Abstract
With an increasing human population access to ruminant products is an important factor in global food supply. While ruminants contribute to climate change, climate change could also affect ruminant production. Here we investigated how the plant response to climate change affects forage quality and subsequent rumen fermentation. Models of near future climate change (2050) predict increases in temperature, CO2, precipitation and altered weather systems which will produce stress responses in field crops. We hypothesised that pre-exposure to altered climate conditions causes compositional changes and also primes plant cells such that their post-ingestion metabolic response to the rumen is altered. This “stress memory” effect was investigated by screening ten forage grass varieties in five differing climate scenarios, including current climate (2020), future climate (2050), or future climate plus flooding, drought or heat shock. While varietal differences in fermentation were detected in terms of gas production, there was little effect of elevated temperature or CO2 compared with controls (2020). All varieties consistently showed decreased digestibility linked to decreased methane production as a result of drought or an acute flood treatment. These results indicate that efforts to breed future forage varieties should target tolerance of acute stress rather than long term climate.
Subject terms: Plant stress responses, Applied microbiology
Introduction
Forage crops for livestock are essential for ruminant production, with grazing land accounting for approximately 60% of global agriculture land1. Continued human population growth predicts an increase to 9.7 billion by 2050, which will lead to an increase in demand for animal products together with pressure to decrease pollutant output. Increases in ruminant production have been achieved to date through continual improvement of the forage feed germplasm focusing mainly on traits such as yield and digestibility2. It can take over 10 years from breeding to release of a new variety and so current varieties are tailored to perform well under current environmental conditions. However, it is recognised that climate is changing; climate change models of the near future (2050) predict increases in temperature of 4–6 °C in the UK, atmospheric CO2 from 400 to 500 ppm3, increases in precipitation (up to 33% more), and altered weather systems (e.g. extreme drought and flooding4). The forage varieties currently used in UK are adapted to current conditions. However, growth under elevated temperature, drought or flood can induce stress responses in the grass5 that could affect not just production but also composition, and thereby nutritive value to ruminants; for instance forage quality has been shown to declines with rising temperatures6. Therefore, to secure future productivity of livestock the development of new forage varieties should take account of the environmental effects on grass production and quality parameters.
Ruminants have the ability to convert fibrous feed unable to be utilised by humans, into easily digestible meat and milk products7,8 due to the presence of a complex rumen microbiome9. However, perturbation of the rumen can be brought about by diet and diet based effects have been observed on both the core microbial community10 and overall rumen microbial community11–14. Previous research has indicated improvement of rumen efficiency could be achieved by manipulating animal diet, improving host-microbial interactions and plant microbial interactions to maximise productivity whilst reducing environmental costs15.
Ingested conserved forages are broken down in the rumen by the action of enzymes from the attached rumen microbiota, but when fresh forage is ingested in addition to microbial activity the possibility exists for a contribution to feed degradation from plant metabolism caused by initiation of innate plant stress responses16–19. When fresh forage is fed to ruminants the cells are still metabolically active, and respond to the conditions of the rumen, including by inducing plant hormone-mediated stress responses resulting in autolytic breakdown20. These post-ingestion changes in plant-based metabolism have the potential to alter substrate composition and as a consequence could affect fermentation profiles in forages with apparently similar chemical composition2. It is currently unclear what the relative contributions of plant and microbial proteolysis to overall rumen function are. However, the possibility that there is a contribution from the plant cells offers an exciting opportunity to exploit natural variation in endogenous plant proteolysis. Selecting for grass varieties that have slow rates of endogenous proteolysis might at least partially mitigate against environmental pollution21,22. However, evidence is limited on the effect of climate change scenarios on the fermentation characteristics of plants which have been challenged by these stresses.
Plants respond to abiotic and biotic stresses by physiological, biochemical, metabolic and molecular mechanisms23. Previous studies have focussed on the characterisation of physiological changes of forage plant varieties under climate change conditions. For example, elevated levels of CO2 have been shown to improve the rate of photosynthesis in plants24 and alleviate the effects of drought stress by conserving water, increasing carbon fixation and increasing fructan accumulation25. However high temperatures have been shown to inhibit photosynthesis26 by altering the structure of chloroplasts and by inactivating chloroplast enzymes through oxidative stress27. Drought stress has also been shown to affect water soluble carbohydrate (WSC) levels. Concentration of fructans in forage grasses had a higher degree of polymerisation in drought than in control conditions, although the increase in WSC reserves to the level of 40 to 50% at the end of drought were not thought to improve drought tolerance in the plants overall28. Previous studies have also demonstrated that drought can decrease the protein concentration due to an increase in protein degradation, a decrease in nitrogen assimilation and a decrease in protein synthesis29. During flooding oxidative pathways within the plant move towards fermentation30 and CO2 levels within plants can become reduced. This leads to a decreased electron sink for light energy and, potentially, oxidative damage of plant cells31 from excess light energy not used in CO2 fixation32. In flooding scenarios where both root and shoot are submerged, aerobic respiration and photosynthesis is reduced due to low light levels and limited gaseous exchange leading to an energy crisis within the plant33.
Plants have the ability to “remember” past occurrences to adapt to new environments34. Pre-exposure to environmental stresses primes the plant cells such that the subsequent response is altered35. This stress memory is now understood to be a key factor determining progression of microbial disease in crops mediated by salicylic acid36 and abiotic responses to temperature37. Salicylic acid has been implicated in the response of plant cells to the rumen20, and thus, pre-exposure of forage plants to stress during growth may prime the cells, such that the stress response is altered once ingested and in the rumen19. Hence an active response may have important effects on rumen fermentation efficiency and subsequently host nutrient uptake in addition to direct effects of climate on the chemical composition of the forage.
Despite extensive study of responses of crop and forage species to adverse environmental conditions, there has been relatively little research into how forage adaptation to climate change will affect ruminant production systems, and the appropriateness of current and future forage varieties for animal production in the future. The aim of this work was therefore to determine how responses to the growth environment, including “stress memory” may affect the subsequent forage degradation in the rumen and, therefore, to understand how ruminant feeding strategies might be affected by climate change.
Materials and methods
Plant material
Ten different current commercial varieties of forage grasses (Table 1) were grown for 3 months in five different climate scenarios, replicated three times at 1-week intervals. Studies on these plant materials were undertaken in compliance with local and national regulations. Climate conditions were simulated in growth chambers and consisted of present-day conditions and future potential climate scenarios as described by Ref.38 (Table 2).
Table 1.
Forage grass varieties.
| Forage grass | Type |
|---|---|
| AberClyde | Tetraploid perennial ryegrass, high sugar (Lolium perenne) |
| AberDart | Diploid perennial ryegrass, high sugar (Lolium perenne) |
| AberEcho | Tetraploid hybrid ryegrass, high sugar (Lolium x boucheanum) |
| AberGlyn | Tetraploid perennial ryegrass (Lolium perenne) |
| AberNiche | Tetraploid festulolium (Italian ryegrass × meadow fescue) (Lolium multiflorum × Festuca pratense) |
| AberRoot | Perennial tetraploid festulolium, high sugar (perennial ryegrass × Atlas fescue) (Lolium perenne × Festuca mairei) |
| AberZeus | Diploid perennial ryegrass, high sugar (Lolium perenne) |
| Barolex | Allohexaploid, tall fescue (Festuca arundinacea) |
| Davinci | Diploid Italian ryegrass (Lolium multiflorum) |
| Premium | Diploid perennial ryegrass (Lolium perenne) |
Table 2.
Climate conditions.
| Climate condition | Description | |
|---|---|---|
| 1 | Control 2020 | 400 ppm CO2, 16–18 °C night/day temperature for an 8-h photoperiod and watered regularly |
| 2 | Control 2050 | 500 ppm CO2, 21–23 °C night/day temperature for an 8-h photoperiod and watered regularly |
| 3 | Flood | As for control 2050 but flooded for 1 week prior to harvesting |
| 4 | Drought | As for control 2050 but no water for 1 week prior to harvesting |
| 5 | Heat Shock | As for control 2050 but temperature increased to 35 °C 2 days prior to harvesting |
Forage characterisation
For each climate scenario, after the 3-month growth period and before harvest, leaves of each variety were analysed for Fv/Fm (Handy PEA, Hansatech Instruments, UK) to determine photochemical efficiency of each plant in each environment39. Chlorophyll content was determined by the addition of 80% acetone to 1 g of fresh plant tissue, which was placed in the dark for 1 h. The sample was then centrifuged for 5 min at 15,000×g and the absorbance at λ = 663 nm (A663) and 645 nm (A645) of the supernatant was determined spectrophotometrically (Pharmacia Biotech). Chlorophyll content was calculated from absorbencies according to Ref.40 and also used as proxy for stress response. Plant dry matter and crude protein were determined according to methods 930.15 and 968.06 respectively (AOAC41), and water-soluble carbohydrates (WSC) spectrophotometrically using the anthrone method42. Dried plant material was analysed sequentially for neutral detergent fibre (NDF), acid detergent fibre (ADF) and acid detergent lignin (ADL)43,44 by using a fibre analyser (ANKOM). Leaf samples of each grass were also freeze dried and ground to a fine powder in a ball mill (MM 30, Retsch Gmbh, Haan,Germany) at speed 30 for 2 min with the inclusion of 2 tungsten beads previously washed in acetone. Ground leaf material was subjected to Fourier transform infrared spectroscopy (FTIR) (Equinox 55 HTS-XT FTIR Spectrophotometer, Bruker UK Ltd, Coventry, UK). This enabled non-targeted analysis (profiling) of biochemical changes within the plant tissue during growth conditions.
