Abstract
Gonadotropin-releasing hormone (GnRH) analogues are commonly used in clinical practice to prevent premature luteinizing hormone (LH) surge during In-Vitro Fertilization/ Intra-Cytoplasmic Sperm Injection (IVF/ICSI) cycles. This review aimed to summarize the available evidence comparing the effects of conventional GnRH antagonist protocols, the most commonly used GnRH antagonist protocols, and GnRH agonist protocols on IVF/ICSI outcomes in women with polycystic ovary syndrome (PCOS). A comprehensive electronic search was carried out in Pubmed, Cochrane CENTRAL, Scopus, Web of Science, CINAHL, TRIP, ClinicalTrials.gov and ISRCTN registry from inception until 24 November 2020 without any language or date restrictions. In addition, reference lists of eligible studies and previous meta-analyses were hand-searched to identify relevant studies. Eligible randomized controlled trials were those designed to compare the effects of conventional GnRH antagonist protocols and GnRH agonist protocols on IVF/ICSI outcomes in PCOS subjects. The Cochrane ROB 2.0 tool was used to assess the risk of bias of each study, and the GRADE assessment was used to evaluate the overall quality of evidence. Data synthesis and analyses were done using Review Manager 5.3 with the assistance of Revman Web. A random-effects model was used for all meta-analysis. Dichotomous outcomes were reported as Relative Risk (RR) and continuous outcomes as Weighted Mean Difference (WMD), both with 95% CIs. The primary outcomes were Live birth rate, Ongoing pregnancy rate, and Ovarian hyperstimulation syndrome (OHSS) rate. Other IVF outcomes were considered secondary outcomes. We included ten studies with 1214 randomized PCOS women. Using GnRH antagonist protocols led to a significantly lower OHSS rate (RR = 0.58; 95% CI: [0.44 to 0.77], P = 0.0002), shorter stimulation duration (WMD = − 0.91; 95% CI: [-1.45 to − 0.37] day, P = 0.0009), lower gonadotropin consumption (WMD = − 221.36; 95% CI: [− 332.28 to − 110.45] IU, P < 0.0001), lower E2 levels on hCG day (WMD = − 259.21; 95% CI: [− 485.81 to − 32.60] pg/ml, P = 0.02), thinner endometrial thickness on hCG day (WMD = − 0.73; 95% CI: [− 1.17 to − 0.29] mm, P = 0.001), and lower number of retrieved oocytes (WMD = − 1.82; 95% CI: [− 3.48 to − 0.15] oocytes, P = 0.03). However, no significant differences in live birth rate, ongoing pregnancy rate, clinical pregnancy rate, multiple pregnancy rate, miscarriage rate and cycle cancellation rate were seen between the GnRH antagonist protocols and the long GnRH agonist one. Although more cycles were cancelled due to poor ovarian response in the GnRH antagonist protocol (RR = 4.63; 95% CI: [1.49 to 14.41], P = 0.008), similar rates of cancellation due to risk of OHSS were noticed in both groups. The differences in IVF/ICSI outcomes may arise from the different patterns of gonadotropins suppression that the GnRH analogues exhibit during the early follicular phase of IVF/ICSI cycles and the divergent direct impacts of these analogues on ovaries and endometrial receptivity. The main evidence limitation was Imprecision. Conventional GnRH antagonist protocols represent a safer and more cost-effective treatment choice for PCOS women undergoing IVF/ICSI cycles than the standard long GnRH agonist protocol without compromising the IVF/ICSI clinical outcomes. The study had no sources of financial support and was prospectively registered at PROSPERO (International Prospective Register of Systematic Reviews) under registration number (CRD42021242476).
Subject terms: Infertility, Health care
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder among females of reproductive age1 and is considered the principal cause of anovulation cases referring to infertility clinics2. Based on the current recommendations, In-vitro Fertilization/ Intra-Cytoplasmic Sperm Injection (IVF/ICSI) can be offered to PCOS women as a third-line treatment choice after the failure of other approaches of ovulation induction3. However, it poses challenges considering the hormonal imbalance, obesity, hyperandrogenism, and insulin resistance observed in this population4. In addition, pregnancy enhances the low-grade inflammation usually noted in PCOS women, which in combination with the subclinical impairment of vascular structure and function could result in a hypoxic state that alters remodeling of spiral arteries and subsequently leads to a reduction in uterine artery impedance, a reduction in the depth of endovascular trophoblast and eventually abnormal placentation5,6. All of this puts PCOS women who conceived using IVF cycles at higher risk of developing adverse pregnancy and neonatal complications like miscarriage, gestational diabetes mellitus, pregnancy-induced hypertension, preterm birth, and giving birth of large-for-gestational-age babies4,7. Since the 1980s, Gonadotropin-releasing hormone (GnRH) agonists have been added to the controlled ovarian hyperstimulation (COS) protocols of IVF/ICSI to prevent premature luteinizing hormone (LH) surge while the follicles are still immature, resulting in a higher clinical pregnancy rate (CPR), lower cycle cancellation rate (CCR) and higher number of the retrieved oocytes8,9. However, due to the flare-up phase, GnRH agonist protocols are associated with some disadvantages, e.g., prolonged protocol duration, higher risk of formation ovarian cysts, and developing hypo-estrogenic side effects10,11. In addition, their use is associated with an increased risk of developing ovarian hyperstimulation syndrome (OHSS)8, an iatrogenic complication of COS characterized by cystic enlargement of the ovaries and fluid shifting from the intravascular to the third space due to increased capillary permeability and ovarian neo-angiogenesis12. This harmful effect may be more detrimental among PCOS women, who are already prone to developing OHSS4,7,13 due to the increased antral follicular counts, increased anti-müllerian hormone (AMH) levels, and increased estradiol levels, which exaggerates their response and sensibility to COS14,15. Thus, attempting to introduce more patient-friendly and cost-effective protocols, GnRH antagonists have been introduced to routine practice as alternatives to GnRH agonists. Unlike the indirect pituitary suppression induced by GnRH agonists, GnRH antagonists immediately and competitively occupy the GnRH receptors16, which helps to overcome the unfavorable effects of GnRH agonist protocols11. GnRH antagonists are usually scheduled during COS based on the progression of follicles development; detecting a leading follicle ≥ 12–14 mm diameter (Flexible protocol)17, or they are used from Day 5/ Day 6 of stimulation onward (Fixed protocol)18,19. However, recent research revealed that high gonadotropins levels during the early follicular phase of COS might have harmful effects on IVF/ICSI outcomes, as unsuppressed follicle-stimulation hormone (FSH) leads to an uncoordinated development of FSH‐sensitive follicles and a reduction in viable oocytes number20,21. While unsuppressed LH enhances estradiol (E2) production, in consequence, higher exposure of the genital tract to LH, E2, and progesterone might adversely affect the implantation rate mainly by altering endometrial receptivity22. Thus, new GnRH antagonist protocols were introduced to suppress gonadotropin levels, not only during the mid-follicular phase but also during the early follicular phase either as an Early-late Flexible protocol23,24 or an Early Fixed protocol25–27. However, Conventional GnRH antagonist protocols are still more commonly used in clinics than the Early GnRH antagonist ones. Several meta-analyses28–32 compared the safety and efficacy of treating PCOS women with GnRH antagonist protocols and the standard Long GnRH agonist protocol. However, all of them have included all available GnRH antagonist protocols; Early GnRH antagonist protocols, and Conventional GnRH antagonist ones. Moreover, most previous reviews28–30,32 have included different triggering agents in the GnRH antagonist group; GnRH agonist trigger and human chorionic gonadotropin (hCG) trigger. A recent Cochrane review33 demonstrated that using GnRH agonists for final oocyte maturation triggering is associated with a lower live birth rate; lower ongoing pregnancy rate; lower incidence of mild, moderate, and severe OHSS; and a higher rate of early miscarriage compared with hCG trigger in fresh cycles. Thus, we conducted this systematic review and meta-analysis to summarize the available evidence comparing the effects of Conventional GnRH antagonist protocols, the most commonly used GnRH antagonist protocols, and GnRH agonist protocols on IVF/ICSI outcomes in women with PCOS.
Materials and methods
Protocol and registration
This review was performed in accordance with relevant guidelines and regulations. The preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines 202034were followed to assure transparent reporting, see Supplementary Tables S1 and S2. The protocol of this study was registered at PROSPERO under registration number (CRD42021242476).
Eligibility criteria
The Participants, Intervention, Comparison, Outcomes, and Studies (PICOS) framework was as follow:
Participants: PCOS women undergoing controlled ovarian hyperstimulation as part of an IVF or ICSI programme.
Intervention: Conventional GnRH antagonist protocols.
Comparison: GnRH agonist protocols.
Outcomes: Live birth rate (LBR) per randomized woman, Ongoing pregnancy rate (OPR) per randomized woman, Ovarian hyperstimulation syndrome (OHSS) rate per woman randomized, Clinical pregnancy rate (CPR) per randomized woman, Multiple pregnancy rate (MPR) per randomized woman, Miscarriage rate (MR) per randomized woman, Cycle cancellation rate (CCR) per randomized woman, Stimulation duration (days), Gonadotropin consumption (IUs), E2 levels on hCG day, Endometrial thickness (in millimeters), Number of retrieved oocytes.
Study design: RCT.
Limits: human studies.
Studies were only included when data of one outcome of the outcomes of interest at least were available in a manner that allowed their inclusion in a meta-analysis.
Exclusion criteria
Meta-analysis, systematic literature reviews, narrative reviews, case reports, observational studies, animal studies, in-vitro studies.
RCTs comparing Early GnRH antagonist protocols with GnRH agonist protocols.
Studies reporting the desired outcomes per cycle and not per woman. (study authors were contacted to request the data per woman. If no response was obtained, the study was excluded).
Studies comparing more than one variable, like those comparing GnRH agonist versus hCG for triggering, in addition to comparing GnRH antagonist with GnRH agonist for suppression.
Regarding conference abstracts, if there was not enough information to decide their eligibility for inclusion or their results were not available numerically, study authors were contacted to provide missing information and data. If no response was obtained, the study was excluded.
Information sources and search strategies
A comprehensive electronic search was carried out in Pubmed, Cochrane Central Register of Controlled Trials (CENTRAL), Scopus, Web of Science, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Turning Research Into Practice (TRIP), ClinicalTrials.gov and ISRCTN registry from inception up until 24 November 2020 without any language or date restrictions. In addition, reference lists of eligible studies and previous meta-analyses were hand-searched to identify relevant studies. The search terms were modified when required for each database. The full-search strategy is reported in Supplementary Table S3.
Selection process
Duplication removal was conducted using two software independently, Mendeley Desktop 1.19.4 software (Glyph & Cog, LLC) and Microsoft Office Excel 2016 (Microsoft Corporation), to limit false duplicate, i.e. incorrectly identified as duplicate. Two authors (S.K. and A.N.) independently double screened articles by title and abstract. Subsequently, the same reviewers independently completed full-text screening of all potentially eligible studies and examined their compliance with the inclusion criteria. Any discrepancies during the study selection process were resolved by consultation with the third reviewer (M.A.). Where required, study investigators were contacted to clarify study eligibility.
Data collection process and data items
A data extraction form was created in Microsoft Office Excel 2016 (Microsoft Corporation) to facilitate the retrieval and storage of relevant data. The following information was sought from included studies: Publication details (first author's name, year of publication), Methods (study design, study duration, country of conduct), Participant’s characteristics (inclusion criteria, exclusion criteria, baseline characteristics, number of randomized women, number of excluded women after randomization, number of women lost to follow-up/ drop-out with reasons). Intervention/Comparison (Protocol used, type of GnRH antagonist/agonist used and when it was started, protocol pre-treatment drugs, stimulator used (type, when it was started, starting dose), trigger used, luteal phase support, transfer day), and all data that would allow the estimation of the effects of interest were extracted. In addition, we extracted information about the randomization process, allocation concealment, blinding, type of analysis, funding, OHSS diagnosis criteria and cycle cancellation criteria. Two authors (S.K. and A.N) independently extracted the data, and any disagreements were resolved by discussion with the third author (M.A). Where a study had multiple publications, the authors collated multiple reports of the same study so that each study rather than each report was the unit of interest in the review, and such study had a single study ID with multiple references35.
Outcomes of interest:
- Primary outcomes:
- Live birth rate (LBR) per woman randomized, defined as delivery of a live fetus after 20 completed weeks of gestation.
- Ongoing pregnancy rate (OPR) per randomized woman, defined as the number of pregnancies beyond 12 weeks' gestation per number of randomized women.
- Ovarian hyperstimulation syndrome (OHSS) rate per randomized woman.
- Secondary outcomes:
- Clinical pregnancy rate (CPR) per randomized woman, defined as the number of pregnancies with at least one gestational sac ± fetal heartbeat at transvaginal ultrasound per number of randomized women.
- Multiple pregnancy rate (MPR) per randomized woman, defined as the number of pregnancies with two or more gestational sacs on transvaginal ultrasound per number of randomized women.
- Miscarriage rate (MR) per randomized woman, defined as the number of pregnancies ending in the spontaneous loss of the embryo or fetus before 20 weeks' gestation per number of randomized women.
- Cycle cancellation rate (CCR) per woman randomized.
