Abstract
Atmospheric conditions affect the release of anemophilous pollen, and the timing and magnitude will be altered by climate change. As simulated with a pollen emission model and future climate data, warmer end-of-century temperatures (4–6 K) shift the start of spring emissions 10–40 days earlier and summer/fall weeds and grasses 5–15 days later and lengthen the season duration. Phenological shifts depend on the temperature response of individual taxa, with convergence in some regions and divergence in others. Temperature and precipitation alter daily pollen emission maxima by −35 to 40% and increase the annual total pollen emission by 16–40% due to changes in phenology and temperature-driven pollen production. Increasing atmospheric CO2 may increase pollen production, and doubling production in conjunction with climate increases end-of-century emissions up to 200%. Land cover change modifies the distribution of pollen emitters, yet the effects are relatively small (<10%) compared to climate or CO2. These simulations indicate that increasing pollen and longer seasons will increase the likelihood of seasonal allergies.
Subject terms: Atmospheric chemistry, Environmental health, Projection and prediction, Risk factors
Atmospheric conditions affect the release of anemophilous pollen. Zhang et al. use a pollen emission model together with future climate data to simulate changes in pollen emission. The study shows that climate change driven pollen increase and seasonal changes may increase seasonal allergies
Introduction
Anemophilous (or wind-driven) pollen plays an important role in plant fertilization and gene dispersal1, alters climate by interacting with clouds and radiation2–4, and triggers allergic diseases such as allergic rhinitis (also known as hay fever) and asthma5,6. Pollen-induced respiratory allergy affects up to 30% of the world population, particularly children <18 years old5,7, and is a worldwide health concern resulting in large economic loss because of medical expenditures, missed work and school days, and early deaths6,8. Because pollen emission is closely associated with environmental drivers, climate change could influence pollen emission and consequently the incidence of allergic disease9,10. Longer and more intense pollen seasons have been observed over the past few decades11–13, which is expected to contribute to the exacerbation and aggravation of pollen allergic rhinitis and asthma7,8,14.
Pollen emissions from anemophilous vegetation are directly correlated with meteorological conditions, such as temperature and precipitation15,16. Temperature impacts the number of winter chill hours and spring frost-free days and is strongly associated with the timing of pollen seasons, including the start date, peak emission date, end date, and duration12,17–19. Over the past several decades, warmer temperatures have been observed to drive earlier (3–22 days) pollen season start dates11,20,21 for spring-flowering taxa (e.g., deciduous trees such as Betula, Quercus, and Acer), while late-flowering taxa (Artemisia and grasses, which dominate in summer and fall) have delayed pollen season start dates by up to 27 days11,17. Prolonged pollen seasons have been recorded for both trees and weeds including Quercus, Cupressaceae, Oleaceae, Urticaceae, and Asteraceae11,12,22. Precipitation exerts both short-term and long-term effects on pollen emissions. Heavy short-term precipitation significantly reduces atmospheric pollen concentrations via wet deposition15,23,24, while changes in long-term accumulated precipitation may favor or disadvantage plant growth and therefore alter the total pollen production25. In the future, temperature and precipitation are projected to change heterogeneously across the United States (US)26, and both driving climate variables could directly affect future US pollen emission change patterns. Moreover, the distribution and composition of plant communities are likely to change in the future due to the climate change27, and further influence the corresponding pollen emission.
Increasing atmospheric CO2 concentrations can fertilize vegetation, enhancing photosynthetic capacity and likely increasing pollen production28,29. Higher CO2 concentrations have been observed to increase both the quantity of male flowers30 and their allergenic protein contents31, therefore leading to higher pollen and pollen allergen production. Under laboratory conditions, doubling CO2 concentrations increased pollen production by a broad range of 60–1299%31–34, however, these studies are constrained to chamber experiments for limited species. Despite the uncertainty of how CO2 increases will affect plants in the real world, these studies suggest that increasing atmospheric CO2 concentrations may elevate the intensity of pollen production and increase the prevalence and severity of pollen allergic disease and associated health burdens14.
While prior observations indicate that pollen phenology is responding to climate change11,35, large uncertainties remain because pollen observations are sparse in both space and time12. Previous observation-based studies are typically limited to small spatial scales10,12,36 (e.g., in a single city or limited sites) or temporal scales17,37 (e.g., ~10 years). Existing continental-scale (e.g., the United States, Europe) studies of future pollen emissions only include individual taxa or a small subset of allergic pollen taxa7,8,16,38–40 or limited climatic drivers7,20,39. Because the impacts of climatic drivers on pollen emission vary with vegetation type22,41 and the dominant pollen taxa vary among regions42, studying the taxa-specific pollen emission changes is necessary to disentangle the complex influences of climatic drivers on pollen emissions. Here we use a pollen emission model that simulates multiple taxa of pollen emissions at the continental scale41. Using a suite of future climate data from the Coupled Model Intercomparison Project version 6 (CMIP6)43 for two different emissions scenarios44 including the Shared Socioeconomic Pathways (SSP) 245 and 585 (see “Methods”), we project the change of pollen emissions at the end of the century (2081–2100) compared to the historical period (1995–2014) over the United States for 13 of the most prevalent airborne pollen taxa. As increased CO2 concentration and land cover changes could be important drivers of pollen production in the future38,40, we also test how rising CO2 concentrations and species range shifts may influence emissions of pollen.
Results
Phenological shifts driven by future warmer temperatures
Using the Pollen Emissions model for Climate Models (PECM)41, we simulate the daily pollen emissions when including the effects of future temperature and precipitation (without effects of CO2; = 1 in Eq. (1); “Methods”). Pollen emission phenology (defined with the pollen season start day of the year (sDOY) and end day of the year (eDOY)) is estimated directly from temperature (see “Methods”), driving three categories of change in the pollen season at the end-of-century due to greenhouse gas warming (Fig. 1, and conceptualized in Fig. 2a–c). The first category (Category 1) represents a shift of both the future flowering season sDOY and eDOY to an earlier date than the historical period (circles in Fig. 1), where sDOY has a stronger temperature dependence than eDOY thereby increasing pollen emission duration (Fig. 2a). Most of the deciduous tree genera (Acer, Alnus, Fraxinus, Morus, Platanus, Populus, Quercus, spring-flowering Ulmus), both coniferous tree families (Cupressaceae, Pinaceae), and C3 grasses (Poaceae) exhibit Category 1 phenological changes. Absent changes in the pollen production (pfannual in Eq. (1)), this asymmetric increase in the sDOY and longer duration would flatten the pollen emission curve and decrease maximum daily pollen emissions (Epol,max) (Fig. 2a). Because temperature is the driving factor of pollen phenology, the spatial distribution of historical duration for individual taxa in Category 1 (Supplementary Fig. 1b) and their future changes (Supplementary Fig. 1f) correspond to the historical spatial temperature pattern (Supplementary Fig. 1a) and projected temperature changes from the CMIP6 models (Supplementary Fig. 1e).
Fig. 1. Future change in pollen season start date and end date.
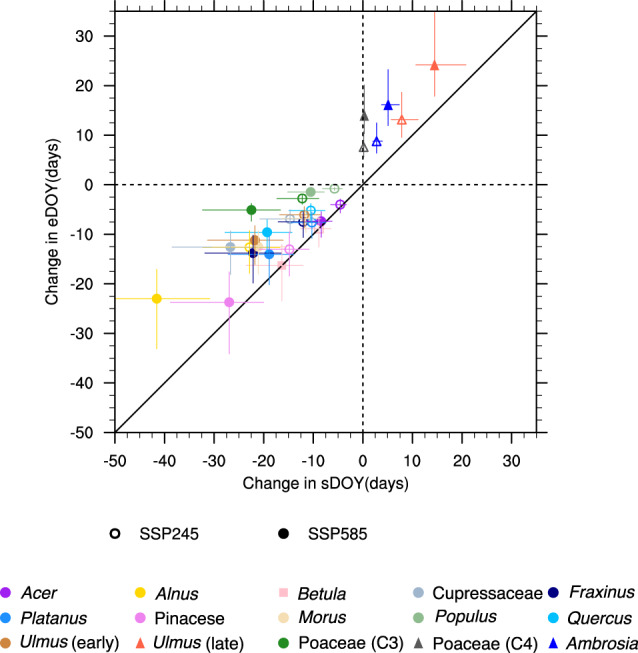
The projected change of start day of year (sDOY) and end day of year (eDOY) of pollen season at the end of century (2081–2100) for individual taxa. Each symbol represents the multi-model mean of the spatially and temporally averaged 15 model members. Error bars represent the minimum and maximum from the 15 CMIP6 (Coupled Model Intercomparison Project Phase 6) model ensembles (n = 15). Open (Shared Socioeconomic Pathway (SSP) 245) and closed (SSP 585) symbols indicate the results from different future emissions scenarios. Circles represent the taxa in Category 1, squares in Category 2, and triangles in Category 3 (visualized in Fig. 1). 1:1 reference in provided with the solid black line. Source data are provided as source data file.