In vitro batch fermentation
Collection and preparation of microbial inoculum
All experiments involving animals were performed in accordance with relevant guidelines and regulations. Experimentation was conducted under the authority of licenses under the U.K. Animals (Scientific Procedures) Act, 1986. Experimentation was approved by the Aberystwyth University Animal Welfare and Ethical Review Body (AWERB). Methods are reported here in accordance with Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines (https://arriveguidelines.org). Rumen fluid was collected on the morning of each plant harvest from each of four non-lactating Holstein–Friesian dairy cows which had been previously prepared with rumen cannulae (Bar Diamond, Parma, ID), and fed ad-lib perennial ryegrass (L. perenne). The rumen samples were mixed and filtered through two layers of muslin under a CO2 stream and the filtrate used to prepare a 10% rumen fluid inoculum in anaerobic buffer44.
Analysis of gas production
Each gas production run consisted of triplicate samples from each of 50 treatments with the addition of inoculum blanks and was repeated over three consecutive weeks. On the day prior to harvest a subsample of each plant was dried to a constant weight to determine DM. Fresh leaves from grass varieties grown in each climate scenario (n = 50) were cut into approx. 1 cm lengths and accurately weighed in triplicate into 140 mL serum bottles to supply fresh matter equivalent to 1 g DM, which were then filled with CO2 prior to and during the addition of 100 mL 10% rumen fluid inoculum. The bottles were back filled with CO2 and sealed with butyl rubber bungs. Bottles were then placed in an incubator at 39 °C in the dark. Measurements of total gas production, CO2 and methane were taken at time intervals of 0, 4, 8, 12, 18, 24, 30 and 48 h. For each sampling point, the head space of the bottles was collected and measured into a 50 mL syringe, informed by a pressure transducer (Bailey and Mackey Ltd, Birmingham, UK)45. The gas composition was analysed by an IRGA (Infra-Red Gas Analyser, ADC, Bioscientific Ltd, UK)45. At the end of the time course (48 h) the contents of the serum bottles were strained, and the remaining grass solid constituents were flash frozen in liquid nitrogen for FTIR analysis and following further freeze drying for dry matter degradation. The pH of the rumen inoculum was measured for each bottle and two aliquots of 1 mL rumen inoculum was sampled for measurements of volatile fatty acid (VFA) and ammonia levels. Samples of the rumen inoculum were also used for end point FTIR analysis in order to explore differences in metabolite production due to the fermentation of the forages incubated at a system level.
Volatile organic compound (VOC) analysis
Non-targeted analysis of volatile organic compounds (VOCs) was used to assess entire gaseous fermentation profiles in addition to targeted measurements of the major volatile fatty acids produced by rumen fermentation (acetate, butyrate, propionate). VOCs were collected from the head space of the serum bottles 24 h after the start of in vitro fermentation using inert coated SafeLok tubes for Odour/Sulphur analysis (Markes International, Llantrisant, UK) fitted to the IRGA. Tubes were dry purged prior to sample desorption for 1 min with 20 mL/min nitrogen at room temperature. Tube desorption (primary desorption) was conducted by thermal desorption using a TD 100 (Markes International, Llantrisant, UK) for 10 min with 40 mL/min at 280 °C to trap at 25 °C. Trap desorption was then conducted for 6 min with 6.5 mL/min helium at 300 °C (max heating rate) 1.5 mL/min onto the column (split ratio 1:4.3). Samples were then re-collected onto sample tubes. Gas Chromatography (GC 7890A, Agilent Technologies) was conducted initially at 40 °C for 4 min followed by 4 °C/min to 250 °C then 20 °C/min to 300 °C and final hold for 2 min (62 min total). Separation was done over 60 m × 0.32 mm × 0.5 mm MEGA-5 MS with 1.5 mL/min helium under a constant flow condition. Mass spectrometry was carried out using a BenchTOFdx (Markes International, Llantrisant, UK) in EI + mode. Spectra were recorded from m/z 35 to 450 (at 1970 scans/scanset) with a transfer line temperature 250 °C and an ion source temperature of 250 °C. A retention time standard (C8–C20, Sigma Aldrich) was used, injecting 1 μL onto a collection tube (Tenax TA, Sulficarb) and analysed as described for the samples.
Chemical analysis
Samples for VFA analysis were acidified by the addition of 4% orthophosphoric acid (final concentration)46 and analysed by gas chromatography with 4 mM ethylbutyric acid as an internal standard47. Ammonia was analysed from end point samples by acidifying with the addition of concentrated hydrochloric acid (5% v/v final concentration)48. Aliquots of incubation buffer and rumen inoculum were analysed by FTIR (Equinox 55 HTS-XT FTIR Spectrophotometer, Bruker UK Ltd, Coventry, UK), where 10 μL of sample was spotted onto a 96 well silicone plate dried to 40 °C before scanning. Remaining plant material was treated as with initial plant material prior to in vitro batch fermentation. All samples were analysed individually with 10% of the samples analysed again for quality control purposes. The FTIR spectra obtained were converted to XY data. The generation of XY data matrices from FTIR analysis were exported in ASCII format. The spectra were mined49 and data were examined using principal components analysis (PCA) using Pychem software50.
Grass physiological characteristics and gas production analyses
Data analysis was conducted in R version 3.6.0 using RStudio51,52. Packages agricolae53, car54, ggplot255, lsmeans56 and rcompanion57 were used. All data were analysed using 2-way ANOVA, further using Tukey mean separation, with the exception of the gas production data. These were analysed using general linear models; in each case, the response was log-transformed to meet parametric assumptions, and the predictors were grass variety, climate scenario, the variety-climate interaction, starting mass of dry matter (DM) and biological replicate number. Pairwise comparisons were performed using least-squares means with Tukey adjustment. Biological replicate number was included in the model to account for non-independence between samples taken on the same date. It was also necessary to include the starting mass of dry matter, as this inevitably varied slightly between samples. The relationship between dry matter and gas production changes over time, such that simply expressing the data as ml gas/g DM does not accurately account for the correlation as fermentation progresses. Instead, using dry matter as an independent variable allows the model to estimate and control for the effect of dry matter at the time of sampling (48 h). The model predictions were obtained for all samples based on 1 g DM.
Analysis of VOC data
Initial deconvolution and peak identification were carried out in AMDIS v2.72, using the NIST database v2.2 (2014). A custom compound library was built using a training dataset of one sample from every treatment-variety combination, distributed across biological replicates. Components were included in the library if they hit against the database (score > 80) and a retention index (RI) ± 30 the database value. Where the top compound produced a convincing spectrum match but fell outside the RI range, the component was added to the library and named by chemical group, e.g. Acid1. All samples were then run against this library with settings as recommended by Ref.58. The AMDIS results were validated and backfilled in Gavin 3.9759 using the parameters given in Table S1; raw outputs from Gavin are given in Table S2. All data analysis was carried out in R version 3.5.2 ‘Eggshell Igloo’ using RStudio51,52 and packages metacoder60,61, mvabund62, randomForest63 and vegan64. R code to reproduce the analyses is available as a Rmarkdown file at https://github.com/ecologysarah/lolium-rumen-voc65. Known contaminant compounds were removed from the peak table, along with compounds corresponding to those present in machine blanks and any compounds that did not occur in all three replicates of at least one treatment-variety combination. Peaks were normalised to percentage area per sample, to account for differences in intensity. Normalised peak data was log10 transformed to meet parametric assumptions, and modelled using a multivariate linear model (manylm62 with grass variety, climate scenario, biological replicate number and the variety-climate scenario interaction as predictors. Pairwise comparisons were conducted by splitting the data into subsets, running manylm on each one, and adjusting the P values with the Benjamini–Hochberg correction66. Data were visualised by organising the compounds into hierarchical groups and plotting heat trees60. Random Forest™ analysis was undertaken to assess classification of data by variety, treatment or experiment, and to identify which compounds were most informative for classification67. The proximity scores created in the process were used as a distance matrix for multi-dimensional scaling (MDS) to plot the outputs. For each forest, the 25 compounds that contributed the largest mean decrease in accuracy when excluded) with a z-ratio ≥ 7 were extracted from the data and used to create a new random forest.
Results
Effect of growth conditions on physiological characteristics of grass varieties at harvest
Overall, the grass varieties were clearly affected by the climate scenarios imposed during growth, indicating that the extent of the stress imposed was sufficient to elicit a physiological response (Fig. S1). Mean biomass values for grasses in each climate scenario (control 2020, control 2050, 2050 + flood, 2050 + drought and 2050 + heat shock) were 14.4 g, 10.2 g, 8.6 g, 6.0 g and 5.8 g DM respectively (SD = 3.61). There was no significant effect of grass variety on Fv/Fm ratio (P = 0.328), chlorophyll (P = 0.118) or crude protein (P = 0.702), with mean values of 0.79 (Fv/Fm), 8.86 ug/mL and 23.8% respectively (Supplementary Table S1). However, there was a significant effect of grass variety on water-soluble carbohydrate (WSC) (P = 0.030), with Aber Niche having a higher (P = 0.031) WSC compared to Aber Root, although there were no differences amongst any of the other grass varieties. The fibre values for NDF, ADF and ADL varied (P < 0.001) between grass varieties, however the mean values were 38.7%, 15.3% and 2.2% respectively (Table S1). Taking all the varieties together, there was an effect (P < 0.05) of climate scenario on crude protein, WSC, chlorophyll, Fv/Fm ratio, NDF, ADF and ADL (Table 3).
Table 3.