- Stimulation duration (days).
- Gonadotropin consumption (IUs).
- E2 levels on hCG day.
- Endometrial thickness (in millimeters) by ultrasound scan on hCG day.
- Number of retrieved oocytes.
Assessment of risk of bias in included studies
Two authors (S.K. and M.A.) independently assessed the risk of bias of each study using the revised Cochrane risk of bias tool for randomized controlled trials (ROB 2.0)36,37. Using ROB 2.0 tool, each study is judged to have either low risk, some concerns, or a high risk of bias for the studied outcome. Any disagreement was resolved by consultation with a third reviewer (N.A.). The risk of bias tool addresses the following domains: Bias arising from the randomization process; Bias due to deviations from intended interventions; Bias due to missing outcome data; Bias in the measurement of the outcome; Bias due to selection of the reported result and overall bias. For each domain, a series of signalling questions with the answers (yes, probably yes, no information, probably no, no) determine the risk of bias (low risk, some concerns and high risk). The overall risk of bias generally corresponds to the worst risk of bias in any of the domains. However, if a study is judged to have “some concerns” risk of bias for multiple domains, it might be judged as at high risk of bias overall36,37.
Summary measures
Dichotomous outcomes were reported as Relative Risk (Risk ratio, RR) and continuous outcomes as Weighted Mean Difference (WMD), both with 95% CIs. When overall results were significant in dichotomous outcomes, the number needed to treat for an additional beneficial outcome (NNTB) and the number needed to treat for an additional harmful outcome (NNTH) were computed from RR based on the Cochrane recommendations by combining the risk ratio with an estimate of the prevalence of the event in the control group of the trials7.
Dealing with missing data
We analyzed data on an intention‐to‐treat basis as far as possible. If any information was missing, the corresponding authors were notified twice via an e-mail to retrieve the missing data. If the authors did not respond within two weeks of the first contact, we assumed that the outcome did not occur in dichotomous outcomes (e.g. clinical pregnancy rate), while we analyzed only available data in continuous ones. If the data were reported as median with (Q25, Q75) or (minimum value, maximum value) and were suspected to be skewed, we contacted study authors to request the results in mean and standard deviation (SD). If no response was obtained, the Box-Cox method of McGrath et al.38 to estimate the mean and SD was used. Where data presented graphically, we contacted the study authors to request the results in mean and SD. If no response was obtained, WebPlotDigitizer (Ankit Rohatgi. WebPlotDigitizer. Version 4.4. 2020. Available at https://automeris.io/WebPlotDigitizer) was used to extract this information. When the miscarriage rate measured at a “12 weeks’ gestation” time-point, and both the number of clinical pregnancies and the number of miscarriage were available, we calculated the ongoing pregnancy rate as [number of clinical pregnancies—number of miscarriage/ number of randomized woman]. Similarly, when the number of clinical pregnancies and the number of ongoing pregnancies were available, we calculated the miscarriage rate as [number of clinical pregnancies—number of ongoing pregnancies/number of randomized woman]. It is worth noting that we considered cycle cancellation cases as missing outcome data for the following outcomes: live birth rate, clinical pregnancy rate, ongoing pregnancy rate, multiple pregnancy rate and miscarriage rate since the participants no longer capable of experiencing those events.
Data synthesis
Data synthesis and analyses were done using the Review Manager 5.3; Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014, with the assistance of Revman Web; Review Manager Web (RevMan Web). Version (2.4.1). The Cochrane Collaboration, (2021). Available at revman.cochrane.org; to create forest plots with ROB assessment since Review Manager v.5.3 only supports ROB 1 tool. A random-effects model was used for all meta-analysis. Heterogeneity amongst the included studies was evaluated qualitatively; through visual inspection of forest plots and comparing the characteristics of included studies, and quantitatively; using the χ2 test of heterogeneity and the I2 statistic. Subgroup analyses were performed to detect potential sources of heterogeneity based on the type of antagonist protocol used (Flexible VS Fixed). The following sensitivity analyses were also performed to determine whether the conclusions are robust to arbitrary decisions made regarding the eligibility criteria and analysis process: the removal of high risk of bias studies and estimated outcome data (converting medians and (Q25, Q75) or medians and (minimum value, maximum value) to means ± standard deviations, and estimating the outcome data from a graph).
Publication bias assessment
Given the difficulty of detecting and correcting publication bias and other reporting biases, we aimed to minimize their potential impact by ensuring a comprehensive search for eligible studies and being alert to duplication of data. If ≥ ten studies were included in a comparison, a funnel plot was produced to graphically explore the possibility of small-study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies) as when there are fewer studies, the power of the tests is weak39.
Assessment of certainty of evidence
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) assessment40 was used to assess the overall quality of evidence for each outcome by two authors (S.K. and M.A.) independently, and a “Summary of findings” table was created using GradePro GDT [GRADEpro Guideline Development Tool. McMaster University, 2020 (developed by Evidence Prime, Inc.). Available from gradepro.org.] to report the certainty of evidence. Disagreements between the two review authors over the quality assessment of the evidence were resolved by discussion, with the involvement of the third author (A.N.) whenever necessary.
Based on the Grade approach, the quality of evidence is assessed based on the risk of bias, inconsistency, indirectness, imprecision, or publication bias. Quality of evidence is rated as:
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: We are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: Our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect.
Very low certainty: We have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect40.
Results
Study selection
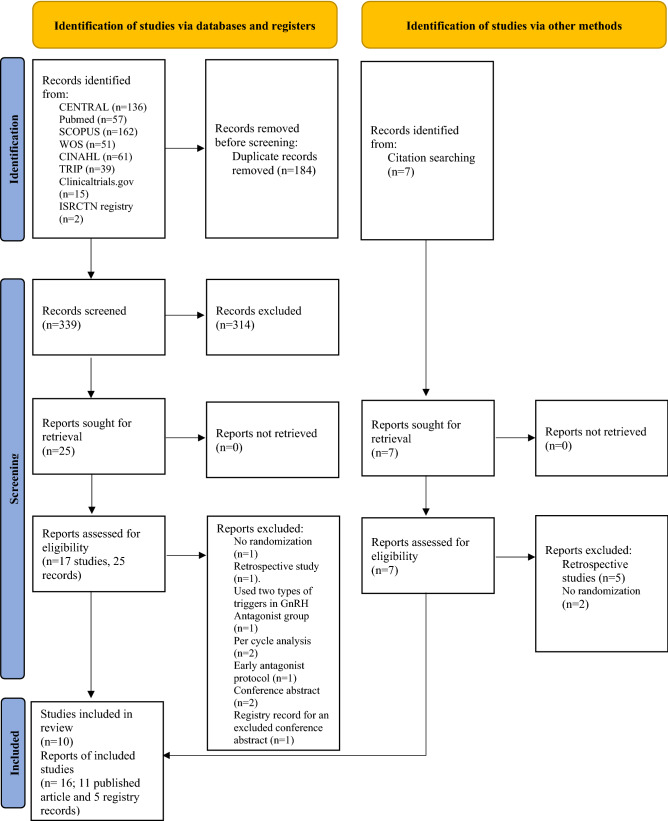
The database search returned a total of 523 records (CENTRAL n = 136, Pubmed n = 57, SCOPUS n = 162, Web of Science n = 51, CINAHL n = 61, TRIP n = 39, Clinicaltrials.gov n = 15, ISRCTN registry n = 2). Additionally, seven records were identified from the hand searches, but none of them met our inclusion criteria. After duplicates removal (n = 184), 339 records were left to be screened, of which 314 were excluded based on the title and abstract. The full-text review of the remaining 25 records (17 studies) led to a rejection of further nine records (7 studies). We only included Conventional GnRH antagonist protocols. Thus, we excluded the Early GnRH antagonist arm from 3 included studies. The excluded studies/arms are shown in Table 1 with reasons for exclusion. A remaining total of 16 records, 11 published article and five registry records (10 studies) met the inclusion criteria and were included in the meta-analysis. (See Fig. 1 for details of this process). The characteristics of included RCTs are summarized in Supplementary Table S4. The included studies randomized a total of 1214 women.
Table 1.
Excluded studies.
| Databases search exclusions: | |
|---|---|
| Study ID | Reasons for exclusion |
| Ashrafi et al.41 | Used two types of triggers in the GnRH antagonist group; hCG trigger in general and GnRH agonist trigger in cases at high risk of OHSS |
| Chen et al.42 | A retrospective study |
| Choi et al. 43 (one arm excluded) | A three-arm study included; an Early Flexible GnRH antagonist protocol, a Conventional Flexible GnRH antagonist protocol, and a Long GnRH agonist protocol. We only included the Conventional Flexible GnRH antagonist arm and the Long GnRH agonist arm |
| Choi et al. 44,45 | Used a Per cycle analysis. Yet, we were not capable of reaching the study corresponding author due to an invalid e-mail |
| Lainas et al.25 | Used an Early GnRH antagonist protocol in the GnRH antagonist arm |
| Mokhtar et al.23,46 (one arm excluded) | A three-arm study included; an Early Flexible GnRH antagonist protocol, a Conventional Flexible GnRH antagonist protocol, and a Long GnRH agonist protocol. We only included the Conventional Flexible GnRH antagonist arm and the Long GnRH agonist arm |
| Moshin et al.47 | A conference abstract. The per-cycle analysis was most likely used in this study. Yet, no response from the author was obtained to confirm its eligibility |
| Shin et al.27,48 (one arm excluded) | A three-arm study included; an Early Fixed GnRH antagonist protocol, a Conventional Fixed GnRH antagonist protocol and a Long GnRH agonist protocol. We only included the Conventional Fixed GnRH antagonist arm and the Long GnRH agonist arm |
| Vrtačnik-Bokal et al.49 | Not a randomized controlled trial |
| Zeinalzadeh et al. 50,51 | A conference abstract with no numerical data, Yet, no response from the author was obtained to provide the study results |
| Hand search exclusions: | |
| Study ID | Reasons for exclusion |
| Orvieto et al.52 | A retrospective case series |
| Orvieto et al.53 | A retrospective case series |
| Kaur et al.54 | Not a randomized controlled trial |
| Kdous et al.55 | A retrospective case–control study |
| Kdous et al.56 | A retrospective case–control study |
| Onofriescu et al.57 | Not a randomized controlled trial |
| Segal et al.58 | A retrospective case series |
GnRH: Gonadotropin-releasing hormone, hCG: Human chorionic gonadotropin, OHSS: ovarian hyperstimulation syndrome.
Figure 1.
Flow diagram selection process.
Study characteristics
Nine studies were single-center RCTs17,18,23,43,59–63, while one study was a multi-center RCT27. All of the included trials had a parallel study design. Inclusion and exclusion criteria were available in all studies. The included studies were published between 2005 and 2018, and were conducted in Greece62, Iran17,23,60, Korea27,43, Poland61, Serbia63 and Turkey18,59. The number of randomized women in the GnRH antagonist arms ranged from 14 to 150 women, while in the GnRH agonist arms from 13 to 150 women. All studies initiated an oral contraceptive pill pre-treatment (OCPs) for all participants.
All included studies compared GnRH antagonist protocols with the Long GnRH agonist protocol. The Flexible GnRH antagonist protocol was used in 8 studies17,23,43,59–63, while the Fixed GnRH antagonist protocol was used in 2 studies18,27. The used GnRH antagonists were Cetrorelix in 8 studies RCTs17,23,27,43,59–61,63, and Ganirelix in 2 studies18,62, while used GnRH agonists were Buserelin in 3 studies17,23,60, Leuprolide in 2 studies18,59, Triptorelin in 5 studies27,43,61–63. The gonadotropin starting dose varied from 150 to 225 IUs of recombinant follicle-stimulating hormone (r-FSH) or human menopausal gonadotropin (hMG) in the GnRH antagonist group while 150 to 300 IUs in the GnRH agonist group. However, only one study did not report gonadotropin starting dose43. All included studies used hCG trigger. All the included studies transferred embryos during the cleavage stage (D2/D3) to the participants except for one studies27 which transferred both cleave stage embryos and blastocyst embryos. Two studies performed intention-to-treat analysis (ITT)60,62, 3 studies performed modified ITT (mITT; ITT with excluding missing outcome data)17,27,59, 4 studies performed ITT/mITT based on the outcome18,23,43,63 and one study performed a per-protocol analysis61. Seven studies reported OHSS diagnosis criteria17,18,23,27,60,62,63, while only three studies reported cycle cancellation criteria17,18,62. For more details about the studies characteristics, see Supplementary Table S4.
Risk of bias of the included studies
Regarding bias arising from the randomization process, four studies17,18,27,61 were at “low risk” of bias, five studies23,43,59,62,63 held “some concern”, and one study was at “high risk” of bias60. Randomization was accomplished using a random numbers table in two studies18,59, a computer-generated random number table in four studies23,61–63 and a web-based system in one study27. However, the remaining studies did not provide any information regarding the methods were used for sequence generation. Allocation concealment was suitably performed using sealed opaque envelopes in 3 studies17,18,61 and using a web-based system in one study27. Nevertheless, the remaining studies did not provide any information about the possibility of allocation concealment or what methods were used to conceal it. Baseline imbalance arises from the randomization process was suspected in one study60 since there was a significant difference in the means of age between the two groups; with younger patients in the GnRH antagonist one, and no information about the randomization process or allocation concealment were provided. However, since ROB 2.0 tool assesses the risk of bias in the remaining domains at the outcome level, the information about the risk of bias regarding the other domains are reported separately for each outcome.
