | C25H46O2 | 1.09 | Undec-10-ynoic acid, tetradecyl ester |
| 11 | 30.211 | 296 | C20H40O | 1.02 | PHYTOL ISOMER |
| 12 | 30.881 | 266 | C16H26O3 | 0.67 | 2-Dodecen-1-yl(-)succinic anhydride |
| 13 | 31.338 | 242 | C16H34O | 2.14 | 1-Decanol, 2-hexyl- |
| 14 | 31.939 | 218 | C16H26 | 2.79 | hexadecahydroPyrene |
| 15 | 32.054 | 240 | C17H36 | 0.7 | Tetradecane, 2,6,10-trimethyl |
| 16 | 34.245 | 250 | C16H26O2 | 7.22 | (Z)-5-(4-tert-Butyl-1-hydroxycyclohexyl)-3-methylpent-2-en-4-yne |
| 17 | 35.092 | 264 | C15H20O4 | 6.6 | 2,3,3a,4,5,5a,6,7,9a,9b-decahydro-3,5a,9-trimethyl-7,9a-peroxy Naphtho[1,2-b]furan-2-one |
| 18 | 35.269 | 264 | C15H20O4 | 4.73 | 2,3,3a,4,5,5a,6,7,9a,9b-decahydro-3,5a,9-trimethyl-7,9a-peroxy Naphtho [1,2-b]furan-2-one |
| 19 | 35.905 | 242 | C16H34O | 2.19 | 2-hexyl-1-Decanol |
| 20 | 37.146 | 266 | C18H34O | 1.89 | Z,E-2,13-Octadecadien-1-ol |
| 21 | 23.923 | 170 | C12H10O | 0.78 | Diphenyl ether |
Figure 3.
GC/MS analysis of the sample Viola odorata L.
Thymus vulgaris oil included 2-Ethynyl-3-hydroxypyridine (12.37%); 2-á-pinene(8.92%),2,5-Dipropoxybenzalde-hyde (7.70%); 5-Amino-8-cyano-7-methoxy-3,4-dihydro-3-methy-l1,6-naphthyridin- (1H)-one (5.05%); à-terpinyl acetate (5.00%); 4-methyl-1-(1-methyl-ethyl)-3-Cyclohexen-1-ol (4.73%), 3-(6,6-Dimethyl-5-oxohept-2-enyl)-cyclo-heptanone (4.54%); 10-Methylnonadecane(4.12%); 9-methyl Nonadecane-(3.55%); n1,1′-oxybis Decane (2.36%); 7,11-Hexadecadienal (2.14%); and (2R,3R)-3- (2-Methoxy-4-methylphenyl)-2,3-dimethylcyclopentanone (2.01%) (Table 10 and Fig. 4).
Table 10.
GC/MS analysis of Thymus vulgaris L.
| Peak no. | Rt (min.) | MW | MF | Area % | Probabilities of the detected compounds |
|---|---|---|---|---|---|
| 1 | 5.1 | 208 | C13H20O2 | 0.86 | TRANS-á-IONON-5,6-EPOXIDE |
| 2 | 5.23 | 122 | C8H15B | 0.79 | 1-Borabicyclo[4.3.0]nonane |
| 3 | 6.46 | 136 | C10H16 | 1.85 | Tricyclene |
| 4 | 6.86 | 136 | C10H16 | 0.69 | Camphene |
| 5 | 7.64 | 136 | C10H16 | 8.92 | 2-á-pinene |
| 6 | 9.07 | 119 | C7H5NO | 12.37 | 2-Ethynyl-3-hydroxypyridine |
| 7 | 11.32 | 196 | C12H20O2 | 0.68 | Linalyl acetate |
| 8 | 12.50 | 152 | C10H16O | 1.27 | (1S) Bicyclo[2.2.1]heptan-2-one, 1,7,7-trimethyl |
| 9 | 13.39 | 156 | C10H20O | 0.78 | 1-Methyl-4-(1-methylethyl)Cyclohexanol |
| 10 | 13.51 | 154 | C10H18O | 4.73 | 4-Methyl-1-(1-methylethyl)-3-Cyclohexen-1-ol |
| 11 | 13.91 | 154 | C10H18O | 1.13 | à,à,4-trimethyl (S) 3-Cyclohexene-1-methanol |
| 12 | 15.67 | 182 | C11H18O2 | 0.63 | linalyl formate |
| 13 | 16.48 | 196 | C12H20O2 | 1.76 | EXOBORNYL ACETATE |
| 14 | 18.17 | 196 | C12H20O2 | 5.00 | à-terpinyl acetate |
| 15 | 20.52 | 142 | C9H18O | 0.56 | 3-Ethylheptanal |
| 16 | 21.94 | 268 | C19H40 | 0.58 | Nonadecane |