Mean values for dry matter% (DM) crude protein (as a % of DM), water soluble carbohydrate (WSC as a % of DM), chlorophyll content, Fv/Fm ratio NDF, ADF and ADL in each climate scenario.
| Analysis | 2020 | 2050 | 2050 + flood | 2050 + drought | 2050 + heat shock | S.D. |
|---|---|---|---|---|---|---|
| Fv/Fm | 0.81b | 0.81b | 0.80ab | 0.78ab | 0.76a | 0.05 |
| Chlorophyll | 11.12c | 9.08ab | 6.19a | 10.05bc | 7.84ab | 2.90 |
| WSC %DM | 4.24b | 3.04ab | 2.57a | 3.52b | 3.64b | 1.17 |
| Crude protein% DM | 23.2ab | 22.5a | 21.6a | 26.7b | 25.0ab | 3.72 |
| Dry matter% | 15.6bc | 13.1ab | 10.2a | 18.3c | 15.7bc | 3.42 |
| NDF %DM | 36.0a | 40.0b | 44.1c | 37.4a | 37.5a | 4.5 |
| ADF %DM | 12.8a | 16.4d | 18.0e | 15.0c | 14.4b | 3.6 |
| ADL %DM | 2.3c | 2.4d | 3.3e | 1.2a | 2.0b | 1.2 |
Lower case letters indicate significant difference based on ls means with Tukey adjustment (P < 0.05).
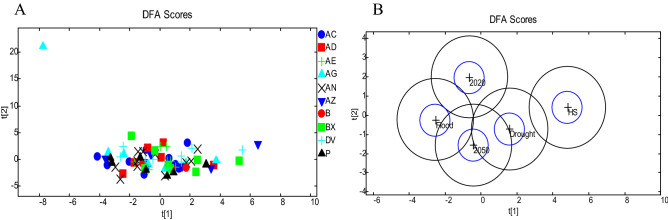
Discriminant functional analysis (DFA) of Fourier Transform infra-red spectroscopy (FTIR) spectra to determine differences in the plant chemistry at harvest showed no clustering by grass variety. However, clustering was observed for climate scenario, with heat shock FTIR spectra differing from all remaining scenarios except drought (Fig. 1).
Figure 1.
DFA analysis of grass variety based on FTIR analysis, showing no distinct clustering between varieties (AC Aber Clyde, AD Aber Dart, AE Aber Echo, AG Aber Glyn, AN Aber Niche, AZ Aber Zeus, B Barolex, BX Aber Root, DV Da vinci, P Premium (A); climate scenarios (B), showing distinct clustering between environmental conditions (DFA circles around the mean group centres with 95% confidence circles for DF1 vs DF2; HS heat shock).
Cumulative gas production
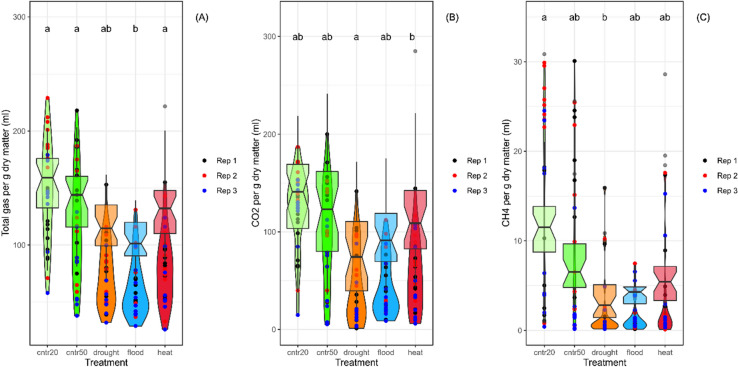
Total cumulative gas production measurements were taken 48 h from the start of the in vitro rumen fermentation experiments (Fig. 2A): a greater amount of gas produced during the 48 h indicates more extensive fermentation. There was a significant effect of grass variety (P = 0.041) on gas production, driven by lower gas production in the Aber Zeus samples (Fig S2). There was a significant effect of climate on gas production (P = 0.002), and pairwise comparisons showed that the flooding treatment produced significantly less gas than the 2020 controls (P = 0.014), 2050 controls (P = 0.038) and the heat shock treatment (P = 0.016) (Fig. 2A). There was no significant interaction between variety and climate scenario.
Figure 2.
Effect of climate scenario on total gas production (A), total CO2 production (B) and total methane production (C) at 48 h fermentation as affected by climate change scenario (all corrected for starting weight of dry matter); HS heat shock). Raw data points are overlaid on boxplots, coloured by replicate experiment. Notches represent 95% confidence intervals. Lower case letters indicate significant difference based on ls means with Tukey adjustment (P < 0.05).
There was no effect of grass variety on CO2 production (P = 0.114), nor any significant interaction between variety and climate scenario. However different climate scenarios did result in differences (P = 0.016) in CO2 production which was lowest in the drought treatment compared to heat shock (Fig. 2B). Methane (CH4) production during in vitro fermentation showed no effect due to grass variety (P = 0.111), nor any significant interaction between variety and climate scenario. However, growth under different climate scenarios did have an effect on methane production (P = 0.016; Fig. 2C), which was again lowest in the drought scenario compared to control 2020.
In vitro rumen fermentation parameters
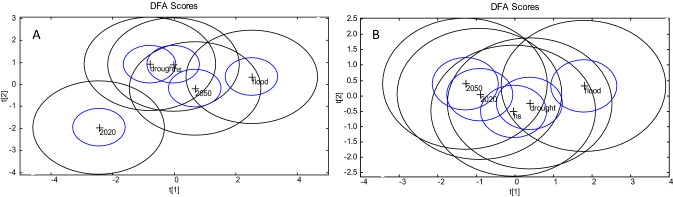
FTIR analysis at the end fermentation after 48 h showed a separation of clusters for the liquid fraction with the flood treatment resulting in differentiation from 2020 climate condition (Fig. 3A). However, there was no differentiation of clusters within the pellet (Fig. 3B).
Figure 3.
DFA analysis of liquid (A) and pellet (B) FTIR as affected for each climate scenario at the end of 48-h fermentation (DFA circles around the mean group centres with 95% confidence circles for DF1 vs DF2; HS heat shock).
There was no effect of grass variety on total VFA production (P = 0.401), ammonia production (P = 0.580) or pH (P = 0.700) with mean values of 251 mM/g DM, 43 mg/g DM and 6.8 respectively. However, climate change scenario affected fermentation profiles of all the parameters analysed (P < 0.001; Table 4).
Table 4.
Fermentation profile values for VFA, ammonia and pH end point analysis.
| 2020 | 2050 | 2050 + flood | 2050 + drought | 2050 + heat shock | S.D. | |
|---|---|---|---|---|---|---|
| DMD g/100 g | 77.5a | 74.2ab | 55.4c | 54.6c | 63.3bc | 18.0 |
| VFA mM/g DM | 315a | 284ab | 229bc | 202c | 227bc | 51.6 |
| Ammonia mg/g DM | 28b | 32b | 52a | 51a | 53a | 26.6 |
| pH | 6.7a | 6.8a | 6.9b | 6.9b | 6.9b | 0.11 |
| Acetate mM/g DM | 174a | 166a | 148a | 109b | 145a | 26.4 |
| Propionate mM/g DM | 66a | 58ab | 40c | 41c | 43bc | 15.6 |
| Isobutyrate mM/g DM | 5a | 4ab | 3b | 3b | 3b | 1.0 |
| Butyrate mM/g DM | 47a | 38ab | 25b | 35a | 23b | 9.7 |
| Acetate: propionate | 2.8a | 3.1ab | 3.9c | 2.9a | 3.6bc | 0.86 |
Lower case letters indicate significant difference based on ls means with Tukey adjustment (P < 0.05).
There was no difference between plant variety Dry Matter Degradation (DMD), mean value of 65.0 g/100 g, however climate scenario significantly affected DMD with future scenarios, flood, drought and heat shock, being significantly less than control 2020 (Table 4). Climate scenario affected VFA production with there being no difference between control 2020 and control 2050 but all other treatments resulted in a lower (P < 0.05) value compared to control 2020. Ammonia concentration was lowest (P < 0.05) in control 2020 and control 2050 compared to all other climate scenarios. The pH in the 2020 and 2050 controls was lower than in all other climate scenarios, however this small change is considered not biologically significant. Acetate production was lowest (P < 0.05) in 2050 + drought compared to all other climate scenarios, whereas propionate concentration was highest (P < 0.05) in control 2020 with decreasing values for control 2050, 2050 + heat shock, 2050 + drought and 2050 + flood respectively. The iso butyrate concentrations were different (P < 0.05) between climate scenarios but a difference of 2 mM was again considered not biologically significant. Butyrate concentration was highest (P < 0.05) in control 2020 and 2050 + drought and lowest in 2050 + flood and 2050 + heat shock with an intermediate value for control 2050. The acetate to propionate ratio (C2:C3) was lowest (P < 0.05) in control 2020 and 2050 + drought and highest in 2050 + flood with intermediate values for control 2050 and 2050 + heat shock.
Volatile organic compounds (VOCs)
Across the entire dataset, 175 distinct VOCs were detected. Climate scenario was a significant predictor of VOC profiles overall (LR4,134 = 5372, P = 0.002), and all pairwise comparisons were also significantly different from each other (P < 0.05) (Table 6).
Table 6.