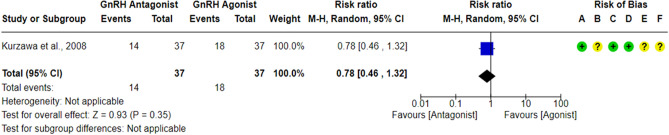
Live birth rate per randomized woman (LBR)
Only one RCT61 compared the LBR between the GnRH antagonist and GnRH agonist protocols in 74 PCOS women, and it did not report any significant differences in LBR between the GnRH antagonist protocol and the Long GnRH agonist protocol (RR = 0.78, 95% CI: [0.46 to 1.32]; P = 0.35; low-quality evidence; Fig. 2). The study was judged to be at “low risk” of bias due to missing outcome data and bias in outcome measurement, taking into account the number of missing outcome data and the event risk. Furthermore, it is an objective outcome, and its assessment would not be influenced by knowledge of the intervention received. However, it held “some concern” regarding the risk of bias due to deviations from intended interventions since a per-protocol analysis was conducted. Yet, the number of excluded participants was unlikely to have a substantial impact on the results. The study had a registry record, and the outcome was specified in it, but it was retrospectively registered. Using GRADE, we judged the certainty of evidence as low; we down-graded it by two levels due to concerns over imprecision.
Figure 2.
Forest Plot: Live Birth Rate Per Randomized Woman. (A) Bias arising from the randomization process; (B) Bias due to deviations from intended interventions; (C) Bias due to missing outcome data;(D) Bias in measurement of the outcome; (E) Bias in selection of the reported result and (F) overall bias.
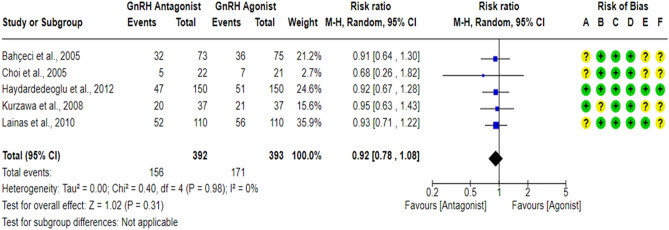
Ongoing pregnancy rate per randomized woman (OPR)
A total of five RCTs18,43,59,61,62 compared the OPR between the GnRH antagonist and GnRH agonist protocols in 785 PCOS women. All included studies were at “low risk” of bias due to deviations from intended interventions except for one study61, which held “some concern” since a per-protocol analysis was conducted. Yet, the number of excluded participants was unlikely to have a substantial impact on the results. All included studies were at “low risk” of bias due to missing outcome data and bias in outcome measurement, taking into account the number of missing outcome data, the causes of missingness, and the event risk. Furthermore, it is an objective outcome, and its assessment would not be influenced by knowledge of the intervention received. Only two studies18,62 had a prospective registry record, and the outcome was pre-specified in it, so they were at “low risk” of bias in selection of the reported result. Pooling the results of these RCTs did not show any significant differences in OPR between the GnRH antagonist protocols and the Long GnRH agonist protocol (RR = 0.92, 95% CI: [0.78 to 1.08]; P = 0.31; I2 = 0%; χ2-P = 0.98; high-quality evidence; Fig. 3).
Figure 3.
Forest Plot: Ongoing Pregnancy Rate Per Randomized Woman. (A) Bias arising from the randomization process; (B) Bias due to deviations from intended interventions; (C) Bias due to missing outcome data;(D) Bias in measurement of the outcome; (E) Bias in selection of the reported result and (F) overall bias.
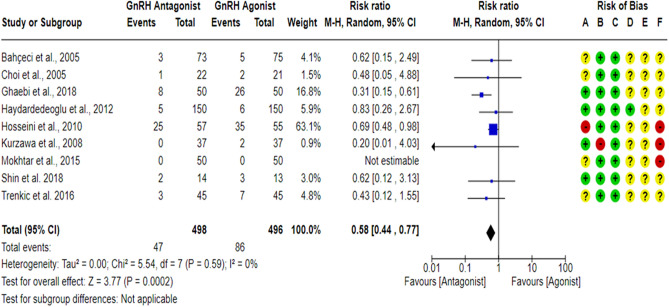
OHSS rate per randomized woman
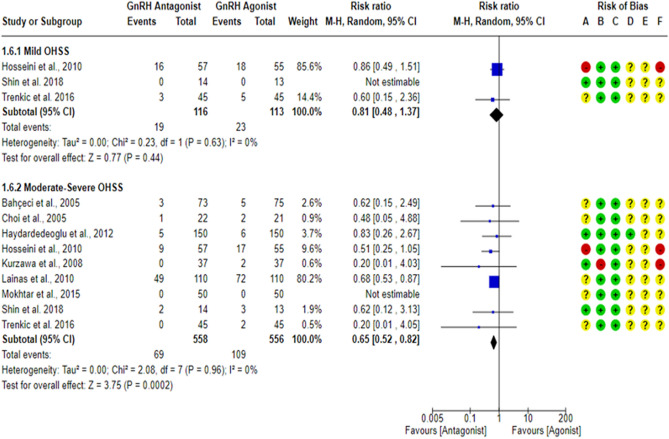
All included studies17,18,23,27,43,59–63 compared the OHSS rate between the GnRH antagonist and GnRH agonist protocols. All included studies were at “low risk” of bias due to missing outcome data and bias due to deviations from intended interventions except for one study61, which was at “high risk” of bias due to deviations from intended interventions since a per-protocol analysis was conducted, and the number of excluded participants was likely to have a substantial impact on the results. One study18 was at “low risk” of bias in outcome measurement since the investigators were blinded (based on the information provided in the registry record), while nine studies17,23,27,43,59–63 held “some concern” of bias due to failure to blind the outcome’ assessors or not reporting whether the outcome assessors were blinded or not. Moreover, OHSS is considered a subjective outcome as it is usually diagnosed based on particular criteria, so we have some concerns about potential bias. Only two studies18,62 had a prospective registry record, and the outcome was pre-specified in it. However, they did not report the outcome measurement time-point. In addition, one study62 reported the OHSS rate based on its grade, with grade I representing “none or mild OHSS”, so we were incapable of determining the overall number of OHSS cases, but we could determine the number of Moderate-Severe cases. Thus, only 9 RCTs17,18,23,27,43,59–61,63 (994 PCOS women) were included in the meta-analysis concerning the overall OHSS rate. Pooling the results of these RCTs showed a significant lower OHSS rate when women were treated with the GnRH antagonist protocols compared to those treated with the Long GnRH agonist protocol (RR = 0.58, 95% CI: [0.44 to 0.77]; P = 0.0002; NNTB 14, NNTB 95% CI: [11 to 26]; I2 = 0%; χ2-P = 0.59; low-quality evidence; Fig. 4). Based on the results of the sensitivity analysis, the results were robust against the exclusion of high risk of bias studies60,61 (RR = 0.44, 95% CI: [0.28 to 0.71]; P = 0.0007; I2 = 0%; χ2-P = 0.76). Using GRADE, we judged the certainty of evidence as low; we down-graded it by two levels, one level due to concerns over imprecision and one due to concerns over the risk of bias. We also conducted a subgroup analysis based on the degree of OHSS [Mild VS Moderate-Severe]. Based on the result of the subgroup analysis, there was not any significant difference in Mild OHSS rate between the GnRH antagonist protocols and the Long GnRH agonist protocol (3 studies; 229 women; RR = 0.81, 95% CI: [0.48 to 1.37]; P = 0.44; I2 = 0%; χ2-P = 0.63; very-low quality evidence; Fig. 5). Using GRADE, we judged the certainty of evidence as very low; we down-graded it by three levels, two levels due to concerns over imprecision and one due to concerns over the risk of bias. On the other hand, a significant lower Moderate-Severe OHSS rate was noticed when women were treated with GnRH antagonist protocols compared to those treated with the Long GnRH agonist protocol (9 studies; 1114 women; RR = 0.65, 95% CI: [0.52 to 0.82]; P = 0.0002; NNTB 15, NNTB 95% CI: [11 to 29]; I2 = 0%; χ2-P = 0.96; very-low quality evidence; Fig. 5). Using GRADE, we judged the certainty of evidence as very low; we down-graded it by three levels, two levels due to concerns over imprecision and one due to concerns over the risk of bias.
Figure 4.
Forest Plot: OHSS Rate Per Randomized Woman. (A) Bias arising from the randomization process; (B) Bias due to deviations from intended interventions; (C) Bias due to missing outcome data;(D) Bias in measurement of the outcome; (E) Bias in selection of the reported result and (F) overall bias.
Figure 5.
Forest Plot: OHSS Rate Per Randomized Woman (Per OHSS Grade). (A) Bias arising from the randomization process; (B) Bias due to deviations from intended interventions; (C) Bias due to missing outcome data;(D) Bias in measurement of the outcome; (E) Bias in selection of the reported result and (F) overall bias.
Clinical pregnancy rate per randomized woman (CPR)
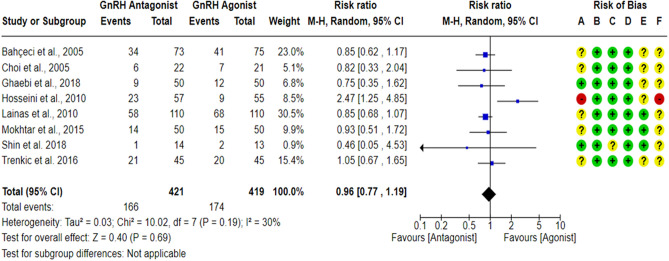
A total of eight RCTs17,23,27,43,59,60,62,63 compared the CPR between the GnRH antagonist and GnRH agonist protocols in 840 PCOS women. All included studies were judged to be at “low risk” of bias due to deviations from intended interventions and bias in outcome measurement taking into account the type of analysis. In addition, CPR is an objective outcome, and its assessment would not be influenced by knowledge of the intervention received. All included studies were at “low risk” of bias due to missing outcome data except for one study27, which held “some concern” based on the number of missing outcome data, the causes of missingness, and the event risk. Only three studies23,27,62 had a prospective registry record, and the outcome was pre-specified in it, so they were at “low risk” of bias in selection of the reported result. Pooling the results of these RCTs did not show any significant differences in CPR between the GnRH antagonist protocols and the Long GnRH agonist protocol (RR = 0.96, 95% CI: [0.77 to 1.19]; P = 0.69; I2 = 30%; χ2-P = 0.19; high-quality evidence; Fig. 6). Based on the results of the sensitivity analysis, the results were robust against the exclusion of the high risk of bias study60 (RR = 0.87, 95% CI: [0.74 to 1.02]; P = 0.09; I2 = 0%; χ2-P = 0.98).
Figure 6.
Forest Plot: Clinical Pregnancy Rate Per Randomized Woman. (A) Bias arising from the randomization process; (B) Bias due to deviations from intended interventions; (C) Bias due to missing outcome data;(D) Bias in measurement of the outcome; (E) Bias in selection of the reported result and (F) overall bias.
Multiple pregnancy rate per randomized woman (MPR)
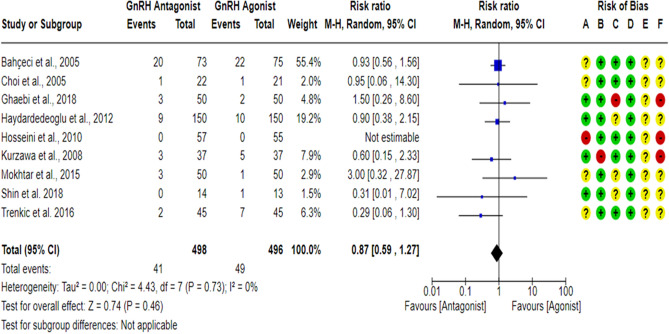
A total of nine17,18,23,27,43,59–61,63 RCTs compared the MPR between the GnRH antagonist and GnRH agonist protocols in 994 PCOS women. All included studies were at “low risk” of bias due to deviations from intended interventions except for one study61, which was at “high risk” of bias since a per-protocol analysis was conducted, and the number of excluded participants was likely to have a substantial impact on the results. All included studies were at “low risk” of bias due to missing outcome data except for three studies18,23,27, which held “some concern”, and one study17 was at “high risk” of bias based on the number of missing data, the causes of missingness, and the event risk. However, we judged the latter as “high risk” of bias since drop-out cases were only observed in the GnRH antagonist group, and the reasons for dropping out were not reported. All included studies were at “low risk” of bias in outcome measurement since it is an objective outcome, and its assessment would not be influenced by knowledge of the intervention received. Pooling the results of these RCTs did not show any significant differences in MPR between the GnRH antagonist protocols and the Long GnRH agonist protocol (RR = 0.87, 95% CI: [0.59 to 1.27]; P = 0.46; I2 = 0%; χ2-P = 0.73; very-low quality evidence; Fig. 7). Based on the results of the sensitivity analysis, the results were robust against the exclusion of high risk of bias studies17,60,61 (RR = 0.87, 95% CI: [0.58 to 1.31]; P = 0.50; I2 = 0%; χ2-P = 0.58). Using GRADE, we judged the certainty of evidence as very low; we down-graded it by three levels, two levels due to concerns over imprecision and one due to concerns over the risk of bias.