| 17 | 22.84 | 199 | C9H13NO4 | 1.87 | 2S,7S Methyl-2-Hydroxy-3-oxotetrahydro-1-Hpyrrolizine-7a-(5H)-carboxylate |
| 18 | 22.97 | 226 | C16H34 | 0.92 | Pentadecane-5-methyl |
| 19 | 23.10 | 212 | C15H32 | 0.75 | 3-ethyl Tridecane |
| 20 | 23.22 | 348 | C19H40O3S | 0.84 | hexyltridecyl ester Sulfurous acid |
| 21 | 23.39 | 226 | C16H34 | 1.09 | 3-methyl Pentadecane |
| 22 | 24.06 | 168 | C8H12N2O2 | 1.52 | 1,6-diisocyanato Hexane |
| 23 | 24.24 | 298 | C20H42O | 2.36 | 1,1′-oxybis Decane, |
| 24 | 24.40 | 282 | C20H42 | 0.81 | Eicosane |
| 25 | 24.65 | 334 | C18H38O3S | 0.57 | Sulfurous acid, butyltetradecyl ester |
| 26 | 25.10 | 282 | C20H42 | 4.12 | 10-Methylnonadecane |
| 27 | 25.24 | 268 | C19H40 | 1.00 | 7-hexyl Tridecane |
| 28 | 25.37 | 334 | C18H38O3S | 1.10 | 6-Tetradecanesulfonic acid, butyl ester |
| 29 | 25.49 | 334 | C18H38O3S | 1.44 | 6-Tetradecanesulfonic acid, butyl ester |
| 31 | 25.68 | 250 | C16H26O2 | 4.54 | 3-(6,6-Dimethyl-5-oxohept-2-enyl)-cycloheptanone |
| 32 | 25.98 | 222 | C13H18O3 | 7.70 | 2,5-Dipropoxybenzaldehyde |
| 33 | 26.30 | 352 | C25H52 | 1.33 | Pentacosane |
| 34 | 26.44 | 282 | C20H42 | 3.55 | 9-methyl, Nonadecane |
| 35 | 26.62 | 224 | C16H32 | 1.08 | 1-Hexadecene |
| 36 | 26.84 | 236 | C16H28O | 2.14 | 7,11-Hexadecadienal |
| 37 | 27.25 | 232 | C11H12N4O2 | 5.05 | 5-Amino-8-cyano-7-methoxy-3,4-dihydro-3-methy-l1,6-naphthyridin-2(1H)-one |
| 38 | 27.32 | 232 | C15H20O2 | 2.01 | (2R,3R)-3-(2-Methoxy-4-methylphenyl)-2,3-dimethylcyclopentanone |
| 39 | 27.42 | 282 | C20H42 | 0.87 | 2,6-dimethyl Octadecane |
| 40 | 27.54 | 310 | C22H46 | 0.77 | 8-heptyl Pentadecane |
| 41 | 27.65 | 376 | C21H44O3S | 0.61 | Sulfurous acid, hexyl pentadecyl ester |
| 42 | 27.82 | 226 | C16H34 | 0.88 | Hexadecane |
| 43 | 28.42 | 164 | C5H9BrO | 0.62 | 1-Bromo-2-methyl-3-Buten-2-ol |
| 44 | 28.54 | 242 | C16H34O | 1.25 | 2-Hexyl-1-decanol |
| 45 | 28.69 | 111 | C7H13N | 1.08 | 1-isocyano Hexane |
| 46 | 29.32 | 116 | C7H16O | 1.94 | 2-ethyl 1-Pentanol |
| 47 | 30.70 | 200 | C13H28O | 0.82 | 2-Propyldecan-1-ol |
| 48 | 31.33 | 197 | C11H19NO2 | 0.98 | 2-Ethylhexyl cyanoacetate |
| 49 | 33.27 | 592 | C41H84O | 0.70 | 1-Hentetracontanol |
| 50 | 36.28 | 324 | C23H48 | 0.57 | 9-hexyl Heptadecane |
| 51 | 37.92 | 366 | C26H54 | 0.58 | 5,14-dibutyl Octadecane |
Figure 4.
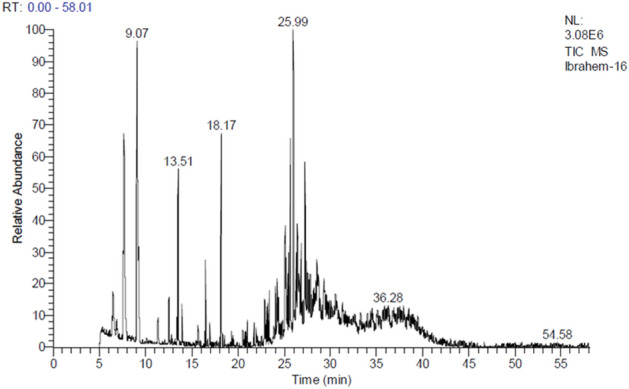
GC/MS analysis of Thymus vulgaris L.
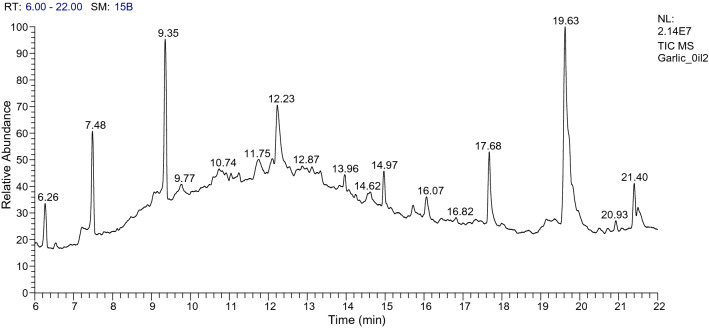
Allium sativum contains many effective chemical compounds including the 9-Octadecenamide, (Z)-(29.07%), Trisulfide, di-2-propenyl (14.86%), and isochiapin B%2 < (8.63%) compounds (Table 11 and Fig. 5).
Table 11.
GC/MS analysis of the Allium sativum.