Top 25 compounds most important for random forest classification of climate scenario, ranked in order of their importance in discriminating each climate scenario at 24 h post incubation (see Table S2 for raw importance scores).
| Control 2020 | Control 2050 | 2050 + drought | 2050 + flood | 2050 + Heat shock | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Abundance | Rank | Abundance | Rank | Abundance | Rank | Abundance | Rank | Abundance | Rank | |
| (2Z,4E)-hexa-2,4-diene | 0.0207 | 13 | 0.00981 | 16 | 0.00573 | 6 | 0.00661 | 7 | 0.00612 | 9 |
| Octa-1,3-diene | 0.0161 | 5 | 0.0532 | 2 | 0.0499 | 5 | 0.0165 | 16 | 0.022 | 14 |
| 3-Ethylocta-1,5-diene | 0.0172 | 14 | 0.0109 | 15 | 0.0115 | 7 | 0.0219 | 9 | 0.008 | 8 |
| Pentan-3-ol | 0.156 | 3 | 0.0652 | 6 | 0.0251 | 16 | 0.0298 | 13 | 0.0168 | 15 |
| Methanethiol | 0.386 | 4 | 0.296 | 3 | 0.152 | 17 | 0.2 | 3 | 0.0959 | 12 |
| Penta-1,3-diene | 0.116 | 1 | 0.0463 | 8 | 0.0149 | 14 | 0.0279 | 18 | 0.0114 | 5 |
| 2,4-Dimethylfuran | 0.237 | 6 | 0.15 | 7 | 0.11 | 9 | 0.12 | 15 | 0.0878 | 4 |
| Acid1 | 0.0217 | 2 | 0.0272 | 23 | 0.0428 | 19 | 0.0471 | 5 | 0.0441 | 3 |
| Pent-1-en-3-ol | 0.0841 | 9 | 0.0392 | 9 | 0.0218 | 4 | 0.0249 | 19 | 0.0152 | 1 |
| Dodecane-1-thiol | 0.00961 | 10 | 0.0113 | 10 | 0.0152 | 3 | 0.016 | 20 | 0.0149 | 2 |
| Methylsulfonylmethane | 1.07 | 8 | 1.82 | 11 | 1.2 | 1 | 1.46 | 8 | 1.38 | 17 |
| 2-Ethylthiophene | 0.0499 | 18 | 0.0669 | 1 | 0.0421 | 18 | 0.0392 | 1 | 0.0353 | 16 |
| 1,1′-Oxydibenzene | 0.0412 | 16 | 0.0528 | 18 | 0.065 | 8 | 0.0723 | 12 | 0.0626 | 7 |
| 2-Methylbut-1-ene | 0.563 | 11 | 0.257 | 24 | 0.144 | 2 | 0.247 | 10 | 0.104 | 6 |
| Ethanol | 2.02 | 15 | 2.27 | 20 | 1.49 | 13 | 1.49 | 6 | 1.15 | 10 |
| 3-Methylpentan-2-one | 5.68 | 17 | 4.97 | 5 | 4.93 | 20 | 4.32 | 11 | 5.46 | 13 |
| Butan-2-one | 5.28 | 20 | 4.72 | 13 | 4.69 | 10 | 4.13 | 4 | 5.2 | 19 |
| Decanal | 0.0546 | 21 | 0.0511 | 22 | 0.0927 | 22 | 0.0844 | 2 | 0.0863 | 20 |
| 3-Methylthiophene | 0.639 | 23 | 0.629 | 12 | 0.195 | 15 | 0.253 | 14 | 0.284 | 11 |
| (3E)-1,3-hexadiene | 0.0192 | 24 | 0.0227 | 17 | 0.0122 | 21 | 0.0115 | 22 | 0.0112 | 21 |
| Dimethyltrisulfane | 0.777 | 7 | 0.903 | 4 | 0.658 | 12 | 0.899 | 17 | 0.371 | 24 |
| 3-Methylbutan-1-ol | 0.0245 | 22 | 0.0281 | 25 | 0.0204 | 23 | 0.0252 | 21 | 0.0172 | 23 |
| (Z)-oct-2-ene | 1.37 | 12 | 1.61 | 14 | 1.71 | 11 | 1.4 | 25 | 1.57 | 25 |
| Heptane | 0.822 | 19 | 0.944 | 21 | 0.648 | 25 | 0.35 | 23 | 1.23 | 18 |
| (E)-oct-4-ene | 1.38 | 25 | 1.61 | 19 | 1.7 | 24 | 1.4 | 24 | 1.57 | 22 |
Based on 100,000 trees. Abundance is the mean relative abundance of the compound in each growth condition (based on total area normalisation). Compounds are shown in order of decreasing importance to the overall classification (the relative reduction in random forest performance when the values for a given compound are randomly permuted). Rank represents order of importance for each growth category.
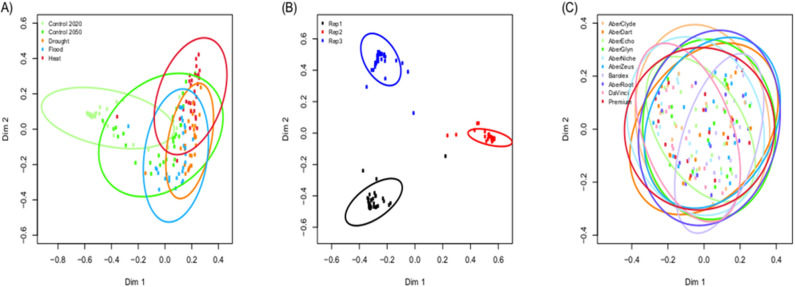
Random forest analysis correctly classified at least 66% of samples in each climate scenario, despite only partial separation on the MDS plot (Table 5, Fig. 4A). Climate scenarios could be successfully differentiated despite marked and significant differences between biological replicates (LR2,134 = 9833, P = 0.002; Table S4; Fig. 4B). No grass varieties could be separated from each other (Fig. 4C).
Table 5.
Results from random forest classification of VOC samples by growth condition at 24 h post incubation.
| Predicted class | Error rate (%) | ||||||
|---|---|---|---|---|---|---|---|
| Control 2020 | Control 2050 | 2050 + drought | 2050 + flood | 2050 + heat shock | |||
| True class | Control 2020 | 26 | 4 | 0 | 0 | 0 | 13 |
| Control 2050 | 1 | 23 | 1 | 2 | 3 | 23 | |
| Drought | 0 | 3 | 21 | 4 | 2 | 33 | |
| Flood | 1 | 2 | 2 | 24 | 1 | 20 | |
| Heat | 1 | 0 | 5 | 0 | 24 | 20 | |
Rows represent the number of samples from each true class that were allocated to each of the predicted classes (columns). The error rate is the percentage of samples that were misclassified from each group. The predicted class for each given sample was determined by a simple majority of votes from the trees in which that sample was out-of-bag (OOB).
Correct classifications are shown in bold.
Figure 4.
MDS ordination of VOC profiles of grass varieties exposed to rumen fluid, based on proximity values from random forest classification by (A) climate scenario; (B) biological replicate; and (C) variety of grass.
Random forests using subsets of only the 25 most discriminatory compounds performed comparably to the originals in their respective classifications (Table 6, Table S4), indicating that these subsets captured much of the relevant information from the full dataset. Within each growth condition, the most important compounds in classification were not necessarily the most abundant and vice versa (Table 6). Only seven compounds (2,4-dimethylfuran, butan-2-one, 3-methylbutan-1-ol, 3-methylpentan-2-one, methylsulfonylmethane, heptane and 1,1'-oxydibenzene) were shared between the discriminatory subsets for climate scenario and biological replicate, further indicating that the effects of climate are robust to background variability (Table S3).
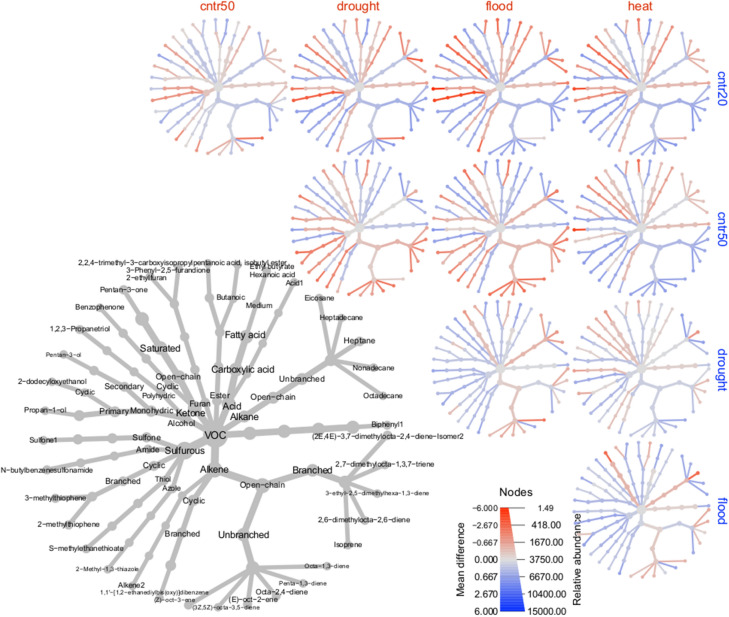
By relative area (normalised per sample), the most abundant group was sulphurous compounds (33% of total area), followed by alkenes (20%), acids (11%) and ketones (11%) (Fig. 5, main panel). By number of compounds, the largest group was alkenes (47 compounds), followed by sulphurous compounds (24 compounds) and alcohols (20 compounds). The relative abundance of alkenes decreased in the future scenarios compared to the 2020 control, while acids increased (Fig. 5).
Figure 5.
Comparisons between VOC profiles from grass varieties exposed to rumen fluid, broken down by climate scenario. Compounds are classified into hierarchical classes, and every node represents on the heat trees one compound or parent class. Node size is proportional to relative abundance for that class/compound. Each of the small heat trees represents a comparison between two climate scenarios. Node colour is on a scale representing the mean abundance change between conditions as a proportion of the total abundance of that compound: orange indicates that the compound had higher relative abundance in the ‘column’ treatment, grey indicates no difference, and blue indicates higher relative abundance in the ‘row’ treatment. Relative abundance is on an arbitrary scale where each sample sums to 100. For ease of viewing, compounds are only included if their relative abundance at least doubled in at least one comparison. For this reason, in some cases a node will show a different direction of change to its parent nodes (i.e. other compounds, not included in the plot, contribute to the parent node’s direction of change).
