Abstract
Bacterial vaginosis (BV) is the most common vaginal infection affecting women worldwide. This infection is characterized by the loss of the dominant Lactobacillus community in the vaginal microbiota and an increase of anaerobic bacteria, that leads to the formation of a polymicrobial biofilm, mostly composed of Gardnerella spp. Treatment of BV is normally performed using broad-spectrum antibiotics, such as metronidazole and clindamycin. However, the high levels of recurrence of infection after treatment cessation have led to a demand for new therapeutic alternatives. Thymbra capitata essential oils (EOs) are known to have a wide spectrum of biological properties, including antibacterial activity. Thus, herein, we characterized two EOs of T. capitata and tested their antimicrobial activity as well as some of their main components, aiming to assess possible synergistic effects. Our findings showed that carvacrol and ρ-cymene established a strong synergistic antimicrobial effect against planktonic cultures of Gardnerella spp. On biofilm, carvacrol and linalool at sub-MIC concentrations proved more efficient in eliminating biofilm cells, while showing no cytotoxicity observed in a reconstituted human vaginal epithelium. The antibiofilm potential of the EOs and compounds was highlighted by the fact cells were not able to recover culturability after exposure to fresh medium.
Subject terms: Microbiology, Diseases
Introduction
Bacterial vaginosis (BV) is the most frequent gynaecological infection affecting women of reproductive age worldwide1. This infection is associated with vaginal discharge, unpleasant smell, an increase in vaginal pH, and the presence of clue cells2,3. Besides these common symptoms, BV has also been related to more serious health complications including preterm delivery4, pregnancy losses5, pelvic inflammatory disease6, and acquisition of sexually transmitted diseases7. BV is described by a decrease in the number of dominant lactic acid-producing species, normally Lactobacillus species, associated with healthy vaginal microbiota, and an overgrowth of strict and facultative anaerobic bacteria, such as Gardnerella spp., Fannyhessea vaginae (former Atopobium vaginae8), Prevotella spp., Mobiluncus spp., and other bacterial vaginosis-associated bacteria (BVAB)9,10. It is well known that BV involves the formation of a polymicrobial biofilm on the vaginal epithelial cells11, where bacterial species interact and develop the infection12. Gardnerella species have been observed as the most common bacteria present in cases of BV however, the role of this pathogen in the infection is still ambiguous13 since Gardnerella colonization does not always cause BV14. Recently a study proposed that what has been previously referred to as Gardnerella vaginalis, in fact, includes 13 different species of the genus Gardnerella, of which 4 have been described: G. vaginalis, G. piotii, G. leopoldii, and G. swidsinskii15. Studies regarding Gardnerella virulence features have suggested that some strains of Gardnerella have a preponderant role in initiating the colonization of the vaginal epithelium, outcompeting other vaginal pathogens16, followed by the formation of biofilm, affecting the progress of BV12,17. However, it is not currently clear if a specific Gardnerella species has higher virulence potential than others.
The treatment of BV is usually performed using the current recommended broad-spectrum antibiotics, namely metronidazole, and clindamycin18. However, a major issue related to BV is the fact that the treatment with conventional antibiotics often results in high recurrence rates19. This has been suggested to be a consequence of the inability of conventional therapies in eradicating the polymicrobial biofilm, which can be attributed to the presence of multidrug resistance species within the consortia20,21, which led to rapid regrowth after treatment cessation22. This poor treatment efficacy has led to an urgent need for new therapies to treat this infection23. As such, alternative therapies using natural products have been proposed, to overcome the resistance to antibiotics and prevent serious complications24–26.
Essential oils (EOs) are complex mixtures of natural volatile compounds extracted from aromatic plants, with low molecular weight, and have been used since ancient times for therapeutic purposes27. They have been recognized for their good antibacterial28, antifungal29, antioxidant30, and antiviral31 properties. In fact, regarding vaginal infections, several EOs have been tested with promising results32–34. However, one of the major disadvantages of using EOs is the fact that, as natural compounds, their compositions might differ between collections, possibly resulting in variations in their activities35. As such, studying the activity of the single compounds from EOs can provide important insights regarding their antimicrobial activity. The Portuguese flora is constituted by approximately 3800 species, of these about 600 taxa are aromatic plants36,37. Lamiaceae family is one of the most representative taxon38, with 29 genera represented in Portugal39. Lavandula, Thymus, and Thymbra are among the most important genera due to their wide use in Portuguese traditional medicines37. The genus Thymbra is represented in Portugal by only one species, Thymbra capitata39. Thymbra capitata (L.) Cav. is a plant widespread on the Mediterranean coast40,41 and its essential oil (EO) is characterized by high carvacrol amounts40,42. Previous studies demonstrated the biological activities of T. capitata EOs, such as antibacterial43, antifungal41, and antioxidant44 effects against different microorganisms. We recently demonstrated the potential of T. capitata EO against Gardnerella spp. growing planktonically and as biofilm45.
In this work, we characterized two samples of T. capitata EO, collected in different locations and tested their antimicrobial activity against the 4 described Gardnerella species. We also assessed potential synergism between some of the EO main components, aiming to develop a future topical application against Gardnerella biofilms.
Results
Essential oils composition
The results from the analysis of EOs revealed that the composition of the two oils was similar, with both EOs being mainly composed of carvacrol (73.9–80.0%) and its biogenetic precursors, γ-terpinene (3.4–7.4%) and p-cymene (4.1–4.9%). The full composition of both oils is detailed in Table 1.
Table 1.
Constituents of two samples of essential oils from Thymbra capitata.
| Compound* | RI SPB-1a | RI SW 10b | Sample Ben. (%) | Sample Car. (%) |
|---|---|---|---|---|
| α-Thujene | 922 | 1029 | 0.4 | 1.4 |
| α-Pinene | 930 | 1030 | 0.3 | 0.6 |
| Oct-1-en-3-ol | 956 | 1440 | t | 0.1 |
| Sabinene | 964 | 1128 | t | t |
| β-Pinene | 970 | 1118 | 0.3 | 0.1 |
| Myrcene | 980 | 1161 | 1.1 | 1.8 |
| α-Phellandrene | 997 | 1171 | 0.1 | 0.2 |
| 3- Carene | 1003 | 1155 | t | 0.1 |
| α-Terpinene | 1010 | 1187 | 0.4 | 1.5 |
| p-Cymene | 1011 | 1275 | 4.1 | 4.9 |
| Limonene | 1020 | 1206 | 0.1 | 0.2 |
| β-Phellandrene | 1020 | 1215 | 0.1 | 0.2 |
| Z-β-Ocimene | 1025 | 1235 | t | t |
| E-β-Ocimene | 1035 | 1253 | t | 0.1 |
| γ-Terpinene | 1046 | 1249 | 3.4 | 7.4 |
| trans-Sabinene hydrate | 1050 | 1459 | 0.4 | 0.6 |
| Cymenene | 1073 | 1440 | 0.1 | 0.1 |
| cis-Sabinene hydrate | 1080 | 1544 | 0.2 | 0.1 |
| Linalool | 1081 | 1543 | 1.0 | 0.3 |
| trans-p-2-Menthen-1-ol | 1122 | 1623 | t | t |
| Borneol | 1144 | 1695 | 0.1 | 0.2 |
| Terpinene-4-ol | 1158 | 1597 | 0.7 | 0.7 |
| trans-Dihydrocarvone | 1167 | 1602 | t | t |
| α-Terpineol | 1169 | 1692 | 0.1 | 0.1 |
| Neral | 1214 | 1679 | t | t |
| Geraniol | 1233 | 1842 | t | 0.1 |
| Geranial | 1242 | 1730 | 0.1 | 0.1 |
| Thymol | 1268 | 2183 | 0.1 | 0.2 |
| Carvacrol | 1275 | 2212 | 80.0 | 73.9 |
| E-Caryophyllene | 1408 | 1590 | 1.9 | 3.4 |
| Aromadendrene | 1425 | 1600 | t | t |
| α-Humulene | 1443 | 1662 | 0.1 | t |
| Allo-aromadendrene | 1445 | 1636 | 0.1 | 0.1 |
| Bicyclogermacrene | 1481 | 1726 | 0.1 | 0.1 |
| Caryophyllene oxide | 1557 | 1968 | 0.2 | 0.2 |
| Total identified | 95.8 | 99.0 |
*Compounds listed in order of elution in the polydimethylsiloxane column (SPB-1 column).
aRI SPB 1: GC retention indices relative to C9-C23 n-alkanes on the SPB-1 column.
bRI SW 10: GC retention indices relative to C9-C23 n-alkanes on the Supelcowax-10 column. t = traces ≤ 0.05%.
Minimal inhibitory concentration and minimal lethal concentration
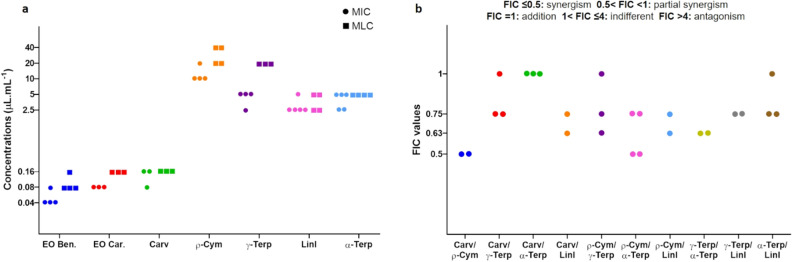
It has been shown that the biological effects of EOs are possibly due to the combined effects of their components, particularly the main ones46. In order to recognize the antimicrobial activity of some of these compounds, that are commercially available, the values of minimal inhibitory concentration (MIC) and minimal lethal concentration (MLC) were first determined. As shown in Fig. 1a, both the EOs had similar antimicrobial activity against the selected Gardnerella isolate. Of the tested individual components, carvacrol was by far the most potent, with a MIC value very similar to the whole EO. Despite having similar high concentrations in both EO, ρ-cymene and γ-terpinene had a fourfold difference in the MIC values, while linalool, which was present in a lower concentration, reported a similar antimicrobial activity as γ-terpinene. Interestingly, for all the active compounds, the values of MLC were almost coincident with the values of MIC.
Figure 1.