| Peak no. | Rt (min.) | MW | MF | Area % | Probabilities of the detected compounds |
|---|---|---|---|---|---|
| 1 | 6.27 | 146 | C6H10S2 | 4.54 | Diallyl disulphide |
| 2 | 7.49 | 152 | C4H8S3 | 9.68 | Trisulfide, methyl 2-propenyl |
| 3 | 9.35 | 178 | C6H10S3 | 14.86 | Trisulfide, di-2-propenyl |
| 4 | 12.22 | 350 | C19H26O6 | 8.63 | ISOCHIAPIN B %2 < |
| 5 | 14.97 | 334 | C20H30O4 | 3.54 | 1,2-Benzenedicarboxylic acid, butyl octyl ester |
| 6 | 16.05 | 346 | C19H22O6 | 3.11 | ISOCHIAPIN B |
| 7 | 17.67 | 387 | C17H37N7O3 | 7.84 | 9-OCTADECENAMIDE |
| 8 | 19.61 | 281 | C18H35NO | 29.07 | 9-Octadecenamide, (Z)- |
| 10 | 21.40 | 208 | C11H12O2S | 4.25 | 3-(Benzylthio)acrylic acid, methyl ester |
| 11 | 23.27 | 300 | C19H24O3 | 5.86 | 3,17-DIOXO-11-à-HYDROXYANDROSTANE-1,4-DIENE |
| 12 | 23.54 | 436 | C26H44O5 | 1.82 | 3 Ethyl iso-allocholate |
| 13 | 23.62 | 490 | C34H50O2 | 6.81 | CHOLEST-5-EN-3-YL BENZOATE |
9-Octadecenamide, (Z)- (29.07), Trisulfide, di-2-propenyl (14.86), and ISOCHIAPIN B %2 < (8.63).
Figure 5.
GC/MS analysis of Allium sativum.
Salvia officinalis oil showed abundance of Terpinen-4-ol (17.35%), Camphor (16.08%), 14-á-H-PREGNA (9.25%), and 1-CHLOROOCTADECANE (6.82%), (Table 12 and Fig. 6). Finally, C. sinensis oil is dissolved in distilled water and its major components include Gallic acid (1674 µg/ml), Catechin (421 µg/ml), Methyl gallate (1076 µg/ml), Coffeic acid (678 µg/ml), Coumaric acid (566 µg/ml), Naringenin (178 µg/ml), and Kaempferol (218 µg/ml), Table 13. Essential oils and the most active ingredients of the analyzed oils were drawn (Fig. 7).
Table 12.
GC/MS analysis of the Salvia officinalis.
| Peak no. | Rt (min.) | MW | MF | Area % | Probabilities of the detected compounds |
|---|---|---|---|---|---|
| 1 | 10.22 | 152 | C10H16O | 16.08 | Camphor |
| 2 | 10.90 | 156 | C10H20O | 5.24 | Cyclohexanol, 1-methyl-4-(1-methylethyl)- |
| 3 | 11.47 | 154 | C10H18O | 17.35 | Terpinen-4-ol |
| 4 | 13.86 | 254 | C13H24O2 | 2.47 | Tridecanedial |
| 5 | 14.50 | 280 | C18H32O2 | 3.43 | 17-Octadecynoic acid |
| 6 | 15.70 | 400 | C28H48O | 0.90 | Cholestan-3-ol, 2-methylene-, (3á,5à)- |
| 7 | 16.68 | 268 | C17H32O2 | 1.80 | 7-Methyl-Z-tetradecen-1-ol acetate |
| 8 | 17.50 | 280 | C19H36O | 1.63 | 12-Methyl-E,E-2,13-octadecadien-1-ol |
| 10 | 17.99 | 288 | C21H36 | 2.03 | 14-á-H-PREGNA |
| 11 | 19.18 | 288 | C18H37Cl | 5.13 | 1-CHLOROOCTADECANE |
| 12 | 19.51 | 288 | C21H36 | 1.77 | 14-á-H-PREGNA |
| 13 | 19.86 | 450 | C32H66 | 4.33 | DOTRIACONTANE |
| 14 | 20.18 | 536 | C37H76O | 1.41 | 1-Heptatriacotanol |
| 15 | 20.32 | 268 | C16H28O3 | 1.15 | Z-(13,14-Epoxy)tetradec-11-en-1-ol acetate |
| 16 | 20.55 | 258 | C16H34S | 1.58 | tert-Hexadecanethiol |
| 17 | 20.80 | 312 | C20H40O2 | 3.17 | Ethanol, 2-(9-octadecenyloxy)-, (Z)- |
| 18 | 20.90 | 288 | C21H36 | 2.18 | 14-á-H-PREGNA |
| 19 | 21.26 | 350 | C19H26O6 | 0.73 | ISOCHIAPIN B %2< |
| 20 | 21.61 | 288 | C18H37Cl | 6.82 | 1-CHLOROOCTADECANE |
| 21 | 21.84 | 294 | C21H36 | 3.7 | 14-á-H-PREGNA |
| 22 | 22.39 | 288 | C21H36 | 0.82 | 1-Heptatriacotanol |
| 23 | 22.47 | 346 | C19H22O6 | 2.74 | ISOCHIAPIN B |
| 24 | 22.73 | 288 | C21H36 | 9.25 | 14-á-H-PREGNA |
| 25 | 23.09 | 280 | C19H36O | 2.20 | 12-Methyl-E,E-2,13-octadecadien-1-ol |
| 26 | 23.23 | 350 | C19H26O6 | 2.05 | ISOCHIAPIN B %2 < |
Figure 6.
GC/MS analysis of Salvia officinalis.
Table 13.
HPLC analysis for Camellia sinensis.
| Standard | Sample green tea | ||||
|---|---|---|---|---|---|
| St. compound | Conc. (µg/ml) | Area | Compound | Area | Conc. (µg/ml = µg/g) |
| allic acid | 16.8 | 179.72 | Gallic acid | 895.77 | 1674.71 |
| Chlorogenic acid | 28 | 335.23 | Chlorogenic acid | 75.30 | 125.79 |
| Catechin | 67.5 | 584.16 | Catechin | 182.42 | 421.56 |
| Methyl gallate | 10.2 | 789.05 | Methyl gallate | 4163.86 | 1076.52 |
| Coffeic acid | 18 | 469.51 | Coffeic acid | 895.98 | 687.01 |
| Syringic acid | 17.2 | 389.86 | Syringic acid | 30.41 | 26.83 |
| Pyro catechol | 29.2 | 451.95 | Pyro catechol | 0.00 | 0.00 |
| Rutin | 61 | 457.55 | Rutin | 71.83 | 191.53 |
| Ellagic acid | 34.3 | 495.60 | Ellagic acid | 37.52 | 51.93 |
| Coumaric acid | 13.2 | 729.56 | Coumaric acid | 1566.70 | 566.93 |
| Vanillin | 12.9 | 543.81 | Vanillin | 0.00 | 0.00 |
| Ferulic acid | 12.4 | 353.45 | Ferulic acid | 71.09 | 49.88 |
| Naringenin | 15 | 266.56 | Naringenin | 158.25 | 178.11 |
| Taxifolin | 13.2 | 189.35 | Taxifolin | 16.08 | 22.42 |
| Cinnamic acid | 5.8 | 573.08 | Cinnamic acid | 0.00 | 0.00 |
| Kaempferol | 12 | 289.35 | Kaempferol | 263.99 | 218.97 |
Figure 7.
