Abstract
Upper extremity access sites are the preferred access sites for hemodialysis. With the improvement of the survival in dialysis population, most patients outlive the dialysis access lifespan. As such, some patients exhaust the vascular access options of the upper extremities, which necessitates the search for new access sites. While lower extremity grafts and hemodialysis reliable outflow devices are potential alternatives, these access sites are plagued with recurrent lesions at the venous anastomosis and subsequent thrombosis leading to poor access survival. Within this framework, the axillary-based dialysis access was developed to address these challenges. In this report, we describe a 70-year-old woman who exhausted her upper extremity access sites and eventually underwent a chest wall arteriovenous graft (AVG) that connected the right axillary artery to the right axillary vein. This chest wall AVG remained functional without any intervention for more than 3 years. In conclusion, chest wall AVG access can be a viable option for hemodialysis patients who have exhausted the access sites of the upper extremities, while potentially minimizing complications seen in other methods of vascular access.
Keywords: End-stage renal disease, Hemodialysis, Arteriovenous graft, Chest, Axillary
Introduction
Functional dialysis access is considered the lifeline for end-stage renal disease (ESRD) patients. Hemodialysis is the most prevalent treatment for ESRD patients. The need for hemodialysis has increased in recent years as the main modality for renal replacement therapy in patients with ESRD [1,2]. Traditionally, constructing a permanent hemodialysis access usually starts with upper extremity autogenous accesses using the non-dominant extremity before the dominant one. However, for patients with poor native vascular fistula options, prosthetic access is considered the next best choice, usually in the brachial-axillary configuration [3].
Due to their survival improvement, most ESRD patients outlive the dialysis access sites in the upper extremities, which necessitates the search for other access sites [2]. When upper extremity sites are exhausted, a subset of dialysis patients are left with either switching to peritoneal dialysis or continuing hemodialysis via other access sites or through the use of a central venous catheter. However, the utilization of peritoneal dialysis remains very low in the ESRD population. On the other hand, central venous catheters are usually complicated with a high rate of infections, and central vein stenoses, and occlusion [4]. As such, the real estate of vascular access among these patients is eventually limited to few options: hemodialysis reliable outflow device or a lower extremity graft access. While lower extremity grafts are associated with a higher risk of infection, both