Figure 7.
Forest Plot: Multiple Pregnancy Rate Per Randomized Woman. (A) Bias arising from the randomization process; (B) Bias due to deviations from intended interventions; (C) Bias due to missing outcome data;(D) Bias in measurement of the outcome; (E) Bias in selection of the reported result and (F) overall bias.
Miscarriage rate per randomized woman (MR)
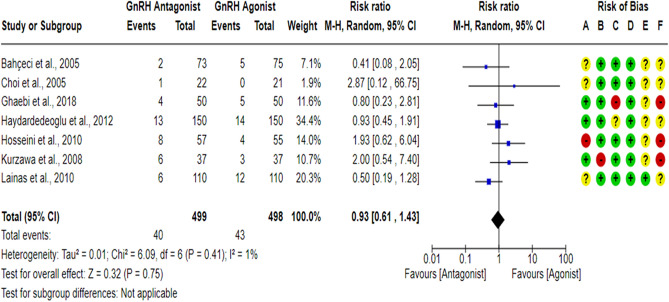
A total of seven RCTs17,18,43,59–62 compared the MR between the GnRH antagonist and GnRH agonist protocols in 997 PCOS women. All included studies were at “low risk” of bias due to deviations from intended interventions except for one study61, which was at “high risk” of bias since a per-protocol analysis was conducted, and the number of excluded participants was likely to have a substantial impact on the results. All included studies were at “low risk” of bias due to missing outcome data except for one study18, which held “some concern”, and one study17 was at “high risk” of bias based on the number of missing outcome data, the causes of missingness, and the event risk. However, we judged the latter as “high risk” of bias since drop-out cases were only observed in the GnRH antagonist group, and the reasons for dropping out were not reported. All included studies were at “low risk” of bias in outcome measurement since it is an objective outcome, and its assessment would not be influenced by knowledge of the intervention received. Pooling the results of these RCTs did not show any significant differences in MR between the GnRH antagonist protocols and the Long GnRH agonist protocol (RR = 0.93, 95% CI: [0.61 to 1.43]; P = 0.75; I2 = 1%; χ2-P = 0.41; very-low quality evidence; Fig. 8). Based on the results of the sensitivity analysis, the results were robust against the exclusion of high risk of bias studies17,60,61 (RR = 0.72, 95% CI: [0.42 to 1.23]; P = 0.23; I2 = 0%; χ2-P = 0.52). Using GRADE, we judged the certainty of evidence as very low; we down-graded it by three levels, two levels due to concerns over imprecision and one due to concerns over the risk of bias.
Figure 8.
Forest Plot: Miscarriage Rate Per Randomized Woman. (A) Bias arising from the randomization process; (B) Bias due to deviations from intended interventions; (C) Bias due to missing outcome data;(D) Bias in measurement of the outcome; (E) Bias in selection of the reported result and (F) overall bias.
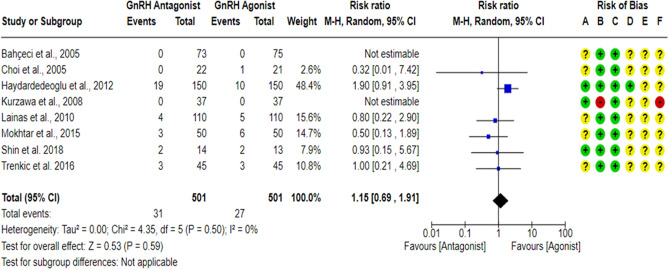
Cycle cancellation rate per randomized woman (CCR)
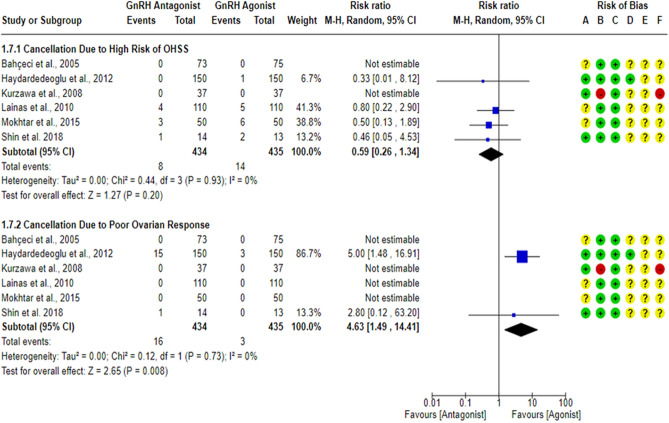
A total of eight studies18,23,27,43,59,61–63 reported cycle cancellation rate between the GnRH antagonist and GnRH agonist protocols in 1002 PCOS women. Although one study17 reported cycle cancellation criteria, it was unclear if any cycle cancellation cases would have been noted in the study’s groups. Thus, this study17 was not included in the meta-analysis. All included studies were at “low risk” of bias due to missing outcome data and bias due to deviations from intended interventions except for one study61, which was at “high risk” of bias due to deviations from intended interventions even though no cycle cancellation cases were reported, but a per-protocol analysis was conducted, and the number of excluded participants was likely to have a substantial impact on the results. One study18 was at “low risk” of bias in outcome measurement since the investigators were blinded (based on the information provided in the registry record), while seven studies23,27,43,59,61–63 held “some concern” of bias due to failure to blind the outcome’ assessors or not being clear in reporting whether the outcome assessors were blinded or not. Cycle cancellation is considered a subjective outcome since the cancellation decision is usually taken based on particular criteria, so we have some concerns about potential bias. Pooling the results of these RCTs did not show any significant differences in CCR between the GnRH antagonist protocols and the Long GnRH agonist protocol (RR = 1.15, 95% CI: [0.69 to 1.91]; P = 0.59; I2 = 0%; χ2-P = 0.50; very-low quality evidence; Fig. 9). Based on the results of the sensitivity analysis, the results were robust against the exclusion of the high risk of bias study61 (RR = 1.15, 95% CI: [0.69 to 1.91]; P = 0.59; I2 = 0%; χ2-P = 0.50). Using GRADE, we judged the certainty of evidence as very low; we down-graded it by three levels, two levels due to concerns over imprecision and one due to concerns over the risk of bias. We also conducted a subgroup analysis based on the causes of cycle cancellation [High Risk of OHSS VS Poor Ovarian Response]. Two studies43,63 did not report the causes of cycle cancellation, so they were excluded from the subgroup analysis. Based on the result of the subgroup analysis, there was not any significant difference in the rate of cycle cancellation due to High Risk of OHSS between the GnRH antagonist protocols and the Long GnRH agonist protocol (6 studies; 869 women; RR = 0.59, 95% CI: [0.26 to 1.34]; P = 0.20; I2 = 0%; χ2-P = 0.93; very-low quality evidence; Fig. 10). Using GRADE, we judged the certainty of evidence as very low; we down-graded it by three levels, two levels due to concerns over imprecision and one due to concerns over the risk of bias. On the other hand, a significant increase in cycle cancellation rate due to Poor Ovarian Response was noticed when women were treated with GnRH antagonist protocol compared to those treated with the Long GnRH agonist protocol (6 studies; 869 women; RR = 4.63, 95% CI: [1.49 to 14.41]; P = 0.008; NNTH 40, NNTH 95% CI: [11 to 292]; I2 = 0%; χ2-P = 0.73; very-low quality evidence; Fig. 10). Using GRADE, we judged the certainty of evidence as very low; we down-graded it by three levels, two levels due to concerns over imprecision and one due to concerns over the risk of bias.
Figure 9.
Forest Plot: Cycle Cancellation Rate Per Randomized Woman. (A) Bias arising from the randomization process; (B) Bias due to deviations from intended interventions; (C) Bias due to missing outcome data;(D) Bias in measurement of the outcome; (E) Bias in selection of the reported result and (F) overall bias.
Figure 10.
Forest Plot: Cycle Cancellation Rate Per Randomized Woman (Per Cause of Cancellation). (A) Bias arising from the randomization process; (B) Bias due to deviations from intended interventions; (C) Bias due to missing outcome data;(D) Bias in measurement of the outcome; (E) Bias in selection of the reported result and (F) overall bias.
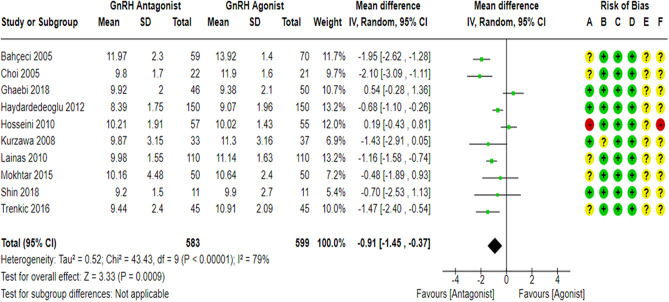
Stimulation duration
All the included studies17,18,23,27,43,59–63 compared the stimulation duration between the GnRH antagonist and GnRH agonist protocols in 1182 PCOS women. All included studies were at “low risk” of bias due to deviations from intended interventions except for one study61, which held “some concern” since a per-protocol analysis was conducted. Yet, the number of excluded participants was unlikely to have a substantial impact on the results. All included studies were at “low risk” of bias due to missing outcome data and bias in outcome measurement, taking into account the number of missing outcome data, and the causes of missingness. In addition, it is an objective outcome, and its assessment would not be influenced by knowledge of the intervention received. Pooling the results of these RCTs showed that the stimulation duration was significantly shorter in the GnRH antagonist protocols compared with the Long GnRH agonist protocol (WMD = − 0.91, 95% CI: [− 1.45 to − 0.37] day; P = 0.0009; I2 = 79%; χ2-P < 0.00001; very low-quality evidence; Fig. 11). Based on the results of the sensitivity analysis, the results were robust against the exclusion of the high risk of bias study60 (WMD = − 1.06, 95% CI: [− 1.59 to − 0.54] day; P < 0.0001; I2 = 74%; χ2-P = 0.0002) and the removal of estimated outcome data61,62 (WMD = − 0.83, 95% CI: [− 1.51 to − 0.14] day; P = 0.02; I2 = 83%; χ2-P < 0.00001). Subgroup analyses were performed to detect potential sources of heterogeneity based on the type of GnRH antagonist protocol used (Flexible VS Fixed). The results of subgroup analysis showed that the stimulation duration was significantly shorter in the GnRH antagonist protocol compared with the Long GnRH agonist protocol either when using Flexible GnRH antagonist protocol (8 RCTs; 860 participants; WMD = − 0.97, 95% CI: [− 1.66 to − 0.28] day; P = 0.006; I2 = 83%; χ2-P < 0.00001) or Fixed GnRH antagonist protocol (2 RCTs; 322 participants; WMD = − 0.68, 95% CI: [-1.09 to − 0.27] day; P = 0.001; I2 = 0%; χ2-P = 0.98) and the result of the subgroup analysis was not significant (χ2-P = 0.49, I2 = 0%). Using GRADE, we judged the certainty of evidence as very low; we down-graded it by three levels, two levels due to concerns over heterogeneity and one over imprecision.
Figure 11.
Forest Plot: Stimulation Duration. (A) Bias arising from the randomization process; (B) Bias due to deviations from intended interventions; (C) Bias due to missing outcome data;(D) Bias in measurement of the outcome; (E) Bias in selection of the reported result and (F) overall bias.
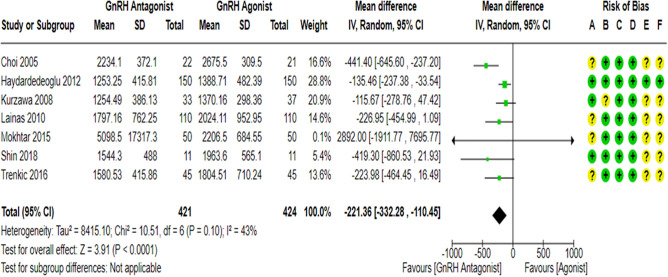
Gonadotropin dose
Seven RCTs18,23,27,43,61–63 compared gonadotropin consumption (IUs) during COS between the GnRH antagonist and GnRH agonist protocols in 845 PCOS women. All of them used r-FSH for ovarian stimulation. All included studies were at “low risk” of bias due to deviations from intended interventions except for one study61, which held “some concern” since a per-protocol analysis was conducted. However, the number of excluded participants was unlikely to have a substantial impact on the results. All included studies were at “low risk” of bias due to missing outcome data and bias in outcome measurement, taking into account the number of missing outcome data, and the causes of missingness. Moreover, it is an objective outcome, and its assessment would not be influenced by knowledge of the intervention received. Only one study18 had a prospective registry record, and the outcome was pre-specified in it, so they were at “low risk” of bias in selection of the reported result. Pooling the results of these RCTs showed that the gonadotropin consumption (IUs) during COS was significantly lower in the GnRH antagonist protocols compared with the Long GnRH agonist protocol (WMD = − 221.36, 95% CI: [− 332.28 to − 110.45] IUs; P < 0.0001; I2 = 43%; χ2-P = 0.1; high-quality evidence; Fig. 12). Based on the results of the sensitivity analysis, the results were robust against the removal of estimated outcome data61,62 (WMD = − 269.12, 95% CI: [− 448.23 to − 0.90] IU; P = 0.003; I2 = 58%; χ2-P = 0.05).