Discussion
Grass physiological changes in current and future environmental conditions
Climate change involves multiple parameters which individually and in combination can alter plant physiology. It is currently unclear if these changes would be beneficial or detrimental to forage quality and hence the impact on ruminant production. During abiotic stresses plants alter their biomass allocation. In this work the lowest total biomass was from the droughted grasses and those subjected to heat stress, which correlates with previous work where biomass allocation decreased with the abiotic stresses of drought and temperature68. Water soluble carbohydrate levels (WSC) in the control 2020, 2050 as well as the drought and heat shock climate scenarios were higher than the flood scenario, which correlates with previous work where drought and heat stress conditions have been shown to generally elevate WSC as a part of a protective mechanism28. However, the combination of drought with elevated levels of CO2 may also have an effect on the drought response resulting in slightly higher WSC and dry matter levels25. Flooding resulted in a negative effect on crude protein levels and water-soluble carbohydrate levels as might be expected, due to reduced photosynthetic capacity and increased the proportion of fibre. However, protein concentrations were found to be in higher in the drought and heat shocked grasses than in controls, in contrast to previous studies69,70. Drought stress reduces photosynthetic activity and Rubisco activity71 and hence lowers protein levels. However, these effects have been demonstrated not to occur until severe or long-term drought stress has occurred72,73 and indeed in the experiments reported here the chlorophyll level and Fv/Fm were not significantly affected by the duration of 2050 drought treatment. The ratio of Fv/Fm showed a significantly lower value for grasses subjected to the heat shock climate scenario. This was found to be below the optimal value for most plants, indicating plant stress by the photoinhibition of photosystem II74. Chlorophyll measurements can also indicate how photosynthesis is affected during stress75. Chlorophyll levels were highest in the control 2020 scenario and the lowest in grasses subjected to heat shock and flood climate scenarios, indicating that photosynthesis was affected by both these treatments, which is consistent with previous studies26,32,33,75.
Again, the elevated CO2 imposed together with the drought, as well as the short drought period may have mitigated the negative effects on protein content. The clustering of the FTIR data based on treatment further indicates that the treatments were sufficient to elicit a response, and the separation of the heat-shocked material from the controls fits with the reduced chlorophyll and Fv/Fm elicited for this treatment indicating a high level of imposed stress.
Effects of climate condition of grass growth on rumen fermentation
Growth of these grasses under different climate conditions was shown to have an impact on fermentation. It is suggested that the plant response to climate has a consequence for the activity of the rumen microbiota; altering the nutrient provision to the colonising microbiota affects colonisation community profiles and in consequence, forage degradation parameters12,76. This is likely to be a result of differences in forage chemistry due to acclimation responses during growth but could also be a result of changes caused by active plant stress responses to the rumen environment, as has been previously demonstrated to occur in grass and clover16–18,76. The effects of pre-harvest climate scenarios on rumen fermentation are in agreement with previous work indicating that plants have a ‘stress memory’ or ‘defence priming’ and that stress responses are influenced by previous exposure34,77,78. The in vitro gas production technique is a method for analysing fermentation of feeds by a rumen microbial inoculum. The analysis of loss of dry matter (DM) and amount of gas (CO2 and CH4) produced as a result of fermentation of the grass feed indicated that the growth conditions could affect how the grasses would be digested by the animal. Flood had the greatest effect in reducing total gas production, compared to both the control climate scenario for 2020 and 2050. This is probably linked to the decreased WSC and increased NDF content of flooded grasses compared with those grown under 2020 or 2050 conditions. This suggests that breeding forage grasses for resilience to a future climate in which there are periods of flooding during the growth period may be important for maximising its nutritional value.
The in vitro DMD values observed in this study for control 2020 are typical of those expected in high quality pastures for ruminants79. The reduced in vitro DMD caused by future climate scenarios, especially periods of flooding or drought, would lead to reduced animal performance and would require higher intakes to maintain the level of production80,81. Interestingly, methane levels were lower from fermentative grasses that had been previously subjected to flood, drought or heat shock compared to the 2020 and 2050 controls, potentially due to physiological damage caused by flood, drought and heat shock. Different silages have been shown to affect rumen fermentation and the microbial community due to differences their chemical composition82. For example, methane production was higher in grass silage compared to corn silage and feeding ruminants with a more digestible forage reduced methane production79,83,84. Thus, the lower levels of methane produced from grass subjected to 2050 + drought, in this study combined with the lower DMD indicate that the stress treatment affected in vitro rumen fermentation compared to the current climate model. Based on the model-predicted data, the acetate: propionate ratio was 3.26 and the propionate: butyrate ratio was 1.54. This closely matched the expected values of 3.25 and 1.33, respectively85. Hydrogen production and therefore methanogenesis was suggested to be altered by the partitioning of degraded DM between microbial synthesis and rumen fermentation86. The manipulation of the level of carbohydrate from feed that goes directly into microbial growth rather than fermentation can alter methanogenesis by the hexose partitioning of the feed87. The volume of methane production in vivo has been shown to be lowest when animals were fed high sugar diets88. Further work confirmed in vivo that partitioning of hexose into fermentation end products (including methane) and microbial biomass is influenced by dietary carbohydrate and by increasing the proportion of WSC in the diet can significantly reduce the amount of methane produced89. Although decreased methane production has been linked to increased WSC89, this does not explain the results obtained in the present study since WSC remained relatively unaffected by the pre-harvest stress treatments.
FTIR profiles after 48 h of digestion discriminated between plants grown in the flood climate scenario compared to the control 2020 but did not discriminate amongst the different grass varieties. This is in contrast with previous work2 where variety did influence the FTIR after 24 h of fermentation. However, it is possible that differences observed after 24 h were not visible after 48 h because of a more extensive degradation by that time. Typically, in fresh forages an increase in acetate is highly correlated to increased methane production, however here while acetate increased, methane was reduced. However, due to the lower DMD values of the stressed forages this may not be reflected in the animal hence further investigation is required.
Changes in VOC profiles during rumen fermentation due to differing climate conditions
This is one of the first experiments analyse VOCs in relation to plant material under simulated rumen conditions, and it showed that it is possible to distinguish an effect of plant growth conditions from rumen VOCs. The unexpected differences between biological replicates most likely represent differences in the rumen fluid on each occasion and suggests that the exact composition of rumen fluid is a key influence on the volatile bouquet. Despite efforts to keep the replicates as consistent as possible, rumen fluid is affected by diet, health, time since last feed and stochastic changes in microbiota10,90 although here animals were on the same diet, fed at the same time, and rumen fluid was also collected at the same time. Thus, changes in VOCs must be affected by very small changes in diet composition or animal physiology. That the effects of stress growth condition could nonetheless be picked out from this high background variability indicates that there are consistent patterns associated with different plant growth conditions. Although none of the ten varieties of grass could be reliably differentiated from the rest, this does not preclude the possibility that a larger sample size would be able to detect subtle differences amongst varieties.
The most abundant VOCs in fresh L. perenne have been reported as benzeneacetaldehyde, 2,5-dimethyl-pyrazine, hexanal and benzaldehyde91. Of these four, all but 2,5-dimethyl-pyrazine were detectable in the present samples, indicating that grass volatiles could be clearly distinguished in the rumen. In total, eleven of the 58 VOCs listed in Ref.91 were also identified here, and for a further seven, closely related compounds were present (i.e. different isomers or branching patterns). In addition, the samples contained 18 of the 50 compounds previously listed in rumen headspace90 with another 10 close matches. This included nine of the 16 compounds which were highlighted as major rumen fermentation products or characteristic components of rumen odour: acetic acid, propanoic acid, pentanoic acid, hexanoic acid, dimethyl sulphide, dimethyl disulphide, dimethyl trisulphide, phenol and 3-methyl-thiophene90. There was a prevalence of dienes in the present study: 16 in total, of which five were among the 25 compounds most discriminatory between treatments. This abundance of unsaturated compounds is indicative of the reducing environment within the rumen.
Eight of the 25 VOCs identified as major discriminators across the stress growth conditions (Table 6) have been previously associated with plant stress responses. The 3-pentanol activates pathogen defence responses via salicylic acid, jasmonic acid and ethylene signalling pathways92,93. The 1,3-octadiene is negatively associated with drought in flowers94, while 3-ethyl-1,5-octadiene is an autolysis product and is possibly linked to herbivore defence95,96. It is known that 1-penten-3-ol is produced following stresses such as wounding, drying or freezing97,98 and has been associated with mown grasslands99. It possesses anti-fungal properties100, but its formation is oxygen-dependent101, suggesting that it was produced prior to rumination. Ethanol, another of the key discriminatory VOCs, is produced by plant cells when anoxia forces them to switch to fermentative metabolism20. Ethanol is also produced by microbial fermentation but is a minor component in the healthy rumen102; it constituted on average 1.7% total area in the present samples. The compound 3-methyl-2-pentanone is associated with herbivore defence103 while 2-ethylthiophene was negatively associated with increased CO2 levels in broccoli104. Also, 2-butenal is a discriminatory compound worth mentioning, even though it was not in the top 25. This is because 2-butenal strongly induces abiotic stress-related transcription factors105 but also causes irreversible damage to chloroplasts106.
Both 2-methyl-1-butene and 3-methyl-1-butene are produced from the reduction of isoprene under anaerobic conditions107. This is of particular interest as isoprene is used as an electron acceptor during acetogenesis and inhibits the production of methane107. Thus, the association of these VOCs with the stress growth conditions and the reduction in methane may be metabolically linked. Isoprene is a major component of plant volatile emissions and offers protection from heat stress and oxidative damage108,109. Although only 2-methyl-1-butene featured in the top 25 compounds, isoprene and both its breakdown products were important in differentiating the control 2020, drought and heat shock growth conditions. Isoprene was most abundant in the control 2020 growth condition, which would fit with the general pattern of inhibited isoprene production at increased CO2 concentrations109.