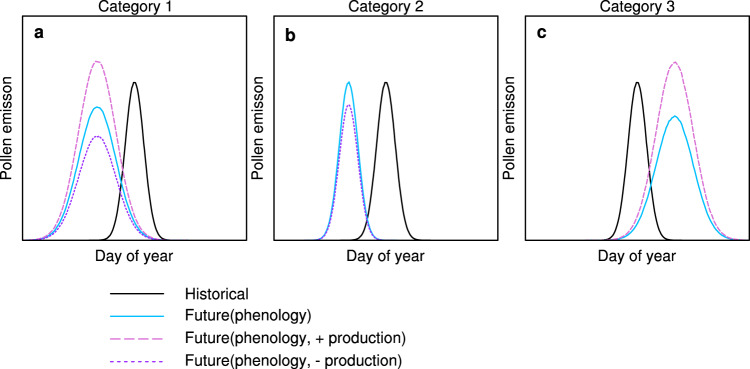
Fig. 2. Three categories of pollen emission changes.
a–c Conceptual schematic for three categories of pollen emission changes, comparing the historical (black) and future (colors) daily pollen emission. Blues lines indicate the future pollen emission change with only temperature-dependent phenology. Pink and purple curves represent future pollen emission change with phenology change and temperature-dependent changes in the annual production factor (pfannual), which can increase (pink) or decrease (purple) production.
The second category (square in Fig. 1) only includes one genus (Betula), where both sDOY and eDOY have a similar temperature dependence and shift earlier at approximately the same rate (Fig. 2b). Therefore, the Betula pollen season duration stays approximately the same with no impacts on the Epol,max (Fig. 2b), and the duration change is decoupled from temperature (e.g., Betula in Supplementary Fig. 1g).
Vegetation in the third category includes short-day species45,46, with maximum emissions in the late summer and early fall (e.g., late-flowering Ulmus, C4 grasses (Poaceae), and Ambrosia) that flower as days shorten in the Northern Hemisphere. Despite seasonal light as a driver, sDOY and eDOY for these taxa was best predicted with temperature41. Both sDOY and eDOY increasing with warmer temperatures (triangles in Fig. 1) and are projected to occur later under end-of-century conditions, with a stronger eDOY temperature dependence than sDOY resulting in a longer duration (Fig. 2c). Taxa in this category experience a longer duration that flattens the pollen emission curve when not considering pollen production changes (Fig. 2c). Similar to Category 1, the duration changes in Category 3 are closely associated with temperature change, with greater increases in duration in the north (up to 16 days) than in the south (up to 12 days) (e.g., Ambrosia in Supplementary Fig. 1h).
Temperature effects on simulated pollen phenology vary substantially for different taxa, leading to a broad range in the increase of pollen season duration. Under scenario SSP 585 (see “Methods”), the duration increases in Category 1 are 2–19 days, where the large variability is a function of the 11 different vegetation taxa in this category (Fig. 1; circles). For the short-day vegetation in Category 3, the duration increases from 10 to 14 days (Fig. 1; triangles). The only genus (Betula) in Category 2 does not exhibit any duration changes as its sDOY and eDOY shift by the same amount (Fig. 1, squares). In addition, the end-of-century sDOY and eDOY changes are a function of future emissions scenarios. Because temperature is the sole driver of modeled pollen phenology change, duration shifts are consistent with the projected temperatures. The duration change with high-end scenario SSP 585 (2–19 days) is approximately twice of that with moderate emission scenario SSP 245 (1–10 days), corresponding to the temperature changes of 4–6 K degrees and 2–3 K, respectively (Supplementary Fig. 2c, e).
Changes in maximum daily pollen emission driven by future climate
In addition to altering phenology, warmer temperatures will also impact vegetation physiology, either facilitating or restraining growth and impacting plant biomass. These changes have the potential to increase or decrease the annual total pollen production37,47 (pfannual; Eq. (5)). Limited observational data suggest that many taxa are projected to have higher pollen production with warmer temperatures (e.g., Alnus, Morus, Platanus, Quercus, spring and late-flowering Ulmus, Cupresseceae, Ambrosia, C3 and C4 Poaceae3), while some taxa will have lower pollen production (e.g., Acer, Betula, Fraxinus, Populus, Pinaceae) (Supplementary Table 1 and Supplementary Fig. 3)37,48. The variations in pfannual in conjunction with phenology can influence the maximum daily pollen emission (Epol,max; grains m−2 d−1), which is a potential health exposure metric. Increasing pfannual under future climate typically increases the Epol,max and counteracts the lengthening season duration for Category 1 and 3 vegetation (Fig. 2a, c, respectively), especially for taxa with a strong dependence on temperature (e.g., Alnus, Platanus, Quercus, late-flowering Ulmus, Cupresseceae, Ambrosia; Supplementary Fig. 3). In contrast, decreasing pfannual will further decrease the Epol,max (Fig. 2a, b), with greater decreases for taxa projected to have longer durations in the future (Acer, Fraxinus, Populus, Pinaceae; Fig. 2a).
As both pollen production and phenology have the potential to impact pollen emission maxima (Epol,max), a sensitivity analysis (see “Methods”) indicates that the temperature-dependent regression and normalization parameters of pollen production (mprod, bprod, andPnorm in Eq. (5)) have the greatest impacts on the simulated pollen amount (Supplementary Table 2). For taxa with pollen season duration sensitive to temperature change (Alnus, Platanus, Populus, late-flowering Ulmus, C4 grass), the regression parameters of phenology can also become important. Overall, the magnitude of annual pollen production is one of the most important parameters in model simulation (pfannual in Eq. (5) and Supplementary Table 2).
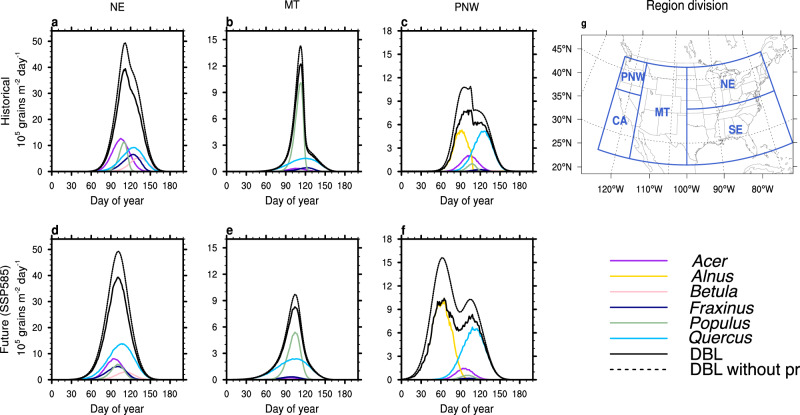
Phenological shifts can create new overlap between different taxa in the future and thereby alter the total Epol,max, with regional variations dependent on the vegetation taxa. In the Northeast (NE; Fig. 3g), many deciduous broadleaf (DBL) taxa (e.g., Acer, Betula, Fraxinus, and Populus) project lower Epol,max driven by temperature-based pfannual reductions and longer pollen season durations (except Betula that is driven only by pfannual). For the three dominant NE DBL genera (Quercus, Populus, and Acer) in the historical period, Acer pollinates first around DOY (day of year) 70, with a peak magnitude of 12 × 105 grains m−2 on DOY 100. This is followed by Populus with a sDOY of 80 and peak magnitude of 11 × 105 grains m−2 on DOY 110, and Quercus with a sDOY of 80 and peak magnitude on DOY 130 of 10 × 105 grains m−2 (Fig. 3a). In the future, the Epol,max increases 4 × 105 grains m−2 (40%) for Quercus but decreases 4 × 105 grains m−2 (33%) and 5.5 × 105 grains m−2 (50%) for Acer and Populus, respectively (Fig. 3d). These three taxa shift to earlier sDOY with longer season durations, driving a convergence of the flowering season. However, the temperature-driven changes in production lead to minimal change in future total Epol,max of DBL (about 5 × 106 grains m−2 on DOY 105 for the historical and future). Similarly in the Mountain (MT) region, the two main taxa (Quercus and Populus) both shift to earlier phenologies and their maximum pollen emissions converge, but the total pollen is dominated by the reduction of Populus pfannual, driving a decrease of total Epol,max of DBL (Fig. 3e). However, the convergence of phenology for the evergreen needleleaf (ENL) vegetation families (Pinaceae and Cupressaceae) leads to an increase of ENL total Epol,max (Supplementary Fig. 4).