Essential oils and their most active ingredients.
Discussion
EOs could serve as suitable alternatives to synthetic insecticides because they are relatively safe, available, and biodegradable15. In this study, 32 oils were evaluated against Cx. pipiens. Thymus vulgare and C. sinensis were the most effective larvicides (100% mortality 24 h PT). The larvicidal effect of the H group could be arranged according to their LT50 values (h) as follows: T. vulgaris (9.67), F. vulgare (10.22), V. odorata (10.31), C. sinensis (13.02), A. sativum (13.95), S. officinalis (15.42), N. sativa (15.67), then An. graveolens (19.90). On the other hand, their LT99 values ranged from 29.77 (F. vulgare) to 55.31 (An. graveolens).
In this study, the most effective oils against adults were An. graveolens and V. odorata followed by T. vulgaris then N. sativa. The data revealed that F. vulgare is a highly potent larvicide. Similarly, its oil controlled Anopheles atroparvus, Culex quinquefasciatus23,24, and Aedes aegypti25. Despite its effectiveness as larvicide in this study, F. vulgare was the least effective adulticide. In contrast, it induced adulticidal properties against Cx. quinquefasciatus23.
Our data indicated that C. sinensis was a highly effective larvicide and the less effective adulticide. Comparatively, the chemical extracts of C. sinensis induced larvicidal and adult repellent effects against Cx. pipiens providing the highest protection (100%) from the bites of starved females at the dose of 6 mg/cm226. Moreover, its leaf extract showed larvicidal effect against Anopheles arabiensis and Anopheles gambiae (s.s.)27.
Thymus vulgarisd An. graveolens showed potent larvicidal and adulticidal effects in this work. Likewise, T. vulgaris has both effects against Cx. quinquefasciatus28 and Ae. aegypti29. Thymus vulgaris exhibited larvicidal properties, 100% mortality, against Cx. pipiens larvae, at 200 ppm, whereas the LC25 and LC50 vlalues indicated no effect on AChE activity, activation of the detoxification system, as indicated by an increase in GST activity and a decrease in GSH rate30.
Our findings agree with another study found that the most potent EOs out of 53 oils against larvae were F. vulgare, T. vulgaris, Citrus medica (lime), and C. sinensis (LC50 = 27.5, 31.6, 51.3, 53.5 ppm, respectively). C. sinensis was the most efficient EOs enhancing the efficacy of deltamethrin, co-toxic factor = 316.67, over than PBO, the positive control, co-toxic factor = 283.35)31.
Some oils applied in this study showed a similar larvicidal effect against Cx. pipiens as N. sativa32,33 and S. officinalis34. Some essential oils such as T. vulgaris, S. officinalis, C. sempervirens and A. graveolens had a larvicidal effect against mosquito larvae and their LC90 values were < 200–300 ppm. This result may be due to several reasons, including the percentages of their principal components compositions that are manipulated according to the origin of plant oil, quality of oil, susceptibility of the strain used, oil storage conditions, and technical conditions35–37.
Likewise our findings, An. graveolens and F. vulgare act as larvicidal, pupicidal, and oviposition deterrent agents against M. domestica38. Moreover, Ocimum basilicum was the most effective extract tested on Cx. pipiens larvae and adults39,40.
Allium sativum showed high potency against larvae in this study. A similar finding was recorded for Cx. pipiens and Culex restuans (LC50 = 7.5 and 2.7 ppm, respectively)41. Argania spinosa oil showed a low larvicidal effect in this study. A similar effect was recorded against Cx. quinquefasciatus larvae42.
Curcuma species was less effective in this study, but its 27 components as curcuminoids and monocarbonyl curcumin derivatives were effective larvicidal agents against Cx. Pipiens and Ae. albopictus43 and hexane extraction of Curcuma longa showed 100% larvicidal activity against Cx. pipiens and Aedes albopictus at 1000 ppm after being treated 24 h44.
Zingiber officinale and Syzygium aromaticum were less effective. In contrast, they were effective against Cx. pipiens (LC50 = as 71.85 and 30.75, respectively)45.
Sesamum indicum is one of the L group in this study. In contrast, petroleum ether extract showed larvidcidal, antifeedant and repellent action against Cx. pipiens33. Furthermore, EOs of N. sativa, Allium cepa, and S. indicum, induced larvicidal effect and their LC50 values against both field and laboratory strains of Cx. pipiens were 247.99 and 108.63; 32.11 and 2.87; and finally, 673.22 and 143.87 ppm, respectively. They influenced the pupation and adult emergence rates besides developmental abnormalities at sublethal concentrations46.
Boswellia serrata (M group) and Brassica carinata (L group) showed relative larvicide against Cx. pipiens in this study. A similar result was reported47,48. The lethal concentration values of Fenugreek (Trigonella foenum-grecum), earth almond (Cyperus esculentus), mustard (Brassica compestris), olibanum (Boswellia serrata), rocket (Eruca sativa), and parsley (Carum ptroselinum) were 32.42, 47.17, 71.37, and 83.36, 86.06, and 152.94 ppm, respectively. Against Cx. pipiens larvae. Furthermore, increasing concentrations were directly proportional to the reduction of both pupation and adult emergences rates48.
