Abstract
Background
Sever acute pancreatitis (SAP) is a critical disease with high mortality, and lack of clinically available treatments with specificity and effectiveness. Bone marrow derived mesenchymal stem cells (BMSCs) exhibited moderate effect on AP which needs further improvement.
Methods
Pancreatic infiltrating lymphocytes were analyzed to demonstrate the intervention of BMSCs on inflammatory cell infiltration of AP. Gene silencing with siRNA and small molecule inhibitor were utilized to determine the key effector molecule of BMSCs on AP. Pharmacological regulation and nanotechnology were introduced to further ameliorate BMSCs action.
Results
It was revealed that BMSCs prevent the progression of acute pancreatitis (AP) by reducing recruitment of macrophages, neutrophils and CD4+T cells in the lesion site. The pivotal role of chemokine–iNOS–IDO axis for BMSCs to intervene AP was confirmed. Compared with any single drug, Chloroquine/Tamoxifen combination together with IFN-γ pronouncedly up-regulated the transcription of several MSC immune regulators such as COX-2, PD-L1, HO-1 especially iNOS/IDO. As expected, BMSCs and human umbilical cord mesenchymal stem cells (UMSCs) pretreated with CQ/TAM/IFN-γ exerted enhanced intervention in AP and SAP mice. Moreover, pretreatment with CQ-LPs/TAM-NPs combination not only counteracted MSCs proliferation inhibition induced by free drugs but also enhanced their efficacy.
Conclusion
Under the background of rapid progress in MSCs clinical translation, this study focuses on the urgent clinical issue and initiates an original mechanism-based strategy to promote intervention on severity progression of SAP, which promises its clinical translation in future.
Keywords: Acute pancreatitis, Mesenchymal stem cells, Chloroquine, Tamoxifen, Nanoparticles
Graphical abstract
1. Introduction
Acute pancreatitis (AP), an acute inflammatory disorder caused by the injured acinar cells, is one of the most common gastrointestinal diseases encountered in emergency departments. When feed-forward inflammatory cascade amplification of AP is so profound that extensive necrosis and organ failure supervene, sever acute pancreatitis (SAP) occurs, which is responsible for 5–20% mortality of acute pancreatitis [1]. However, the therapeutic value of protease inhibitor and trypsin inhibitor like somatostatin against acute pancreatitis is still lack of high-quality clinical evidence [2]. So, there is still no specific drug for SAP until now. The early treatment of SAP includes early fluid resuscitation, analgesia, nutritional support and treatment for etiology and early complications, together with the late treatment comprising of percutaneous catheter drainage and surgeries [3]. Thus, substantial progression in understanding the pathophysiology of AP offers a probability for development of mechanism-based threatment against it.
Mesenchymal stromal cells (MSCs), also known as mesenchymal stem cells, are adult progenitor cells with pleiotropic effects that can be differentiated into cell types of mesodermal origin, such as adipocytes, osteocytes, and chondrocytes. The immunoregulatory properties of MSCs allow for their promising therapeutic applications in many inflammatory disorders [4]. MSCs have manifested obvious therapeutic effect against both acute and chronic pancreatitis reported by many researchers [5,6]. And MSCs exerted the effect through reducing acinar cell degeneration, pancreatic edema, and inflammatory cell infiltration in pancreas [6]. Jing Yang recently reported that UMSCs pretreated with Ang II enhanced the therapeutic effect on SAP by promoting angiogenesis.(J [7]. However, more evidence is stilled needed to decipher the underlying molecular mechanisms to elicit further clinical application[6].
The immune-modulatory capabilities of MSCs are not constitutive but rather are licensed by inflammatory cytokines. The activation of MSCs might vary depending on the levels and the types of inflammation within the residing tissues [4].. MSCs exerts its therapeutic effect through the release of various mediators including immunosuppressive molecules, growth factors, exosomes, chemokines, complement components etc.(L [4,8,9]. The proposed model is that MSCs drive T cell migration into proximity through secreting chemokines and suppress its function by nitric oxide, the product of inducible nitric oxide synthase (iNOS), of which the expression is provoked by cytokines such as IFN-γ, TNF-α, IL-1β etc. [10]. Among the cytokines, IFN-γ seems to play a central role probably through the interferon signaling, and the concomitant cytokines might play a supporting role. However, the promise of MSC-based therapies is somewhat hindered by their apparent modest clinical benefits, highlighting the need for approaches that would increase the efficacy of MSCs through inducing immune modulators secretion.