Figure 12.
Forest Plot: Gonadotropin Dose. (A) Bias arising from the randomization process; (B) Bias due to deviations from intended interventions; (C) Bias due to missing outcome data;(D) Bias in measurement of the outcome; (E) Bias in selection of the reported result and (F) overall bias.
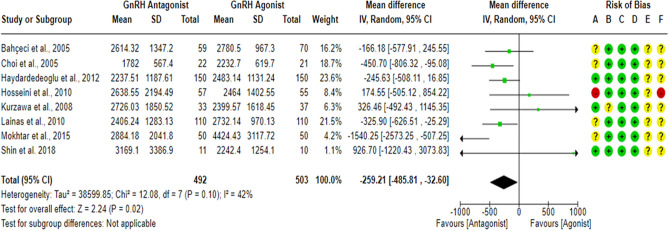
E2 levels on hCG day
Eight RCTs18,23,27,43,59–62 compared E2 levels on hCG day between the GnRH antagonist and GnRH agonist protocols in 995 PCOS women. All included studies were at “low risk” of bias due to deviations from intended interventions except for one study61, which held “some concern” since a per-protocol analysis was conducted. However, the number of excluded participants was unlikely to have a substantial impact on the results. All included studies were at “low risk” of bias due to missing outcome data and bias in outcome measurement, taking into account the number of missing outcome data, and the causes of missingness. Moreover, it is an objective outcome, and its assessment would not be influenced by knowledge of the intervention received. Pooling the results of these RCTs showed a significant lower E2 level on hCG day in the GnRH antagonist protocols compared with the Long GnRH agonist protocol (WMD = − 259.21, 95% CI: [− 485.81 to − 32.60] pg/ml; P = 0.02; I2 = 42%; χ2-P = 0.10; moderate-quality evidence; Fig. 13). Based on the results of the sensitivity analysis, the results were robust against the exclusion of the high risk of bias study60 (WMD = − 298.98, 95% CI: [− 530.64 to − 67.32] pg/ml; P = 0.01; I2 = 42%; χ2-P = 0.11) and the removal of estimated outcome data61,62 (WMD = − 292.87, 95% CI: [− 591.48 to 5.74] pg/ml; P = 0.05; I2 = 49%; χ2-P = 0.08). Using GRADE, we judged the certainty of evidence as moderate; we down-graded it by one level due to concerns over imprecision.
Figure 13.
Forest Plot: E2 Levels on hCG Day. (A) Bias arising from the randomization process; (B) Bias due to deviations from intended interventions; (C) Bias due to missing outcome data;(D) Bias in measurement of the outcome; (E) Bias in selection of the reported result and (F) overall bias.
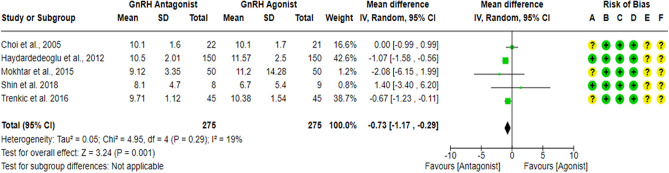
Endometrial thickness on hCG day
Five RCTs18,23,27,43,63 compared Endometrial thickness on hCG day between the GnRH antagonist and GnRH agonist protocols in 550 PCOS women. All included studies were at “low risk” of bias due to missing outcome data, bias due to deviations from intended interventions and bias in outcome measurement, considering the type of analysis, the number of missing outcome data, and the causes of missingness. Moreover, it is an objective outcome, and its assessment would not be influenced by knowledge of the intervention received. Pooling the results of these RCTs showed a significant lower Endometrial thickness on hCG day in the GnRH antagonist protocols compared to the Long GnRH agonist protocol (WMD = -0.73, 95% CI: [-1.17 to -0.29] mm; P = 0.001; I2 = 19%; χ2-P = 0.29; moderate-quality evidence; Fig. 14). Using GRADE, we judged the certainty of evidence as moderate; we down-graded it by one level due to concerns over imprecision.
Figure 14.
Forest Plot: Endometrial Thickness on hCG Day. (A) Bias arising from the randomization process; (B) Bias due to deviations from intended interventions; (C) Bias due to missing outcome data;(D) Bias in measurement of the outcome; (E) Bias in selection of the reported result and (F) overall bias.
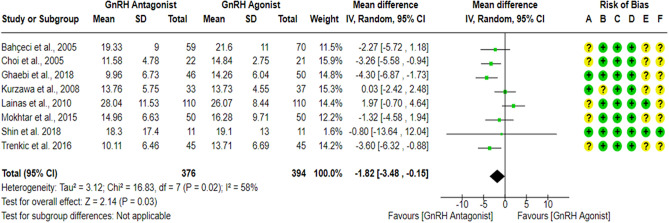
Number of retrieved oocytes
Nine RCTs17,23,27,43,59–63 compared the number of retrieved oocytes between the GnRH antagonist and GnRH agonist protocols in 882 PCOS women. All included studies were at “low risk” of bias due to deviations from intended interventions except for one study61, which held “some concern” since a per-protocol analysis was conducted. Yet, the number of excluded participants was unlikely to have a substantial impact on the results. All included studies were at “low risk” of bias due to missing outcome data and bias in outcome measurement, considering the number of missing outcome data, and the causes of missingness. Moreover, it is an objective outcome, and its assessment would not be influenced by knowledge of the intervention received. Only two studies27,62 had a prospective registry record, and the outcome was pre-specified in it, so they were at “low risk” of bias in selection of the reported result. Pooling the results of these RCTs did not show any differences in the number of retrieved oocytes between the GnRH antagonist protocols and the Long GnRH agonist protocol (WMD = − 1.13, 95% CI: [− 3.11 to 0.84] oocyte; P = 0.26; I2 = 75%; χ2-P < 0.0001). Based on the results of the sensitivity analysis, the results were robust against the removal of estimated outcome data43,61,62 (WMD = − 1.55, 95% CI: [− 4.42 to 1.31] oocyte; P = 0.29; I2 = 78%; χ2-P = 0.0004). Nevertheless, exclusion of high risk of bias studies60 changed the effect, as the results showed a significantly lower number of retrieved oocytes in the GnRH antagonist protocols compared to the Long GnRH agonist protocol (WMD = − 1.82, 95% CI: [− 3.48 to − 0.15] oocytes; P = 0.03; I2 = 58%; χ2-P = 0.02; low-quality evidence; Fig. 15), so we excluded it from the meta-analysis. The results of subgroup analysis showed a significantly lower number of retrieved oocytes in the GnRH antagonist protocol compared with the Long GnRH agonist protocol after using Flexible GnRH antagonist protocol (7 RCTs; 748 participants; WMD = − 1.83, 95% CI: [− 3.56 to − 0.10] oocytes; P = 0.04; I2 = 64%; χ2-P = 0.01), but not Fixed GnRH antagonist protocol (1 RCTs; 22 participants; WMD = -0.80, 95% CI: [− 13.64 to 12.04] oocytes; P = 0.90), but the result of the subgroup analysis was not significant (I2 = 0%, χ2-P = 0.88). Using GRADE, we judged the certainty of evidence as low; we down-graded it by two levels, one level due to concerns over imprecision and one due to concerns over heterogeneity.
Figure 15.
Forest Plot: Number of Retrieved Oocytes. (A) Bias arising from the randomization process; (B) Bias due to deviations from intended interventions; (C) Bias due to missing outcome data;(D) Bias in measurement of the outcome; (E) Bias in selection of the reported result and (F) overall bias.
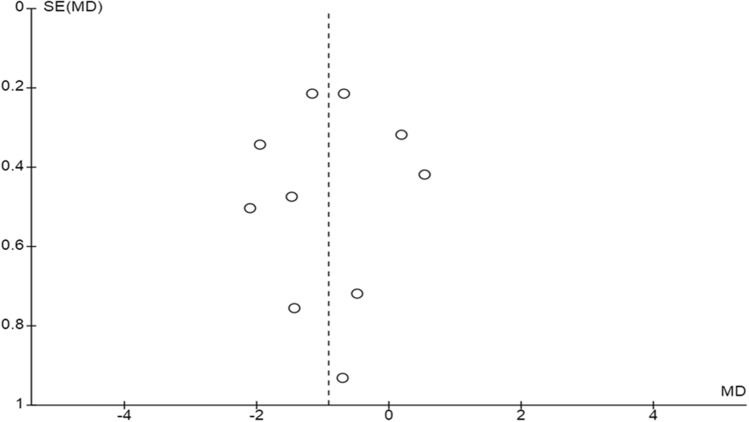
Based on the number of included studies in each comparison, we were able to produce only a funnel plot of the stimulation duration outcome, which revealed no publication bias (Fig. 16). The results of our review are summarized in a summary finding table, see Table 2.
Figure 16.
Funnel Plot: Stimulation duration.
Table 2.
Summary of finding table.
| Outcome | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty | |
|---|---|---|---|---|---|
| Risk with Long GnRH agonist | Risk with Conventional GnRH antagonist | ||||
| Live birth rate | 486 per 1,000 | 379 per 1,000 (224 to 642) | RR 0.78 (0.46 to 1.32) | 74 (1 RCT) | ⨁⨁◯◯ LOW a |
| Ongoing pregnancy rate | 435 per 1,000 | 400 per 1,000 (339 to 470) | RR 0.92 (0.78 to 1.08) | 785 (5 RCTs) | ⨁⨁⨁⨁ HIGH |
| OHSS rate (All grades) | 173 per 1,000 | 101 per 1,000 (76 to 134) | RR 0.58 (0.44 to 0.77) | 994 (9 RCTs) | ⨁⨁◯◯ LOW b,c |
| Mild OHSS rate | 204 per 1,000 | 165 per 1,000 (98 to 279) | RR 0.81 (0.48 to 1.37) | 229 (3 RCTs) | ⨁◯◯◯ VERY LOW a,b |
| Moderate-Severe OHSS rate | 196 per 1,000 | 127 per 1,000 (102 to 161) | RR 0.65 (0.52 to 0.82) | 1114 (9 RCTs) | ⨁◯◯◯ VERY LOW b,d |
| Clinical pregnancy rate | 415 per 1,000 | 399 per 1,000 (320 to 494) | RR 0.96 (0.77 to 1.19) | 840 (8 RCTs) | ⨁⨁⨁⨁ HIGH |
| Multiple pregnancy rate | 99 per 1,000 | 86 per 1,000 (58 to 125) | RR 0.87 (0.59 to 1.27) | 994 (9 RCTs) | ⨁◯◯◯ VERY LOW b,d |
| Miscarriage rate | 86 per 1,000 | 80 per 1,000 (53 to 123) | RR 0.93 (0.61 to 1.43) | 997 (7 RCTs) | ⨁◯◯◯ VERY LOW a,b |
| Cycle cancellation rate | 54 per 1,000 | 62 per 1,000 (37 to 103) | RR 1.15 (0.69 to 1.91) | 1002 (8 RCTs) | ⨁◯◯◯ VERY LOW a,b |
| Cycle cancellation rate due to high risk of OHSS | 32 per 1,000 | 19 per 1,000 (8 to 43) | RR 0.59 (0.26 to 1.34) | 869 (6 RCTs) | ⨁◯◯◯ VERY LOW a,b |
| Cycle cancellation rate due to poor ovarian response | 7 per 1,000 | 32 per 1,000 (10 to 99) | RR 4.63 (1.49 to 14.41) | 869 (6 RCTs) | ⨁◯◯◯ VERY LOW b,e |
| Duration of stimulation | The mean duration of stimulation ranged from 9.07–13.92 Days | MD 0.91 Days. Lower (1.45 lower to 0.37 lower) | – | 1182 (10 RCTs) | ⨁◯◯◯ VERY LOW f,g |
| Gonadotropin Dose | The mean gonadotropin Dose ranged from 2675.5–1370.16 IUs | MD 221.36 IUs. Lower (332.28 lower to 110.45 lower) | – | 845 (7 RCTs) | ⨁⨁⨁⨁ HIGH |
| E2 levels hCG day | The mean E2 levels day of hCG ranged from 2232.7–4424.43 pg/ml | MD 259.21 pg/ml. lower (485.81 lower to 32.6 lower) | – | 995 (8 RCTs) | ⨁⨁⨁◯ MODERATE g |
| Endometrial thickness on hCG day | The mean endometrial thickness on day of hCG ranged from 6.7–11.57 mm | MD 0.73 mm. lower (1.17 lower to 0.29 lower) | – | 550 (5 RCTs) | ⨁⨁⨁◯ MODERATE g |
| Number of retrieved oocytes | The mean no. of retrieved oocytes ranged from 13.71–26.07 oocytes | MD 1.82 oocytes. Lower (3.48 lower to 0.15 lower) | – | 770 (8 RCTs) | ⨁⨁◯◯ LOW g,h |
E2: Estradiol, GnRH: Gonadotropin-releasing hormone, hCG: Human chorionic gonadotropin, OHSS: ovarian hyperstimulation syndrome.
aEvidence downgraded by two levels for very serious imprecision – 95% CI includes both appreciable benefit and harm or no effect and very low number of events (total number of events < 300).
bEvidence downgraded by one level for serious risk of bias – the majority of the RCTs have some concern or high risk of bias.
cEvidence downgraded by one level for serious imprecision – low number of events (total number of events < 300).
dEvidence downgraded by two levels for very serious imprecision – 95% CI includes both appreciable effect and little or no effect and very low number of events (total number of events < 300).
eEvidence downgraded by two levels for very serious imprecision—low number of events (total number of events < 300) and the effect estimate has a wide confidence interval.
fEvidence downgraded by two levels for very serious inconsistency (unexplained heterogeneity).
gEvidence downgraded by one level for serious imprecision – 95% CI includes both appreciable effect and little or no effect.
hEvidence downgraded by one level for serious inconsistency (unexplained heterogeneity).