Some oil-resins as Commiphora molmol, Araucaria heterophylla, Eucalyptus camaldulensis, Pistacia lentiscus, and Boswellia sacra showed larvicidal activity against Cx pipiens larvae. The larvicidal effect 24 and 48 h PT, respectively, were for acetone extracts, 1500 ppm, of C. molmol (83.3% and 100% and LC50 = 623.52 and 300.63 ppm) and A. heterophylla (75% and 95% and LC50 = 826.03 and 384.71 ppm). On the other hand, the aqueous extract of A. heterophylla induced higher moralities (LC50 = 2819.85 ppm and 1652.50 ppm), followed by C. molmol, (LC50 = 3178.22 and 2322.53 ppm)49.
A similar larvicidal effect was recorded for Rosmarinus officinalis, hexane extract (80 and 160 ppm), reduced 100% mortality against 3rd and 4th instars larvae of Cx. pipiens and the toxicity increased in the pupal and adult stages50.
Out of 36 essential oils, red moor besom leaf oil has strong fumigation activity against Cx. pipiens pallens adults51. Similar to the adulticidal effect of the applied oils in this work, some other oils have adulticidal activities against mosquitoes as Cedrus deodara, Eucalyptus citriodora, Cymbopogon flexuous, Cymbopogon winterianus, Pinus roxburghii, S. aromaticum, and Tagetes minuta52. The Leaf Oils of Cinnamomum species had adulticidal activities against Ae. aegypti and Aedes albopictus53. EOs have adulticidal effects against Musca domestica54 as A. sativum, S. aromaticum, and F. vulgare55. Essential oils of Melaleuca leucadendron (L.) and Callistemon citrinus (Curtis) showed 100% adult mortality against Aedes aegypti (L.) and Cx. quinquefasciatus (Say), 24 h exposure56.
The results showed that A. sativum, and S. officinalis oils were effective against mosquito larvae, maybe due to the presence of a number of active secondary compounds such as ISOCHIAPIN B%2 < (sesquiterpene lactone) and 9-Octadecenamide, (Z)-that are anti-inflammatory activity57, also, Terpinen-4-ol and Camphor in Sage oil that these are excellent natural insecticide58, but these oils garlic and Sage did not show the required efficacy against adult mosquitoes.
The phytochemical analysis of this study revealed the major activated compounds of the analyzed oils. Green tea oil is a highly effective larvicide in this study contains a high amount of polyphenols that have antioxidant activity. A similar finding was reported59. Our data indicated that green tea oil also contains polyphenols as Gallic acid, Catechin, Methyl gallate, Coffeic acid, Coumaric acid, Naringenin, and Kaempferol which might aid in its insecticidal effect.
This study indicated that F. vulgare contains Estragole (70.36%) and Limonene (8.96%). Similarly, Limonene as a cyclic monoterpene has a viable insecticidal effect60. Besides, Estragole induced toxicity to adult fruit flies, Ceratitis capitata61. Moreover, An. graveolens contains thiosemicarbazone (32.13%) in this study. Likewise, thiosemicarbazide is a major component An. graveolens with insecticidal effect62. Also, Dauco and carotol are essential oils documented for An. graveolens in this work have repellent activity against adult Ae. aegypti, Ae. albopictus, and Anopheles quadrimaculatus Say63. Furthermore, V. odorata in the present analysis contains alpha-ionone, which revealed anti-inflammatory and analgesic effects64. Thymus vulgaris showed good alpha-pinene and pyridine derivatives that play an important role as larvicidal and adulticidal effects against Ae. aegypti and growth regulator, respectively65,66. In addition, the combination of all constituents may promote their individual larvicidal and adulticidal effects.
The biochemical compositions showed that T. vulgaris oil affected the energy reserves with a marked effect on proteins and lipids30. The differences between our findings and those of the others could be attributed to the biological activities and the chemical composition for EOs, which could vary between plant age, tissues, geographical origin, the part used in the distillation process, distillation type, and the species. Therefore, types and levels of active constituents in each oil may be responsible for the variability in their potential against pests16.
Conclusions
Diseases transmitted by mosquitoes represent global concerns. Our findings demonstrate the potential of F. vulgare and C. sinensis as the most potent larvicides and N. sativa, V. odorata, and An. graveolens as the most effective adulticides as they contain good command of different essential oils. EOs could be used for integrated mosquito control programs as larvicides or synergists for enhancing the efficacy of current adulticides31. Further studies are needed to develop nanoformulations that improve the efficacy and minimize applications after revealing their ecotoxicological side views.
Acknowledgements
This work was funded by the Science, Technology, Innovation Funding Authority, Egypt, entitled: “Lumpy Skin Disease in Cattle and Development of Sustainable Pest Management Tools”, Project ID: 37024.
Author contributions
Conceptualization, A.A., A.M. and M.B.; methodology, H.K., M.B., I.R.; validation, M.B., I.R. and A.A.; formal analysis, A.A. and H.K.; resources, A.A.; writing—original draft preparation, M.B., I.R., H.K. and A.A.; writing—review and editing, H.K., A.A., A.M. and A.S.; supervision, H.K.; project administration, A.S.; funding acquisition, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jones, R. T., Ant, T. H., Cameron, M. M. & Logan, J. G. Vol. 376 (The Royal Society, 2021). [DOI] [PMC free article] [PubMed]
- 2.Abdel-Shafi IR, et al. Mosquito identification and molecular xenomonitoring of lymphatic filariasis in selected endemic areas in Giza and Qualioubiya Governorates, Egypt. J. Egypt. Soc. Parasitol. 2016;46:93–100. doi: 10.12816/0026153. [DOI] [PubMed] [Google Scholar]
- 3.Selim, A., Radwan, A., Arnaout, F. & Khater, H. The recent update of the situation of west nile fever among equids in Egypt after three decades of missing information. Pakistan Veterinary J. 40 (2020).