Several of the discriminatory compounds were also associated with microbial metabolism. Methanethiol is produced by the microbial breakdown of S-containing amino acids110 and also plays an important role in anaerobic methane cycling110,111. The compound 1-butanol, 3-methyl is a by-product of amino acid fermentation112. This compound has also been detected in plant leaves100 but given that it is produced by various fungal endophytes it is not necessarily endogenous113,114, indeed, it is inhibitory to germination and growth in Arabidopsis114.
Summary and conclusions
It is currently not clear what the impact of climate change on rumen fermentation will be. Consideration of the effect of altered environmental conditions on both plant growth and animal physiology is required. Here we have investigated the former, to explore whether forage growth under an altered climate would produce legacy effects in forage that would impact on rumen fermentation when ingested. Although there was relatively little variation between grass varieties, consistent effects due to growth under severe weather events were detected. Notably acute flood and drought caused decreased digestibility in those forages. As these conditions are predicted to increase in frequency over the next decades, unless addressed this will have a limiting effect on production efficiency. The detection of VOCs associated with plant stress responses during fermentation is further evidence that post-ingestion plant metabolism is a component of the functional rumen system. Together, these data indicate that forage breeding strategies should consider response to future as well as current climates to ensure economic and environmental sustainability of production.
Supplementary Information
Acknowledgements
We would like to acknowledge funding from BBSRC (BB/R019185/1 and BB/R018464/1; Future forages: Implications of forage response to climate change for ruminant production) and BBS/E/W/0012843D (Core Strategic Programme in Resilient Crops). Additionally, we thank Dr KJ Hart for his help with chemical analysis.
Author contributions
A.K.S., H.J.R., E.H.H., S.R.C., C.T.M. devised the experiment. E.H.H., T.E.D., P.R.S. and S.R.C. performed the experimentation. E.H.H., S.R.C. and C.J.C. analysed the data and prepared the figures. E.H.H., S.R.C., A.K.S., H.J.R. and C.T.M. wrote the manuscript. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: The original version of this Article contained errors in Table 1, where the “Type” values of the “Forage grass” were incorrect for “AberDart”, “AberEcho” and “AberGlyn”, “AberNiche”, “AberRoot”, “Barolex” and “Davinci”. Full information regarding the corrections made can be found in the correction notice for this Article.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
10/31/2022
A Correction to this paper has been published: 10.1038/s41598-022-21958-y
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-08309-7.
References
- 1.FAO . Livestock & the Environment—Meeting the challenge. 7. FAO; 1997. [Google Scholar]
- 2.Kingston-Smith AH, Davies TE, Rees Stevens P, Mur LAJ. Comparative Metabolite fingerprinting of the rumen system during colonisation of three forage grass (Lolium perenne L.) varieties. PLoS One1. 2013;8:e828012013. doi: 10.1371/journal.pone.0082801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith MR, Myers SS. Impact of anthropogenic CO2 emissions on global human nutrition. Nat. Clim. Change. 2018;8:834–839. doi: 10.1038/s41558-018-0253-3. [DOI] [Google Scholar]
- 4.Murphy JM, Brown S, Fung F. UKCP Factsheet: Probabilistic Projections of Climate Extremes. Met Office; 2020. [Google Scholar]
- 5.Loka D, et al. Impacts of abiotic stresses on the physiology and metabolism of cool-season grasses: A review. Food Energy Secur. 2019;8:e00152. doi: 10.1002/fes3.152. [DOI] [Google Scholar]
- 6.Lee MA, Davis AP, Chagunda MG, Manning P. Forage quality declines with rising temperatures, with implications for livestock production and methane emissions. Biogeoscience. 2017;14:1403–1417. doi: 10.5194/bg-14-1403-2017. [DOI] [Google Scholar]
- 7.Flachowsky G, Meyer U, Südekum K. Land use for edible protein of animal origin—A review. Animals. 2017;7:25. doi: 10.3390/ani7030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kingston-Smith AH, Edwards JE, Huws SA, Kim EJ, Abberton MT. Plant-based strategies towards minimising livestock's long shadow. The Proceedings of the Nutrition Society. 2010;69:613–620. doi: 10.1017/S0029665110001953. [DOI] [PubMed] [Google Scholar]
- 9.Hungate R. The Rumen and Its Microbes. New York Academic Press; 1966. [Google Scholar]
- 10.Henderson G, et al. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Scientific Reports. 2015;5:14567. doi: 10.1038/srep14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belanche A, et al. Maternal versus artificial rearing shapes the rumen microbiome having minor long-term physiological implications. Environmental Microbiology. 2019;21:4360–4377. doi: 10.1111/1462-2920.14801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belanche A, Kingston-Smith AH, Griffith GW, Newbold CJ. A multi-kingdom study reveals the plasticity of the rumen microbiota in response to a shift from non-grazing to grazing diets in sheep. Frontiers in Microbiology. 2019;10:122. doi: 10.3389/fmicb.2019.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belanche A, et al. Shifts in the rumen microbiota due to the type of carbohydrate and level of protein ingested by dairy cattle are associated with changes in rumen fermentation. The Journal of Nutrition. 2012;142:1684–1692. doi: 10.3945/jn.112.159574. [DOI] [PubMed] [Google Scholar]
- 14.Kong Y, Teather R, Forster R. Composition, spatial distribution, and diversity of the bacterial communities in the rumen of cows fed different forages. FEMS Microbiology Ecology. 2010;74:612–622. doi: 10.1111/j.1574-6941.2010.00977.x. [DOI] [PubMed] [Google Scholar]
- 15.Huws SA, et al. Addressing global ruminant agricultural challenges through understanding the rumen microbiome: Past, present, and future. Frontiers in Microbiology. 2018;9:2161. doi: 10.3389/fmicb.2018.02161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beha EM, Theodorou MK, Thomas BJ, Kingston-Smith AH. Grass cells ingested by ruminants undergo autolysis which differs from senescence: Implications for grass breeding targets and livestock production. Plant, Cell & Environment. 2002;25:1299–1312. doi: 10.1046/j.1365-3040.2002.00908.x. [DOI] [Google Scholar]
- 17.Kingston-Smith AH, Bollard AL, Armstead I, Thomas BJ, Theodorou MK. Proteolysis and programmed cell death in clover leaves is induced by grazing. Protoplasma. 2003;220:119–129. doi: 10.1007/s00709-002-0044-5. [DOI] [PubMed] [Google Scholar]
- 18.Kingston-Smith AH, Merry RJ, Leemans DK, Thomas H, Theodorou MK. Evidence in support of a role for plant-mediated proteolysis in the rumens of grazing animals. Brit. J. Nutr. 2005;93:73–79. doi: 10.1079/BJN20041303. [DOI] [PubMed] [Google Scholar]
- 19.Kingston-Smith AH, Marshall AH, Moorby JM. Breeding for genetic improvement of forage plants in relation to increasing animal production with reduced environmental footprint. Animal. 2012;7:79–88. doi: 10.1017/S1751731112000961. [DOI] [PubMed] [Google Scholar]
- 20.Kingston-Smith AH, Davies TE, Edwards J, Gay A, Mur LA. Evidence of a role for foliar salicylic acid in regulating the rate of post-ingestive protein breakdown in ruminants and contributing to landscape pollution. Journal of Experimental Botany. 2008;63:3243–3255. doi: 10.1093/jxb/ers048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamau S, et al. A route to decreasing N pollution from livestock: Use of Festulolium hybrids improves efficiency of N flows in rumen simulation fermenters. Food Energy Secur. 2020;9:e209. doi: 10.1002/fes3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Humphreys MW, O’Donovan SA, Farrell MS, Gay AP, Kingston-Smith AH. The potential of novel Festulolium (2n = 4x = 28) hybrids as productive, nutrient-use-efficient fodder for ruminants. Food Energy Secur. 2014;3:98–110. doi: 10.1002/fes3.50. [DOI] [Google Scholar]