Antimicrobial activity of two EOs from Thymbra capitata, carvacrol, ρ-cymene, γ-terpinene, linalool, and α-terpinene against Gardnerella sp. UM241. (a) Determination of the minimum inhibitory and minimum lethal concentration. (b) Results from checkerboard assays of combinations between the selected individual compounds from the EO. Individual points represent distinct checkerboard combinations. In both panels, each point represents the results from independent assays.
Combined effects of individual compounds of EOs
It has been previously shown that the combined effect of some individual compounds can enhance their antimicrobial activities47,48. Taking into consideration the MIC determinations, we assessed possible synergism between the individual components tested. As shown in Fig. 1b, different effects were observed wherein combinations of carvacrol and ρ-cymene resulted in the highest synergistic activity. No antagonistic effects were observed.
Activity of EOs and compounds on a Gardnerella biofilm
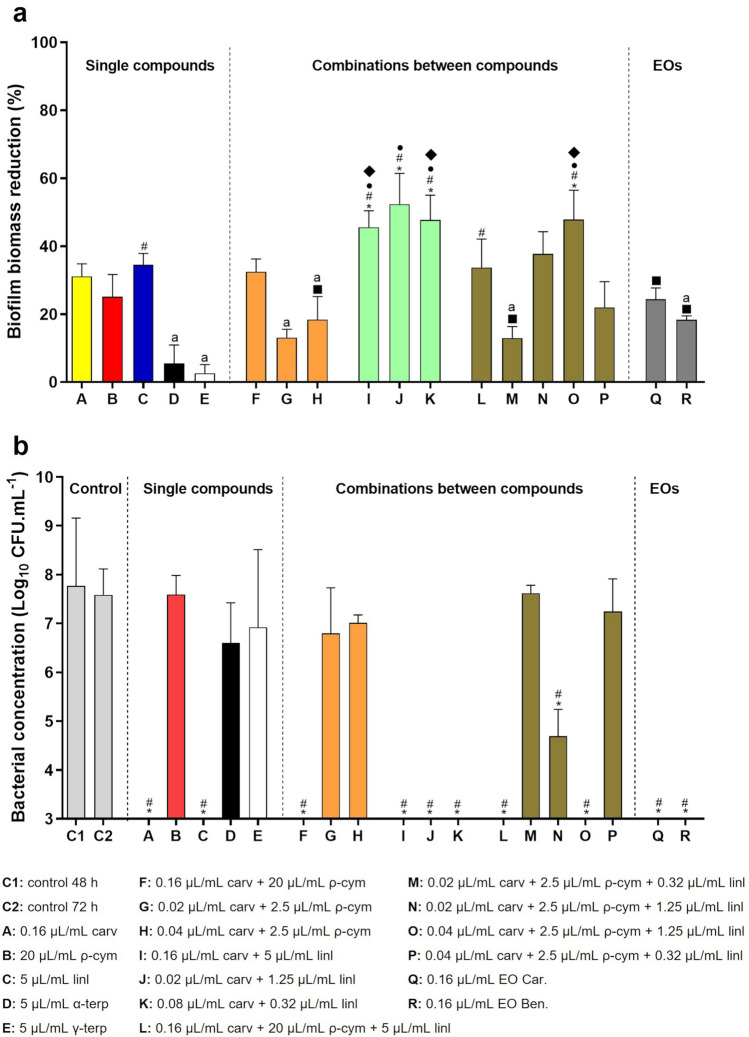
Since BV is well-known to be associated with biofilm formation, mainly constituted by Gardnerella11, we further tested the EO and some of their components' anti-biofilm activity. As shown in Fig. 2a, α- and γ-terpinene had no effect in reducing the biomass of the biofilm, despite their antimicrobial activity assessed against planktonic cultures. Carvacrol, ρ-cymene, and linalool add a similar effect at either of the two EOs tested. Interestingly, several combinations of carvacrol and linalool, at sub-mic concentrations, revealed a significantly higher ability to reduce the biofilm biomass, as compared with the MLC concentrations of the EOs. The addition of ρ-cymene did not enhance the carvacrol and linalool synergistic effect. Interestingly, when assessing cell culturability, the total reduction of CFU’s was observed for both EOs, carvacrol, linalool, or any mixture of these 2 components (Fig. 2b).
Figure 2.
Activity of single components, combinations between compounds and EOs from Thymbra capitata on a 48 h Gardnerella sp. UM241 biofilm. (a) Effect on biofilm biomass reduction. Results are presented in mean percentage (%) of biofilm biomass reduction. (b) Effect on biofilm cells culturability. Results are represented as mean of Log (CFU.mL−1) with a limit of detection of Log=3. Primary colors were selected to represent the individual components and the mixtures represent the color combination of the individual components. Statistical analysis was performed using one-way ANOVA (n ≥ 3). In panel (a), differences are showed, when p < 0.05, by asterisk symbol when comparing with 5 µL/mL α-terp; hash symbol when comparing with 5 µL/mL γ-terp; bullet symbol when comparing with 0.02 µL/mL carv + 2.5 µL/mL ρ-cym; filled square symbol when comparing with 0.02 µL/mL carv + 1.25 µL/mL linl and filled diamond symbol when comparing with 0.02 µL/mL carv + 2.5 µL/mL ρ-cym + 0.32 µL/mL linl. All conditions are statistically different from the 48 h-biofilm control except for the cases marked (a). In panel (b), differences are represented by asterisk symbol when comparing with control 48 h and hash symbol when comparing with control 72 h, when p < 0.05.
The effect of DMSO on Gardnerella sp. UM241 cells culturability was also evaluated and the results are described in Supplementary Fig. 1. DMSO did not show any negative effect on cells viability at the different concentrations tested.
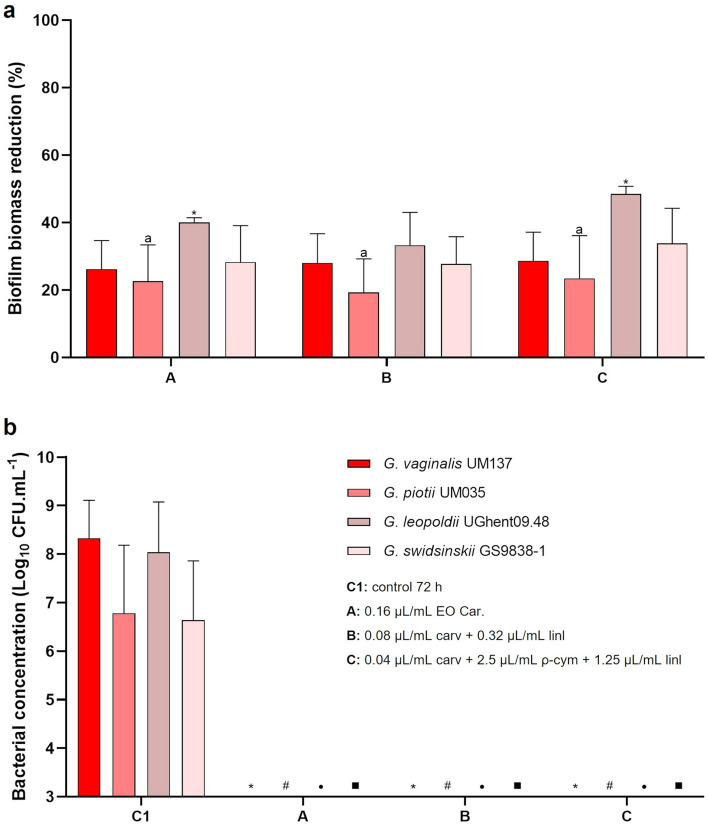
Other four Gardnerella species were used to assess the activity of all components on biofilms. We have chosen the conditions that were able to eliminate the growth of Gardnerella sp. UM241, namely the EO sample from Carvoeiro, the combination between carvacrol and linalool with the lowest amount of linalool, and a combination of 3 selected compounds (at their lowest concentrations tested). The results are shown in Fig. 3. The capacity of the compounds to reduce biofilm biomass (Fig. 3a) was similar to that observed when tested in Gardnerella sp. UM241 biofilm. When evaluating the results of bacterial culturability (Fig. 3b) it is possible to verify the inhibition of growth for all the species in all the tested conditions.
Figure 3.
Activity of EO Car. and two combinations among compounds on a 48 h biofilm of G. vaginalis UM137, G. piotii UM035, G. leopoldii UGent 09.48 and G. swidsinskii GS 9838–1. a: Effect on biofilm biomass reduction. Results are presented in percentage (%) of biofilm biomass reduction. Error bars represent s.e.m. Statistical differences were found for all the conditions when compared with the 72 h-biofilm control, except for G. leopoldii where no differences were found (a) (t-test, n = 4). Statistical differences were found in G. leopoldii, between conditions A and C (asterisk symbol) (t-test, n = 4). (b) Effect on biofilm cells culturability. Results are represented as mean of Log (CFU.mL−1) with a limit of detection of Log = 3. Error bars represent s.d. Statistical analysis was performed using t-test (n = 4). Differences are represented when p < 0.05, when compared with control 72 h of G. vaginalis UM137 (asterisk symbol), G. piotii UM035 (hash symbol), G. leopoldii UGent 09.48 (bullet symbol) and G. swidsinskii GS 9838-1 (filled square symbol).