Fig. 3. Simulated regional pollen seasonal magnitude and timing in the historical and future.
a–f Twenty-year average time series of daily pollen emission flux (grains m−2 d−1) of the six dominant individual tree taxa in the deciduous broadleaf forest (DBL) and the total DBL emission with (solid black line) and without (dashed black line) precipitation effects. a–c Historical (1995–2014) emission and (d–f) end of the century (2081–2100) emissions from multi-model average simulation for shared socioeconomic pathway (SSP) 585. g We define five geographic regions: Northeast (NE; 38–48° N and 70–100° W), Southeast (SE; 25–38° N and 70–100° W), Mountain (MT; 25–48° N and 100–116° W), California (CA; 25–40° N and 116–125° W) and Pacific Northwest (PNW; 40–48° N and 116–125° W). Three regions are selected for DBL daily pollen emission analysis: Northeast, NE (a, d); Mountain, MT (b, e); Pacific Northwest, PNW (c, f).
In contrast, the phenology of the two dominant emitters in Pacific Northwest (PNW) (Alnus and Quercus) diverge. Alnus emissions start on DOY 60 and overlap with Quercus around DOY 100 in the historical period (Fig. 3c). In the future, Alnus sDOY shifts earlier at a faster rate than Quercus, thereby reducing coincident emissions (Fig. 3f). Both Alnus and Quercus pfannual are positively impacted by warmer future temperatures, yet the divergence of phenology mitigates the production increases and results in a modest 30% increase of the total Epol,max in the PNW.
Future precipitation changes can also influence pollen emissions. In the historical period, precipitation decreases the mean daily pollen emissions by up to 30% (Fig. 3a–c), especially in the regions with higher daily precipitation intensity (NE, SE, and PNW, Supplementary Fig. 2b). In the future, monthly averaged daily precipitation is projected to increase up to 30% during spring and winter (Supplementary Fig. 5), with the most significant changes occurring in NE and PNW under SSP 585 (Supplementary Fig. 2f). Because spring is the flowering season for most high-emitting anemophilous vegetation, increased future precipitation decreases Epol,max up to 40% of in the NE and PNW regions (Fig. 3d, f).
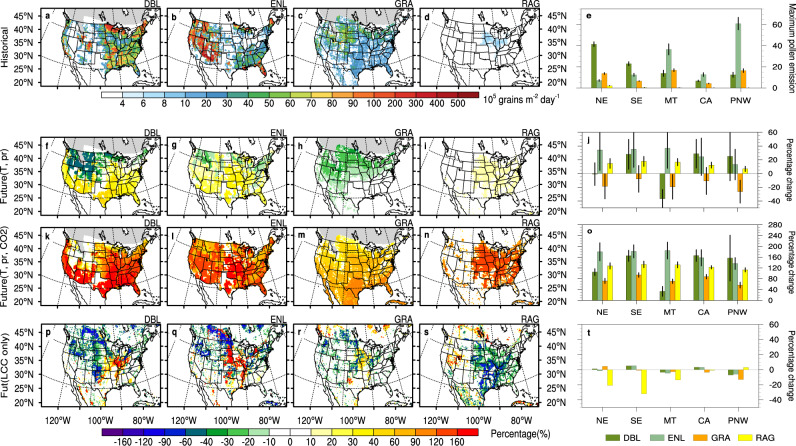
Overall, temperature and precipitation future climate effects alter Epol,max from −35 to 40% under the future SSP 585 scenario (Fig. 4j), driven by the competing effects of duration lengthening, pfannual change, and precipitation scavenging. The Epol,max of DBL increase in the southern regions by 10–30% but are reduced up to 20% in the NE and 40% MT regions (Fig. 4f). Due to the greatest magnitude of warming in the north (Supplementary Fig. 2e), Epol,max decreases are caused by both duration lengthening and changes in the dominant taxa pfannual, as several northern DBL taxa exhibit a negative relationship with temperature (Supplementary Fig. 3a, c, d, g). ENL Epol,max increase 10–40% over most of the US with some decreases in high latitude and mountain regions (Fig. 4g). The geographic distribution and composition of plant community plays an important role of the spatial differences of DBL and ENL pollen emissions, with the interactions between different taxa phenology and effects of temperature on pfannual (Supplementary Fig. 3). Emissions from grasses (Poaceae) and ragweed (Ambrosia) lack the intra-PFT interaction in pollen phenology. Therefore, the future Epol,max change for grasses and ragweed are determined by the competing effects of the temperature-based pfannual and pollen season duration changes. For grasses, the longer duration dominates and decreases Epol,max by 10–40% over all regions, with greater reductions in the north due to the larger temperature increases and longer season durations (Fig. 4h). In contrast, ragweed exhibits a stronger dependence of pfannual with temperature (Supplementary Fig. 3m) and future Epol,max increases about 10–20% over the continental US (Fig. 4i).
Fig. 4. Historical and future changes of maximum daily pollen emissions (Epol,max).
a–e Multi-model historical average (1995–2014) maximum daily pollen emission flux (Epol,max) over the United States (grains m−2 d−1). f–j Projected multi-model average future Epol,max change (%) at the end of century (2081–2100) for shared socioeconomic pathway (SSP) 585, with the effects of temperature (T) and precipitation (pr) only, and (k–o) projected future Epol,max change (%) due to temperature, precipitation, and CO2. Panels a–o use the taxa-based pollen emission model (PECM) driven by meteorology input data from each CMIP6 model to calculate the multi-model average. p–t Plant functional type (PFT)-based model Epol,max change (%) with land cover change (LLC) effects only. The simulation is conducted using PFT-based pollen emission model (PECM) with historical (2015) and future (2100) PFT land cover and driven by the multi-model average climate input data. Columns represent different PFTs: deciduous broadleaf forest (DBL) (a, f, k, p), evergreen needleleaf forest (ENL) (b, g, l, q), grasses (GRA) (c, h, m, r), ragweed (RAG) (d, i, n, s). Bar charts (e, j, o, t) show the spatial averages in five subregions (Fig. 3g) with error bars representing the standard deviation from the average of 15 independent CMIP6 (Coupled Model Intercomparison Project Phase 6) model ensembles (n = 15) in each region (e, j, o).