There have been many reports about strategies to enhance MSCs therapeutic effect using active stimulators, biomaterial vectors as well as hypoxic preconditioning etc. [11] MSCs with pretreatment of IFN-γ can augment inhibition over T-cell proliferation dramatically [12] Tamoxifen (TAM), chloroquine (CQ) and KYNA have been reported to regulate anti-inflammatory molecules of BMSCs including iNOS, IDO, TSG-6 etc. [13]; G [14]. As IFN-γ seems to be indispensable in the immune regulation of MSCs, it is speculated that interferon signaling might be very important for MSCs to realize its immunosuppressive function. Beyond the engagement of canonical JAK-STAT signaling pathways that promote production of interferon responsive genes, the IFNs participate in multiple other signaling cascades to generate products that mediate biological responses and outcomes including MAPK, Akt/mTOR, ULK1 and NF-κB pathways etc. And TAM and CQ have been reported to regulate one or more of these pathways, which is probably responsible for the regulation of MSCs immune regulation effect [[15], [16], [17], [18], [19]]. As spheroid formation of MSCs contributes to maintaining their stemness and enhancing their differentiation capacities, biomaterials has been used to promote the spheroid formation. Hydrogels tethered with IFN-γ encapsulating hMSCs has increased IFN-γ retention time, preserves its biological activity, and eventually strengthened colonic mucosal wounds healing effect of hMSCs. Nano-hydroxyapatite [20] and silk fibroin containing scaffold exert osteoinductive effects on BMSCs via IL-1α. In a word, the function of MSCs is adjustable and rational use of immune stimulator as well as biomaterials will contribute to its function.
In this study, firstly, we further explore the molecular mechanism for the therapeutic effect of MSCs against AP on the base of existing literature. Then, by integrating immune regulators and biomaterials into MSC-based cell therapy, we attempt to find new ideas and strategies for SAP therapy. And an advantage of this strategy is the application of biomaterials in vitro without introducing any biomaterials into human body.
2. Materials and methods
2.1. 1Materials and reagents
Chloroquine (CQ) was purchased from J&K Scientific, Ltd. (Beijing, China). Tamoxifen was purchased from sigma-Aldrich (America). Amylase detection kit was purchased from Nanjing Jiancheng Institute of Biotechnology (Nanjing, China). Cerulein was purchased from Shanghai Yuanye Bio-Technology CO., Ltd (Shanghai, China).
2.2. Experimental animal model and treatment
Male C57BL/6 mice (6–8 weeks old) were purchased from Beijing HFK Bioscience Co., Ltd. (Beijing, China). All animal experiments involved in the present study were approved by the Animal Care and Use Committee of Sichuan University (Chengdu, Sichuan, China).
For acute pancreatitis (AP) model, mice were sequentially given 10 times of intraperitoneal injections of caerulein (75 μg/kg) with 1 h-interval for each time. For severe acute pancreatitis (AP) model, LPS (15 mg/kg) was intraperitoneally injected immediately after the last cerulein injection. For treatment groups, BMSCs or UMSCs cells were slowly infused via the tail vein immediately after the second cerulein injection, with a dose of 2 × 10^6 cells/kg for AP and 5 × 10^6/kg cells for SAP. Then animals were sacrificed at 24-h after the first cerulein injection and pancreases were harvested.
2.3. Isolation of pancreatic infiltrating immune cells
The pancreas was minced and digested with 0.01 mg/ml DNase and 0.5 mg/ml collagenase D (Sigma-Aldrich, St. Louis, MO) for 15 min. The cell suspension after digestion was filtered with a 40-μm cell strainer to obtain single cells and washed with 2% FBS-containing 1640 medium. Next, the suspension was centrifuged at 1200 rpm for 5min to eliminate the major debris followed by further purification with 40–70% Percoll gradient centrifugation. The isolated cells were incubated with an anti-CD16/CD32 antibody (BD Pharmingen, Tokyo, Japan) to prevent non-specific antibody binding. Then, the cells were stained with the following antibodies: CD45-FITC, CD11b-PB, Ly6C-PECy7, Ly6G-APC, CD3-PECy5.5, CD4-PECy7, CD8-APC (Biolegend Inc, San Diego, CA), and analyzed by BD Fortessa flow cytometry system (BD Biosciences Pharmingen, San Diego, CA). The data was analyzed with Flowjo software.
2.4. Statistical analysis
All results were expressed as means ± standard deviation. Statistical analysis was according to SPSS statistical software (IBM Corp., Armonk, NY, USA), followed by Student-Newman-Keuls as a post hoc test. A value of P < 0.05 was considered to be statistically significant.