Effect of EOs and compounds on Gardnerella biofilm structure and viability
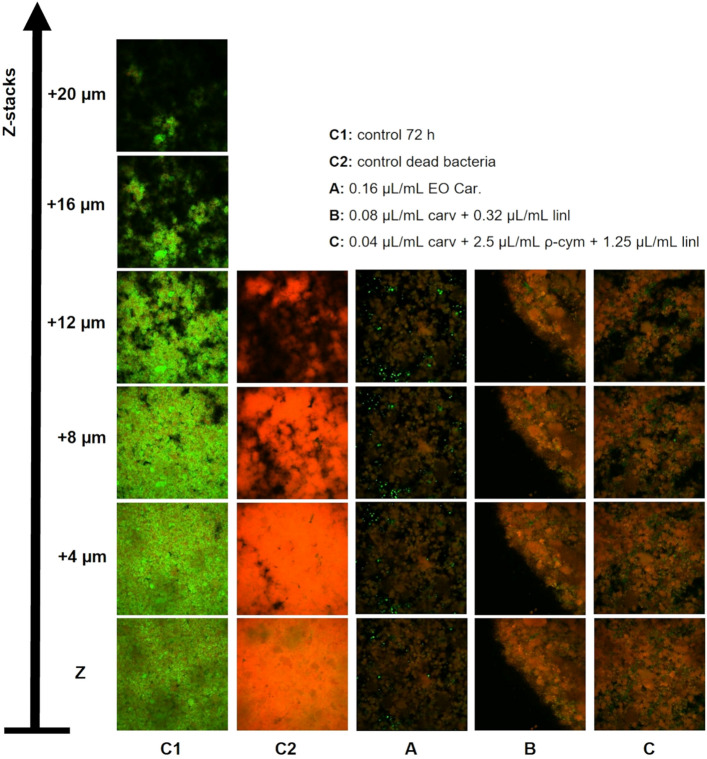
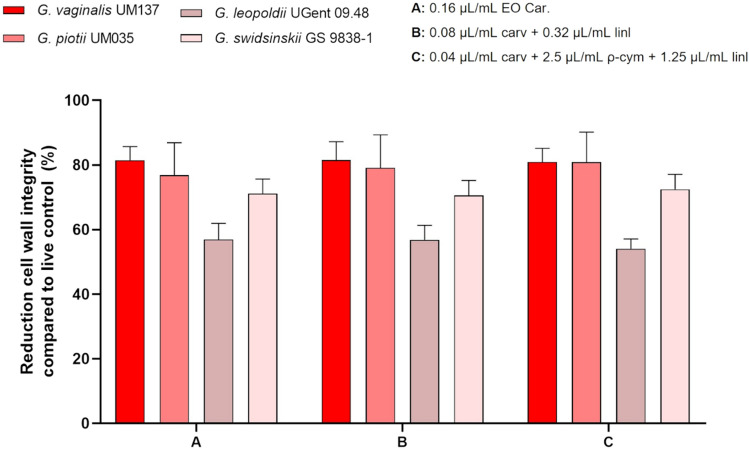
Since we observed a total reduction of cultivable bacteria without completely removing the biofilm structure, we decided to analyze the biofilm structure after the antimicrobial challenge, by using LIVE/DEAD staining and CSLM observation. As shown in Fig. 4, the big majority of biofilm cells treated with the EO or the two most promising combinations of components, presented a damaged cell wall, similar to the dead cells from biofilm control. Nonetheless, some cells still keep an intact cell wall, but they cannot be recovered (Fig. 3b), which suggests the presence of viable but not cultivable cells (VBNC)49. To confirm the CLSM observation and to quantitatively determine the % of viable or dead cells in biofilms formed by the 4 species of Gardnerella, after antimicrobial challenge, we performed a fluorimeter assay, based on LIVE/DEAD staining. As shown in Fig. 5, despite no culturable cells being recovered, 20 to 40% of biofilm cells maintained their cell wall intact, being G. leopoldii UGent 09.48 the strain with higher tolerance to the tested antimicrobial agents.
Figure 4.
Representative images of CLSM analysis of G. vaginalis UM137 72 h biofilm after the activity of EO Car. and some specific combinations using LIVE/DEAD staining. The images were acquired using an objective of 10 × and z-stacks of 4 µm.
Figure 5.
Results from LIVE/DEAD quantitative analysis of G. vaginalis UM137, G. piotii UM035, G. leopoldii UGent 09.48 and G. swidsinskii GS 9838-1 48 h biofilms after the activity of EO Car. and two combinations of compounds. The results are shown in percentage of reduction of cell wall integrity compared to live control. Error bars represent s.d. No statistical differences were found when using a t-test (n = 3) comparing the different conditions within the same tested species. All conditions are statistically different from the control.
Recovery of Gardnerella spp. cells culturability
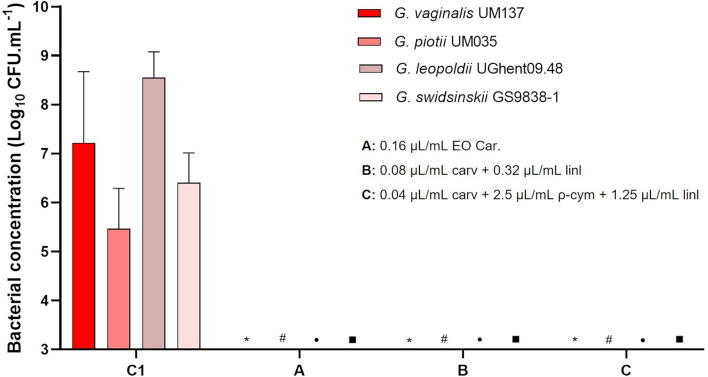
With the detection of a variable percentage of VBNC in all tested species, we performed another experiment, wherein we attempt to recover the culturability of the biofilm cells, by allowing the remaining biofilm to be incubated in fresh sBHI, after removing the antimicrobial agents. As shown in Fig. 6, no recovery of culturability was detected, after 24 h of incubation in fresh medium.
Figure 6.
Analysis of biofilm cells culturability recovery of different Gardnerella species after the effect of EO Car. and specific combinations of compounds. The experiments were performed after 96 h of biofilm formation with sBHI medium. Results are represented as mean of Log (CFU.mL−1) with a limit of detection of Log = 3, and the error bars represent s.d. Statistical analysis was performed using a t-test (n = 4). Differences are represented when p < 0.05, when compared with control of G. vaginalis UM137 (asterisk symbol), G. piotii UM035 (hash symbol), G. leopoldii UGent 09.48 (bullet symbol) and G. swidsinskii GS 9838-1 (filled square symbol). C1: control (48 h-biofilm + 24 h sBHI + 24 h sBHI); A: 0.16 µL/mL EO Car; B: 0.08 µL/mL carv + 0.32 µL/mL linl; C: 0.04 µL/mL carv + 2.5 µL/mL ρ-cym + 1.25 µL/mL linl.
Colonization of reconstructed human vaginal epithelium
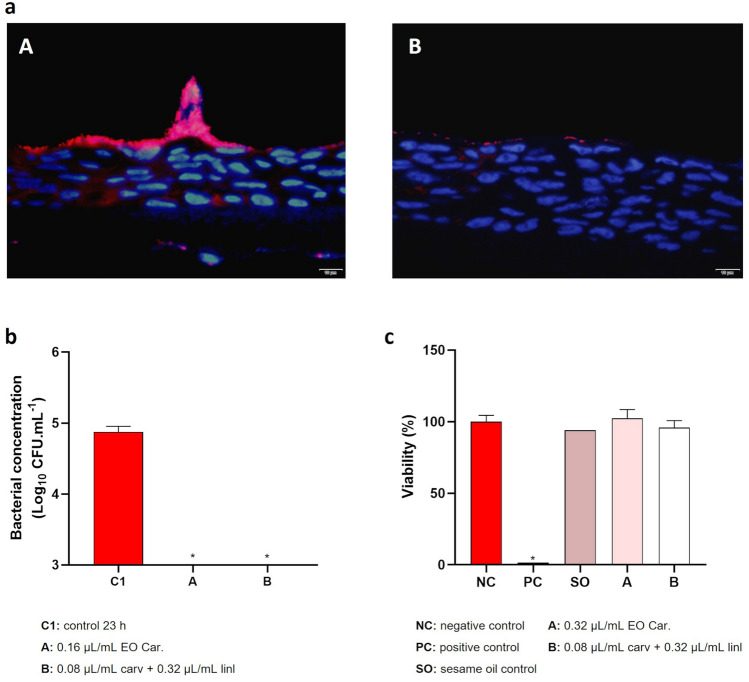
To assess the antimicrobial potential of the EO or carvacrol + linalool, G. vaginalis early-stage biofilms were grown on a human vaginal epithelium tissue, using a chemically defined medium that simulates genital tract secretions. The biofilms were then challenged at the EO MLC concentration or the compounds at the concentrations where synergism was observed. As represented in Fig. 7a, G. vaginalis not exposed to the antimicrobial agents was able to form a biofilm on the vaginal epithelium, and the biofilm was drastically reduced after the antimicrobial challenge. Like the previous experiments, no culturable cells were recovered after the antimicrobial challenge (Fig. 7b). Interestingly, at this concentration, neither the EO nor the carvacrol + linalool combinations demonstrated significant cytotoxicity (Fig. 7c).
Figure 7.
Colonization of reconstituted human vaginal epithelium and cytotoxicity of T. capitata EO. (a) Microscopic images of reconstituted human vaginal epithelium after 23 h of colonization with G. vaginalis UM137 (A) and after 9 h of G. vaginalis UM137 colonization with 14 h of contact with EO Car. (B). (b) Results of cells culturability after colonization of vaginal epithelium. Results are represented as mean of Log (CFU.mL−1) with a limit of detection of Log = 3. Error bars represent s.d. Statistical analysis was performed using t-test (n = 3 for control and EO assay; n = 2 for carvacrol + linalool assay). Differences are represented by * when comparing with control 23 h, when p < 0.05. (c) Results of cytotoxicity of EO and combination carvacrol + linalool on the vaginal tissue are the mean of percentage (%) of viability. Error bars represent s.d. Statistical analysis was performed using a t-test (n = 4 for EO assay; n = 3 for controls and carvacrol + linalool assay). Differences are represented by * when comparing with the negative control. Statistical analysis did not include sesame oil control (n = 1).
Discussion
The increasing levels of bacterial resistance associated with commonly used antimicrobial agents are one of the problems that most affect public health50. In BV, increased resistance to metronidazole and clindamycin have been reported51–53, and this led to seek for new alternatives of treatment54. As far as we are aware, the use of EOs as alternatives for the treatment of vaginal infections is still limited, however, some EOs have been tested on Candida species associated with vaginal candidiasis55,56, in BV45,57,58, trichomoniasis59 or other vaginal infection-related pathogens60. One possible criticism of the utilization of EO as a reliable therapeutically option is the variability that is often observed between different batches of the same EO, which can affect its properties and biological activities61. Thus, it is extremely important to characterize the essential oils to ensure their chemical homogeneity. According to our results, the EOs analyzed were very homogeneous, characterized by high contents in carvacrol. Interestingly, the antimicrobial potential of the two EOs was also very similar. Furthermore, assessing the antimicrobial activity of single compounds or mixtures of compounds present in the EOs, has the potential to develop a more standardized product, despite having increased costs of production. In this study, we decided to study single compounds and combinations of compounds from T. capitata EO, in order to find a composition that has good antimicrobial activity against biofilms formed by Gardnerella spp.