Climate-driven increases in annual total pollen emissions
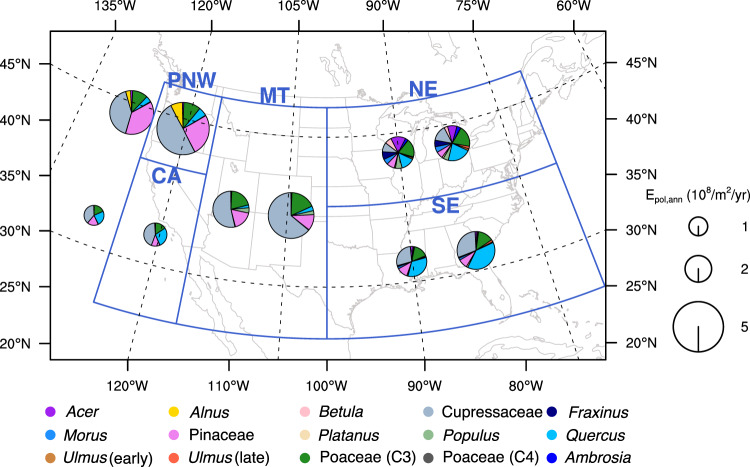
In addition to the maximum daily pollen emission (Epol,max), future climate also impacts the simulated annual total pollen emission (Epol,ann; the sum of simulated daily pollen emission). The magnitude of Epol,ann is impacted by pfannual, precipitation, and land cover fraction of each vegetation types. Due to the variation in vegetation geographic distribution, the composition and contribution of Epol,ann from different taxa vary significantly across the US (Fig. 5). In the historical, Epol,ann is the highest in the PNW (4.2 × 108 grains m−2 yr−1), because of the extensive vegetation coverage as well as large pollen production of the dominant taxa (Pinaceae, Cupressaceae). NE has relatively lower Epol,ann (2.7 × 108 grains m−2 yr−1), with a greater number of contributing taxa that is dominated by Acer, Populus, and Quercus.
Fig. 5. Average annual total pollen emission in the historical and future.
Regional average annual total pollen emission (Epol,ann, unit: 108 grains m−2 yr−1) in the historical (left; 1995-2014) and future shared socioeconomic pathway (SSP) 585 scenario (right; 2081-2100) for the 5 geographic regions (Northeast (NE), Southeast (SE), Mountain (MT), California (CA), and Pacific Northwest (PNW). Chart size indicates the amount of total pollen emitted and different colors represent the contribution of different taxa. The data used here are provided as source data file.
Assuming the land cover fraction and CO2 concentration are the same as the historical period, future pollen emission composition from different taxa changes substantially among the regions (e.g., relative contributions from Quercus increase up to 8% in the NE, and Cupressaceae increase up to 10% in the MT and PNW in the future; Fig. 5). While temperatures are warming, the total Epol,ann over NE and PNW regions show relatively small increases (16 and 26%, respectively) as the pfannual of the several regional taxa exhibit negative correlations with temperature (e.g., Acer, Betula, Populus and Pinaceae; Supplementary Fig. 3a, c, g, l). In contrast, the SE and MT regions have larger increases in Epol,ann (up to 40%), where the dominant taxa (Quercus and Cupressaceae) have a strong positive dependence on temperature (Supplementary Fig. 3h, k).
Overall, simulated Epol,ann increases 16–40% over the United States when considering future climate effects only (Fig. 5). Because Epol,ann integrates pollen emissions over the entire year, its magnitude is driven by the pfannual and precipitation, and phenological shifts do not impact the integrated total. Therefore, although the pollen emission peak (Epol,max) decreases over some high latitude and Mountain (MT) regions for DBL, ENL, and grasses (Fig. 4f–h) due to phenological shifts, the temperature-dependent pfannual and precipitation are the drivers for an increase in total accumulation (Epol,ann) (Fig. 5).
CO2 and land cover effects on pollen emissions
When we allow future CO2 to affect pollen production ( = 2 in Eq. (1); “Methods”), Epol,ann increases up to 250% and Epol,max increases by up to 200% throughout the continental U.S. (Fig. 4k–o). This suggests that increased CO2 could counteract the simulated daily maximum pollen emission decreases due to climate-only effects in some regions. For DBL and grasses, the increases are spatially consistent with temperature changes and are greatest in the South (Fig. 4k, m). ENL and RAG increase throughout the US (Fig. 4l, n), with ENL affected by the convergence of intra-taxa phenologies (Supplementary Fig. 4). Because multiple taxa and subsequent overlap changes are not simulated for GRA and RAG, their regional variations are related to the spatial distribution of future temperature and precipitation changes (Supplementary Fig. 2). With warmer projected temperatures in the south and decreased precipitation intensity in the Southwestern US, the future maximum emissions for GRA are about 30% greater in the south than in the north (Fig. 4m, o). For RAG, the pollen maximum emission in the southern latitudes is also slightly higher (10%) than northern latitudes (Fig. 4n, o).
Future climate change and anthropogenic impacts are likely to shift the spatial distribution of plant communities27 and therefore impact pollen emissions. Because gridded taxa-specific land cover change data are not available, we test the impact of land cover change using projections of plant functional types (PFTs) from GCAM-Demeter land use dataset49 (Supplementary Fig. 6). PECM1.0 can estimate pollen emissions based on PFT with greater uncertainties than the taxa-specific method employed for the previous simulations41. We simulate the future maximum pollen emission with PFT-based pollen emission model using both the historical and future land cover (see “Methods”) (Supplementary Fig. 7). The difference between the two PFT simulations indicates the impact of future land cover change on maximum pollen emissions over the US, noting that the pfannual vary between the PFT and taxa-based models (Supplementary Table 1).
Future PFT changes projected by the Global Change Analysis Model (GCAM)49 simulate an increase in tree coverage in the Central US and Mississippi River Valley at the expense of crop, and some decrease in tree coverage at high altitudes of the Rockies and the Pacific Northwest (Supplementary Fig. 6). Compared to tree PFTs, changes to grassland are relatively small and occur in smaller patches (Supplementary Fig. 6c). Ragweed coverage is based on urban and crop land cover, and the future reduction of the cropland due to the expansion of grasses or trees49 drives large decreases (up to 80%) of potential ragweed land cover over the eastern US (Supplementary Fig. 6d). For the two dominant US tree types (DBL and ENL), the regional pollen maximum emission increases up to 6% in SE and CA (California) while decreasing up to 7% in the MT and PNW regions (Fig. 4p, q, t). Future changes to grass cover change grass pollen emissions by -18–5% (Fig. 4t). Ragweed emissions have the largest pollen emission maxima decreases over NE and SE (up to 32%; Fig. 4t) because of cropland reduction, however, we note large uncertainties in the spatial distribution of ragweed. Compared to emission changes due to climate or CO2 effects, the maximum pollen emission changes due to land cover changes at the regional scale are relatively small (−32 to 6%).
Discussion
Taken together, we project that under the SSP 585 future climate scenario the pollen season will start earlier (up to 40 d) and become longer (+19 d) with temperature change, and the annual total pollen emission will also increase (16–40%) over the United States. Climate-only driven changes are relatively small (−35 to 40%) compared to large maximum emission increases (up to 200%) when accounting for increased CO2 on pollen production (Fig. 4j, o), although we note the large uncertainties in the CO2 effects on production50. Land cover change can either increase or decrease the future pollen emission maxima, but the regional impacts are smaller (−32 to 6%) than other factors and we conclude that the land cover change influence on pollen emission is likely to have less of an impact than the influence of meteorological factors or CO2.
These projected trends correspond to previous observational studies based on ~30-year historical data analysis, which have identified a 20 d advance, 8 d lengthen of pollen season13, a 46% increase of annual total pollen emission, and a 42.4% enhance of peak pollen emission11. While these prior studies have evaluated total pollen changes in the historical period, this work highlights the importance of studying taxa-specific pollen emission and finds that the influence of climate change on daily pollen emissions varies for different regional forest compositions. We also demonstrate that climate could drive the convergence or divergence of individual taxa pollination, which can magnify or mitigate the climate change impacts and have significant implications for evaluating the consequences of future pollen emissions. While pollen is one of the main causes of human allergies, these projected future changes may lead to growing population’s exposure and the severity of symptoms in individuals with allergic rhinitis and allergic asthmas induced by pollen.