Discussion
The results of our review suggest that using Conventional GnRH antagonist protocol in PCOS women is associated with lower gonadotropins consumption (high-quality evidence), shorter stimulation duration (very low-quality evidence), thinner endometrial thickness on hCG day (moderate-quality evidence), lower E2 levels on hCG day (moderate-quality evidence), lower number of retrieved oocytes (low-quality evidence), and fewer OHSS incidence (low-quality evidence) without compromising clinical pregnancy rate (high-quality evidence), ongoing pregnancy rate (high-quality evidence) or live birth rate (low-quality evidence). Moreover, similar MPR (very low-quality evidence) and MR (very low-quality evidence) have been noticed in the GnRH antagonist protocols and the Long GnRH agonist protocol. Similarly, the overall risk of cycle cancellation is comparable between the two groups (very low-quality evidence). Nevertheless, more cycles have been cancelled due to poor ovarian response in the GnRH antagonist protocols (very low-quality evidence), while similar rates of cancellation due to risk of OHSS have been observed in both groups (very low-quality evidence).
Lack of gonadotropins suppression during the early follicular phase of Conventional GnRH antagonist protocols may lay behind the shorter stimulation duration, the lower gonadotropins consumption, and the lower number of retrieved oocytes noted in the GnRH antagonist group compared to the Long GnRH agonist one since higher LH levels may improve FSH sensibility61, while higher FSH leads to an uncoordinated and a heterogeneous development of FSH‐sensitive follicles20,21, which may, as a result, reduce the number of retrieved oocytes. However, it is worth mentioning that all included women were pre-treated with oral contraceptive pills (OCPs). Although OCPs effects on IVF outcomes are still unclear and controversial, they may prevent the early rise of endogenous gonadotropins during the Conventional GnRH antagonist protocol and improve the homogeneity of the follicular development64. However, Although it was suggested that a 5-days OCPs free interval is enough for gonadotropins recovery65, Kolibianakis et al.66 observed significantly lower levels of LH, E2 and progesterone after five days of OCPs cessation compared with the levels observed in the same patients at the time of OCPs initiation, and compared with the levels seen in patients underwent GnRH antagonist COS without OCPs on Day2 cycle. Further, the LH levels remained lower in the OCPs group till the day of hCG triggering66. However, differences in the ovarian microenvironment can also be suspected. Arising evidence highlight the possibility of GnRH analogues affecting ovarian cells directly67,68. Several studies have reported different steroidogenesis pattern in GnRH antagonist and GnRH agonist protocols69–72. However, It is unlikely that the differences in steroidogenesis arise from differences in the steroidogenic cell numbers, as similar levels of granulosa cells apoptosis were observed in the GnRH antagonist protocols and the GnRH agonist ones69,73,74. Previously, Khalaf et al.69 noted that granulosa lutein cells obtained from GnRH antagonist-treated women showed significantly lower aromatase activity, lower aromatase expression, and higher expression of FSH receptors compared to those obtained from GnRH agonist-treated women. Similarly, Winkler et al.70 noted that GnRH antagonist led to a dose-dependent reduction in granulosa cell aromatase in a granulosa cell culture model. On the contrary, the GnRH agonist stimulated aromatase in a dose-dependent manner. These effects combined with the possible ability of GnRH antagonists to reduce the number of retrieved oocytes may be responsible for their protective effect in reducing OHSS incidences during COS. In addition, Vrtačnik-Bokal et al. study49 reported lower estradiol and vascular endothelial growth factors (VEGF) levels in the follicular fluids (FF) of PCOS women treated with the GnRH antagonist protocol compared to those treated with the Long GnRH agonist protocol. On the contrary, Ferrari et al. study75 showed higher FF VEGF levels in the GnRH antagonist group compared to the Long GnRH agonist one. On the other side, Malhotra et al.76 observed higher FF VEGF levels in normo-responder women treated with the GnRH antagonist protocol compared to the Long GnRH agonist protocol. Yet, the ratio of the soluble form of VEGF receptor-1 (sVEGFR-1) to VEGF did not differ between the two protocols significantly. VEGF, also called vascular permeability factor, is the founding member of the VEGFs family, which is known for its role in regulating angiogenesis and vasculogenesis77. The soluble form of VEGF receptor-1 (sVEGFR-1) acts as an anti-angiogenic factor by sequestering VEGF and decreasing its free form availability78. Arising evidence supports VEGF pivotal role in regulation follicle angiogenesis79, ovulation80,81, placentation and implantation82,83. Moreover, VEGF plays an important role in the pathophysiology of OHSS13,84. PCOS ovaries exhibit higher vascularization and lower impedance to flow in ovarian stromal vessels compared to control85–87, which may be arisen from the differences in the levels of ovarian angiogenesis regulation factors as PCOS women showed higher VEGF levels and lower sVEGFR-1 levels compared to control both in serum and follicular fluid samples88,89. Based on the abovementioned results, GnRH antagonist effects on reducing VEGF levels during COS may be more noticeable in the PCOS subjects due to the ovarian angiogenesis abnormality seen in this population. However, with such limited data, it is risky to conclude that similar effects unlikely to be seen in non-PCOS subjects. Thus, Further research is needed to confirm this hypothesis and provide a better understanding of the mechanism that underlies GnRH antagonists’ protective effects in reducing OHSS incidences during COS.
The effect of GnRH antagonists on endometrial receptivity is still questionable. Several studies suggested a detrimental impact on endometrial receptivity90–93. Some reports noticed a significant reduction in the endometrial expression of HOXA-10 during GnRH antagonist cycles compared with GnRH agonist cycles or natural cycles90,91. HOXA10 is a transcription factor that belongs to the homeobox gene family. It is highly expressed in endometrial stromal cells and plays essential roles in embryo implantation and endometrium proliferation, differentiation, and receptivity94. Infertility due to implantation failure was noted in female mice with HOXA-10 mutation. However, these mice gave viable embryos that could implant and develop normally in a wild-type surrogate95. Moreover, a recent study showed an upregulation of endometrial Allograft inflammatory factor-1 (AIF-1) expression, a cytokine associated with inflammation and allograft rejection, in the GnRH antagonist group compared with the GnRH agonist one, which might be unfavorable for embryo implantation as increased AIF-1 might inhibit adhesion during implantation via raised Tumor necrosis factor-α (TNF-α)92. Furthermore, although treating mice with both GnRH agonist and GnRH antagonist was associated with a reduction in the endometrial expression of the endometrial receptivity markers; leukemia-inhibitory factor (LIF) and integrin β3, GnRH agonist-treated mice showed a higher implantation rate and a higher endometrial expression of LIF and integrin β3 subunit93. On the other hand, several clinical studies revealed that the endometrial development during GnRH antagonist cycles mimics the natural endometrium more closely than GnRH agonist cycles96,97. However, it still unknown whether the GnRH antagonists may have similar effects on PCOS subjects who suffered from abnormal endometrial receptivity98,99. Our review showed a significant reduction in endometrial thickness in the GnRH antagonist group compared with the GnRH agonist one. In practice, CPR and LBR decrease for each millimeter of endometrial thickness declines below 8 mm in fresh IVF cycles100. Yet, endometrial thickness means in the GnRH antagonist group range from 8.1 to 10.5 mm VS 6.7 to 11.57 mm in the GnRH agonist group, with mean differences -0.73 mm and 95% CI [-1.17 to -0.29] mm. Thus, it is unlikely that the effect on endometrial thickness will lead to clinically meaningful adverse effects on CPR, OPR, MR or LBR in general cases.
The results of previous reviews showed no differences in CPR28–32, OPR28,29, LBR29, MR30,31, and CCR due to high risk of OHSS31 between PCOS subjects treated with GnRH antagonist protocols and the Long GnRH agonist protocol, which comes along with the results of our review. In agreement with our results, Lambalk et al.29 and Xiao et al.30 studies showed that using GnRH antagonist in PCOS subjects is associated with lower OHSS incidences. Moreover, Pundir et al.31 also reported lower incidences of Severe-Moderate OHSS but not Mild OHSS in the GnRH antagonist protocols, which also comes along with our results. Furthermore, we noted lower gonadotropins consumption and shorter stimulation duration in GnRH antagonist protocols compared with the Long GnRH agonist protocol, which follows previous review results28,31. However, Lin et al.28 and Griesinger et al.32 did not note significant differences in OHSS rate between GnRH antagonist protocols and the Long GnRH agonist protocol. Moreover, Griesinger et al.32 did not observe any significant differences in the gonadotropins consumption between different GnRH analogues protocols. This may be due to the limited number of studies were included in the meta-analyses that investigated these effects in those reviews. On the contrary with our results, previous reviews did not show any significant differences between GnRH antagonist protocols and GnRH agonist protocols on E2 levels on hCG day30,31 or the number of retrieved oocytes29–32 except for Lin et al.28 review, which reported lower retrieved oocytes number in the GnRH antagonist protocols, which may arise from the differences in studies inclusion and exclusion criteria since all previous reviews included both Early and Conventional GnRH antagonist protocols, while ours only included Conventional protocols.
Strengths, limitations and future research
The main strength of the present study is being the first systematic review and meta-analysis to investigate the effects of Conventional GnRH antagonist protocols and the Long GnRH agonist protocol on IVF/ICSI outcomes in women with PCOS. In addition, we examined the effects of GnRH analogues on twelve IVF/ICSI outcomes and assessed the quality of the obtained evidence using the GRADE approach, which gives a better understanding of the overall effects and aids the health care providers to improve the plan therapy offered to patients. However, there were also some limitations. Although we conducted a comprehensive search of relevant databases besides the reference lists hand-search with no language restrictions, we may have missed trials that would have been eligible for inclusion. Furthermore, despite the results tables of the non-English published studies could directly be obtained and interpreted, assessing the methodological quality was a bit challenging. Another limitation is the number of included studies since it was relatively small, and the obtained evidence was downgraded for imprecision for several outcomes. For the same reason, we were incapable of assessing the risk of publication bias using funnel plots except for one outcome (stimulation duration). Above that, the diagnosis criteria of OHSS and CCR were poorly defined among the included trials. Also, despite our attempts to obtain the missing outcome data from the study authors, some data were still missing, and they possibly caused some bias. In addition, all included studies focused on fresh cycles. Thus, the reproducibility of obtained results in frozen cycles is still unknown. Therefore, more well-designed RCTs following the CONSORT statement are required to be more confidant in the results obtained on PCOS women during fresh cycles and to further compare the effects of the Conventional GnRH antagonist protocols and the GnRH agonist protocols on IVF/ICSI outcomes during frozen ones.
Conclusions
Conventional GnRH antagonist protocols represent a safer and more cost-effective choice for PCOS women undergoing IVF/ICSI cycles than the standard Long GnRH agonist protocol without compromising the IVF/ICSI clinical outcomes.
Supplementary Information
Acknowledgements
We would like to thank Dr Jae Jun Shin (Seoul, Korea) and Dr Sara Mokhtar (Tehran, Iran) for their support and precious help in providing additional information about their studies.
Author contributions
S.K. conceptualized and designed the study protocol, performed the statistical analysis, contacted the studies’ authors for additional information, and drafted the manuscript. S.K. and A.N. performed the literature search and extracted the data with the help of M.A. as a consultor. S.K. and M.A. assessed the studies’ risk of bias and evaluated the quality of the evidence with the help of A.N. as a consultor. A.N. and M.A. revised the manuscript. All authors approved the final manuscript.
Data availability
All the data supporting the findings of this study are available within the article and its supplementary material.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-08400-z.