Due to the wide-ranging biological properties attributed to T. capitata EO, several studies have been conducted to assess the antibacterial activity of T. capitata EO and carvacrol, and to less extent the activity of their compounds62. As a result, it has been shown that the antimicrobial activity of EOs is dependent on their chemical compositions63,64. The high content of carvacrol, a phenolic monoterpene, in T. capitata EO is pointed to be largely responsible for the antimicrobial activity65. Not surprisingly, herein, carvacrol also presented the highest antimicrobial activity of the five compounds tested. It has also been described that monoterpenes have low antimicrobial activity and are usually ineffective as antimicrobial agents when used alone66,67, as also observed in this study. Nevertheless, components present in small amounts in EOs can still play an important role in antimicrobial activity due to the possible synergistic effects68,69.
It has been previously shown that interactions between major and minor constituents can have a critical influence on global antimicrobial activity27. The most common interactions between different components include synergistic, antagonistic, additive, and indifferent effects70. Noticeably, the strongest effect happened in the combination of carvacrol and ρ-cymene resulting in synergism. This effect is expected to result from the interaction of ρ-cymene with the lipidic membrane of the cells causing the expansion of the membrane, which facilitates and increases the transportation of carvacrol into the cells66,71. Other studies already reported this synergistic effect between carvacrol and ρ-cymene, particularly against Listeria monocytogenes72 and Escherichia coli73.
Interestingly, when testing the antimicrobial activity against biofilms, α- and γ-terpinene had no detectable effect, and while ρ-cymene was able to reduce ~ 30% of the total biomass, its effect on bacterial culturability was neglectable. Furthermore, despite carvacrol + ρ-cymene having a better synergistic effect against planktonic cultures, on biofilms, the most active combination was carvacrol + linalool. This last combination was more effective in reducing the total biomass of the biofilm (~ 50%) than the whole EO (~ 30%). This further demonstrates that biofilm cultures susceptibility to antimicrobials cannot be predicted from the standardized MIC determinations74,75. Despite not being able to completely eradicate the biofilm biomass, cell culturability was significantly affected, with no colony detected in many of the tested conditions. A similar effect was also reported with T. capitata EO against a biofilm of Candida glabrata where despite the low reduction of biofilm biomass, the cells were not metabolic active, remaining inactive or dead41. In another study with Staphylococcus aureus and E. coli biofilms, five different EOs also promoted the reduction of cells metabolic activity inpre-established biofilms and even the complete elimination of viability in some of the cases76.
To further understand the impact of the EO and some of the most promising mixtures, we also performed a resuscitation attempt77, whereby stimulating the cells without the antimicrobial stress, they can recover the full active metabolic state and culturability. However, in our tested conditions, no cell recovery was observed. This observation suggests that these antimicrobial agents have a lasting effect on Gardnerella, and this might improve the recurrence rates currently observed after treatment with metronidazole or clindamycin78,79.
Herein, we also determined the feasibility of using reconstituted vaginal epithelial tissue, to establish a Gardnerella spp. biofilm. While this vaginal model has been used before to study Candida infection80 or vaginal dryness81, as far as we are aware, this is the first study that reports Gardnerella spp. colonization in this model. By using a PNA-FISH specific probe, we were able to observe intact biofilms formed on the vaginal epithelium. Interestingly, after the antimicrobial challenge, most of the bacterial cells were killed, and the small clusters of cells observed were non-cultivable. Importantly, at the tested concentration used herein, we did not observe any significant cytotoxicity in the vaginal tissue.
In summary, this study revealed the potential of T. capitata EO and some of its components to be used as potential new therapeutic agents in the treatment of BV. Nonetheless, this study has some limitations and further work should be performed to test these components on multi-species biofilms, that better mimic what occurs in BV82. Furthermore, it would be important to assess their activity against Lactobacillus species common colonizers of the healthy vaginal microbiota. In addition, similar to what is described in other EOs83, it would be also important to analyze the effect of the tested components with conventional antibiotics. Apart from it, there are some limitations in the methods, namely in the biofilm biomass quantification by CV, since it is known that one of the disadvantages of this method is the lack of reproducibility84.
Materials and methods
Plant material and commercial compounds
Aerial parts of T. capitata plant were collected at the flowering stage in two different locations of the Algarve region (south of Portugal): Benagil (sample Ben., Benagil beach: 37°05′13.1″N 8°25′35.5″W) and Carvoeiro (sample Car., Carvoeiro: 37°05′33.9″N 8°27′55.5″W), to obtain oils from two different populations. The collection site access has been approved by Mr. Daniel Prado, the local owner. Dr. Jorge Paiva, a taxonomist at the University of Coimbra, confirmed species authenticity and plant names were checked with https://www.theplantlist.org. Voucher specimens were included in the Herbarium of the University of Coimbra (COI), with the accession number Salgueiro 19, and in the Herbarium of the Faculty of Pharmacy of UC, with the accession numbers Salgueiro 135 and Salgueiro 196. All plant collection procedures complied with relevant institutional, national and european legislation. Furthermore, five commercial compounds, including the main compound of the EOs (carvacrol (carv), γ-terpinene (γ-terp), ρ-cymene (ρ-cym), Sigma-Aldrich, Missouri, USA). Other minor monoterpenes (α-terpinene (α-terp) and linalool (linl), Sigma-Aldrich), that previously showed effective antimicrobial activity85–87, were also tested against Gardnerella species.
Essential oil extraction and analysis
The EOs were isolated by hydrodistillation for 3 h, using a Clevenger-type apparatus according to the European Pharmacopoeia88. The oils were preserved in a sealed vial at 4ºC. The compositions of the T. capitata EO were established by the combination of gas chromatography with FID detectors (GC-FID) and gas chromatography-mass spectroscopy (GC/MS) analysis as previously reported89. GC-FID analysis was performed in a Hewlett-Packard 6890 gas chromatograph (Agilent Technologies, Palo Alto, CA, USA) set with a single injector and two flame ionization detectors (FID). A divider (Agilent Technologies, part no. 5021-7148) was used for simultaneous sampling on two fused silica capillary columns: a SPB-1 (polydimethylsiloxane 30 m × 0.20 mm i.d., film thickness 0.20 µm) and a SupelcoWax-10 (polyethyleneglycol 30 m × 0.20 mm i.d., film thickness 0.20 µm). Oven temperature program: 70–220 °C (3 °C min−1), 220 °C (15 min); injector temperature: 250 °C; mobile phase: helium, with flow adjusted to maintain a linear velocity of 30 cm s−1; split ratio 1:40; detectors temperature: 250 °C. GC–MS analysis was performed in an Hewlett-Packard 6890 gas chromatograph interfaced with an Hewlett-Packard mass selective detector MSD 5973 (Agilent Technologies). An HP1 (Agilent Technologies) fused silica column (polydimethylsiloxane 30 m × 0.25 mm i.d., film thickness 0.25 µm) was used. GC parameters as described above; interface temperature: 250 °C; MSD parameters: interface temperature: 250 °C; MS source temperature: 230 °C; MS quadrupole temperature: 150 °C; ionization energy: 70 eV; ionization current: 60 µA; scan range: 35–350 units; scans s−1: 4.51.
The oils components were identified by considering, concurrently: (i) the acquired retention indices on two columns with different phases SPB-1 (polydimethylsiloxane) and SupelcoWax-10 (polyethyleneglycol) determined by linear interpolation relative to the retention times of C8–C23 of n-alkanes and compared with reference data from authentic products (available in the laboratory database of the Faculty of Pharmacy, University of Coimbra) and literature data90; (ii) the acquired mass spectra compared with reference data from the laboratory database, the Wiley/NIST library91 and literature92. The relative amount of each component was estimated from GC peaks areas without any correction regarding FID responses.
Bacterial growth conditions
Various strains from the genus Gardnerella were analyzed in this study. Gardnerella sp. UM241, previously recovered from patients diagnosed with BV93, G. vaginalis UM137, G. piotii UM035, G. leopoldii UGent 09.48, and G. swidsinskii GS 9838-1, previously identified by MALDI-TOF15,94, were used herein. The strains were kept frozen in Brain Heart Infusion medium (BHI, Liofilchem, Roseto degli Abruzz, Italy) with 23% (v/v) of glycerol (Panreac, Barcelona, Spain) and plated in Columbia Blood Agar (CBA) [Columbia Base Agar medium (Liofilchem) supplemented with 5% (v/v) of defibrinated horse blood (Oxoid Ltd, Hampshire, UK)] and incubated for 48 h at 37 °C and 10% CO2. For each experiment, planktonic cells were grown in BHI supplemented (sBHI) with 2% (w/v) gelatin (Liofilchem), 0.1% (w/v) starch (Panreac) and 0.5% (w/v) yeast extract (Liofilchem) and incubated for 24 h at 37 °C and 10% CO2 (CO2 Incubator MCO-18AC, Panasonic, Bracknell, UK).
Determination of the minimal inhibitory concentration and minimal lethal concentration
The MIC and MLC of both EOs, as well as five individual components of the oil, were evaluated by the macrodilution method, using the isolate Gardnerella sp. UM241, as described previously45 with some modifications. Briefly, each component was dissolved in the same proportion (1:1) in dimethyl-sulfoxide (DMSO, Scharlau, Barcelona, Spain) to improve its solubility. Therefore, serial dilutions in glass test tubes were prepared in sBHI to obtain a range of concentrations between 0.04 and 40 µL/mL in the experiments with the compounds. Regarding the experiments with the EOs, a range of concentrations between 0.02 and 2.5 µL/mL was tested. Afterwards, 500 µL of the bacterial inoculum, adjusted to the concentration of 106 colony forming units (CFU)/mL, was added to each tube performing a total volume of 1 mL. Positive control with DMSO at the highest concentration used in the dilutions was included. A second positive control was used with bacterial suspension without any compounds or EOs. The negative control only included sBHI medium. After 48 h of incubation at 37 °C and 10% CO2, the MIC value was determined by reading the optical density (OD) of all the dilutions at 620 nm. MLC was determined by plating 10 µL of each dilution on CBA plates and defined as the lowest concentration where no growth was detected. Each experiment was repeated at least three independent times.