hemodialysis reliable outflow device and the lower extremity graft are complicated with access dysfunction and thrombosis, which eventually shorten the access survival [3]. It is within this context that chest wall arteriovenous grafts were developed to address these challenges and unmet needs of this subset of dialysis patients. In this article, we report the interesting case of a 70-year-old woman on hemodialysis who exhausted her upper extremities access sites that underwent the insertion of a chest loop AVG. Surprisingly, the chest graft stayed functional over the last 3.4 years and required no intervention to preserve patency.
Case report
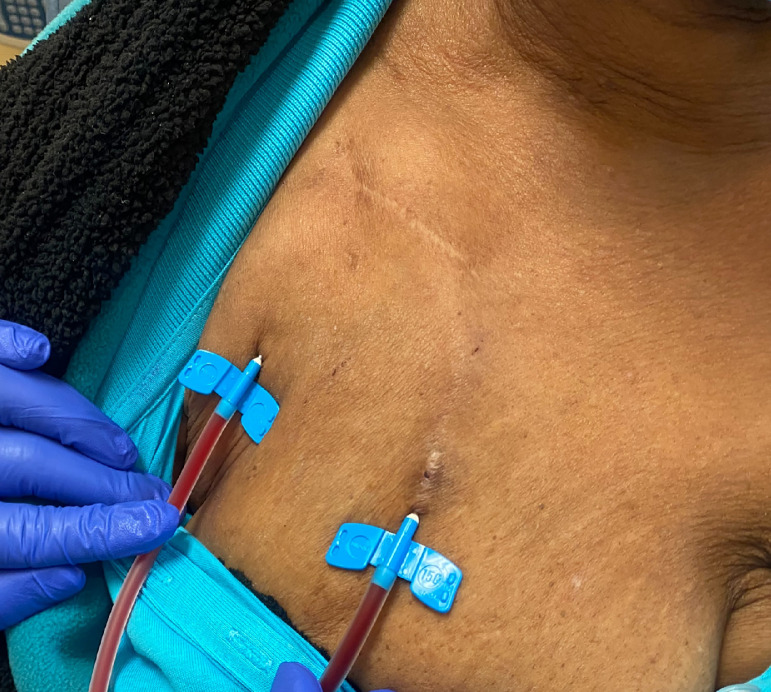
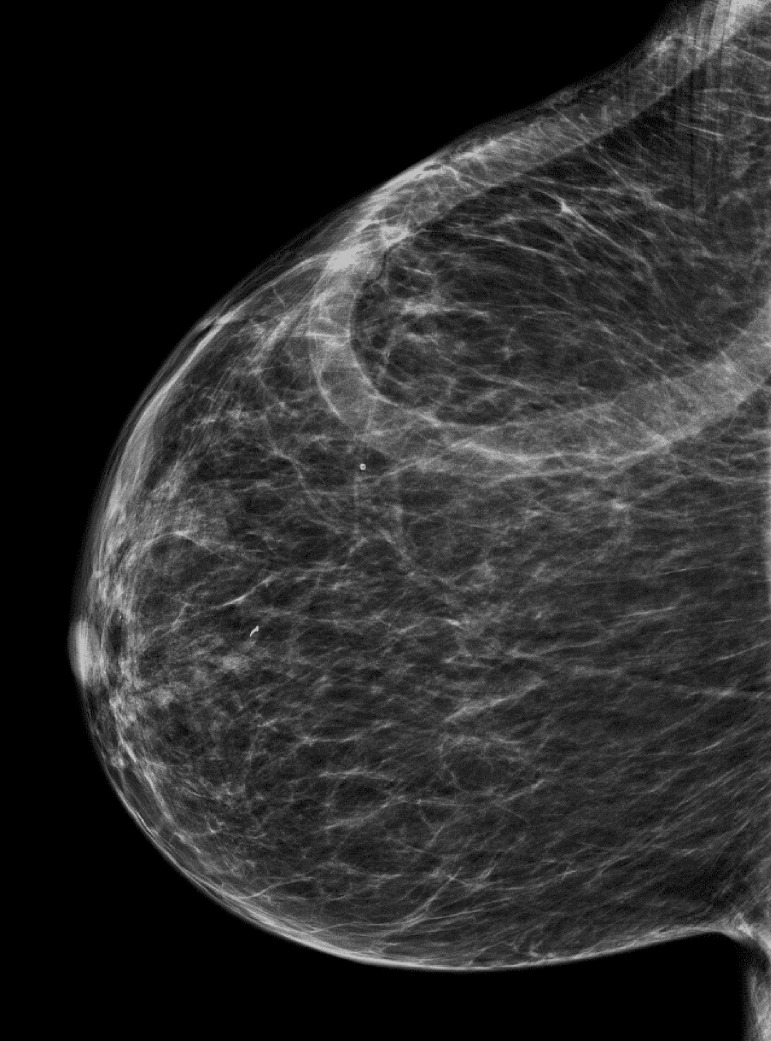
A 70-year-old African American woman with a known history of ESRD attributed to focal segmental glomerulosclerosis presented for dialysis access evaluation. Her past medical history was remarkable for hypertension, peripheral arterial disease, treated hepatitis C, stroke with residual right-sided weakness, gout, and diabetes. Her medications included metoprolol, amlodipine, cinacalcet, esomeprazole, and allopurinol. Physical exam was significant for multiple scars of old access surgeries on the upper extremities and a loop right chest AV graft with good bruit and thrill. In the background, the patient started dialysis in 2004 using a tunneled venous catheter; in late 2005, she underwent left upper arm arteriovenous (AV) fistula creation that lasted for 7 years till 2013 at which she received a deceased donor kidney transplant. With no further follow-up on its functionality, the AV fistula clotted. However, in 2016, the kidney transplant graft failed due to chronic rejection, and subsequently, the patient resumed hemodialysis via a tunneled venous catheter. Between 2016-2018, multiple attempts to create reliable dialysis access failed including upper extremity AV fistula and grafts. The etiology of access failure was attributed to non-maturation, immediate thrombosis, or severe steal syndrome that required access ligation. Notably, the patient declined peritoneal dialysis option. With her severe peripheral arterial disease and history of steal syndrome, the thigh AV graft option was excluded. Subsequently, she underwent right axillary artery to right axillary vein loop AV graft in September 2018 (post-operative access ultrasound is shown Fig. 1) that was used immediately for cannulation. Up till January 31, 2022, the chest AV graft has been functional without any intervention (Fig. 2). Interestingly, during a routine screening mammogram, the chest AV graft was seen very clearly (Fig. 3).
Fig. 1.
Post-operative access ultrasound of the chest arteriovenous graft (AVG).
Fig. 2.
The chest AVG is being cannulated during dialysis session.
Fig. 3.
Mammogram showing clearly the chest loop AVG.