References
- 1.Azziz R, et al. Polycystic ovary syndrome. Nat. Rev. Dis. Primers. 2016;2:16057. doi: 10.1038/nrdp.2016.57. [DOI] [PubMed] [Google Scholar]
- 2.Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8:41. doi: 10.1186/1741-7015-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costello MF, et al. Evidence summaries and recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome: assessment and treatment of infertility. Hum. Reprod. Open. 2019;2019:hoy021. doi: 10.1093/hropen/hoy021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tandulwadkar SR, Lodha PA, Mangeshikar NT. Obstetric complications in women with IVF conceived pregnancies and polycystic ovarian syndrome. J. Hum. Reprod. Sci. 2014;7:13–18. doi: 10.4103/0974-1208.130802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonnell R, Hart RJ. Pregnancy-related outcomes for women with polycystic ovary syndrome. Women’s Heal. 2017;13:89–97. doi: 10.1177/1745505717731971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palomba S, et al. Pregnancy complications in women with polycystic ovary syndrome. Hum. Reprod. Update. 2015;21:575–592. doi: 10.1093/humupd/dmv029. [DOI] [PubMed] [Google Scholar]
- 7.Sha T, Wang X, Cheng W, Yan Y. A meta-analysis of pregnancy-related outcomes and complications in women with polycystic ovary syndrome undergoing IVF. Reprod. Biomed. Online. 2019;39:281–293. doi: 10.1016/j.rbmo.2019.03.203. [DOI] [PubMed] [Google Scholar]
- 8.Hughes EG, et al. The routine use of gonadotropin-releasing hormone agonists prior to in vitro fertilization and gamete intrafallopian transfer: a meta-analysis of randomized controlled trials. Fertil. Steril. 1992;58:888–896. doi: 10.1016/s0015-0282(16)55430-2. [DOI] [PubMed] [Google Scholar]
- 9.Copperman AB, Benadiva C. Optimal usage of the GnRH antagonists: a review of the literature. Reprod. Biol. Endocrinol. 2013;11:20. doi: 10.1186/1477-7827-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eryılmaz OG, et al. Ovarian cyst formation following Gonadotropin-Releasing Hormone-Agonist administration decreases the oocyte quality in IVF cycles. Balkan Med. J. 2012;29:197–200. doi: 10.5152/balkanmedj.2011.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devroey P, et al. Improving the patient’s experience of IVF/ICSI: a proposal for an ovarian stimulation protocol with GnRH antagonist co-treatment. Hum. Reprod. 2009;24:764–774. doi: 10.1093/humrep/den468. [DOI] [PubMed] [Google Scholar]
- 12.Kumar P, Sait SF, Sharma A, Kumar M. Ovarian hyperstimulation syndrome. J. Hum. Reprod. Sci. 2011;4:70–75. doi: 10.4103/0974-1208.86080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Namavar Jahromi B, et al. Ovarian hyperstimulation syndrome: a narrative review of its pathophysiology, risk factors, prevention, classification, and management. Iran. J. Med. Sci. 2018;43:248–260. [PMC free article] [PubMed] [Google Scholar]
- 14.Sun B, et al. Factors associated with ovarian hyperstimulation syndrome (OHSS) severity in women with polycystic ovary syndrome undergoing IVF/ICSI. Front. Endocrinol. (Lausanne). 2020;11:615957. doi: 10.3389/fendo.2020.615957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer D, et al. Avoiding OHSS: controlled ovarian low-dose stimulation in women with PCOS. Geburtshilfe Frauenheilkd. 2016;76:718–726. doi: 10.1055/s-0042-100206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behery MA, Hasan EA, Ali EA, Eltabakh AA. Comparative study between agonist and antagonist protocols in PCOS patients undergoing ICSI: a cross-sectional study. Middle East Fertil. Soc. J. 2020;24:2. [Google Scholar]
- 17.Ghaebi NK, et al. Pregnancy outcomes in PCOS patients undergoing IVF with long GnRH agonist protocol versus flexible GnRH antagonist. Iran. J. Obstet. Gynecol. Infertil. 2018;21:1–9. [Google Scholar]
- 18.Haydardedeoglu B, Kilicdag EB, Parlakgumus AH, Zeyneloglu HB. IVF/ICSI outcomes of the OCP plus GnRH agonist protocol versus the OCP plus GnRH antagonist fixed protocol in women with PCOS: a randomized trial. Arch. Gynecol. Obstet. 2012;286:763–769. doi: 10.1007/s00404-012-2348-9. [DOI] [PubMed] [Google Scholar]
- 19.Devroey P, et al. A double-blind, non-inferiority RCT comparing corifollitropin alfa and recombinant FSH during the first seven days of ovarian stimulation using a GnRH antagonist protocol. Hum. Reprod. 2009;24:3063–3072. doi: 10.1093/humrep/dep291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fanchin R, et al. Luteal estradiol pre-treatment coordinates follicular growth during controlled ovarian hyperstimulation with GnRH antagonists. Hum. Reprod. 2003;18:2698–2703. doi: 10.1093/humrep/deg516. [DOI] [PubMed] [Google Scholar]
- 21.Fanchin R, Méndez Lozano DH, Schonäuer LM, Cunha-Filho JS, Frydman R. Hormonal manipulations in the luteal phase to coordinate subsequent antral follicle growth during ovarian stimulation. Reprod. Biomed. Online. 2005;10:721–728. doi: 10.1016/s1472-6483(10)61115-7. [DOI] [PubMed] [Google Scholar]
- 22.Kolibianakis EM, et al. Exposure to high levels of luteinizing hormone and estradiol in the early follicular phase of gonadotropin-releasing hormone antagonist cycles is associated with a reduced chance of pregnancy. Fertil. Steril. 2003;79:873–880. doi: 10.1016/s0015-0282(02)04920-8. [DOI] [PubMed] [Google Scholar]
- 23.Mokhtar S, et al. ART outcomes in GnRH antagonist protocol (flexible) and long GnRH agonist protocol during early follicular phase in patients with polycystic ovary syndrome: a randomized clinical trial. J. Reprod. Infertil. 2015;16:148–154. [PMC free article] [PubMed] [Google Scholar]
- 24.Kim C-H, et al. Effectiveness of GNRH antagonist multiple dose protocol applied during early and late follicular phase compared with GNRH agonist long protocol in non-obese and obese patients with polycystic ovary syndrome undergoing IVF/ICSI. Clin. Exp. Reprod. Med. 2012;39:22–27. doi: 10.5653/cerm.2012.39.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lainas TG, et al. Initiation of GnRH antagonist on Day 1 of stimulation as compared to the long agonist protocol in PCOS patients. A randomized controlled trial: Effect on hormonal levels and follicular development. Hum. Reprod. 2007;22:1540–1546. doi: 10.1093/humrep/dem033. [DOI] [PubMed] [Google Scholar]
- 26.Hwang J-L, et al. Ovarian stimulation by concomitant administration of cetrorelix acetate and HMG following Diane-35 pre-treatment for patients with polycystic ovary syndrome: a prospective randomized study. Hum. Reprod. 2004;19:1993–2000. doi: 10.1093/humrep/deh375. [DOI] [PubMed] [Google Scholar]
- 27.Shin JJ, et al. Early gonadotropin-releasing hormone antagonist protocol in women with polycystic ovary syndrome: a preliminary randomized trial. Clin. Exp. Reprod. Med. 2018;45:135–142. doi: 10.5653/cerm.2018.45.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin H, et al. Is a GnRH antagonist protocol better in PCOS patients? A Meta-Analysis of RCTs. PLoS ONE. 2014;9:e91796. doi: 10.1371/journal.pone.0091796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lambalk CB, et al. GnRH antagonist versus long agonist protocols in IVF: a systematic review and meta-analysis accounting for patient type. Hum. Reprod. Update. 2017;23:560–579. doi: 10.1093/humupd/dmx017. [DOI] [PubMed] [Google Scholar]
- 30.Xiao J, Chen S, Zhang C, Chang S. Effectiveness of GnRH antagonist in the treatment of patients with polycystic ovary syndrome undergoing IVF: a systematic review and meta analysis. Gynecol. Endocrinol. 2013;29:187–191. doi: 10.3109/09513590.2012.736561. [DOI] [PubMed] [Google Scholar]
- 31.Pundir J, Sunkara SK, El-Toukhy T, Khalaf Y. Meta-analysis of GnRH antagonist protocols: do they reduce the risk of OHSS in PCOS? Reprod. Biomed. Online. 2012;24:6–22. doi: 10.1016/j.rbmo.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 32.Griesinger G, Diedrich K, Tarlatzis BC, Kolibianakis EM. GnRH-antagonists in ovarian stimulation for IVF in patients with poor response to gonadotrophins, polycystic ovary syndrome, and risk of ovarian hyperstimulation: a meta-analysis. Reprod. Biomed. Online. 2006;13:628–638. doi: 10.1016/s1472-6483(10)60652-9. [DOI] [PubMed] [Google Scholar]
- 33.Youssef MAFM, et al. Gonadotropin-releasing hormone agonist versus HCG for oocyte triggering in antagonist-assisted reproductive technology. Cochrane Database Syst. Rev. 2014 doi: 10.1002/14651858.CD008046.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li, T., Higgins, J. & Deeks, J. (editors). Chapter 5: Collecting data. in Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). (eds. Higgins, J. et al.) (Cochrane, 2021.).
- 36.Higgins, J., Savović, J., Page, M., Elbers, R. & Sterne, J. Chapter 8: Assessing risk of bias in a randomized trial. in Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021) (eds. Higgins, J. et al.) (Cochrane, 2021).
- 37.Sterne JAC, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 38.McGrath S, Zhao X, Steele R, Thombs BD, Benedetti A. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat. Methods Med. Res. 2020 doi: 10.1177/0962280219889080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Page, M., Higgins, J. & Sterne, J. Chapter 13: Assessing risk of bias due to missing results in a synthesis. in Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021) (eds. Higgins, J. et al.) (Cochrane, 2021).
- 40.Schünemann, H., Brożek, J., Guyatt, G. & Oxman, A. GRADE handbook for grading quality of evidence and strength of recommendations. (Updated October 2013). (The GRADE Working Group, 2013).
- 41.Ashrafi M, et al. A comparative study of GnRH antagonist and GnRH agonist in PCO patients undergoing IVF/ICSI cycles. Int. J. Reprod. Biomed. IRANIAN J. Reprod. Med. 2005;3:14–18. [Google Scholar]
- 42.Chen Y, Zhao J, Zhang H. Comparative effectiveness of three ovarian hyperstimulation protocol in In Vitro Fertilization (IVF) cycles for women with Polycystic Ovary Syndrome. Med. Sci. Monit. 2018;24:9424–9428. doi: 10.12659/MSM.913757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi JW, et al. Efficacy of controlled ovarian hyperstimulation using GnRH antagonist in women with polycystic ovary syndrome undergoing IVF-ET. Korean J Obs. Gynecol. 2005;48:716–725. [Google Scholar]
- 44.Choi MH, et al. Comparison of assisted reproductive technology outcomes in infertile women with polycystic ovary syndrome: In vitro maturation, gnrh agonist, and gnrh antagonist cycles. Clin. Exp. Reprod. Med. 2012;39:166–171. doi: 10.5653/cerm.2012.39.4.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi MH, et al. IVF comparison of ART outcomes in infertile PCOS women; In Vitro Maturation (IVM) vs. GnRH agonist vs. GnRH antagonist cycles. Fertil. Steril. 2012;98:S210. [Google Scholar]
- 46.Iranian Registry of Clinical Trials. IRCT2012120311653N1. Comparing IVF outcomes in GnRH antagonist protocol during early and late follicular phase and GnRH antagonist protocol (flexible) and long GnRH agonist protocol in patients with polycystic ovary syndrome. (2013).
- 47.Moshin, V., Croitor, M. & Hotineanu, A. GnRH antagonist versus long GnRH agonists protocol in PCOS patients undergoing IVF treatment. Abstr. 23rd Annu. Meet. ESHRE, lyon, Fr.22 Suppl 1, i121 (2007).
- 48.ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). NCT01402336. GnRH antagonist versus GnRH agonist in Polycystic Ovary Syndrome during In Vitro Fertilization - Embryo Transfer. (2011).
- 49.Vrtačnik-Bokal E, et al. Follicular oestradiol and VEGF after GnRH antagonists or GnRH agonists in women with PCOS. Reprod. Biomed. Online. 2009;18:21–28. doi: 10.1016/s1472-6483(10)60420-8. [DOI] [PubMed] [Google Scholar]
- 50.Zeinalzadeh M, et al. Comparison of GnRH agonists and antagonists in the outcome of IVF/ICSI in women with polycystic ovary syndrome. Iran. J. Reprod. Med. 2014;12:58. [Google Scholar]
- 51.Iranian Registry of Clinical Trials. IRCT201402041760N30. Comparison of GnRH agonists and antagonists the outcome IVF/ICSI in women with polycystic ovary syndrome. https://www.irct.ir/trial/1294.