- 4.Selim A, Megahed A, Kandeel S, Alouffi A, Almutairi MM. West Nile virus seroprevalence and associated risk factors among horses in Egypt. Sci. Rep. 2021;11:1–9. doi: 10.1038/s41598-021-00449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selim A, Radwan A. Seroprevalence and molecular characterization of West Nile Virus in Egypt. Compar. Immunol. Microbiol. Infectious Diseases. 2020;71:101473. doi: 10.1016/j.cimid.2020.101473. [DOI] [PubMed] [Google Scholar]
- 6.Jones, R. T., Ant, T. H., Cameron, M. M. & Logan, J. G. (The Royal Society, 2021).
- 7.Selim, A., Manaa, E., Abdelhady, A., Ben Said, M. & Sazmand, A. Serological and molecular surveys of Anaplasma spp. in Egyptian cattle reveal high A. marginale infection prevalence. [DOI] [PMC free article] [PubMed]
- 8.Selim A, et al. Seroprevalence and risk factors associated with Canine Leishmaniasis in Egypt. Veterinary Sci. 2021;8:236. doi: 10.3390/vetsci8100236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selim A, Megahed AA, Kandeel S, Abdelhady A. Risk factor analysis of bovine leukemia virus infection in dairy cattle in Egypt. Compar. Immunol. Microbiol. Infectious Diseases. 2020;72:101517. doi: 10.1016/j.cimid.2020.101517. [DOI] [PubMed] [Google Scholar]
- 10.Selim A, Abdelhady A. The first detection of anti-West Nile virus antibody in domestic ruminants in Egypt. Trop. Anim. Health Prod. 2020;52:3147–3151. doi: 10.1007/s11250-020-02339-x. [DOI] [PubMed] [Google Scholar]
- 11.Selim, A., Abdelhady, A. & Alahadeb, J. Prevalence and first molecular characterization of Ehrlichia canis in Egyptian dogs. Pak. Vet. J. (2020).
- 12.Khater HF, et al. Malaria. IntechOpen; 2019. [Google Scholar]
- 13.Baz, M. M. Strategies for mosquito control. PhD thesis, faculty of Science, Benha University, Egypt (2013).
- 14.Khater HF. Prospects of botanical biopesticides in insect pest management. Pharmacologia. 2012;3:641–656. doi: 10.5567/pharmacologia.2012.641.656. [DOI] [Google Scholar]
- 15.Khater HF. Bioactivity of essential oils as green biopesticides: Recent global scenario. Recent Progress Med. Plants. 2013;37:151–218. [Google Scholar]
- 16.Khan N, Mukhtar H. Tea and health: Studies in humans. Curr. Pharm. Des. 2013;19:6141–6147. doi: 10.2174/1381612811319340008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Govindarajan M, Rajeswary M, Hoti S, Bhattacharyya A, Benelli G. Eugenol, α-pinene and β-caryophyllene from Plectranthus barbatus essential oil as eco-friendly larvicides against malaria, dengue and Japanese encephalitis mosquito vectors. Parasitol. Res. 2016;115:807–815. doi: 10.1007/s00436-015-4809-0. [DOI] [PubMed] [Google Scholar]
- 18.Khater H, Geden C. Potential of essential oils to prevent fly strike by Lucilia sericata, and effects of oils on longevity of adult flies. J. Vector Ecol. 2018;43:261–270. doi: 10.1111/jvec.12310. [DOI] [PubMed] [Google Scholar]
- 19.Noutcha, M. A., Edwin-Wosu, N. I., Ogali, R. E. & Okiwelu, S. N. The role of plant essential oils in mosquito (Diptera: Culicidae) control. Annu. Res. Rev. Biol. 1–9 (2016).
- 20.WHO. Larval source management: A supplementary malaria vector control measure: An operational manual. (2013).
- 21.Vatandoost H, et al. Comparison of CDC bottle bioassay with WHO standard method for assessment susceptibility level of malaria vector, Anopheles stephensi to three imagicides. J. Arthropod. Borne Dis. 2019;13:17. [PMC free article] [PubMed] [Google Scholar]
- 22.Shafaie F, Aramideh S, Valizadegan O, Safaralizadeh MH, Pesyan NN. GC/MS analysis of the essential oils of Cupressus arizonica Greene, Juniperus communis L. and Mentha longifolia L. Bull. Chem. Soc. Ethiopia. 2019;33:389–400. doi: 10.4314/bcse.v33i3.1. [DOI] [Google Scholar]
- 23.Modise SA, Ashafa AOT. Larvicidal, pupicidal and insecticidal activities of Cosmos bipinnatus, Foeniculum vulgare and Tagetes minuta against Culex quinquefasciatus mosquitoes. Trop. J. Pharm. Res. 2016;15:965–972. doi: 10.4314/tjpr.v15i5.10. [DOI] [Google Scholar]
- 24.Pavela R, Žabka M, Bednář J, Tříska J, Vrchotová N. New knowledge for yield, composition and insecticidal activity of essential oils obtained from the aerial parts or seeds of fennel (Foeniculum vulgare Mill.) Ind. Crops Products. 2016;83:275–282. doi: 10.1016/j.indcrop.2015.11.090. [DOI] [Google Scholar]
- 25.Rocha DK, et al. Larvicidal activity against Aedes aegypti of Foeniculum vulgare essential oils from Portugal and Cape Verde. Nat. Product Commun. 2015;10:1934578X1501000438. [PubMed] [Google Scholar]
- 26.Hassan MI, Atwa WA, Moselhy WA, Mahmoud DA. Efficacy of the green tea, Camellia sinensis leaves extract on some biological activities of Culex pipiens and the detection of its phytochemical constituents. Egypt. Acad. J. Biol. Sci. F. Toxicol. Pest Control. 2020;12:59–70. doi: 10.21608/eajbsf.2020.78354. [DOI] [Google Scholar]