- 23.Gagne-Bourque F, Bertrand A, Claessens A, Aliferis KA, Jabaji S. Alleviation of drought stress and metabolic changes in timothy (Phleum pratense L.) Colonized with Bacillus subtilis B26. Frontiers in Plant Science. 2016;7:584. doi: 10.3389/fpls.2016.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taub D. Effects of rising atmospheric concentrations of carbon dioxide on plants. Nat. Edu. Knowl. 2010;3:10. [Google Scholar]
- 25.Volaire F, et al. The resilience of perennial grasses under two climate scenarios is correlated with carbohydrate metabolism in meristems. Journal of Experimental Botany. 2020;71:370–385. doi: 10.1093/jxb/erz424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui L, Li J, Fan Y, Xu S, Zhang Z. High temperature effects on photosynthesis, PSII functionality and antioxidant activity of two Festuca arundinacea cultivars with different heat susceptibility. Botanical Studies. 2006;47:61–69. [Google Scholar]
- 27.Dekov I, Tsonev T, Yordanov I. Effects of water stress and high-temperature stress on the structure and activity of photosynthetic apparatus of Zea mays and Helianthus annuus. Photosynthetica. 2000;38:361–366. doi: 10.1023/A:1010961218145. [DOI] [Google Scholar]
- 28.Volaire F, Thomas H, Lelievre F. Survival and recovery of perennial forage grasses under prolonged Mediterranean drought. The New Phytologist. 1998;140:439–449. doi: 10.1111/j.1469-8137.1998.00288.x. [DOI] [PubMed] [Google Scholar]
- 29.Bista DR, Heckathorn SA, Jayawardena DM, Mishra S, Boldt JK. Effects of drought on nutrient uptake and the levels of nutrient-uptake proteins in roots of drought-sensitive and -tolerant grasses. Plants. 2018;7:28. doi: 10.3390/plants7020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kato-Noguchi H, Morokuma M. Ethanolic fermentation and anoxia tolerance in four rice cultivars. Journal of Plant Physiology. 2007;164:168–173. doi: 10.1016/j.jplph.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 31.Allen JF. Redox control of transcription: Sensors, response regulators, activators and repressors. FEBS Letters. 1995;332:203–207. doi: 10.1016/0014-5793(93)80631-4. [DOI] [PubMed] [Google Scholar]
- 32.Arbona V, Hossain Z, López-Climent MF, Pérez-Clemente RM, Gómez-Cadenas A. Antioxidant enzymatic activity is linked to waterlogging stress tolerance in citrus. Physiologia Plantarum. 2008;132:452–466. doi: 10.1111/j.1399-3054.2007.01029.x. [DOI] [PubMed] [Google Scholar]
- 33.Yeung E, Bailey-Serres J, Sasidharan R. After the deluge: Plant revival post-flooding. Trends in Plant Science. 2019;24:443–454. doi: 10.1016/j.tplants.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Kinoshita T, Seki M. Epigenetic memory for stress response and adaptation in plants. Plant and Cell Physiology. 2014;55:1859–1863. doi: 10.1093/pcp/pcu125. [DOI] [PubMed] [Google Scholar]
- 35.Hilker M, Schmülling T. Stress priming, memory, and signalling in plants. Plant, Cell & Environment. 2019;42:753–761. doi: 10.1111/pce.13526. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka S, Han X, Kahmann R. Microbial effectors target multiple steps in the salicylic acid production and signalling pathway. Frontiers in Plant Science. 2015;6:349. doi: 10.3389/fpls.2015.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohama N, Sato H, Shinozaki K, Yamaguchi-Shinozaki K. Transcriptional regulatory network of plant heat stress response. Trends in Plant Science. 2017;22:53–65. doi: 10.1016/j.tplants.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Fung F, Gawith M. UKCP18 for UKCP09 Users, UKCP18 Guidance. Met. Office, Hadley Centre; 2018. [Google Scholar]
- 39.Murchie EH, Lawson T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. Journal of Experimental Botany. 2013;64:3983–3998. doi: 10.1093/jxb/ert208. [DOI] [PubMed] [Google Scholar]
- 40.Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiology. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.AOAC . Official Methods of Analysis. 21. AOAC International; 2019. [Google Scholar]
- 42.Yemm EW, Willis AJ. The estimation of carbohydrates in plant extracts by anthrone. The Biochemical Journal. 1954;57:508–514. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber, and nonstartch polysaccharides in relation to animal nutrition. Journal of Dairy Science. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- 44.Van Soest P. Development of a comprehensive system of feed analyses and its application to forages. Journal of Animal Science. 1967;26:119–128. doi: 10.2527/jas1967.261119x. [DOI] [Google Scholar]
- 45.Theodorou MK, Williams BA, Dhanoa MS, McAllan AB, France J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Animal Feed Science and Technology. 1994;48:185–197. doi: 10.1016/0377-8401(94)90171-6. [DOI] [Google Scholar]
- 46.Richardson AJ, Calder AG, Steward CS, Smith A. Simultaneous determination of volatile and non-volatile acidic fermentation products of anaerobes by capillary gas chromatography. Letters in Applied Microbiology. 1989;9:5–8. doi: 10.1111/j.1472-765X.1989.tb00278.x. [DOI] [Google Scholar]
- 47.Jouany JP. Volatile fatty acid and alcohol determination in digestive contents, silage juices, bacterial cultures and anaerobic fermentor contents. Sciences des Aliments. 1982;2:131–144. [Google Scholar]
- 48.Weatherburn MW. Phenol-hypochlorite reaction for determination of ammonia. Analytical Chemistry. 1967;39:971–974. doi: 10.1021/ac60252a045. [DOI] [Google Scholar]
- 49.Allwood JW, Clarke A, Goodacre R, Mur LAJ. Dual metabolomics: A novel approach to understanding plant–pathogen interactions. Phytochemistry. 2010;71:590–597. doi: 10.1016/j.phytochem.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 50.Jarvis RM, Broadhurst D, Johnson H, O’Boyle NM, Goodacre R. PYCHEM: A multivariate analysis package for python. Bioinformatics. 2006;22:2565–2566. doi: 10.1093/bioinformatics/btl416. [DOI] [PubMed] [Google Scholar]
- 51.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria (2020). https://www.R-project.org/. Accessed 10 Nov 2021.
- 52.RStudio Team . RStudio: Integrated Development for R. RStudio, PBC; 2020. [Google Scholar]
- 53.Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.3-1 (2019). https://CRAN.R-project.org/package=agricolae.
- 54.Fox J, Weisberg S. An R Companion to Applied Regression. 3. Sage; 2019. [Google Scholar]
- 55.Wickham H. Ggplot2: Elegant Graphics for Data Analysis. 2. Springer; 2009. [Google Scholar]
- 56.Lenth R. Least-squares means: The R Package lsmeans. Journal of Statistical Software. 2016;69:1–33. doi: 10.18637/jss.v069.i01. [DOI] [Google Scholar]
- 57.Mangiafico, S. Rcompanion: Functions to Support Extension Education Program Evaluation. R package version 2.3.25 (2020).
- 58.Lu H, Liang Y, Dunn WB, Shen H, Kell DB. Comparative evaluation of software for deconvolution of metabolomics data based on GC-TOF-MS. TrAC Trends Anal. Chem. 2008;27:215–227. doi: 10.1016/j.trac.2007.11.004. [DOI] [Google Scholar]
- 59.Behrends V, Tredwell GD, Bundy JG. A software complement to AMDIS for processing GC-MS metabolomic data. Analytical Biochemistry. 2011;415:206–208. doi: 10.1016/j.ab.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 60.Foster, Z. metacoder: Tools for Parsing, Manipulating, and Graphing Hierarchical Data(2016). https://cran.r-project.org/web/packages/metacoder/index.html. Accessed 10 Nov 2021.
- 61.Foster ZSL, Sharpton TJ, Grünwald NJ. Metacoder: An R package for visualization and manipulation of community taxonomic diversity data. PLoS Comp Biol. 2017;13:e1005404. doi: 10.1371/journal.pcbi.1005404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y, Naumann U, Wright ST, Warton DI. mvabund—An R package for model-based analysis of multivariate abundance data. Brit. Ecol. Soc. 2012;3:471–474. [Google Scholar]
- 63.Liaw A, Wiener M. Classification and regression by randomForest. R News. 2002;2:18–22. [Google Scholar]
- 64.Oksanen, J. et al. vegan: Community Ecology Package. R Package Version 2.3-5 (2016). http://CRAN.R-project.org/package=vegan. Accessed 10 Nov 2021.
- 65.Allaire, J. et al. rmarkdown: Dynamic Documents for R. R Package Version 2.10 (2016).https://rmarkdown.rstudio.com. Accessed 10 Nov 2021.