One important limitation to this study is the uncertainties associated with the pollen emission parameterizations. PECM is based on geographically constrained historical pollen counts to derive the relationships between pollen and climate, and sparse data coverage with an urban site focus may limit our ability to parameterize pollen emissions from natural forests41. The sensitivity analysis of model parameters (see “Methods”) indicates the dominant uncertainty is related to the pollen production and the climate-relevant production parameters, which is derived from a limited suite of field-based studies (Supplementary Table 1). More measurements across space and time could improve our understanding of pollen production and better constrain the model simulations. In addition, limited experimental chamber data for a small number of vegetation taxa31–34,38 create uncertainty in the parameterization of the effects of higher CO2 concentrations on pollen production. We broadly applied a doubling of pollen production for the end-of-century CO2 concentrations projected by the SSP 585 scenario, and additional studies that examine the role of CO2 and climate on pollen emission interannual variability are greatly needed. Finally, large uncertainties in future plant community shifts51,52 also limit the simulations of land cover change effects on pollen emission. Recent advances in species distribution modeling include the development of new approaches (e.g., regression-based and machine learning)53, yet there are large uncertainties connected to climate change projection and biotic stresses (e.g., insects, fungi, bacterial)53. Gridded taxa-specific land cover change data for multiple taxa over the entire CONUS is still lacking54–56. Our simulations using PFT-based land cover change data provide overall estimates of vegetation shifts, but the development of the spatially resolved taxa-specific land cover data over a large scale will be crucial to evaluate the effects of plant community composition change on future pollen emission.
Despite these limitations, this study quantifies the potential climate change impacts from CO2, temperature, and precipitation on pollen emission over the US. In the historical period, temperature is the dominant driver of continental-scale pollen emissions and the CO2 effect is relatively small with about 50 ppm increase in the past 30 years13. However, in the future, CO2 concentrations are projected to increase dramatically, especially under the high-emission scenario utilized here (e.g., an increase of about 700 ppm at the end of the century under SSP 58557), and its effects on pollen production have the potential to lead to large pollen increases. Our approach to simulate individual taxa pollen emissions highlights that changing climate will alter the pollen phenology and total production of individual taxa, potentially increasing the seasonal overlap and overall increasing pollen emissions. Land cover change has relatively smaller effects in our simulations, suggesting that the climate drivers may be more important and occurring faster than the shifts in vegetation distribution, however our approach does not account for the spatial shifts of individual taxa ranges, which will certainly be important to assess future regional pollen emission composition. This study provides an important predictive tool to start to investigate the consequences of climate change on future plant communities and their corresponding health effects.
Methods
Pollen emissions model
We utilize the Pollen Emissions model for Climate Models (PECM1.041), which is a prognostic model developed from historical pollen count data from the National Allergy Bureau (NAB) of the American Academy of Allergy, Asthma and Immunology (AAAAI). It simulates the phenology of the 13 most prevalent wind-pollinating taxa (including Acer, Alnus, Ambrosia, Betula, Cupressaceae, Fraxinus, Poaceae, Morus, Pinaceae, Platanus, Populus, Quercus, and Ulmus) over the United States, which accounts for 77% of the total pollen counts across the United States during 2003–201041. It predicts pollen emission for a broad range of taxa at a large geographic scale (25-km resolution in this study), and it is able to capture up to 57% of the variance of pollen season.
The pollen emission model simulates the pollen emission flux of individual taxon (Epol; grains m−2 d−1) over the continental United States as a function of land cover and meteorological factors:
| 1 |
where A is the vegetation land cover fraction (m2 vegetated m−2 total area) based on the observed land cover data (see “Model input data” section), and pfannual (grains m−2 d−1) is the annual pollen productivity factor. Pollen phenology is dictated by γphen, which determines the seasonal pollen emission and is empirically calculated with a Gaussian distribution (Eq. (2)):
| 2 |
where μ and σ are the mean and half-width of the Gaussian, respectively. They are determined by the pollen season to start day of the year (sDOY) and end day of the year (eDOY):
| 3 |
| 4 |
The timing of pollen emission (sDOY and eDOY) for individual taxon is linearly related to the previous-year annual average temperature (PYAAT) with a linear regression between observed first or last day of pollen count and the corresponding temperature (e.g., Supplementary Fig. 8), with a constant factor controlling the width (a =3) based on evaluation versus observed pollen counts.
Here we make three modifications to PECM1.0, including (1) a modification to the annual pollen production (pfannual), (2) the introduction of a precipitation factor (γprecip) to account for the reduction in emissions during wet conditions, and (3) a carbon dioxide factor () that scales the pollen production to CO2 concentrations.
In PECM1.0, taxa-dependent pollen production values by taxa (Pannual; grains tree−1 converted to grains m−2) are derived from limited literature field surveys47,58–66 and are an important input (see “Model sensitivity analysis”). Pannual values are updated from Wozniak et al.41 to include new literature (Supplementary Table 1). However, in PECM1.0, Pannual was held constant for each taxon and does not account for the potential interannual variation of pollen production with time. A few studies have examined the interannual variability of pollen count67,68, but a complication of using atmospheric pollen count to determine the interannual variability of production is that many meteorological factors influence the count (e.g., wind, precipitation, and other meteorological conditions such as boundary layer height). Therefore we scale the literature production factors (Pannual) to a temperature-dependent annual production factor (pfannual) with the observed linear relationship between log-transformed observed annual total pollen counts and previous-year annual average temperature (PYAAT) for each taxa (Eq. (5)) normalized to the temperature-dependent production (Pnorm):
| 5 |
mprod and bprod are the slope and intercept of the linear regression, respectively, of the observed pollen count and temperature and vary for each taxon (Supplementary Table 1). Pnorm is the pollen count at the historical average temperature over the US. One exception is for the genus Alnus, which is has a limited spatial range in the Pacific Northwest (PNW) and we use the historical average temperature in the PNW.
Nine of the 15 total PYAAT-pollen count relationships are statistically significant (P < 0.05, Supplementary Fig. 3), suggesting that this regression captures the influence of prior year temperature on the subsequent year’s pollen production. While there are likely many other factors that influence pollen production (e.g., moisture resources, soil nitrogen, etc.), this provides a simple method to account for year over year pollen production. The annual total pollen counts of a majority of taxa (10 of 15) are positively correlated to temperature (mprod > 0) (including Alnus, Morus, Platanus, Quercus, spring, and late-flowering Ulmus, Cupresseceae, Ambrosia, C3 and C4 Poaceae), suggesting annual pollen production will increase with the warmer temperatures and therefore lead to higher pollen counts. However, several taxa with broad spatial coverage over the US show negative correlations (Acer, Betula, Fraxinus, Populus, Pinaceae), with only Acer and Betula exhibiting statically significant correlations as well as high sensitivity to temperature change (|mprod| >0.05). Overall, the observed relationship between pollen counts and temperature suggests mostly positive correlations with similar statistics demonstrated in Schramm et al.48, with exceptions for some vegetation that exhibits broad spatial coverage (e.g., Acer, Populus, Pinaceae).
Precipitation exerts a dual effect on pollen emission11. High precipitation will remove emitted pollen grains from the atmosphere and can prevent pollen release, while low annual precipitation may limit plant growth and may restrain pollen production. However, due to the complexity of precipitation effects, we did not find robust correlations between precipitation and pollen production at longer time scales. Therefore, we only include the precipitation scavenging effects in this study through a precipitation factor (γprecip). If the precipitation exceeds a minimum intensity of 5 mm d−1 69, all emitted pollen is removed from the atmosphere15,16,23,24, and the precipitation factor (γprecip) is set to zero for that day. While other atmospheric conditions such as wind and humidity are linked to precipitation changes and may inversely lead to higher pollen concentration before or during the rainfall events69,70, this study does not account for meteorological factors other than temperature and precipitation, as this requires coupling with an atmospheric model as in Wozniak et al.41.
The impact of atmospheric CO2 on pollen emissions is based on laboratory studies for limited taxa, which exhibit a wide range of responses to CO2 increases31–34,38. Despite these uncertainties, we consider CO2 effects in the model as an additional sensitivity test to understand the potential of this feedback on future pollen emissions. For the SSP 585 emissions scenario at the end of the century, we conduct an additional simulation where , based on the previous studies31–34,38,50, which essentially assumes a doubling of the pollen production factor for all taxa.