3. Results
3.1. Reduction of pancreatic proinflammatory cells recruitment by BMSCs in AP mice
Bone marrow derived mesenchymal stem cells abbreviated as BMSCs had fibroblast-like morphology and proliferated fast in complete media (Figure. S1A). BMSCs were positive for CD90 and CD29, but negative for CD45 (Figure. S1B), and had the potential to differentiate into adipogenesis, osteogenesis and chondrogenesis (Figure. S1C). AP model was established through repeated intraperitoneal injection of cerulein as described previously [21] together with early intervention of BMSCs intravenously. Pathological score of pancreatic HE sections indicated BMSCs intervention improved pathological indicators including edema, inflammation and necrosis (Fig. 1A and B). BMSCs also down-regulated serum amylase and pancreatic cytokines at RNA level including TNF-α, IL-6 and IL-1β (Fig. 1C and D). Macrophages are believed to be the most likely source of proinflammatory agents (IL-1, IL-6, and TNF-α), and decrease of macrophage infiltration would consequently decrease cytokine levels [22]. As these cytokines will increase capillary permeability and promote leukocyte adherence and extravasation [22], BMSCs decreased the pathological scores including edema and inflammation probably by lowering the cytokine level. Above all, BMSCs effectively alleviated pancreatitis inflammation, which was consistent with previous studies.(F [23,24].
Fig. 1.
BMSCs reduced pancreatic proinflammatory cells recruitment in AP mice. (A,B) Pathological scoring including edema, inflammation and necrosis of HE staining sections,scale bar = 50 μm; (C) Serum amylase activities (U/L); (D) Pancreatic inflammatory cytokine at mRNA level; (E,F) Flow cytometry diagram of myeloid cells (CD45 + CD11b+), CD4+T cells (CD3+CD4+), macrophages (CD11b + F4/80+) and neutrophils (CD11b + Ly6G+) gated from CD45+ cells in PIL of three groups including healthy NT, AP and BMSCs treated group at 24 h after the first cerulein injection; (G) Immunofluorescence images of pancreatic sections stained with CD11b + Gr-1+ (macrophages and neutrophils) and CD4+T cells, scale bar = 100 μm Ⅰ: healthy control group; Ⅱ: AP group; Ⅲ: AP pretreated with BMSCs group. Scale = 100 μm. All the data are expressed as the means ± SD (n = 3 per group) ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
To study the effect of BMSCs intervention on acute pancreatitis, mouse pancreatic infiltrating lymphocytes (abbreviated as PIL) of three groups including healthy control group, acute pancreatitis group and BMSCs treated acute pancreatitis group were analyzed (n = 3). It has been reported that rapid migration of macrophage, neutrophils and T cells especially CD4+T cells to pancreas had detrimental effect in cerulein induced pancreatitis in mice [25,26]. Therefore, these leukocyte subtypes of macrophage (CD11b + F4/80+), neutrophil (CD11b + Ly6G+) and CD4+T cells in PIL at 24 h after the first injection of cerulein were analyzed (Figure.S2). For pancreatitis mice, the percentage of infiltrating myeloid cells (CD45 + CD11b+) in pancreas dramatically increased from 1.37% (healthy control) to 46.6% including 16.2% of macrophages and 29.0% of neutrophils. And CD4+T cells percentage increased from 16.7% to 26.0%. The result is consistent with the previous report that CD11bhigh cells cannot be detected in healthy pancreas and will migrate from bone marrow to damaged pancreas after cerulein administration [26].
With intervention of BMSCs, percentage of macrophages, neutrophils and CD4+T cells in PIL all decreased significantly without exception (Fig. 1E and F). The result suggested that intervening of pancreatitis by BMSCs was achieved by reducing proinflammatory cell infiltration. Proinflammatory cell recruitment in pancreas including myeloid cell (CD11b + Gr-1+) and CD4+T cell (CD4+) was confirmed by immunofluorescent staining (Fig. 1G), which was consistent with the flow cytometry result. Separation of PIL uncovered the influence of BMSCs on pancreatitis-related inflammatory cells, which was more advantageous to the conventional immunohistochemical method.
3.2. Role of iNOS on BMSCs anti-inflammatory effect for AP
By analyzing lymphocyte subtypes in PIL, the cell populations regulated by MSCs were identified in this study. As the recruitment of all three subsets including neutrophil, macrophage and CD4+T cells have been influenced, so we focus on chemokine–iNOS–IDO axis which has been reported to regulate neutrophil, macrophage as well as T cells. [27]; G. [4,28,29]; H. F [30].