- 27.Muema JM, Bargul JL, Nyanjom SG, Mutunga JM, Njeru SN. Potential of Camellia sinensis proanthocyanidins-rich fraction for controlling malaria mosquito populations through disruption of larval development. Parasit. Vectors. 2016;9:1–10. doi: 10.1186/s13071-016-1789-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pavela R. Larvicidal property of essential oils against Culex quinquefasciatus Say (Diptera: Culicidae) Ind. Crops Prod. 2009;30:311–315. doi: 10.1016/j.indcrop.2009.06.005. [DOI] [Google Scholar]
- 29.de Oliveira AA, et al. Larvicidal, adulticidal and repellent activities against Aedes aegypti L. of two commonly used spices, Origanum vulgare L. and Thymus vulgaris L. S. Afr. J. Bot. 2021;140:17–24. doi: 10.1016/j.sajb.2021.03.005. [DOI] [Google Scholar]
- 30.Bouguerra N, Tine-Djebbar F, Soltani N. Effect of Thymus vulgaris L. (Lamiales: Lamiaceae) essential oil on energy reserves and biomarkers in Culex pipiens L. (Diptera: Culicidae) from Tebessa (Algeria) J. Essential Oil Bearing Plants. 2018;21:1082–1095. doi: 10.1080/0972060X.2018.1504696. [DOI] [Google Scholar]
- 31.Sheng Z, et al. Screening of larvicidal activity of 53 essential oils and their synergistic effect for the improvement of deltamethrin efficacy against Aedes albopictus. Ind. Crops Products. 2020;145:112131. doi: 10.1016/j.indcrop.2020.112131. [DOI] [Google Scholar]
- 32.Alkenani NA, et al. Molecular identification and bio-control of mosquitoes using black seeds extract in Jeddah. Pak. Vet. J. 2021 doi: 10.29261/pakvetj/2021.025. [DOI] [Google Scholar]
- 33.Farag M. Larvicidal and repellent potential of Sesamum indicum hull peels extracts against Culex pipiens L. (Diptera: Culicidae) Egypt. J. Aquat. Biol. Fisheries. 2021;25:995–1011. doi: 10.21608/ejabf.2021.170345. [DOI] [Google Scholar]
- 34.Abd El Meguid AD, Mahmoud SH, Baz MM. Toxicological activity of four plant oils against Aedes caspius and Culex pipiens (Diptera: Culicidae) Int. J. Mosq. Res. 2019;6:86–94. [Google Scholar]
- 35.El Ouali Lalami A, El-Akhal F, Ez Zoubi Y, Taghzouti K. Study of phytochemical screening and larvicidal efficacy of ehtanolic extract of Salvia officinalis (Lamiaceae) from North Center of Morocco against Culex pipiens (Diptera: Culicidae) vector of serious human diseases. Int. J. Pharmacog. Phytochem. Res. 2016;8:1663–1668. [Google Scholar]
- 36.Hayouni EA, et al. Tunisian Salvia officinalis L. and Schinus molle L. essential oils: Their chemical compositions and their preservative effects against Salmonella inoculated in minced beef meat. Int. J. Food Microbiol. 2008;125:242–251. doi: 10.1016/j.ijfoodmicro.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Nabti I, Bounechada M. Larvicidal activities of essential oils extracted from five Algerian medicinal plants against Culiseta longiareolata Macquart. Larvae (Diptera: Culicidae) Eur. J. Biol. 2019;78:133–138. [Google Scholar]
- 38.Chantawee A, Soonwera M. Larvicidal, pupicidal and oviposition deterrent activities of essential oils from Umbelliferae plants against house fly Musca domestica. Asian Pac. J. Trop. Med. 2018;11:621. doi: 10.4103/1995-7645.246338. [DOI] [Google Scholar]
- 39.Belong P, Ntonga PA, Fils E, Dadji GAF, Tamesse JL. Chemical composition and residue activities of Ocimum canum Sims and Ocimum basilicum L. essential oils on adult female Anopheles funestus. J. Anim. Plant Sci. 2013;19:2854–2863. [Google Scholar]
- 40.El Zayyat EA, Soliman MI, Elleboudy NA, Ofaa SE. Bioefficacy of some Egyptian aromatic plants on Culex pipiens (Diptera: Culicidae) adults and larvae. J. Arthropod. Borne Dis. 2017;11:147. [PMC free article] [PubMed] [Google Scholar]
- 41.Muturi EJ, Ramirez JL, Zilkowski B, Flor-Weiler LB, Rooney AP. Ovicidal and larvicidal effects of garlic and asafoetida essential oils against West Nile virus vectors. J. Insect Sci. 2018;18:43. doi: 10.1093/jisesa/iey036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alerwi ST, et al. Molecular identification and bio-control of Culex quinquefasciatus from Yanbu region. J. Entomol. Zool. Stud. 2019;7:1081–1086. [Google Scholar]
- 43.Matiadis D, et al. Curcumin derivatives as potential mosquito larvicidal agents against two mosquito vectors, Culex pipiens and Aedes albopictus. Int. J. Mol. Sci. 2021;22:8915. doi: 10.3390/ijms22168915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prak, J.-W., Yoo, D.-H., Kim, H. K., Koo, H.-N. & Kim, G.-H. in 2014 Larvicidal and repellent activities of 33 plant extracts against two mosquitoes as Culex pipiens and Aedes albopictus. 181–181.
- 45.Jabbar, A., Tariq, M., Gulzar, A., Mukhtar, T. & Zainab, T. Lethal and sub lethal effects of plant extracts and green silver nanoparticles against Culex pipiens. (2021).