Model input data
PECM can calculate pollen emissions based on two different types of land cover: taxa-based land cover or plant functional type (PFT)-based land cover41. Generally, the taxa-specific model showed better agreement with observed pollen counts than the PFT model41 and we use taxa-based model for the assessment of future climate changes in this work. The taxa-based land cover data of 11 dominant tree taxa are defined by the Biogenic Emission Landuse Dataset version 3 (BELD)71, with satellite-derived land cover data from the Community Land Model 4 (CLM4)72 to provide spatial distributions of Poaceae (C3 and C4 categories) and Ambrosia (calculated using the urban and crop categories)41. While emissions are simulated at the taxa level, for analysis we group the taxa into 4 PFTs: deciduous broadleaf forest (DBL), evergreen needleleaf forest (ENL), grasses (GRA), and ragweed (RAG). DBL contains 9 tree taxa (Acer, Alnus, Betula, Fraxinus, Morus, Platanus, Populus, Quercus, and Ulmus), and ENL includes two tree families (Cupressaceae and Pinaceae). GRA (Poaceae) has two types (C3 and C4 grass) with distinctly different flowering times, and RAG has one taxon (Ambrosia). The emission of each PFT is the aggregate of the modeling emission of all taxa belonging to that PFT.
In the future, the distribution and composition of plant communities can be altered by climate change (e.g., increase the relative abundances of heat-tolerant species, change the distribution of water-demanding species30) or human activities (e.g., impact land cover change and seed dispersal44), yet the effects of climatic drivers on plant communities are poorly understood and further constrain predictive model development55,56. Due to the large uncertainties and the lack of available data for taxa-level spatial distribution shifts, we include a sensitivity test of future land cover change effects on pollen emission using the PFT-based PECM model41. In the climate-based simulations of this study with the taxa-based model, we do not consider the changes to the land cover and land use data in the future and hold vegetation land cover in the model constant. For the sensitivity test, we simulated the future maximum daily pollen emission both using historical (2015) and future (2100) PFT land cover data from GCAM-Demeter land use dataset49, which is driven by the same climate forcing data used for PECM. Compared to the taxa-based model, the PFT version of the model extends to all of North America and simulates higher ENL pollen emissions over the US (Supplementary Fig. 7).
Daily temperature and precipitation data are from 15 models from the Coupled Model Intercomparison Project Phase 6 (CMIP6) (https://esgf-node.llnl.gov/search/cmip6/), with individual model information included in Supplementary Table 3. Climate data are regridded to a 25 km Lambert Conformal Conic projection41 using the Earth System Modeling Framework (ESMF) higher-order patch regridding method over the United States to match the spatial resolution of PECM. We calculate the previous-year annual average temperature (PYAAT) for each model grid cell from daily temperature as a dependent variable to model the pollen season phenology. Because pollen grains are formed in the year previous to flowering, their amount is determined by the photosynthates accumulated in the past summer and correlated with previous-year annual average temperature (PYAAT)73. Pollen emissions are simulated using the meteorology data input from each CMIP6 model and then an evenly weighted multi-model average from 15 PECM simulations is calculated for analysis.
We analyze pollen emissions at a 25-km resolution over the continental US for two periods: the historical (1995–2014) and end-of-century future (2081–2100) and compare differences in pollen season timing and pollen emission magnitude. For the future, we utilize model simulations for two emissions scenarios combining both shared socioeconomic pathways (SSPs) and representative concentration pathways (RCPs), specifically, SSP 245 is the “middle of the road” development pathway (SSP2) with a 4.5 W/m2 radiative forcing level by 2100 corresponding to RCP4.5 scenario, and SSP 585 is the high fossil-fueled development (SSP5) with a higher (8.5 W/m2) radiative forcing level by 2100 (RCP8.5)44,57.
Model sensitivity analysis
To evaluate the uncertainties of the PECM model, we conducted a sensitivity analysis using the Morris method74. The Morris method is a “one-at-a-time” approach, allowing a computationally efficient uncertainty evaluation for a large number of model parameters. Nine parameters for each of the fifteen taxa used in the model are studied in this analysis (Supplementary Table 4). The uncertainty ranges of each parameter are determined by literature values (Pannual) or computed by the 95% confidence level (the linear regression slope (m) and intercept (b) used to calculate start date (msDOY, bsDOY) and end date (meDOY, beDOY) of pollen season, and the pollen production (mprod, bprod)). Because the normalized pollen production (Pnorm) is calculated using the linear regression with mprod and bprod, its uncertainty range is also determined by the range of mprod and bprod. For the phenological Gaussian width a, the maximum and minimum value is obtained by ± 0.2 of the original value (3).
Using the method of Morris from the Sensitivity Analysis Library (SALib) in Python (https://salib.readthedocs.io/en/latest/), we conducted 1000 (N) model runs for each taxon, where the N is determined by the trajectories (p = 100) generated and the number of parameters (k = 9) for each taxa (N = p × (k + 1)). For each run, we compute the regional average maximum pollen emission over the US for 1 year (2015). Analyzing the value of input parameters and the model outputs, the Morris sensitivity package calculates the ratios of model output changes to the parameter variation, then computes the absolute values of mean (μ*) and standard deviation (σ) for each input parameter. The magnitude of μ* shows the overall influence on the model output, where a large μ* indicates the input parameters important in determining the model output. σ is used to detect the non-linearity and interaction of the input parameters, where a large σ suggests the parameter has a nonlinear effect on the model or this parameter is interacting with other parameters.
For each taxon, we computed the ranks of the Morris indices (μ* and σ) for the input parameters and evaluated their relative importance (Supplementary Table 2). The four highest-ranked variables indicate a larger overall influence on model output. Generally, the top four ranked parameters of μ* and σ are similar between taxa, although with slightly different orders. Overall, the production-related parameters have the highest μ* and σ for most taxa, where the annual pollen production (Pannual) and normalization parameter(Pnorm) are the two most important factors. This rank is expected as these two factors directly impact the magnitude of simulated pollen emission. Phenology factors (e.g., msDOY, bsDOY, meDOY, and beDOY) control the timing and variation of daily pollen emissions and are relatively less influential on the simulated pollen. However, for the taxa that exhibit a strong temperature dependence on the pollen season duration (e.g., Alnus, Platanus, Populus, late-flowering Ulmus, C4 grass), the pollen phenology factors are more important for the simulated maximum pollen emission (Supplementary Table 2).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This work was supported by the National Science Foundation grant AGS-1821173 to ALS. Climate data are provided by the CMIP6 archive (https://esgf-node.llnl.gov/search/cmip6/; see detailed model information in “Methods”). We gratefully acknowledge the AAAAI United States pollen count data in the development of the PECM model. We thank Manish Verma at the University of Michigan Consulting for Statistics, Computing and Analytics Research (CSCAR) for guidance on model uncertainty analysis.
Source data
Author contributions
Y.Z. performed all of the research included in the paper. A.S. designed and discussed the outline of the research with Y.Z. and advised on the methodology and the visualizations produced by this work. Designing and writing of the paper was completed by Y.Z. A.S. contributed to the improvement of the writing clarity and grammar of the manuscript.
Peer review
Peer review information
Nature Communications thanks William Anderegg and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
Raw data of the simulated historical and future daily pollen emission for 15 CMIP6 models generated in this study are available from www.deepblue.lib.umich.edu under access code 10.7302/1s0g-b46875. The processed data used to produce all figures are available at 10.7302/628t-r41676. Source data are provided with this paper.
Code availability
The source code of the pollen emission model (PECM) used to produce pollen data is available in the Github repository (https://github.com/steiner-lab/pecm) under access code 10.5281/zenodo.587417777.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yingxiao Zhang, Email: yingxz@umich.edu.
Allison L. Steiner, Email: alsteine@umich.edu
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-28764-0.