To reveal the role of iNOS on BMSCs anti-inflammatory effect, BMSCs transfected with control siRNA/iNOS siRNA and BMSCs with or without iNOS inhibitor L-NMMA pretreatment were studied for their intervention on AP mice. The mice were divided into five groups: AP, AP mice intervened with BMSCs only (abbreviated as BMSCs), AP mice intervened with BMSCs and administered with iNOS inhibitor L-NMMA (L-NMMA), AP mice intervened with BMSCs transfected with control siRNA (si-NC), and AP mice intervened with BMSCs transfected with iNOS siRNA (si-iNOS). Both mRNA and protein levels showed that the expression of iNOS in BMSCs decreased after transfection with iNOS siRNA (Fig. 2A). The pathological indicators including histological score of HE sections, serum amylase level and pancreatic cytokine mRNA levels suggested that the symptoms of the disease for the mice in both BMSCs and si-NC group were obviously relieved. On the contrary, this effect was reversed for mice in both si-iNOS and L-NMMA groups (Fig. 2B–E). In addition, T cell activating factor FasL was also up-regulated, suggesting that the activation of T cells was not inhibited in the state of disease. Immunofluorescence of tissue section also showed that the infiltration of CD4 + T cells in pancreatic tissue did not significantly decrease. The primer sequences were shown in supplementary Table 1.
Fig. 2.
Influence of iNOS on BMSCs' anti-inflammatory effect. (A) iNOS mRNA and protein level in BMSCs transfected with negative control siRNA (si-NC) or iNOS siRNA (si-iNOS); (B, C) HE staining of pancreatic section and corresponding statistical results of pathological scoring including edema, infiltration, necrosis.
The effect of iNOS molecule of BMSCs on PIL was shown in Fig. 2F and G. BMSCs without any treatment inhibited the infiltration of macrophages, neutrophils and CD4+ T cells, while this effect was reversed after intravenous administration of L-NMMA. The CD45 + CD11b + cells increased from 15.4% to 38.0%, the CD11b + F4/80+ cells increased from 9.38% to 23.7%, the CD11b + Ly6G + cells increased from 6.49% to 14.8%, and the CD3+CD4+ T cells increased from 14.2% to 20.9%. Like BMSCs, the si-NC BMSCs also inhibited the infiltration of myeloid cells and CD4+T cells recruitment. On the contrary, si-iNOS BMSCs lost the ability of immunosuppression, and the number of CD45 + CD11b + cells increased to 33.5% which included 17.3% of macrophages and 18.6% of neutrophils, and CD3+ CD4+ T cells increased to 28.8%. As expected, this phenomenon was also demonstrated by immunofluorescence staining of pancreatic tissue (Figure S3). These results above suggest that iNOS is the key molecule of BMSCs inhibiting the infiltration of inflammatory cells in AP (Fig. 2H).
Scale bar = 50 μm; (D) Serum amylase level (U/L); (E) Pancreatic cytokine mRNA expression levels, results are presented as the mean ± SD obtained from three independent experiments; (F,G) Flow cytometry diagram of myeloid cells (CD45 + CD11b+), CD4+T cells (CD3+CD4+), macrophages (CD11b + F4/80+) and neutrophils (CD11b + Ly6G+) gated from CD45+ cells in PIL of four groups including healthy control, AP and BMSCs treated group at 24 h after the first cerulein injection; (H) Schematic illustration shows the efficacy of BMSCs for prevention of proinflammatory cells recruitment through iNOS. All the data are expressed as the means ± SD (n = 3 per group). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
3.3. Synergistic upregulation of BMSCs and UMSCs anti-inflammatory molecule by combination of Chloroquine and Tamoxifen in vitro
Many key immune regulators of MSCs including PGE2, TSG-6, IDO, iNOS, TGFβ, HO-1 and PD-L1 have been reported previously for MSCs to exert the anti-inflammation effect [31,32]. As described above, iNOS played a central role for intervention of AP by BMSCs. In this part, the hypothesis of enhanced AP intervention by up-regulating iNOS in BMSCs would be verified. It was revealed in our study that iNOS mRNA expression level of MSCs was positively correlated with CQ/TAM concentration (Fig. 3A). As there was a trade-off between the benefits of stimulation effect and the risk of side effects originated from the drugs, relatively low concentration of CQ 40 μM and TAM 20 μM were used in the following experiments. Among the anti-inflammatory molecules influenced by CQ/TAM, iNOS and IDO were the most up-regulated (Fig. 3B). The KYNA, a metabolite of IDO, has been reported to augment IDO and TSG-6. And the rise of iNOS, IDO as well as COX2 induced by KYNA was also observed (Figure S4A). It is noteworthy that mRNA expression level of iNOS and IDO were also the highest among the molecules measured. Based on the results above, we hypothesized that combination of these stimulators might achieve better effect. As CQ and TAM are clinically available drugs, they were chosen for further study instead of KYNA. It was found that CQ/TAM stimulated the RNA level of iNOS synergistically (Fig. 3C, E), and had no effect on cell surface markers (Fig. 3F). Besides, pretreatment with CQ/TAM alone or jointly together with IFN-γ would not cause MSCs apoptosis (Fig. 3G(a)). Primer sequences were listed in supplementary Table 1.
Fig. 3.