Determination of the fractional inhibitory concentration by the checkerboard method
The checkerboard method was performed to study the interactions and the resultant effect from the combination of pairs of compounds from T. capitata against the isolate Gardnerella sp. UM241. Serial dilutions of each compound were prepared as described above in a range of concentrations appropriated to include the value of MIC for each compound. The experiments were performed on glass tubes for a final volume of 1 mL. For one of the compounds, serial dilutions were added along the lines and for the other compound were added along with the columns. Thereafter, 500 µL of a bacterial inoculum, prepared as described above and adjusted to the concentration of 106 CFU/mL, was added to all the tubes. Then, the tubes were incubated for 48 h at 37 °C and 10% CO2. To analyze the effect of the interactions between the compounds, the OD of the content of each tube was measured at 620 nm, and the value of the fractional inhibitory concentration (FIC) index was determined according to the lowest concentrations in which bacterial growth was not observed. The FIC was calculated as previously described95, by , where and 96. The effect was considered synergistic when FIC was ≤ 0.5, partial synergistic when FIC was > 0.5 and < 1, additive when FIC = 1, indifferent when FIC was > 1 and ≤ 4, and antagonistic when FIC was > 497. In each assay, the lowest value of FIC was selected, and the FIC of each combination was presented by the range of values from all assays. For each combination, experiments were performed at least two independent times.
Biofilm formation and activity of EOs and components from T. capitata
Biofilm formation experiments were performed as previously described98 for all the Gardnerella strains used in this study. Briefly, the cell concentration of 24 h-old cultures was assessed by OD at 620 nm and the inoculums were further diluted to obtain a final concentration of 108 CFU/mL. Biofilms were incubated in 96-well flat-bottom tissue culture plate (Orange Scientific, Braine-l’Alleud, Belgium) in 100 µL of sBHI for 48 h at 37 °C and 10% CO2, with fresh medium replacement after 24 h. The antimicrobial challenge occurred for another 24 h at the required concentrations of compounds. In a first assessment, the biofilm of Gardnerella sp. UM241 was challenged with the EOs at MLC concentration and single components at the highest MIC concentration. The sub-MIC concentrations for combinations between compounds were chosen based on the results from checkerboard assays, where some type of interaction between the components was observed. The following experiments were performed for the other 4 species of Gardnerella spp. and the conditions tested were EO Car. at 0.16 µL/mL, and the combinations of 0.08 µL/mL carvacrol + 0.32 µL/mL linalool and 0.04 µL/mL carvacrol + 2.5 µL/mL ρ-cymene + 1.25 µL/mL linalool. Two controls were included in each experiment. A positive control included a biofilm that was incubated for a total of 72 h without any antimicrobial challenge. Another control included the final incubation with DMSO in the maximum concentration used to dissolve the compounds. Biofilms were then quantified after 72 h of incubation. Each experiment was repeated at least three independent times with seven technical replicates.
Biofilm biomass quantification by crystal violet staining method
Biofilm quantification by crystal violet (CV) was performed as previously reported99. Briefly, after the washing and fixation step, 100 µL of 1% (w/v) CV solution (Acros Organics, New Jersey, USA) was added to each well for 20 min. The CV solution was removed, and the biofilms were washed twice with 200 µL of H2O. Finally, 150 µL of 33% (v/v) acetic acid (Thermo Fisher Scientific, Lenexa, KS, USA) was dispensed into each well and the OD was measured at 595 nm using a microplate reader. The reduction in biofilm biomass was determined by comparing the values of OD of each condition with the OD values of the positive control without DMSO.
Biofilm cells culturability analysis by colony-forming units
Biofilm formation was performed as described above, with the difference that 24-well flat-bottom tissue culture plates (Orange Scientific) were used, in which a coverslip (Thermo Scientific™ Nunc™ Thermanox™ Coverslips) was distributed in each one of the wells. After that, 1 mL of 24 h-bacterial inoculum, adjusted to the concentration of 108 CFU/mL, was dispensed in each well and the plate was incubated at 37 °C and 10% CO2 with replacement by the fresh medium at 24 h. After 48 h, the growth medium was removed and the coverslips were transferred to a new 24-well plate. Thereafter, 300 µL of the desirable compounds in sBHI were dispensed into the wells and the plate was incubated for more 24 h at 37 °C and 10% CO2. After that time, the medium was removed, and each coverslip was washed with NaCl 0.9% (w/v). 300 µL of fresh sBHI was added to the wells and the biofilm was mechanically detached from the coverslips. Then, 100 µL of each well content was removed and serial dilutions were performed in NaCl 0.9% (w/v) and 20 µL of each dilution was plated in a CBA plate and incubated for 48 h at 37 °C and 10% CO2. The experiments were repeated at least three independent times.
Biofilms assessed by LIVE/DEAD staining combined with confocal laser scanning microscopy
In order to evaluate the structure of the biofilm after the effect of T. capitata EOs and compounds, the biofilm was analyzed by confocal laser scanning microscopy (CLSM), as previously described49. Briefly, the biofilm of 4 Gardnerella species was performed in 24-well plates with coverslips, as described above and the conditions EO Car. at 0.16 µL/mL, and the combinations of 0.08 µL/mL carvacrol + 0.32 µL/mL linalool and 0.04 µL/mL carvacrol + 2.5 µL/mL ρ-cymene + 1.25 µL/mL linalool were tested. After 72 h, the biofilm was washed with NaCl 0.9% (w/v) and then stained using a LIVE/DEAD™ BacLight™ Bacterial Viability Kit (Invitrogen, Thermo Fisher Scientific), consisting of SYTO 9 and propidium iodide (PI). After incubation, the biofilms coating the coverslips were gently washed with 1 × PBS and then, the coverslips were removed from the wells and placed on microscope glass slides (VWR, Lisbon, Portugal). Two types of controls represented by untreated and dead biofilm cells were considered for this experiment and were used to define the CLSM laser intensity threshold. The dead control was obtained by covering the coverslips with the biofilms with 200 µL of 100% (v/v) methanol (Thermo Fisher Scientific) for 30 min. Then, all the coverslips with the live, dead, and treated biofilms were covered with 100 µL of the LIVE/ DEAD staining mix, with SYTO 9 and PI used each in a concentration of 3 µL/mL. Subsequently, the coverslips were incubated for 15 min. in the dark at room temperature. Biofilm image stacks were acquired with an Olympus™ FluoView FV1000 (Olympus, Lisbon, Portugal) confocal laser scanning microscope, using a 10 × objective. SYTO9 was detected using a filter with an excitation wavelength of 485 nm and an emission filter of 498 nm. PI was detected using a filter with an excitation wavelength of 536 nm and an emission filter of 617 nm. The CLSM images were analyzed using the FV10-ASW 4.0 Viewer Software (Olympus). This experiment was performed twice with two technical duplicates.
LIVE/DEAD quantification of biofilm cells by fluorometry
To analyze the biofilm cells viability after the action of the compounds and EO, a quantitative LIVE/DEAD analysis was performed of the 4 Gardnerella species biofilm challenged with EO Car. at 0.16 µL/mL, and the combinations of 0.08 µL/mL carvacrol + 0.32 µL/mL linalool and 0.04 µL/mL carvacrol + 2.5 µL/mL ρ-cymene + 1.25 µL/mL linalool. After 72 h of biofilm formation as described above, the medium was removed, and the biofilm was washed with NaCl 0.9% (w/v) and then detached from the 24 well-plate using NaCl 0.85% (w/v) and centrifuged for 5 min at maximum velocity. Subsequently, the concentration of biofilm suspension was adjusted to 107 CFU/mL and the LIVE/DEAD staining was added to biofilms as described in the CLSM section. Afterwards, the absorbance was measured using a multi-label microplate reader (Cytation 3, Bio Tek, Maine, USA) using the wavelengths of 485/530 nm and 485/630 nm (excitation/emission wavelength). Of note that before these experiments, a calibration curve was performed for each Gardnerella spp. according to the manufacturer’s instructions in each independent assay. In brief, from a fresh bacterial suspension collected from a CBA plate, suspensions with live and dead cells were prepared, as well as artificial dilutions of live/dead cells (33% live + 67% dead cells; 50% live + 50% dead cells; 67% live + 33% dead cells). A linear equation was obtained from the relation between the percentage of live/dead bacteria from the suspension and the green/red fluorescence ratio. Finally, for each biofilm assay, the obtained LIVE/DEAD ratios were then corrected according to the equation obtained from the calibration of the respective experiment. The percentage of reduction in cell integrity was obtained by comparison to the live control. Three independent assays were performed with two technical replicates.
Biofilm cells recovery after removing the effect of EO and compounds from T. capitata
In an attempt to verify if biofilm cells can recover culturability after the activity of EOs and compounds, an experiment was performed as described for quantification of biofilm cells viability, in 24-well plates, without coverslips. After 48 h of biofilm formation, the medium was removed, and 1 mL of compounds was added. After 24 h of action of compounds and EOs on the biofilm, the medium from the biofilm was removed and the biofilm was washed with 1 mL of NaCl 0.9% (w/v). Then, 1 mL of fresh sBHI or sBHI supplemented with 0.25% (w/v) of maltose (Fisher Bioreagents, Fair Lawn, New Jersey, USA) was added to each well, and the plate was incubated for more 24 h at 37 °C and 10% CO2. After this time, the medium was removed, biofilm was washed again with NaCl 0.9% (w/v) and mechanically detached from the plate in 300 µL of sBHI. Serial dilutions in NaCl 0.9% (w/v) were performed and 20 µL of each dilution were plated in a CBA and incubated for 48 h at 37 °C and 10% CO2. The experiment was performed four independent times.