- 52.Orvieto R, et al. What is the preferred GnRH analogue for polycystic ovary syndrome patients undergoing controlled ovarian hyperstimulation for in vitro fertilization? Fertil. Steril. 2009;91:1466–1468. doi: 10.1016/j.fertnstert.2008.07.1711. [DOI] [PubMed] [Google Scholar]
- 53.Orvieto R, et al. Does day-3 LH/FSH ratio influence in vitro fertilization outcome in PCOS patients undergoing controlled ovarian hyperstimulation with different GnRH-analogue. Gynecol. Endocrinol. 2012;28:422–424. doi: 10.3109/09513590.2011.633661. [DOI] [PubMed] [Google Scholar]
- 54.Kaur H, et al. A prospective study of GnRH long agonist versus flexible GnRH antagonist protocol in PCOS: Indian experience. J. Hum. Reprod. Sci. 2012;5:181–186. doi: 10.4103/0974-1208.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kdous M, M’solly S, Zhioua F, Meriah S. Use of GnRH antagonist (cetrorelix®) in controlled ovarian hyperstimulation in women with polycystic ovary disease [Utilisation des antagonistes de la GnRH (cetrorelix®) dans la stimulation plurifolliculaire chez les patientes porteuses d’une dystrophie o. Tunisie Med. 2008;86:1060–1065. [PubMed] [Google Scholar]
- 56.Kdous M, Chaker A, Bouyahia M, Zhioua F, Zhioua A. Increased risk of earlypregnancy loss and lower live birth rate with GnRH antagonist vs long GnRH agonist protocol in PCOS women undergoing controlled ovarian hyperstimulation [Augmentation du taux de fausses couches spontanees precoces et diminution du t. Tunisie Med. 2009;87:834–842. [PubMed] [Google Scholar]
- 57.Onofriescu A, et al. GnRH Antagonist IVF Protocol in PCOS. Curr. Heal. Sci. J. 2013;39:20–25. [PMC free article] [PubMed] [Google Scholar]
- 58.Segal S, et al. Comparison of outcomes between controlled ovarian stimulation with GnRH-agonist vs GnRH-antagonist for in vitro fertilization cycles in women with polycystic ovarian syndrome. Fertil. Steril. 2008;90:S232. [Google Scholar]
- 59.Bahçeci M, et al. Use of a GnRH antagonist in controlled ovarian hyperstimulation for assisted conception in women with polycystic ovary disease: A randomized, prospective, pilot study. J. Reprod. Med. Obstet. Gynecol. 2005;50:84–90. [PubMed] [Google Scholar]
- 60.Hosseini MA, et al. Comparison of gonadotropin-releasing hormone agonists and antagonists in assisted reproduction cycles of polycystic ovarian syndrome patients. J. Obstet. Gynaecol. Res. 2010;36:605–610. doi: 10.1111/j.1447-0756.2010.01247.x. [DOI] [PubMed] [Google Scholar]
- 61.Kurzawa R, Ciepiela P, Baczkowski T, Safranow K, Brelik P. Comparison of embryological and clinical outcome in GnRH antagonist vs. GnRH agonist protocols for in vitro fertilization in PCOS non-obese patients. A prospective randomized study. J. Assist. Reprod. Genet. 2008;25:365–374. doi: 10.1007/s10815-008-9249-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lainas TG, et al. Flexible GnRH antagonist protocol versus GnRH agonist long protocol in patients with polycystic ovary syndrome treated for IVF: A prospective randomised controlled trial (RCT) Hum. Reprod. 2010;25:683–689. doi: 10.1093/humrep/dep436. [DOI] [PubMed] [Google Scholar]
- 63.Trenkić MS, et al. Flexible GnRH antagonist protocol vs. long GnRH agonist protocol in patients with polycystic ovary syndrome treated for IVF: Comparison of clinical outcome and embryo quality. Ginekol. Pol. 2016;87:265–270. doi: 10.17772/gp/62205. [DOI] [PubMed] [Google Scholar]
- 64.Huirne JAF, et al. Effect of an oral contraceptive pill on follicular development in IVF/ICSI patients receiving a GnRH antagonist: a randomized study. Reprod. Biomed. Online. 2006;13:235–245. doi: 10.1016/s1472-6483(10)60621-9. [DOI] [PubMed] [Google Scholar]
- 65.Cédrin-Durnerin I, et al. Effects of oral contraceptive, synthetic progestogen or natural estrogen pre-treatments on the hormonal profile and the antral follicle cohort before GnRH antagonist protocol. Hum. Reprod. 2007;22:109–116. doi: 10.1093/humrep/del340. [DOI] [PubMed] [Google Scholar]
- 66.Kolibianakis EM, et al. Effect of oral contraceptive pill pretreatment on ongoing pregnancy rates in patients stimulated with GnRH antagonists and recombinant FSH for IVF. A randomized controlled trial. Hum. Reprod. 2006;21:352–357. doi: 10.1093/humrep/dei348. [DOI] [PubMed] [Google Scholar]
- 67.Maggi R, et al. GnRH and GnRH receptors in the pathophysiology of the human female reproductive system. Hum. Reprod. Update. 2016;22:358–381. doi: 10.1093/humupd/dmv059. [DOI] [PubMed] [Google Scholar]
- 68.Metallinou C, Asimakopoulos B, Schröer A, Nikolettos N. Gonadotropin-releasing hormone in the ovary. Reprod. Sci. 2007;14:737–749. doi: 10.1177/1933719107310707. [DOI] [PubMed] [Google Scholar]
- 69.Khalaf M, et al. GnRH agonist and GnRH antagonist protocols in ovarian stimulation: Differential regulation pathway of aromatase expression in human granulosa cells. Reprod. Biomed. Online. 2010;21:56–65. doi: 10.1016/j.rbmo.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 70.Winkler N, Bukulmez O, Hardy DB, Carr BR. Gonadotropin releasing hormone antagonists suppress aromatase and anti-Müllerian hormone expression in human granulosa cells. Fertil. Steril. 2010;94:1832–1839. doi: 10.1016/j.fertnstert.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 71.Garcia-Velasco JA, et al. Human ovarian steroid secretion in vivo: Effects of GnRH agonist versus antagonist (cetrorelix) Hum. Reprod. 2001;16:2533–2539. doi: 10.1093/humrep/16.12.2533. [DOI] [PubMed] [Google Scholar]
- 72.Minaretzis D, et al. Gonadotropin-releasing hormone antagonist versus agonist administration in women undergoing controlled ovarian hyperstimulation: cycle performance and in vitro steroidogenesis of granulosa-lutein cells. Am. J. Obstet. Gynecol. 1995;172:1518–1525. doi: 10.1016/0002-9378(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 73.Giampietro F, Sancilio S, Tiboni GM, Rana RA, Di Pietro R. Levels of apoptosis in human granulosa cells seem to be comparable after therapy with a gonadotropin-releasing hormone agonist or antagonist. Fertil. Steril. 2006;85:412–419. doi: 10.1016/j.fertnstert.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 74.Lavorato HL, et al. GnRH agonist versus GnRH antagonist in IVF/ICSI cycles with recombinant LH supplementation: DNA fragmentation and apoptosis in granulosa cells. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012;165:61–65. doi: 10.1016/j.ejogrb.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 75.Ferrari B, Pezzuto A, Barusi L, Coppola F. Follicular fluid vascular endothelial growth factor concentrations are increased during GnRH antagonist/FSH ovarian stimulation cycles. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006;124:70–76. doi: 10.1016/j.ejogrb.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 76.Malhotra N, et al. Physiological balance between fVEGF and sVEGFR1 is maintained within ovarian follicles in normoresponder women irrespective of GnRH-agonist and GnRH-antagonist protocols. J. Reprod. Heal. Med. 2015;1:41–43. [Google Scholar]
- 77.Holmes DI, Zachary I. The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease. Genome Biol. 2005;6:209. doi: 10.1186/gb-2005-6-2-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eddy AC, Bidwell GL, III, George EM. Pro-angiogenic therapeutics for preeclampsia. Biol. Sex Differ. 2018;9:36. doi: 10.1186/s13293-018-0195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hou L, Taylor RN, Shu Y, Johnston-MacAnanny EB, Yalcinkaya TM. Vascular endothelial growth factor (VEGF) and placental growth factor (PLGF) directly correlate with ovarian follicle size in women undergoing in vitro fertilization (IVF) Fertil. Steril. 2014;102:e256. [Google Scholar]
- 80.Xu F, et al. Intraovarian actions of anti-angiogenic agents disrupt periovulatory events during the menstrual cycle in monkeys. Contraception. 2005;71:239–248. doi: 10.1016/j.contraception.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 81.Hazzard TM, Xu F, Stouffer RL. Injection of soluble vascular endothelial growth factor receptor 1 into the preovulatory follicle disrupts ovulation and subsequent luteal function in rhesus monkeys. Biol. Reprod. 2002;67:1305–1312. doi: 10.1095/biolreprod67.4.1305. [DOI] [PubMed] [Google Scholar]
- 82.Chen D, Zheng J. Regulation of placental angiogenesis. Microcirculation. 2014;21:15–25. doi: 10.1111/micc.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ghosh D, et al. Expression of vascular endothelial growth factor (VEGF) and placental growth factor (PlGF) in conceptus and endometrium during implantation in the rhesus monkey. Mol. Hum. Reprod. 2000;6:935–941. doi: 10.1093/molehr/6.10.935. [DOI] [PubMed] [Google Scholar]
- 84.Naredi N, Talwar P, Sandeep K. VEGF antagonist for the prevention of ovarian hyperstimulation syndrome: current status. Med. J. Armed Forces India. 2014;70:58–63. doi: 10.1016/j.mjafi.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pan H, Wu M, Cheng Y, Li C, Chang F. Quantification of Doppler signal in polycystic ovary syndrome using three-dimensional power Doppler ultrasonography: a possible new marker for diagnosis. Hum. Reprod. 2002;17:201–206. doi: 10.1093/humrep/17.1.201. [DOI] [PubMed] [Google Scholar]
- 86.Ultrasonography T, et al. Polycystic ovarian syndrome: assessment with color Doppler angiography and three-dimensional ultrasonography. J. Ultrasound Med. 1999;18:303–313. doi: 10.7863/jum.1999.18.4.303. [DOI] [PubMed] [Google Scholar]
- 87.Alcázar JL, Kudla MJ. Ovarian stromal vessels assessed by spatiotemporal image correlation–high definition flow in women with polycystic ovary syndrome: a case–control study. Ultrasound Obstet. Gynecol. 2012;40:470–475. doi: 10.1002/uog.11187. [DOI] [PubMed] [Google Scholar]
- 88.Kudsy M, Alhalabi M, Al-quobaili F. Follicular fluid Vascular Endothelial Growth Factor (VEGF) could be a predictor for pregnancy outcome in normo-responders and polycystic ovary syndrome women undergoing IVF/ICSI treatment cycles. Middle East Fertil. Soc. J. 2016;21:52–56. [Google Scholar]
- 89.Artini PG, et al. Vascular endothelial growth factor and its soluble receptor in patients with polycystic ovary syndrome undergoing IVF. Hum. Fertil. 2009;12:40–44. doi: 10.1080/14647270802621358. [DOI] [PubMed] [Google Scholar]
- 90.Rackow BW, Kliman HJ, Taylor HS. GnRH antagonists may affect endometrial receptivity. Fertil. Steril. 2008;89:1234–1239. doi: 10.1016/j.fertnstert.2007.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen Q, et al. GnRH antagonist alters the migration of endometrial epithelial cells by reducing CKB. Reproduction. 2020;159:733–743. doi: 10.1530/REP-19-0578. [DOI] [PubMed] [Google Scholar]
- 92.Xu B, et al. Increased AIF-1-mediated TNF-α expression during implantation phase in IVF cycles with GnRH antagonist protocol. Hum. Reprod. 2018;33:1270–1280. doi: 10.1093/humrep/dey119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ruan H, et al. Ovarian stimulation with GnRH agonist, but not GnRH antagonist, partially restores the expression of endometrial integrin β3 and leukaemia-inhibitory factor and improves uterine receptivity in mice. Hum. Reprod. 2006;21:2521–2529. doi: 10.1093/humrep/del215. [DOI] [PubMed] [Google Scholar]
- 94.Wang Y, Hu S, Yao G, Sun Y. Identification of HOXA10 target genes in human endometrial stromal cells by RNA-seq analysis. Acta Biochim. Biophys. Sin. (Shanghai) 2021;53:365–371. doi: 10.1093/abbs/gmaa173. [DOI] [PubMed] [Google Scholar]
- 95.Satokata I, Benson G, Maas R. Sexually dimorphic sterility phenotypes in Hoxa10-deficient mice. Nature. 1995;374:460–463. doi: 10.1038/374460a0. [DOI] [PubMed] [Google Scholar]
- 96.Simon C, et al. Similar endometrial development in oocyte donors treated with either high- or standard-dose GnRH antagonist compared to treatment with a GnRH agonist or in natural cycles. Hum. Reprod. 2005;20:3318–3327. doi: 10.1093/humrep/dei243. [DOI] [PubMed] [Google Scholar]
- 97.Haouzi D, et al. Controlled ovarian hyperstimulation for In Vitro Fertilization alters endometrial receptivity in humans: protocol effects. Biol. Reprod. 2010;82:679–686. doi: 10.1095/biolreprod.109.081299. [DOI] [PubMed] [Google Scholar]
- 98.Kara M, Ozcan SS, Aran T, Kara O, Yilmaz N. Evaluation of endometrial receptivity by measuring HOXA-10, HOXA-11, and leukemia inhibitory factor expression in patients with polycystic ovary syndrome. Gynecol. Minim. Invasive Ther. 2019;8:118–122. doi: 10.4103/GMIT.GMIT_112_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schulte MMB, Tsai J, Moley KH. Obesity and PCOS: the effect of metabolic derangements on endometrial receptivity at the time of implantation. Reprod. Sci. 2015;22:6–14. doi: 10.1177/1933719114561552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu KE, Hartman M, Hartman A, Luo Z-C, Mahutte N. The impact of a thin endometrial lining on fresh and frozen-thaw IVF outcomes: an analysis of over 40 000 embryo transfers. Hum. Reprod. 2018;33:1883–1888. doi: 10.1093/humrep/dey281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data supporting the findings of this study are available within the article and its supplementary material.