Synergistic upregulation of BMSCs and UMSCs anti-inflammatory molecules by combination of Chloroquine and Tamoxifen in vitro. The concentrations for CQ, TAM and IFN-γ were 40 μM, 20 μM and 20 ng/ml if there's no special indication. CQ/TAM was used in combination with IFN-γ. NT: BMSCs/UMSCs with no treatment; IFN-γ: BMSCs/UMSCs pretreated with IFN-γ only; IC: BMSCs/UMSCs pretreated with IFN-γ/CQ; IT: BMSCs/UMSCs pretreated with IFN-γ/TAM; ICT:BMSCs/UMSCs pretreated with IFN-γ/CQ/TAM;Both RNA and protein samples were collected after 24 h of treatment; (A) BMSCs were stimulated with different concentrations of CQ or TAM for 24 h; (B) BMSCs anti-inflammatory molecule mRNA level stimulated with CQ or TAM for 24 h; (C) BMSCs iNOS mRNA level stimulated individually or jointly with CQ and TAM; (D) UMSCs IDO mRNA level stimulated individually or jointly with CQ/TAM; (E) INOS and IDO protein levels after stimulation by various drugs; (F) Detection of surface markers on BMSCs pretreated with CQ/TAM combination for 24 h; (G) Detection of cell apoptosis of BMSCs (a) and UMSCs (b) pretreated with CQ/TAM combination for 24 h. All the data are expressed as the means ± SD (n = 3 per group). ∗p < 0 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Indoleamine 2,3-dioxygenase (IDO) in human and inducible nitric oxide synthase (iNOS) in mouse are among the key molecules mediating immunosuppression by MSCs, which are species dependent [33]. MSCs from human employ IDO to suppress immune responses, and MSCs from mouse/rat utilize iNOS [33]. So, the role of IDO on UMSCs anti-inflammatory effect for AP was also studied. Human umbilical cord mesenchymal stem cells were successfully extracted according to previous method [34], which expressed CD73, CD105, but not CD34 (Figure S5), and had the potential to differentiate into adipogenesis, osteogenesis and chondrogenesis (Figure. S5C). Similarly, pretreatment of UMSCs with CQ and TAM in vitro showed up-regulation of IDO expression both in mRNA and protein level (Fig. 3 D, E) and had no effect on cell apoptosis (Fig. 3G(b). So, further study would be performed to verify the intervention on AP mice by MSCs pretreated with CQ/TAM.
3.4. Enhanced intervention of MSCs pretreated with CQ/TAM combination in AP model
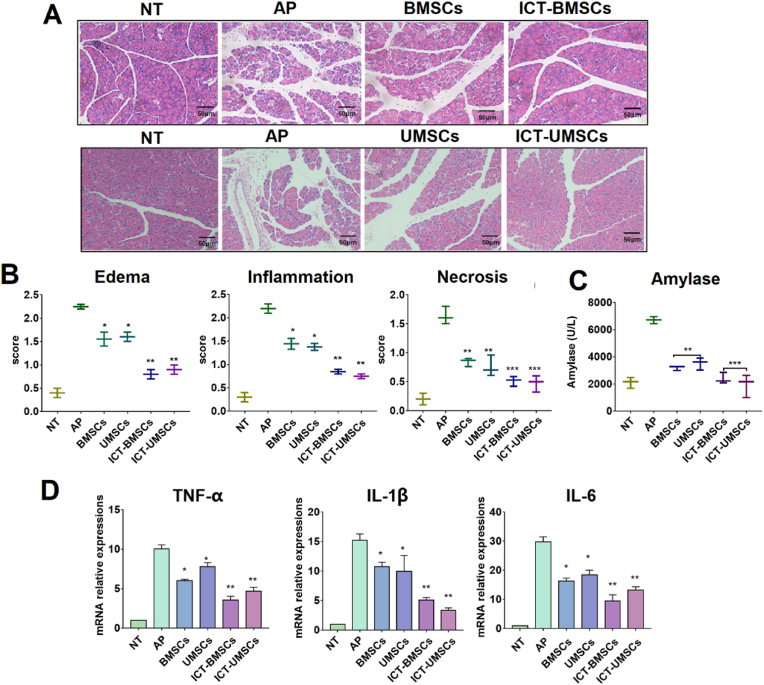
There are six groups: NT (healthy group with no treatment), AP group without treatment (AP), BMSCs/UMSCs treatment group (BMSCs/UMSCs), BMSCs/UMSCs pretreated with IFN-γ in combination with CQ/TAM group (ICT-BMSCs/UMSCs). As expected, ICT-BMSCs/UMSCs more effectively protected the pancreatic structure by reducing acinar cell apoptosis as well as alleviating pancreatic edema and inflammation (Fig. 4A). The histopathological score was also shown in Fig. 4B. In addition, ICT-BMSCs/UMSCs significantly reduced the serum amylase level and pancreatic inflammatory cytokine expression level including TNF-α, IL-1β and IL-6 (Fig. 4C and D). And there was no significant difference between BMSCs and UMSCs group.