Colonization of human vaginal epithelial tissue and activity of EO and compounds from T. capitata
To mimic the vaginal epithelial environment, a Reconstructed Human Vaginal Epithelium (HVE-SkinEthic®, Episkin, France) was used. A 24 h inoculum of G. vaginalis UM137 was prepared in sBHI medium. After 24 h the inoculum was centrifuged for 20 min, 3134 g, and the pellet was resuspended in a medium simulating genital tract secretions (mGTS)100. After that, the OD was adjusted to a concentration of 107 CFU/mL and 1 mL was dispensed on the tissues for colonization of the vaginal epithelium. The tissues were then incubated at 37˚C and 10% CO2, for 9 h. Then, mGTS with either EO Car. at a concentration of 0.16 μL/mL or a combination of 0.08 μL/mL carvacrol + 0.32 μL/mL linalool was added to each tissue, after removal of the spent media, and incubated for a further 14 h. For microscopic analysis, the tissues were placed in 4% paraformaldehyde (Thermo Fisher Scientific) and then embedded in paraffin (Leica TP1020, Leica Biosystems, Nussloch, Germany). Paraffin tissue blocks were prepared (Leica EG 1140 H, Leica Biosystems, Nussloch, Germany) and 3-μm-thick sections were obtained using a microtome (Microm HM 325, Thermo Fisher Scientific, Walldorf, Germany). For the deparaffinization step, sections were placed in xylene (Thermo Fisher Scientific) twice for 5 min, followed by a hydration step with 100% and 50% of ethanol (Thermo Fisher Scientific) for 5 min each and a final step in distilled water for 5 min. The samples were allowed to air-dry and the PNA-FISH procedure was performed using a previously developed PNA probe for Gardnerella101, with a hybridization step at 60 °C for 90 min. The samples were analyzed using an Olympus BX51 epifluorescence microscope (Olympus, Lisbon, Portugal) equipped with a TRITC filter (BP 530–550, FT 570, LP 591 sensitive to the Alexa Fluor 594 molecule). To perform the culturability assays the tissues were washed once with NaCl 0.9% (w/v) and 500 µL of mGTS was added. A cycle of sonication was performed to displace the cells from the tissues, for 10 s with an amplitude of 33%. Serial dilutions in NaCl 0.9% (w/v) were then performed in duplicates from the content of each well, and each dilution was plated on CBA plates and incubated at 37ºC and 10% CO2, for 72 h. The experiment was performed twice, with technical duplicates.
Vaginal irritation test
A toxicity study was performed using the Reconstructed Human Vaginal Epithelium (HVE-SkinEthic®, Episkin, France) model. This reconstructed epithelium reveals a strong histologic resemblance with human vaginal tissue. Upon arrival, the tissues were incubated overnight, at 37 °C, ≥ 90% humidity, 5% CO2 (Binder APT.lineTM C150E2 Incubator, Binder, NY, USA), in 6-well plates (VWR) using 1 mL of Maintenance Medium (provided by Episkin together with the tissues). Tissues were then transferred to new 24-well plates (one plate per condition) containing 300 μL of Maintenance Medium, and then 30 μL of the test substances or controls were gently dispersed over the entire tissue surface. EO Car. at 0.32 μL/mL and 0.08 μL/mL carvacrol + 0.32 μL/mL linalool tested concentrations were prepared in sesame oil (Ph Eur. Grade, Sigma), since this is the oily vehicle recommended for skin irritation testing according to ISO 10993-23:2021. Phosphate Buffer Saline without Ca2+ and Mg2+ (DPBS, VWR) and Sodium Lauryl Sulfate 1% w/v, were used as negative and positive controls, respectively. Solvent control (sesame oil) was also included. A different plate was used for each study substance (n = 3 tissues per substance). After 24 h of incubation at 37 °C, ≥ 90% humidity, 5% CO2, tissue integrity was assessed by the MTT assay, as previously described102. Briefly, the tissues were washed with PBS and gently dried. Then, the tissues were transferred to a 24-well plate containing 300 µL per well of a 0.5 mg/mL MTT (Alfa Aeser) solution (in PBS, VWR) and incubated for 3 h, at 37 °C, ≥ 90% humidity, 5% CO2, protected from light. After this period, the tissues were transferred to a single 24-well plate containing 750 µL of isopropyl alcohol and more 750 µL were further added at the top of each tissue to allow for the extraction of formazan for ≥ 2 h, in a sealed plastic bag, under agitation in a plate stirrer. Absorbance was then measured at 570 nm without a reference filter, according to the tissues provider instructions (Promega GloMax® Explorer System, USA). The background was deducted from all measured absorbance using isopropyl alcohol in free wells of the same reading plate. The absorbance of the negative control was considered 100% viability reference for products toxicity calculation.
Statistical analysis
Data were analyzed with a one-way analysis of variance (ANOVA) and t-test with an alpha level of 0.05, using GraphPad Prism version 8 (GraphPad Software, California, USA). Results are presented as the mean + standard deviation (s.d.) or mean + standard error of the mean (s.e.m.). Statistical differences were considered significant when p-values were less than 0.05.
Supplementary Information
Acknowledgements
This work was supported by the Portuguese Foundation for Science and Technology (FCT) by the research project [PTDC/BIA-MIC/28271/2017] under the scope of COMPETE 2020 [POCI-01-0145-FEDER-028271] and by the strategic funding of unit [UID/BIO/04469/2020]. RPO was supported by fellowship SFRH/BPD/124437/2016 from FCT, which is co-financed by the European Social Fund (ESF) and the European Regional Development Fund (ERDF), through the Centro 2020 Regional Operational Programme.
Author contributions
N.C., L.S., R.P.O., J.M.O. designed the experiments; L.S. collected the plants; L.S. and C.C. extracted and characterize the EO; L.G.V.S. performed the MIC and MLC and checkerboard experiments; L.G.V.S. and J.C. performed the biofilm quantification assays, recovery of cells culturability and colonization of RHVE experiments; N.C. performed the CLSM experiments; R.P.O. and M.T. performed the vaginal irritation assays; L.G.V.S. and N.C. drafted the manuscript. All authors critically revised the draft and approved the final manuscript.
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
Competing interests
All authors of this manuscript are authors of the patent submitted.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Lúcia G. V. Sousa and Joana Castro.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-08217-w.
References
- 1.Peebles K, Velloza J, Balkus JE, McClelland RS, Barnabas RV. High global burden and costs of bacterial vaginosis: A systematic review and meta-analysis. Sex. Transm. Dis. 2019;46:304–311. doi: 10.1097/OLQ.0000000000000972. [DOI] [PubMed] [Google Scholar]
- 2.Sobel JD. Bacterial vaginosis. Annu. Rev. Med. 2000;51:349–356. doi: 10.1146/annurev.med.51.1.349. [DOI] [PubMed] [Google Scholar]
- 3.Livengood CH. Bacterial vaginosis: An overview for 2009. Rev. Obstet. Gynecol. 2009;2:28–37. [PMC free article] [PubMed] [Google Scholar]
- 4.Svare JA, Schmidt H, Hansen BB, Lose G. Bacterial vaginosis in a cohort of Danish pregnant women: Prevalence and relationship with preterm delivery, low birthweight and perinatal infections. BJOG An Int. J. Obstet. Gynaecol. 2006;113:1419–1425. doi: 10.1111/j.1471-0528.2006.01087.x. [DOI] [PubMed] [Google Scholar]
- 5.Isik G, Demirezen Ş, Dönmez H, Beksaç M. Bacterial vaginosis in association with spontaneous abortion and recurrent pregnancy losses. J. Cytol. 2016;33:135–140. doi: 10.4103/0970-9371.188050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ness RB, et al. A cluster analysis of bacterial vaginosis-associated microflora and pelvic inflammatory disease. Am. J. Epidemiol. 2005;162:585–590. doi: 10.1093/aje/kwi243. [DOI] [PubMed] [Google Scholar]
- 7.Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: A meta-analysis of published studies. AIDS. 2008;22:1493–1501. doi: 10.1097/QAD.0b013e3283021a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nouioui I, et al. Genome-based taxonomic classification of the phylum actinobacteria. Front. Microbiol. 2018;9:2007. doi: 10.3389/fmicb.2018.02007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ceccarani C, et al. Diversity of vaginal microbiome and metabolome during genital infections. Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-019-50410-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onderdonk AB, Delaney ML, Fichorova RN. The human microbiome during bacterial vaginosis. Clin. Microbiol. Rev. 2016;29:223–238. doi: 10.1128/CMR.00075-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swidsinski A, et al. Adherent biofilms in bacterial vaginosis. Obstet. Gynecol. 2005;106:1013–1023. doi: 10.1097/01.AOG.0000183594.45524.d2. [DOI] [PubMed] [Google Scholar]
- 12.Muzny CA, et al. An updated conceptual model on the pathogenesis of bacterial vaginosis. J. Infect. Dis. 2019;220:1399–1405. doi: 10.1093/infdis/jiz342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosca AS, Castro J, Sousa LGV, Cerca N. Gardnerella and vaginal health: the truth is out there. FEMS Microbiol. Rev. 2020;44:73–105. doi: 10.1093/femsre/fuz027. [DOI] [PubMed] [Google Scholar]
- 14.Hickey RJ, Forney LJ. Gardnerella vaginalis does not always cause bacterial vaginosis. J. Infect. Dis. 2014;210:1682–1683. doi: 10.1093/infdis/jiu303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaneechoutte M, et al. Emended description of Gardnerella vaginalis and description of Gardnerella leopoldii sp. nov., Gardnerella piotii sp. nov. and Gardnerella swidsinskii sp. nov., with delineation of 13 genomic species within the genus Gardnerella. Int. J. Syst. Evol. Microbiol. 2019;69:679–687. doi: 10.1099/ijsem.0.003200. [DOI] [PubMed] [Google Scholar]
- 16.Castro J, et al. Reciprocal interference between Lactobacillus spp. and Gardnerella vaginalis on initial adherence to epithelial cells. Int. J. Med. Sci. 2013;10:1193–1198. doi: 10.7150/ijms.6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwebke JR, Muzny CA, Josey WE. Role of Gardnerella vaginalis in the pathogenesis of bacterial vaginosis: A conceptual model. J. Infect. Dis. 2014;210:338–343. doi: 10.1093/infdis/jiu089. [DOI] [PubMed] [Google Scholar]