References
- 1.Zhang J, et al. Sperm cells are passive cargo of the pollen tube in plant fertilization. Nat. Plants. 2017;3:1–5. doi: 10.1038/nplants.2017.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Després V, et al. Primary biological aerosol particles in the atmosphere: a review. Tellus B Chem. Phys. Meteorol. 2012;64:15598. [Google Scholar]
- 3.Steiner AL, et al. Pollen as atmospheric cloud condensation nuclei. Geophys. Res. Lett. 2015;42:3596–3602. [Google Scholar]
- 4.Li W, et al. Overview of primary biological aerosol particles from a Chinese boreal forest: insight into morphology, size, and mixing state at microscopic scale. Sci. Total Environ. 2020;719:137520. doi: 10.1016/j.scitotenv.2020.137520. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt CW. Pollen overload: seasonal allergies in a changing climate. Environ. Health Perspect. 2016;124:A71–A75. doi: 10.1289/ehp.124-A70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Amato G, et al. The effects of climate change on respiratory allergy and asthma induced by pollen and mold allergens. Allergy. 2020;75:2219–2228. doi: 10.1111/all.14476. [DOI] [PubMed] [Google Scholar]
- 7.Neumann JE, et al. Estimates of present and future asthma emergency department visits associated with exposure to oak, birch, and grass pollen in the United States. GeoHealth. 2019;3:11–27. doi: 10.1029/2018GH000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anenberg SC, et al. Impacts of oak pollen on allergic asthma in the United States and potential influence of future climate change. GeoHealth. 2017;1:80–92. doi: 10.1002/2017GH000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beggs PJ. Impacts of climate change on aeroallergens: past and future. Clin. Exp. Allergy. 2004;34:1507–1513. doi: 10.1111/j.1365-2222.2004.02061.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim SH, Park HS, Jang JY. Impact of meteorological variation on hospital visits of patients with tree pollen allergy. BMC Public Health. 2011;11:1–8. doi: 10.1186/1471-2458-11-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, et al. Allergenic pollen season variations in the past two decades under changing climate in the United States. Glob. Chang. Biol. 2015;21:1581–1589. doi: 10.1111/gcb.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziska LH, et al. Temperature-related changes in airborne allergenic pollen abundance and seasonality across the northern hemisphere: a retrospective data analysis. Lancet Planet. Heal. 2019;3:e124–e131. doi: 10.1016/S2542-5196(19)30015-4. [DOI] [PubMed] [Google Scholar]
- 13.Anderegg WRL, et al. Anthropogenic climate change is worsening North American pollen seasons. Proc. Natl Acad. Sci. USA. 2021;118:e2013284118. doi: 10.1073/pnas.2013284118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lake IR, et al. Climate change and future pollen allergy in Europe. Environ. Health Perspect. 2017;125:385–391. doi: 10.1289/EHP173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruffaerts N, et al. Comparative long-term trend analysis of daily weather conditions with daily pollen concentrations in Brussels, Belgium. Int. J. Biometeorol. 2018;62:483–491. doi: 10.1007/s00484-017-1457-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sofiev M, et al. A numerical model of birch pollen emission and dispersion in the atmosphere. Description of the emission module. Int. J. Biometeorol. 2013;57:45–58. doi: 10.1007/s00484-012-0532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grewling, et al. Variation in Artemisia pollen seasons in Central and Eastern Europe. Agric. Meteorol. 2012;160:48–59. [Google Scholar]
- 18.Recio M, et al. Intensity and temporality of airborne Quercus pollen in the southwest Mediterranean area: Correlation with meteorological and phenoclimatic variables, trends and possible adaptation to climate change. Agric. Meteorol. 2018;250–251:308–318. [Google Scholar]
- 19.Fu YH, Campioli M, Deckmyn G, Janssens IA. The impact of winter and spring temperatures on temperate tree budburst dates: results from an experimental climate manipulation. PLoS ONE. 2012;7:e47324. doi: 10.1371/journal.pone.0047324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Vliet AJH, Overeem A, De Groot RS, Jacobs AFG, Spieksma FTM. The influence of temperature and climate change on the timing of pollen release in the Netherlands. Int. J. Climatol. 2002;22:1757–1767. [Google Scholar]
- 21.Emberlin J, et al. Responses in the start of Betula (birch) pollen seasons to recent changes in spring temperatures across Europe. Int. J. Biometeorol. 2002;46:159–170. doi: 10.1007/s00484-002-0139-x. [DOI] [PubMed] [Google Scholar]
- 22.Ariano R, Canonica GW, Passalacqua G. Possible role of climate changes in variations in pollen seasons and allergic sensitizations during 27 years. Ann. Allergy, Asthma Immunol. 2010;104:215–222. doi: 10.1016/j.anai.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Green BJ, Dettmann M, Yli-Panula E, Rutherford S, Simpson R. Atmospheric Poaceae pollen frequencies and associations with meteorological parameters in Brisbane, Australia: A 5-year record, 1994–1999. Int. J. Biometeorol. 2004;48:172–178. doi: 10.1007/s00484-004-0204-8. [DOI] [PubMed] [Google Scholar]
- 24.Wang, Y. et al. Disproportionate control on aerosol burden by light rain. Nat. Geosci. 1–5. 10.1038/s41561-020-00675-z (2021).
- 25.Makra, L., Matyasovszky, I., Páldy, A. & Deák, Á. J. The influence of extreme high and low temperatures and precipitation totals on pollen seasons of Ambrosia, Poaceae and Populus in Szeged, southern Hungary. Grana51, 215–227 (2012).
- 26.Collins, M. et al. Climate Change 2013-The Physical Science Basis: Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. 1029–1136 (Cambridge University Press, 2013).
- 27.Feeley, K. J., Bravo-Avila, C., Fadrique, B., Perez, T. M. & Zuleta, D. Climate-driven changes in the composition of New World plant communities. Nat. Clim. Chang. 10, 965–970 (2020).
- 28.Drake, B. G., Gonzàlez-Meler, M. A. & Long, S. P. MORE EFFICIENT PLANTS: a consequence of rising atmospheric CO2? Annu. Rev. Plant Physiol. Plant Mol. Biol.48, 609–639 (1997). [DOI] [PubMed]
- 29.LaDeau S, Clark J. Pollen production by Pinus taeda growing in elevated atmospheric CO2. Funct. Ecol. 2006;20:541–547. [Google Scholar]
- 30.Darbah JNT, et al. Effects of decadal exposure to interacting elevated CO2 and/or O3 on paper birch (Betula papyrifera) reproduction. Environ. Pollut. 2008;155:446–452. doi: 10.1016/j.envpol.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 31.Kim KR, et al. Does the increase in ambient CO2 concentration elevate allergy risks posed by oak pollen? Int. J. Biometeorol. 2018;62:1587–1594. doi: 10.1007/s00484-018-1558-7. [DOI] [PubMed] [Google Scholar]
- 32.Wayne P, Foster S, Connolly J, Bazzaz F, Epstein P. Production of allergenic pollen by ragweed (Ambrosia artemisiifolia L.) is increased in CO2-enriched atmospheres. Ann. Allergy, Asthma Immunol. 2002;88:279–282. doi: 10.1016/S1081-1206(10)62009-1. [DOI] [PubMed] [Google Scholar]
- 33.Rogers CA, et al. Interaction of the onset of spring and elevated atmospheric CO2 on ragweed (Ambrosia artemisiifolia L.) pollen production. Environ. Health Perspect. 2006;114:865–869. doi: 10.1289/ehp.8549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albertine, J. M. et al. Projected carbon dioxide to increase grass pollen and allergen exposure despite higher ozone levels. PLoS ONE9, e111712 (2014). [DOI] [PMC free article] [PubMed]
- 35.Ahlholm, J. U., Helander, M. L. & Savolainen, J. Genetic and environmental factors affecting the allergenicity of birch (Betula pubescens ssp. czerepanovii [Orl.] Hämet-Ahti) pollen. Clin. Exp. Allergy28, 1384–1388 (1998). [DOI] [PubMed]
- 36.Clot B. Trends in airborne pollen: an overview of 21 years of data in Neuchâtel (Switzerland) Aerobiologia (Bologna). 2003;19:227–234. [Google Scholar]