- 66.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995;57:289–300. [Google Scholar]
- 67.Breiman L. Random forests. Machine Learning. 2001;45:5–32. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- 68.Alhaithloul HAS. Impact of combined heat and drought stress on the potential growth responses of the desert grass Artemisia sieberi alba: Relation to biochemical and molecular adaptation. Plants. 2019;8:416. doi: 10.3390/plants8100416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fariaszewska A, et al. Mild drought stress-induced changes in yield, physiological processes and chemical composition in Festuca, Lolium and Festulolium. Journal of Agronomy and Crop Science. 2017;203:103–116. doi: 10.1111/jac.12168. [DOI] [Google Scholar]
- 70.Bista DR, Scott A, Heckathorn SA, Jayawardena DM, Mishra S, Boldt JK. Effects of drought on nutrient uptake and the levels of nutrient-uptake proteins in roots of drought-sensitive and -tolerant grasses. Plants. 2018;7:28. doi: 10.3390/plants7020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saud S, et al. Silicate application increases the photosynthesis and its associated metabolic activities in Kentucky bluegrass under drought stress and post-drought recovery. Environmental Science and Pollution Research. 2016;23:17647–17655. doi: 10.1007/s11356-016-6957-x. [DOI] [PubMed] [Google Scholar]
- 72.Medrano H, Parry MA, Socias X, Lawlor DW. Long term water stress inactivates Rubisco in subterranean clover. The Annals of Applied Biology. 1997;131:491–501. doi: 10.1111/j.1744-7348.1997.tb05176.x. [DOI] [Google Scholar]
- 73.Flexas J, et al. Decreased Rubisco activity during water stress is not induced by decreased relative water content but related to conditions of low stomatal conductance and chloroplast CO2 concentration. The New Phytologist. 2006;172:73–82. doi: 10.1111/j.1469-8137.2006.01794.x. [DOI] [PubMed] [Google Scholar]
- 74.Maxwell K, Johnson GN. Chlorophyll fluorescence—A practical guide. Journal of Experimental Botany. 2000;51:659–668. doi: 10.1093/jexbot/51.345.659. [DOI] [PubMed] [Google Scholar]
- 75.Caudle KL, Maricle BR. Effects of flooding on photosynthesis, chlorophyll fluorescence, and oxygen stress in plants of varying flooding tolerance. Trans. Kansas Acad. Sci. 2012;115:5–18. doi: 10.1660/062.115.0102. [DOI] [Google Scholar]
- 76.Mayorga OL, et al. Temporal metagenomic and metabolomic characterization of fresh perennial ryegrass degradation by rumen bacteria. Frontiers in Microbiology. 2016 doi: 10.3389/fmicb.2016.01854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pastor V, Luna E, Mauch-Mani B, Ton J, Flors V. Primed plants do not forget. Environmental and Experimental Botany. 2013;94:46–56. doi: 10.1016/j.envexpbot.2012.02.013. [DOI] [Google Scholar]
- 78.Ding Y, Fromm M, Avramova Z. Multiple exposures to drought ‘train’ transcriptional responses in Arabidopsis. Nature Communications. 2012;3:740. doi: 10.1038/ncomms1732. [DOI] [PubMed] [Google Scholar]
- 79.Hart KJ, Martin PG, Foley PA, Kenny DA, Boland TM. Effect of sward dry matter digestibility on methane production, ruminal fermentation, and microbial populations of zero-grazed beef cattle. Journal of Animal Science. 2009;87:3342–3350. doi: 10.2527/jas.2009-1786. [DOI] [PubMed] [Google Scholar]
- 80.AFRC . Energy and Protein Requirements of Ruminants. CAB International; 1993. [Google Scholar]
- 81.Van Soest PJ. Nutritional Ecology of the Ruminant. Cornell University Press; 1994. [Google Scholar]
- 82.Lengowski MB, et al. Changes in rumen microbial community composition during adaption to an in vitro system and the impact of different forages. PLoS One1. 2016;11:e0150115. doi: 10.1371/journal.pone.0150115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hegarty R. Reducing rumen methane emissions through elimination of rumen protozoa. Crop & Pasture Science. 1999;50:1321–1328. doi: 10.1071/AR99008. [DOI] [Google Scholar]
- 84.Benchaar C, Pomar C, Chiquette J. Evaluation of dietary strategies to reduce methane production in ruminants: A modelling approach. Canadian Journal of Animal Science. 2001;81:563–574. doi: 10.4141/A00-119. [DOI] [Google Scholar]
- 85.Wolin MJ. A theoretical fermentation balance. Journal of Dairy Science. 1960;43:1452–1459. doi: 10.3168/jds.S0022-0302(60)90348-9. [DOI] [Google Scholar]
- 86.Beever DE. Rumen function. In: Forbes JM, France J, editors. Quantitative Aspects of Ruminant Digestion and Metabolism. CAB International; 1993. pp. 187–215. [Google Scholar]
- 87.Meeks G, Bates J. Cost Effectiveness of Options for Reducing UK Methane Emissions—Final Report. AEA Technology Environment; 1999. [Google Scholar]
- 88.Moss AR, Jouany JP, Newbold J. Methane prediction by ruminants: Its contribution to global warming. Ann. Zootechnol. 2000;49:231–253. doi: 10.1051/animres:2000119. [DOI] [Google Scholar]
- 89.Moss AR, Newbold CJ. The impact of hexose partitioning on methane production in vitro. Reproduction, Nutrition, Development. 2000;40:201–202. [Google Scholar]
- 90.Cai L, Koziela JA, Davisa J, Loa Y-C, Xina H. Characterization of volatile organic compounds and odors by in-vivo sampling of beef cattle rumen gas, by solid-phase microextraction, and gas chromatography–mass spectrometry–olfactometry. Analytical and Bioanalytical Chemistry. 2006;386:1791–1802. doi: 10.1007/s00216-006-0799-1. [DOI] [PubMed] [Google Scholar]
- 91.Hong S, Kang W, Su Y, Guo Y-L. Analysis of trace-level volatile compounds in fresh turf crop (Lolium perenne L.) by gas chromatography quadrupole time-of-flight mass spectrometry. Chinese Journal of Chemistry. 2013;31:1329–1335. doi: 10.1002/cjoc.201300414. [DOI] [Google Scholar]
- 92.Song GC, Ryu C-M. Two volatile organic compounds trigger plant self-defence against a bacterial pathogen and a sucking insect in cucumber under open field conditions. International Journal of Molecular Sciences. 2013;14:9803–9819. doi: 10.3390/ijms14059803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Song GC, Choi HK, Ryu C-M. Gaseous 3-pentanol primes plant immunity against a bacterial speck pathogen, Pseudomonas syringae pv. tomato via salicylic acid and jasmonic acid-dependent signaling pathways in Arabidopsis. Frontiers in Plant Science. 2015;6:821. doi: 10.3389/fpls.2015.00821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Campbell DR, Sosenski P, Raguso RA. Phenotypic plasticity of floral volatiles in response to increasing drought stress. Annals of Botany. 2019;123:601–610. doi: 10.1093/aob/mcy193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rohloff J, Bones AM. Volatile profiling of Arabidopsis thaliana—Putative olfactory compounds in plant communication. Phytochemistry. 2005;66:1941–1955. doi: 10.1016/j.phytochem.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 96.Mumm R, Posthumus MA, Dicke M. Significance of terpenoids in induced indirect plant defence against herbivorous arthropods. Plant, Cell & Environment. 2008;31:575–585. doi: 10.1111/j.1365-3040.2008.01783.x. [DOI] [PubMed] [Google Scholar]
- 97.Fall R, Karl T, Jordan A, Lindinger W. Biogenic C5 VOCs: Release from leaves after freeze–thaw wounding and occurrence in air at a high mountain observatory. Atmospheric Environment. 2001;35:3905–3916. doi: 10.1016/S1352-2310(01)00141-8. [DOI] [Google Scholar]
- 98.Matsui K, Sugimoto K, Mano J, Ozawa R, Takabayashi J. Differential metabolisms of green leaf volatiles in injured and intact parts of a wounded leaf meet distinct ecophysiological requirements. PLoS One1. 2012;7:e36433. doi: 10.1371/journal.pone.0036433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.König G, et al. Relative contribution of oxygenated hydrocarbons to the total biogenic VOC emissions of selected mid-European agricultural and natural plant species. Atmospheric Environment. 1995;29:861–874. doi: 10.1016/1352-2310(95)00026-U. [DOI] [Google Scholar]
- 100.Cruz AF, et al. Phytochemicals to suppress Fusarium head blight in wheat–chickpea rotation. Phytochemistry. 2012;78:72–80. doi: 10.1016/j.phytochem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 101.Fisher A, Grimes H, Fall R. The biochemical origin of pentenol emissions from wounded leaves. Phytochemistry. 2003;62:159–163. doi: 10.1016/S0031-9422(02)00521-6. [DOI] [PubMed] [Google Scholar]
- 102.Lettat A, et al. Rumen microbial and fermentation characteristics are affected differently by bacterial probiotic supplementation during induced lactic and subacute acidosis in sheep. BMC Microbiology. 2012;12:142. doi: 10.1186/1471-2180-12-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Giron-Calva PS, Li T, Blande JD. Volatile-mediated interactions between cabbage plants in the field and the impact of ozone pollution. Journal of Chemical Ecology. 2017;43:339–350. doi: 10.1007/s10886-017-0836-x. [DOI] [PubMed] [Google Scholar]
- 104.Krumbein A, Kläring H, Schonhof I, Schreiner M. Atmospheric carbon dioxide changes photochemical activity, soluble sugars and volatile levels in broccoli (Brassica oleracea var. italica) Journal of Agricultural and Food Chemistry. 2010;58:3747–3752. doi: 10.1021/jf903280w. [DOI] [PubMed] [Google Scholar]
- 105.Yamauchi Y, Kunishima M, Mizutani M, Sugimoto Y. Reactive short-chain leaf volatiles act as powerful inducers of abiotic stress-related gene expression. Scientific Reports. 2015;5:8030. doi: 10.1038/srep08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mano J, Miyatake F, Hiraoka E, Tamoi M. Evaluation of the toxicity of stress-related aldehydes to photosynthesis in chloroplasts. Planta. 2009;230:639–648. doi: 10.1007/s00425-009-0964-9. [DOI] [PubMed] [Google Scholar]
- 107.Kronen M, Lee M, Jones ZL, Manefield MJ. Reductive metabolism of the important atmospheric gas isoprene by homoacetogens. The ISME Journal. 2019;13:1168–1182. doi: 10.1038/s41396-018-0338-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sharkey TD, Wiberley AE, Donohue AR. Isoprene emission from plants: Why and how. Annals of Botany. 2008;101:5–18. doi: 10.1093/aob/mcm240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Loreto F, Schnitzler J-P. Abiotic stresses and induced BVOCs. Trends in Plant Science. 2010;15:154–166. doi: 10.1016/j.tplants.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 110.Lomans BP, van der Drift C, Pol A, Op den Camp HJM. Microbial cycling of volatile organic sulfur compounds. Cellular and Molecular Life Sciences. 2002;4:575–588. doi: 10.1007/s00018-002-8450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moran JJ. Methyl sulfides as intermediates in the anaerobic oxidation of methane. Environmental Microbiology. 2007;10:162–173. doi: 10.1111/j.1462-2920.2007.01441.x. [DOI] [PubMed] [Google Scholar]
- 112.Boumba VA, Ziavrou KS, Vougiouklakis T. Biochemical pathways generating post-mortem volatile compounds co-detected during forensic ethanol analyses. Forensic Science International. 2008;67(174):133–151. doi: 10.1016/j.forsciint.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 113.Kanchiswamy CN, Malnoy M, Maffei ME. Chemical diversity of microbial volatiles and their potential for plant growth and productivity. Frontiers in Plant Science. 2015;6:151. doi: 10.3389/fpls.2015.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Siri-udom S, Suwannarach N, Lumyong S. Applications of volatile compounds acquired from Muscodor heveae against white root rot disease in rubber trees (Hevea brasiliensis Müll. Arg.) and relevant allelopathy effects. Fungal Biology. 2017;121(6–7):573–581. doi: 10.1016/j.funbio.2017.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.