- 18.Workowski KA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm. Rep. 2021;70:1–187. doi: 10.15585/mmwr.rr7004a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vodstrcil LA, Muzny CA, Plummer EL, Sobel JD, Bradshaw CS. Bacterial vaginosis: Drivers of recurrence and challenges and opportunities in partner treatment. BMC Med. 2021;19:194. doi: 10.1186/s12916-021-02077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferris MJ, et al. Association of Atopobium vaginae, a recently described metronidazole resistant anaerobe, with bacterial vaginosis. BMC Infect. Dis. 2004;4:5. doi: 10.1186/1471-2334-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beigi RH, Austin MN, Meyn LA, Krohn MA, Hillier SL. Antimicrobial resistance associated with the treatment of bacterial vaginosis. Am. J. Obstet. Gynecol. 2004;191:1124–1129. doi: 10.1016/j.ajog.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 22.Swidsinski A, et al. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am. J. Obstet. Gynecol. 2008;198(97):e1–97.e6. doi: 10.1016/j.ajog.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 23.Muzny CA, Kardas P. A narrative review of current challenges in the diagnosis and management of bacterial vaginosis. Sex. Transm. Dis. 2020;47:441–446. doi: 10.1097/OLQ.0000000000001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Machado D, Castro J, Palmeira-de-Oliveira A, Martinez-de-Oliveira J, Cerca N. Bacterial vaginosis biofilms: Challenges to current therapies and emerging solutions. Front. Microbiol. 2016;6:1528. doi: 10.3389/fmicb.2015.01528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sobel JD, Sobel R. Current and emerging pharmacotherapy for recurrent bacterial vaginosis. Expert Opin. Pharmacother. 2021;22:1593–1600. doi: 10.1080/14656566.2021.1904890. [DOI] [PubMed] [Google Scholar]
- 26.Falconi-McCahill A. Bacterial vaginosis: A clinical update with a focus on complementary and alternative therapies. J. Midwifery Womens. Health. 2019;64:578–591. doi: 10.1111/jmwh.13013. [DOI] [PubMed] [Google Scholar]
- 27.Burt S. Essential oils: Their antibacterial properties and potential applications in foods: A review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 28.Hammer KA, Carson CF, Riley TV. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999;86:985–990. doi: 10.1046/j.1365-2672.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 29.Khan MSA, Malik A, Ahmad I. Anti-candidal activity of essential oils alone and in combination with amphotericin B or fluconazole against multi-drug resistant isolates of Candida albicans. Med. Mycol. 2012;50:33–42. doi: 10.3109/13693786.2011.582890. [DOI] [PubMed] [Google Scholar]
- 30.Bozin B, Mimica-Dukic N, Samojlik I, Jovin E. Antimicrobial and Antioxidant Properties of Rosemary and Sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) Essential Oils. J. Agric. Food Chem. 2007;55:7879–7885. doi: 10.1021/jf0715323. [DOI] [PubMed] [Google Scholar]
- 31.Garozzo A, et al. In vitro antiviral activity of Melaleuca alternifolia essential oil. Lett. Appl. Microbiol. 2009;49:806–808. doi: 10.1111/j.1472-765X.2009.02740.x. [DOI] [PubMed] [Google Scholar]
- 32.Hammer KA, Carson CF, Riley TV. In vitro susceptibilities of lactobacilli and organisms associated with bacterial vaginosis to Melaleuca alternifolia (tea tree) oil. Antimicrob. Agents Chemother. 1999;43:196. doi: 10.1128/aac.43.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pina-Vaz C, et al. Antifungal activity of Thymus oils and their major compounds. J. Eur. Acad. Dermatol. Venereol. 2004;18:73–78. doi: 10.1111/j.1468-3083.2004.00886.x. [DOI] [PubMed] [Google Scholar]
- 34.Karaman M, et al. Origanum vulgare essential oil affects pathogens causing vaginal infections. J. Appl. Microbiol. 2017;122:1177–1185. doi: 10.1111/jam.13413. [DOI] [PubMed] [Google Scholar]
- 35.Hyldgaard M, Mygind T, Meyer RL. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012;3:12. doi: 10.3389/fmicb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barata AM, Rocha F, Lopes V, Bettencourt E, Figueiredo AC. Medicinal and aromatic plants: Portugal. In: Ozturk M, Ameenah G-FB, editors. Medicinal and Aromatic Plants of the World. Eolss Publishers; 2011. pp. 1–46. [Google Scholar]
- 37.da Cunha AP, Nogueira MT, Roque OR. Plantas Aromáticas e Óleos Essenciais Composição e Aplicações. Fundação Calouste Gulbenkian; 2012. [Google Scholar]
- 38.Raja RR. medicinally potential plants of labiatae (Lamiaceae) family: An overview. Res. J. Med. Plant. 2012;6:203–213. [Google Scholar]
- 39.Carapeto, A. et al. Lamiaceae: Espécies. Flora-On: Flora de Portugal Interactiva, Sociedade Portuguesa de Botânica. http://www.flora-on.pt/ (2022).
- 40.Figueiredo AC, et al. Portuguese Thymbra and Thymus species volatiles: Chemical composition and biological activities. Curr. Pharm. Des. 2008;14:3120–3140. doi: 10.2174/138161208786404218. [DOI] [PubMed] [Google Scholar]
- 41.Palmeira-de-Oliveira A, et al. The anti-Candida activity of Thymbra capitata essential oil: Effect upon pre-formed biofilm. J. Ethnopharmacol. 2012;140:379–383. doi: 10.1016/j.jep.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 42.Neves A, et al. Characterization of Portuguese Thymbra capitata, Thymus caespititius and Myrtus communis essential oils in topical formulations. Flavour Fragr. J. 2017;32:392–402. [Google Scholar]
- 43.Benbelaïd F, et al. Antimicrobial activity of some essential oils against oral multidrug-resistant Enterococcus faecalis in both planktonic and biofilm state. Asian Pac. J. Trop. Biomed. 2014;4:463–472. doi: 10.12980/APJTB.4.2014C1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miguel MG, et al. Antioxidant and antiproliferative activities of the essential oils from Thymbra capitata and Thymus Species grown in Portugal. Evid. Based. Complement. Alternat. Med. 2015;2015:851721. doi: 10.1155/2015/851721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Machado D, et al. Thymbra capitata essential oil as potential therapeutic agent against Gardnerella vaginalis biofilm-related infections. Future Microbiol. 2017;12:407–416. doi: 10.2217/fmb-2016-0184. [DOI] [PubMed] [Google Scholar]
- 46.Aljaafari MN, et al. An overview of the potential therapeutic applications of essential oils. Molecules. 2021;26:628. doi: 10.3390/molecules26030628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Basavegowda N, Baek K-H. Synergistic antioxidant and antibacterial advantages of essential oils for food packaging applications. Biomolecules. 2021;11:1267. doi: 10.3390/biom11091267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chouhan S, Sharma K, Guleria S. Antimicrobial activity of some essential oils-present status and future perspectives. Medicines. 2017;4:58. doi: 10.3390/medicines4030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carvalhais V, et al. Tetracycline and rifampicin induced a viable but nonculturable state in Staphylococcus epidermidis biofilms. Future Microbiol. 2018;13:27–36. doi: 10.2217/fmb-2017-0107. [DOI] [PubMed] [Google Scholar]
- 50.Giurazza R, et al. Emerging treatment options for multi-drug-resistant bacterial infections. Life. 2021;11:519. doi: 10.3390/life11060519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Backer E, et al. Antibiotic susceptibility of Atopobium vaginae. BMC Infect. Dis. 2006;6:51. doi: 10.1186/1471-2334-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Austin MN, Beigi RH, Meyn LA, Hillier SL. Microbiologic response to treatment of bacterial vaginosis with topical clindamycin or metronidazole. J. Clin. Microbiol. 2005;43:4492–4497. doi: 10.1128/JCM.43.9.4492-4497.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li T, et al. Antimicrobial susceptibility testing of metronidazole and clindamycin against Gardnerella vaginalis in planktonic and biofilm formation. Can. J. Infect. Dis. Med. Microbiol. 2020;2020:1361825. doi: 10.1155/2020/1361825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tomás M, Palmeira-de-Oliveira A, Simões S, Martinez-de-Oliveira J, Palmeira-de-Oliveira R. Bacterial vaginosis: Standard treatments and alternative strategies. Int. J. Pharm. 2020;587:119659. doi: 10.1016/j.ijpharm.2020.119659. [DOI] [PubMed] [Google Scholar]
- 55.Pietrella D, et al. Beneficial effect of Mentha suaveolens essential oil in the treatment of vaginal candidiasis assessed by real-time monitoring of infection. BMC Complement. Altern. Med. 2011;11:18. doi: 10.1186/1472-6882-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mondello F, De Bernardis F, Girolamo A, Cassone A, Salvatore G. In vivo activity of terpinen-4-ol, the main bioactive component of Melaleuca alternifolia Cheel (tea tree) oil against azole-susceptible and -resistant human pathogenic Candida species. BMC Infect. Dis. 2006;6:158. doi: 10.1186/1471-2334-6-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trinh H-T, Lee I-A, Hyun Y-J, Kim D-H. Artemisia princeps Pamp. essential oil and its constituents eucalyptol and α-terpineol ameliorate bacterial vaginosis and vulvovaginal candidiasis in mice by inhibiting bacterial growth and NF-κB activation. Planta Med. 2011;77:1996–2002. doi: 10.1055/s-0031-1280094. [DOI] [PubMed] [Google Scholar]
- 58.Masoudi M, Miraj S, Rafieian-Kopaei M. Comparison of the effects of Myrtus Communis L, Berberis Vulgaris and metronidazole vaginal gel alone for the treatment of bacterial vaginosis. J. Clin. Diagn. Res. 2016;10:04–07. doi: 10.7860/JCDR/2016/17211.7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abdali K, et al. Comparison of the effect of vaginal Zataria multiflora cream and oral metronidazole pill on results of treatments for vaginal infections including trichomoniasis and bacterial vaginosis in women of reproductive age. Biomed. Res. Int. 2015;2015:683640. doi: 10.1155/2015/683640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwiertz A, Duttke C, Hild J, Müller HJ. In vitro activity of essential oils on microorganisms isolated from vaginal infections. Int. J. Aromather. 2006;16:169–174. [Google Scholar]