Fig. 4.
Improved intervention of BMSCs and UMSCs pretreated with ICT against AP. There are six groups in this study including NT (healthy mice with no treatment), AP (AP mice with no treatment), BMSCs (AP mice treated with BMSCs), AP mice treated with UMSCs (UMSCs), AP mice treated with BMSCs intervened with the CQ/TAM combination plus IFN-γ (ICT-BMSCs), AP mice treated with UMSCs intervened with CQ/TAM combination plus IFN-γ (ICT-UMSCs). The concentrations for CQ, TAM and IFN-γ were 40uM, 20uM and 20 ng/ml if there's no special indication. CQ/TAM was used in combination with IFN-γ. (A) Photographs of pancreatic sections with HE staining; (B) Pathological statistics of pancreatic HE staining sections including edema, infiltration, necrosis; (C) Serum amylase level; (D) Pancreatic cytokine mRNA expression level. Statistical analysis was performed between the control group and the treated groups. Results are presented as the mean ± SD obtained from three independent experiments ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (Treatment groups vs. AP group). Scale bar = 100 μm.
3.5. Intervention of MSCs pretreated with CQ/TAM combination in SAP model
Previously, SAP rat model has been induced by the injection of taurocholic acid (TCA) into pancreatic duct, which is traumatic and has high mortality [24]. Recently, SAP mouse model has been established by intraperitoneal injection of cerulein in combination with lipopolysaccharide (LPS). [35]; X [36]. For classic cerulein-induced mouse acute pancreatitis model, cerulein acts directly on the acinar cells and causes their apoptosis. To simulate the acinar cell necrosis in SAP, LPS has been used in combination with cerulein to exacerbate pancreatic inflammation through activating TLR4-related signaling.(X [36]. In this study, mice in SAP group showed much higher levels of amylase and cytokines than mice in AP group, and there were more necrotic cells as shown in HE pathological section (Figure S6). In AP group, interlobular space widened, edema and inflammatory cell infiltration were found, and no obvious necrosis was seen. In SAP group, interlobular arrangement was disordered, edema and a large number of inflammatory cells infiltrated were found, and local acinar cell fusion necrosis was found. These phenomena were consistent with the previous report [37], and confirmed the severity of LPS + cerulein induced SAP model.
As shown in Fig. 5, the pathological scores and the levels of inflammatory factors in the CQ/TCM pretreated MSCs groups (ICT-BMSCs) were lowered relative to the MSCs only groups, although inflammation score and IL-1β mRNA level had no statistical difference.
Fig. 5.
Intervention of BMSCs and UMSCs pretreated with ICT against SAP. There are six groups in this study including Control, SAP, SAP mice treated with BMSCs (BMSCs), SAP mice treated with UMSCs (UMSCs); SAP mice treated with BMSCs intervened with the CQ/TAM combination plus IFN-γ (ICT-BMSCs), SAP mice treated with UMSCs intervened with CQ/TAM combination plus IFN-γ (ICT-UMSCs). (A) Photograph of pancreatic section with HE staining; (B) Statistics of pathological score of pancreas (edema, infiltration, necrosis); (C) Pancreatic cytokine mRNA expression level. Results are presented as the mean ± SD obtained from three independent experiments ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (Treatment groups vs. SAP group). Scale bar = 100 μm.