- 37.Ziello C, et al. Changes to airborne pollen counts across Europe. PLoS ONE. 2012;7:e34076. doi: 10.1371/journal.pone.0034076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Isukapalli SS, Bielory L, Georgopoulos PG. Bayesian analysis of climate change effects on observed and projected airborne levels of birch pollen. Atmos. Environ. 2013;68:64–73. doi: 10.1016/j.atmosenv.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang R, et al. Development of a regional-scale pollen emission and transport modeling framework for investigating the impact of climate change on allergic airway disease. Biogeosciences. 2014;11:1461–1478. doi: 10.5194/bgd-10-3977-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamaoui-Laguel L, et al. Effects of climate change and seed dispersal on airborne ragweed pollen loads in Europe. Nat. Clim. Chang. 2015;5:766–771. [Google Scholar]
- 41.Wozniak MC, Steiner AL. A prognostic pollen emissions model for climate models (PECM1.0) Geosci. Model Dev. 2017;10:4105–4127. [Google Scholar]
- 42.Lo F, Bitz CM, Battisti DS, Hess JJ. Pollen calendars and maps of allergenic pollen in North America. Aerobiologia (Bologna). 2019;35:613–633. doi: 10.1007/s10453-019-09601-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eyring V, et al. Overview of the Coupled Model Intercomparison Project Phase 6 (CMIP6) experimental design and organization. Geoscientific Model Development. Eur. Geosci. Union. 2016;9:1937–1958. [Google Scholar]
- 44.Riahi K, et al. The shared socioeconomic pathways and their energy, land use, and greenhouse gas emissions implications: an overview. Glob. Environ. Chang. 2017;42:153–168. [Google Scholar]
- 45.Garner WW. Comparative responses of long-day and short-day plants to relative length of day and night. Plant Physiol. 1933;8:347–356. doi: 10.1104/pp.8.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reekie JYC, Hicklenton PR, Reekie EG. Effects of elevated CO2 on time of flowering in four short-day and four long-day species. Can. J. Bot. 1994;72:533–538. [Google Scholar]
- 47.Fumanal, B., Chauvel, B. & Bretagnolle, F. Estimation of the pollen and seed production of common ragweed in Europe. Ann. Agric. Environ. Med. 14, 233–236 (2007). [PubMed]
- 48.Schramm PJ, et al. A systematic review of the effects of temperature and precipitation on pollen concentrations and season timing, and implications for human health. Int. J. Biometeorol. 2021;65:1615–1628. doi: 10.1007/s00484-021-02128-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen M, et al. Global land use for 2015–2100 at 0.05° resolution under diverse socioeconomic and climate scenarios. Sci. Data. 2020;7:1–11. doi: 10.1038/s41597-020-00669-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ziska LH, Caulfield FA. Rising CO2 and pollen production of common ragweed (Ambrosia artemisiifolia), a known allergy-inducing species: Implications for public health. J. Plant Physiol. 2000;27:893–898. [Google Scholar]
- 51.Alexander JM, et al. Lags in the response of mountain plant communities to climate change. Glob. Change Biol. 2018;24:563–579. doi: 10.1111/gcb.13976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Komatsu KJ, et al. Global change effects on plant communities are magnified by time and the number of global change factors imposed. Proc. Natl Acad. Sci. USA. 2019;116:17867–17873. doi: 10.1073/pnas.1819027116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pecchi M, et al. Species distribution modelling to support forest management. A literature review. Ecol. Modell. 2019;411:108817. [Google Scholar]
- 54.Iverson, L. R., Prasad, A. M., Matthews, S. N. & Peters, M. Estimating potential habitat for 134 eastern US tree species under six climate scenarios. For. Ecol. Manag. 254, 390–406 (2008).
- 55.Noce S, Collalti A, Santini M. Likelihood of changes in forest species suitability, distribution, and diversity under future climate: the case of Southern Europe. Ecol. Evol. 2017;7:9358–9375. doi: 10.1002/ece3.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garzón MB, et al. Predicting habitat suitability with machine learning models: the potential area of Pinus sylvestris L. in the Iberian Peninsula. Ecol. Modell. 2006;197:383–393. [Google Scholar]
- 57.Meinshausen M, et al. The SSP greenhouse gas concentrations and their extensions to 2500. Geosci. Model Sev. Discuss. 2019;2019:1–77. [Google Scholar]
- 58.Katz DSW, Morris JR, Batterman SA. Pollen production for 13 urban North American tree species: allometric equations for tree trunk diameter and crown area. Aerobiologia (Bologna). 2020;36:401–415. doi: 10.1007/s10453-020-09638-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Molina, R. T., López, F. G., Rodríguez, A. M. & Palaciso, I. S. Pollen production in anemophilous trees. Grana35, 38–46 (1996).
- 60.Helbig N, Vogel B, Vogel H, Fiedler F. Numerical modelling of pollen dispersion on the regional scale. Aerobiol. 2004;20:3–19. [Google Scholar]
- 61.Jato V, Rodriguez-Rajo FJ, Aira MJ. Use of phenological and pollen-production data for interpreting atmospheric birch pollen curves. Ann. Agric. Environ. Med. 2007;14:271–280. [PubMed] [Google Scholar]
- 62.Gómez-Casero, M. T., Hidalgo, P. J., García-Mozo, H., Domínguez, E. & Galán, C. Pollen biology in four Mediterranean Quercus species. Grana43, 22–30 (2010).
- 63.Hidalgo, P. J., Galán, C. & Domínguez, E. Pollen production of the genus Cupressus. Grana38, 296–300 (2010).
- 64.Damialis, A., Fotiou, C., Halley, J. M. & Vokou, D. Effects of environmental factors on pollen production in anemophilous woody species. Trees25, 253–264 (2011).
- 65.Aboulaïch, N., Bouziane, H., El Kadiri, M. & Riadi, H. Male phenology and pollen production of Cupressus sempervirens in Tetouan (Morocco). Grana47, 130–138 (2008).
- 66.Prieto-Baena, J. C., Hidalgo, P. J., Domínguez, E. & Galán, C. Pollen production in the Poaceae family. Grana42, 153–159 (2011).
- 67.McLauchlan KK, Barnes CS, Craine JM. Interannual variability of pollen productivity and transport in mid-North America from 1997 to 2009. Aerobiologia (Bologna). 2011;27:181–189. [Google Scholar]
- 68.Latorre, F., Rotundo, C., Sierra, M. L. & Fassola, H. Daily, seasonal, and interannual variability of airborne pollen of Araucaria angustifolia growing in the subtropical area of Argentina. Aerobiologia36, 277–290 (2020)..
- 69.Kluska K, Piotrowicz K, Kasprzyk I. The impact of rainfall on the diurnal patterns of atmospheric pollen concentrations. Agric. Meteorol. 2020;291:108042. [Google Scholar]
- 70.Huffman JA, et al. High concentrations of biological aerosol particles and ice nuclei during and after rain. Atmos. Chem. Phys. 2013;13:6151–6164. [Google Scholar]
- 71.Kinnee E, Geron C, Pierce T. United State land use inventory for estimating biogenic ozone precursor emissions. Ecol. Appl. 1997;7:46–58. [Google Scholar]
- 72.Oleson, K. et al. Technical Description of version 4.5 of the Community Land Model (CLM) Report NCAR/TN-503+STR. 10.5065/D6RR1W7M (2013).
- 73.Emberlin JC, Norris-Hill J, Bryant RH. A calendar for tree pollen in London. Taylor Fr. 1990;29:301–309. [Google Scholar]
- 74.Morris MD. Factorial sampling plans for preliminary computational experiments. Technometrics. 1991;33:161–174. [Google Scholar]
- 75.Zhang, Y. & Steiner, A. Simulated historical (1995-2014) and future (2081-2100) pollen emission using PECM2.0 Raw data [Data set], University of Michigan - Deep Blue Data. 10.7302/1s0g-b468 (2022).
- 76.Zhang, Y. & Steiner, A. Simulated pollen emission using PECM Averaged data [Data set], University of Michigan - Deep Blue Data. 10.7302/628t-r416 (2022).
- 77.Zhang, Y. & Steiner, A. Projected climate-driven changes in pollen emission season length and magnitude over the continental United States. Github 10.5281/ZENODO.5874177 (2022) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data of the simulated historical and future daily pollen emission for 15 CMIP6 models generated in this study are available from www.deepblue.lib.umich.edu under access code 10.7302/1s0g-b46875. The processed data used to produce all figures are available at 10.7302/628t-r41676. Source data are provided with this paper.
The source code of the pollen emission model (PECM) used to produce pollen data is available in the Github repository (https://github.com/steiner-lab/pecm) under access code 10.5281/zenodo.587417777.