- 61.Demuner AJ, et al. Seasonal variation in the chemical composition and antimicrobial activity of volatile oils of three species of Leptospermum (Myrtaceae) grown in Brazil. Molecules. 2011;16:1181. doi: 10.3390/molecules16021181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Castro J, Salgueiro L, Cerca N. Essential oils as potential antibiofilm agents: Insights into the key role of thymbra capitata to fight biofilm-associated infections. In: Singh S, editor. Volatile Oils: Production, Composition and Uses. Nova Science Publishers; 2021. [Google Scholar]
- 63.Dorman HJ, Deans SG. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 64.Guimarães AC, et al. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules. 2019;24:2471. doi: 10.3390/molecules24132471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Casiglia S, Bruno M, Scandolera E, Senatore F, Senatore F. Influence of harvesting time on composition of the essential oil of Thymus capitatus (L.) Hoffmanns. & Link. growing wild in northern Sicily and its activity on microorganisms affecting historical art crafts. Arab. J. Chem. 2019;12:2704–2712. [Google Scholar]
- 66.Ultee A, Slump RA, Steging G, Smid EJ. Antimicrobial activity of carvacrol toward Bacillus cereus on rice. J. Food Prot. 2000;63:620–624. doi: 10.4315/0362-028x-63.5.620. [DOI] [PubMed] [Google Scholar]
- 67.Bagamboula C, Uyttendaele M, Debevere J. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. Food Microbiol. 2004;21:33–42. [Google Scholar]
- 68.Merghni A, et al. Assessment of the antibiofilm and antiquorum sensing activities of Eucalyptus globulus essential oil and its main component 1,8-cineole against methicillin-resistant Staphylococcus aureus strains. Microb. Pathog. 2018;118:74–80. doi: 10.1016/j.micpath.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 69.Herman A, Tambor K, Herman A. Linalool affects the antimicrobial efficacy of essential oils. Curr. Microbiol. 2016;72:165–172. doi: 10.1007/s00284-015-0933-4. [DOI] [PubMed] [Google Scholar]
- 70.Bassolé IHN, Juliani HR. Essential oils in combination and their antimicrobial properties. Molecules. 2012;17:3989–4006. doi: 10.3390/molecules17043989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rattanachaikunsopon P, Phumkhachorn P. Assessment of factors influencing antimicrobial activity of carvacrol and cymene against Vibrio cholerae in food. J. Biosci. Bioeng. 2010;110:614–619. doi: 10.1016/j.jbiosc.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 72.Periago PM, Delgado B, Fernández PS, Palop A. Use of carvacrol and cymene to control growth and viability of Listeria monocytogenes cells and predictions of survivors using frequency distribution functions. J. Food Prot. 2004;67:1408–1416. doi: 10.4315/0362-028x-67.7.1408. [DOI] [PubMed] [Google Scholar]
- 73.Kiskó G, Roller S. Carvacrol and p-cymene inactivate Escherichia coli O157:H7 in apple juice. BMC Microbiol. 2005;5:36. doi: 10.1186/1471-2180-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rafaque Z, et al. In-vitro investigation of antibiotics efficacy against uropathogenic Escherichia coli biofilms and antibiotic induced biofilm formation at sub-minimum inhibitory concentration of ciprofloxacin. Infect. Drug Resist. 2020;13:2801. doi: 10.2147/IDR.S258355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lagha R, Abdallah FB, Al-Sarhan BO, Al-Sodany Y. Antibacterial and biofilm inhibitory activity of medicinal plant essential oils against Escherichia coli isolated from UTI patients. Molecules. 2019;24:1161. doi: 10.3390/molecules24061161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Borges A, Lopez-Romero JC, Oliveira D, Giaouris E, Simões M. Prevention, removal and inactivation of Escherichia coli and Staphylococcus aureus biofilms using selected monoterpenes of essential oils. J. Appl. Microbiol. 2017;123:104–115. doi: 10.1111/jam.13490. [DOI] [PubMed] [Google Scholar]
- 77.Pasquaroli S, et al. Antibiotic pressure can induce the viable but non-culturable state in Staphylococcus aureus growing in biofilms. J. Antimicrob. Chemother. 2013;68:1812–1817. doi: 10.1093/jac/dkt086. [DOI] [PubMed] [Google Scholar]
- 78.Bradshaw CS, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J. Infect. Dis. 2006;193:1478–1486. doi: 10.1086/503780. [DOI] [PubMed] [Google Scholar]
- 79.Bradshaw CS, et al. The association of Atopobium vaginae and Gardnerella vaginalis with bacterial vaginosis and recurrence after oral metronidazole therapy. J. Infect. Dis. 2006;194:828–836. doi: 10.1086/506621. [DOI] [PubMed] [Google Scholar]
- 80.Alves CT, et al. Candida albicans promotes invasion and colonisation of Candida glabrata in a reconstituted human vaginal epithelium. J. Infect. 2014;69:396–407. doi: 10.1016/j.jinf.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 81.Stabile G, Ricci G, Scalia MS, Seta FD. Induced dryness stress on human vaginal epithelium: The efficacy of a new vaginal gel. Gels. 2021;7:157. doi: 10.3390/gels7040157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Verstraelen H, Swidsinski A. The biofilm in bacterial vaginosis. Curr. Opin. Infect. Dis. 2013;26:86–89. doi: 10.1097/QCO.0b013e32835c20cd. [DOI] [PubMed] [Google Scholar]
- 83.Nafis A, et al. New insight into the chemical composition, antimicrobial and synergistic effects of the moroccan endemic Thymus atlanticus (Ball) roussine essential oil in combination with conventional antibiotics. Molecules. 2021;26:5850. doi: 10.3390/molecules26195850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Azeredo J, et al. Critical review on biofilm methods. Crit. Rev. Microbiol. 2017;43:313–351. doi: 10.1080/1040841X.2016.1208146. [DOI] [PubMed] [Google Scholar]
- 85.Liu X, et al. Antibacterial activity and mechanism of linalool against Pseudomonas aeruginosa. Microb. Pathog. 2020;141:103980. doi: 10.1016/j.micpath.2020.103980. [DOI] [PubMed] [Google Scholar]
- 86.Guo F, et al. Antimicrobial activity and proposed action mechanism of linalool against Pseudomonas fluorescens. Front. Microbiol. 2021;1:49. doi: 10.3389/fmicb.2021.562094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang S-K, et al. Combinatorial antimicrobial efficacy and mechanism of linalool against clinically relevant Klebsiella pneumoniae. Front. Microbiol. 2021;1:382. doi: 10.3389/fmicb.2021.635016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Council of Europe. European Pharmacopoeia. (European Directorate for the Quality of Medicines and Healthcare of the Council of Europe, 2010).
- 89.Cavaleiro C, Salgueiro LR, Miguel MG, Da Cunha AP. Analysis by gas chromatography-mass spectrometry of the volatile components of Teucrium lusitanicum and Teucrium algarbiensis. J. Chromatogr. A. 2004;1033:187–190. doi: 10.1016/j.chroma.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 90.Wallace, W. E. Retention Indices. in NIST Chemistry WebBook, NIST Standard Reference Database Number 69 (eds. Linstrom, P. J. & Mallard, W. G.) (National Institute of Standards and Technology, 2022).
- 91.McLafferty FW. Wiley Registry of Mass Spectral Data. Wiley; 2010. [Google Scholar]
- 92.Adams RP. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. Allured Publishing Corporation; 2007. [Google Scholar]
- 93.Castro J, et al. Using an in-vitro biofilm model to assess the virulence potential of bacterial vaginosis or non-bacterial vaginosis Gardnerella vaginalis isolates. Sci. Rep. 2015;5:11640. doi: 10.1038/srep11640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Castro J, Rosca AS, Cools P, Vaneechoutte M, Cerca N. Gardnerella vaginalis enhances Atopobium vaginae viability in an in vitro model. Front. Cell. Infect. Microbiol. 2020;10:83. doi: 10.3389/fcimb.2020.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Climo MW, Patron RL, Archer GL. Combinations of vancomycin and beta-lactams are synergistic against staphylococci with reduced susceptibilities to vancomycin. Antimicrob. Agents Chemother. 1999;43:1747–1753. doi: 10.1128/aac.43.7.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bonapace CR, Bosso JA, Friedrich LV, White RL. Comparison of methods of interpretation of checkerboard synergy testing. Diagn. Microbiol. Infect. Dis. 2002;44:363–366. doi: 10.1016/s0732-8893(02)00473-x. [DOI] [PubMed] [Google Scholar]
- 97.Marques MB, Brookings ES, Moser SA, Sonke PB, Waites KB. Comparative in vitro antimicrobial susceptibilities of nosocomial isolates of Acinetobacter baumannii and synergistic activities of nine antimicrobial combinations. Antimicrob. Agents Chemother. 1997;41:881–885. doi: 10.1128/aac.41.5.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Machado D, Palmeira-de-Oliveira A, Cerca N. Optimization of culture conditions for Gardnerella vaginalis biofilm formation. J. Microbiol. Methods. 2015;118:143–146. doi: 10.1016/j.mimet.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 99.Peeters E, Nelis HJ, Coenye T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods. 2008;72:157–165. doi: 10.1016/j.mimet.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 100.Stingley RL, Liu H, Mullis LB, Elkins CA, Hart ME. Staphylococcus aureus toxic shock syndrome toxin-1 (TSST-1) production and Lactobacillus species growth in a defined medium simulating vaginal secretions. J. Microbiol. Methods. 2014;106:57–66. doi: 10.1016/j.mimet.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 101.Machado A, et al. Fluorescence in situ Hybridization method using Peptide Nucleic Acid probes for rapid detection of Lactobacillus and Gardnerella spp. BMC Microbiol. 2013;13:82. doi: 10.1186/1471-2180-13-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ayehunie S, Wang YY, Landry T, Bogojevic S, Cone RA. Hyperosmolal vaginal lubricants markedly reduce epithelial barrier properties in a three-dimensional vaginal epithelium model. Toxicol. Rep. 2018;5:134–140. doi: 10.1016/j.toxrep.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).