3.6. Counteraction of proliferation inhibition and improvement of efficacy achieved by nanoparticle encapsulation of CQ/TAM
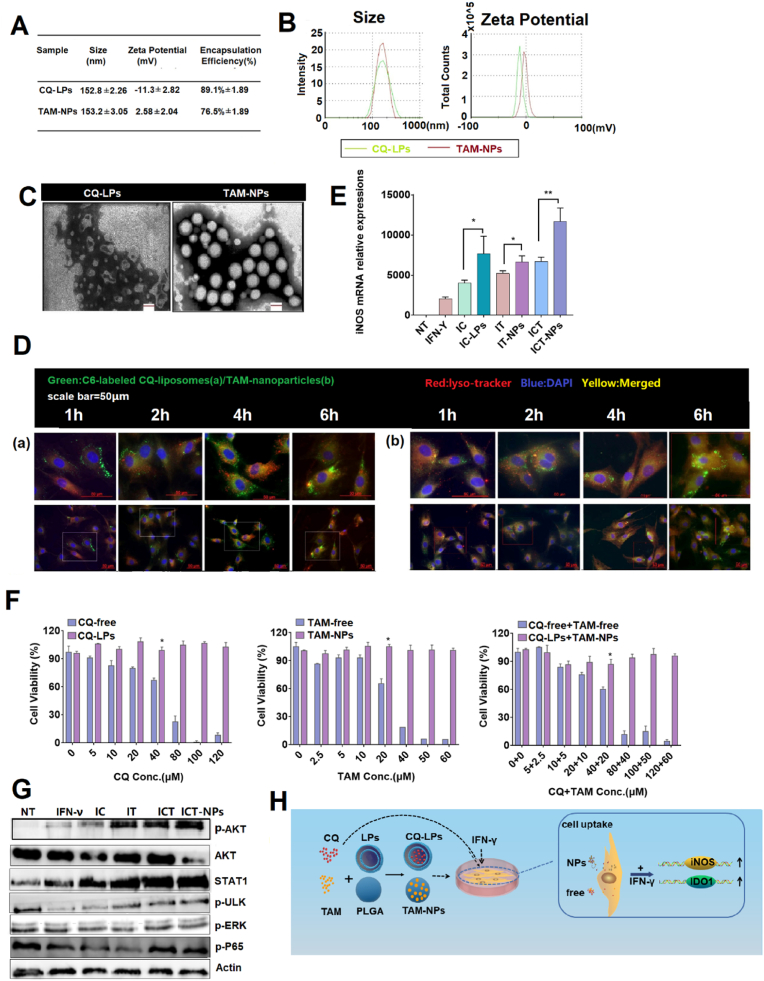
The potential impact of nanotechnology in MSC-driven regenerative medicine has drawn more and more attention as nanoparticles play an important role in exploiting MSCs advantage to achieve an ideal therapeutic outcome [38]. So, nanoparticle encapsulation of the drugs was implemented in this study. PLGA-based nanoparticle and liposome with satisfying biocompatibility and biodegradability were adopted as vectors for the drugs [39]; L. M [40,41]. The prescriptions for both drugs were screened using a single factor screening method with encapsulation efficiency and loading efficiency as the screening indicators (data not shown). The pharmaceutical and biological characteristics of the optimal prescriptions for the CQ loaded liposomes (CQ-LPs) and the TAM loaded PLGA nanoparticles (TAM-NPs) are shown in Fig. 6, Fig. 7.
Fig. 6.
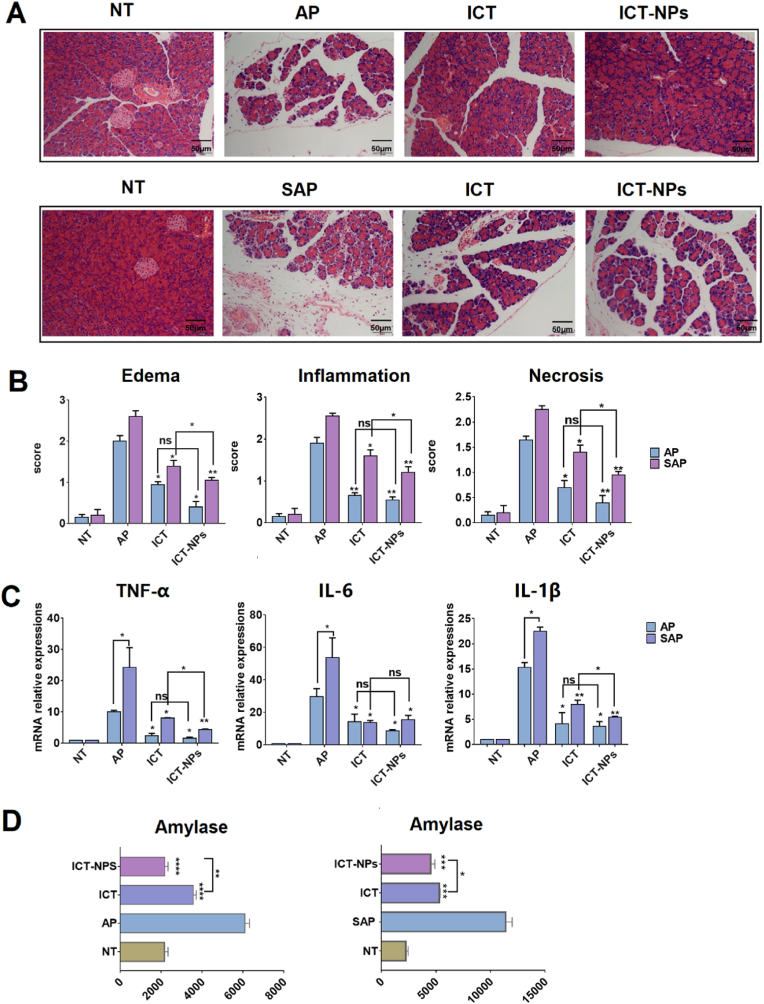
Characterization of the drug loaded nanoparticles: CQ-LPs and TAM-NPs. The concentrations for CQ, TAM and IFN-γ were 40 μM, 20 μM and 20 ng/ml if there's no special indication. Both CQ and TAM were used in combination with IFN-γ. NT: no treatment; IFN-γ:BMSCs treated with IFN-γ only; IC: BMSCs treated with IFN-γ/CQ; IC-LPs: BMSCs pretreated with IFN-γ/CQ-LPs; IT: BMSCs pretreated with IFN-γ/TAM; IT-NPs: BMSCs pretreated with IFN-γ/TAM-NPs; ICT: BMSCs pretreated with IFN-γ/CQ/TAM; ICT-NPs: BMSCs pretreated with IFN-γ/CQ-LPs/TAM-NPs. Both RNA and protein samples were collected at 24 h after treatment. (A, B) Particle size and zeta potential; (C) Transmission electron microscopy photographs; (D) Cellular uptake of CQ-LPs (a) and TAM-NPs (b) by BMSCs at 1 h, 2 h, 4 h, 6 h. Red: Lysosome tracker, Green: Coumarin loaded in CQ-LPs and TAM-NPs, Blue: DAPI; (E) BMSCs iNOS mRNA level stimulated individually or jointly with free CQ/TAM or the drug loaded nanoparticles. (F) BMSCs proliferation inhibition at various concentrations of the free drugs and the nanoparticles determined by MTT; (G) Schematic diagram describes the synthesis of CQ-LPs/TAM-NPs as well as the enhanced cellular uptake of the nanoparticles in BMSCs relative to the free drugs. All the data is expressed as the means ± SD (n = 3 per group). ∗p < 0.05, ∗∗p < 0.01. Scale bar = 50 μm.As expected, CQ-LPs/TAM-NPs combination pretreatment guaranteed the efficient intervention of BMSCs in both AP and SAP mice model (Fig. 7). In AP mouse model, CQ-LPs/TAM-NPs combination pretreated BMSCs (ICT-NPs) minimized acinar cell damage, reduced inflammatory