- 46.Khater HF. Biocontrol of Some Insects. Benha University; 2003. [Google Scholar]
- 47.Baz MM, Hegazy MM, Khater HF, El-Sayed YA. Comparative evaluation of five oil-resin plant extracts against the mosquito larvae, Culex pipiens Say (Diptera: Culicidae) Pak. Vet. J. 2021 doi: 10.29261/pakvetj. [DOI] [Google Scholar]
- 48.Khater HF, Shalaby AA-S. Potential of biologically active plant oils to control mosquito larvae (Culex pipiens, Diptera: Culicidae) from an Egyptian locality. Rev. Inst. Med. Trop. Sao Paulo. 2008;50:107–112. doi: 10.1590/S0036-46652008000200008. [DOI] [PubMed] [Google Scholar]
- 49.Baz MM, Hegazy MM, Khater HF, El-Sayed YA. Comparative evaluation of five oil-resin plant extracts against the mosquito larvae, Culex pipiens Say (Diptera: Culicidae) Pak. Vet. J. 2021;41:191–196. [Google Scholar]
- 50.Shalaby A, Khater H. Toxicity of certain solvent extracts of Rosmarinus officinalis against Culex pipiens larvae. J. Egypt. German Soc. Zool. E. 2005;48:69–80. [Google Scholar]
- 51.Chen W, Wu H, Ma Z, Feng J, Zhang X. Evaluation of fumigation activity of thirty-six essential oils against Culex pipiens pallens (Diptera: Culicidae) Acta Entomol. Sin. 2018;61:86–93. [Google Scholar]
- 52.Makhaik, M., Naik, S. N. & Tewary, D. K. Evaluation of anti-mosquito properties of essential oils. (2005).
- 53.Jantan IB, Yalvema MF, Ahmad NW, Jamal JA. Insecticidal activities of the leaf oils of eight cinnamomum species against Aedes aegypti and Aedes albopictus. Pharm. Biol. 2005;43:526–532. doi: 10.1080/13880200500220771. [DOI] [Google Scholar]
- 54.Khater HF, Geden CJ. Efficacy and repellency of some essential oils and their blends against larval and adult house flies, Musca domestica L. (Diptera: Muscidae) J. Vector Ecol. 2019;44:256–263. doi: 10.1111/jvec.12357. [DOI] [PubMed] [Google Scholar]
- 55.Levchenko, M. A., Silivanova, E. A., Khodakov, P. E. & Gholizadeh, S. Insecticidal efficacy of some essential oils against adults of Musca domestica L. (Diptera: Muscidae). Int. J. Trop. Insect Sci. 1–9 (2021).
- 56.Pushpalatha E, Viswan KA. Adulticidal and repellent activities of Melaleuca leucadendron (L.) and Callistemon citrinus (Curtis) against filarial and dengue vectors. Assoc. Advancement Entomol. 2013;38:149–154. [Google Scholar]
- 57.Sahi NM. Evaluation of insecticidal activity of bioactive compounds from Eucalyptus citriodora against Tribolium castaneum. Int. J. Pharm. Phytochem. Res. 2016;8:1256–1270. [Google Scholar]
- 58.Fu J, et al. Fumigant toxicity and repellence activity of camphor essential oil from Cinnamonum camphora Siebold against Solenopsis invicta workers (Hymenoptera: Formicidae) J. Insect Sci. 2015;15:129. doi: 10.1093/jisesa/iev112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zulhussnain M, et al. Insecticidal and Genotoxic effects of some indigenous plant extracts in Culex quinquefasciatus Say Mosquitoes. Sci. Rep. 2020;10:1–13. doi: 10.1038/s41598-020-63815-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sutthanont N, et al. Chemical composition and larvicidal activity of edible plant-derived essential oils against the pyrethroid-susceptible and-resistant strains of Aedes aegypti (Diptera: Culicidae) J. Vector Ecol. 2010;35:106–115. doi: 10.1111/j.1948-7134.2010.00066.x. [DOI] [PubMed] [Google Scholar]
- 61.Ling Chang C, Kyu Cho I, Li QX. Insecticidal activity of basil oil, trans-anethole, estragole, and linalool to adult fruit flies of Ceratitis capitata, Bactrocera dorsalis, and Bactrocera cucurbitae. J. Econ. Entomol. 2009;102:203–209. doi: 10.1603/029.102.0129. [DOI] [PubMed] [Google Scholar]
- 62.da Silva JBP, et al. Thiosemicarbazones as Aedes aegypti larvicidal. Eur. J. Med. Chem. 2015;100:162–175. doi: 10.1016/j.ejmech.2015.04.061. [DOI] [PubMed] [Google Scholar]
- 63.Ali A, Radwan MM, Wanas AS, Khan IA. Repellent activity of carrot seed essential oil and its pure compound, carotol, against mosquitoes. J. Am. Mosq. Control Assoc. 2018;34:272–280. doi: 10.2987/18-6751.1. [DOI] [PubMed] [Google Scholar]
- 64.Branquinho LS, et al. Anti-inflammatory and toxicological evaluation of essential oil from Piper glabratum leaves. J. Ethnopharmacol. 2017;198:372–378. doi: 10.1016/j.jep.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 65.Sarma R, Adhikari K, Mahanta S, Khanikor B. Combinations of plant essential oil based terpene compounds as larvicidal and adulticidal agent against Aedes aegypti (Diptera: Culicidae) Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-019-45908-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gad M, Aref S, Abdelhamid A, Elwassimy M, Abdel-Raheem S. Biologically active organic compounds as insect growth regulators (IGRs): Introduction, mode of action, and some synthetic methods. Curr. Chem. Lett. 2021;10:393–412. doi: 10.5267/j.ccl.2021.5.004. [DOI] [Google Scholar]