Discussion
Until recent years, the upper extremity native AV fistulae is considered the access route of choice [1,2]. However, these fistulas are plagued with poor maturation and limited lifespans that usually require the construction of AVGs in the upper extremities, which are associated with poor survival due to venous anastomosis lesions and thrombosis [2]. On the other hand, femoral AVGs have been associated with a significant risk of infection, leg ischemia, and graft failure [1,6]. Further, it is worth mentioning that creating a prosthetic femoral access is technically challenging in obese patients and is usually associated with an increased risk of dysfunction and subsequent failure [6]. In order to mitigate these challenges, the implantation of an anterior chest wall AV graft became a plausible option in this group of patients. It is well acknowledged that obese patients have less subcutaneous fat covering the axillary vessels than the femoral vessels, a finding that favors the placement of chest wall AVG in obese patients [6]. Further, it is reasonable to speculate that chest wall AVG could provide a viable alternative in dialysis patients who failed non-dominant upper extremity access before using the dominant arm, a merit that can prevent potential complications that could disable the functionality of the dominant arm [6].
It is important to note that patients should undergo an evaluation of the central venous system before undergoing the operation of creating an anterior chest wall graft. This is to ensure that the patient has viable patent central veins. Venography is considered the gold standard for such an evaluation, but magnetic resonance imaging (MRI) and Duplex ultrasound have been used. However, MRI and ultrasounds are not as sensitive in detecting stenosis [1]. Prosthetic axillary access placement has been documented between the axillary artery and ipsilateral axillary vein, contralateral axillary vein, and the ipsilateral jugular vein [5]. Our patient underwent right axillary artery to ipsilateral axillary vein AVG.
After losing her functional AV fistula after kidney transplantation, our patient underwent multiple attempts to create a reliable dialysis access with limited success due to fistula non-maturation, AVG immediate thrombosis, and severe steal syndrome that required access ligation. Consequently, lower extremity AVG was excluded due to severe peripheral arterial disease. At this point, an anterior chest wall AV graft was created by connecting the right axillary artery with the right axillary vein. The chest wall loop AVG of our patient has been functional without any intervention for more than 3 years.
In conclusion, functional dialysis access is considered the lifeline for ESRD population. Over time, most of these patients outlive the dialysis access sites in the upper extremities, which necessitates the search for other access sites. One such access site is the femoral position, which is usually associated with a significant risk of infection, leg ischemia, and graft failure [1,6]. In order to overcome these risks, creating an axillary-based dialysis access could be considered a viable alternative in this subset of ESRD patients. In addition to their satisfactory patency rate, these grafts could significantly minimize the infectious and ischemic complications encountered with long-term catheter use and femoral loop access.
Author Contributions
All authors contributed equally to this article. All authors read and approved the final manuscript.
Ethics Approval
Our institution does not require ethical approval for reporting individual cases or case series.
Footnotes
Competing Interests: The authors declare no conflicts of interest associated with this manuscript.
Patient Consent: Written informed consent was obtained from the patient for all procedures and publication of this case and accompanying images.
References
- 1.Niyyar V.D. Anterior chest wall arteriovenous grafts: An underutilized form of hemodialysis access. Seminars Dial. 2008;21(6):578–580. doi: 10.1111/j.1525-139X.2008.00491.x. [DOI] [PubMed] [Google Scholar]
- 2.Stephenson M.A., Norris J.M., Mistry H., Valenti D. Axillary-axillary interarterial chest loop graft for successful early hemodialysis access. J Vasc Access. 2012;14(3):291–294. doi: 10.5301/jva.5000121. [DOI] [PubMed] [Google Scholar]
- 3.Glickman M.H. Hero vascular access device. Seminars Vasc Surg. 2011;24(2):108–112. doi: 10.1053/j.semvascsurg.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Lazarides M.K., Georgakarakos E.I., Schoretsanitis N. Extra- and Intrathoracic Access. J Vasc Access. 2014;15(7_suppl):125–129. doi: 10.5301/jva.5000231. [DOI] [PubMed] [Google Scholar]
- 5.Teruya T.H., Schaeffer D., Abou-Zamzam A.M., Bianchi C. Arteriovenous graft with outflow in the proximal axillary vein. Ann Vasc Surg. 2009;23(1):95–98. doi: 10.1016/j.avsg.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 6.Seeger J.M. The role of the prosthetic axilloaxillary loop access as a tertiary arteriovenous access procedure. Yearbook Surg, 2009. 2009:353–354. doi: 10.1016/j.jvs.2008.03.030. [DOI] [PubMed] [Google Scholar]