cell infiltration, and there was no obvious difference between the normal pancreatic tissue and the pancreatitis tissue after the nanoparticle combination treatment (Fig. 7A and B). In addition, the frequency of macrophage (CD11b + F4/80+) and neutrophil (CD11b + Ly6G+) in PIL of ICT-NPs group was almost comparable to that of NT group (Figure S7). In SAP mouse model, although ICT-NPs group did not completely prevent inflammation progression as it did in AP mouse model, ICT-NPs further mitigated the pancreatic tissue damage and decreased inflammatory cytokine expression relative to that of ICT group (Fig. 7B and C).
Fig. 7.
Maximum intervention against AP and SAP by the drug loaded nanoparticle combinations in vivo. The concentrations for CQ, TAM and IFN-γ were 40 μM, 20 μM and 20 ng/ml if there's no special indication. CQ/TAM was used in combination with IFN-γ. There are six groups in this study including NT (healthy mice with no treatment), AP (AP mice with no treatment), SAP (SAP mice with no treatment), BMSCs (AP mice or SAP mice intervened with BMSCs), ICT (AP mice or SAP mice intervened with IFN-γ/CQ/TAM combination), ICT-NPs (AP mice or SAP mice intervened with IFN-γ/CQ-LPs/TAM-NPs combination). (A) Photographs of pancreatic sections with HE staining; (B) Statistics of pathological score of pancreas (edema, infiltration, necrosis); (C) Pancreatic cytokine mRNA expression level; (D) Serum amylase activities (U/L). Results are presented as the mean ± SD obtained from three independent experiments ∗p < 0.05, ∗∗p < 0.01. Scale bar = 100 μm.
Particle size, zeta potential, encapsulation efficiency and loading efficiency of CQ-LPs and the TAM-NPs were shown in Fig. 6A and B. Morphology of the nanoparticles was observed with TEM (Fig. 6C). CQ-LPs showed a typical liposome structure with a particle size of 150 nm and neutral charge. The TAM-NPs were solid nanoscaled-spheres around 150 nm with slightly negative-charged. To track CQ-LPs and the TAM-NPs inside of the cells, fluorescent probe coumarin (green) was encapsulated in the nanoparticles together with the drugs. Fast intracellular accumulation of green fluorescence between 1 h and 2 h indicates the quickly uptake of the vectors. Scattered yellow dots inside of the cells was the overlapping of the nanoparticle green fluorescence and the lysosome-tracker red fluorescence suggesting entering of the nanoparticles in lysosome (Fig. 6D). Relatively fast escape of CQ-LPs from the lysosomes after 2 h was proved by the enhanced cytoplasmic green fluorescence, which was probably attributed to the endo-lysosome inhibition effect of CQ.(C [42]. It has also been reported previously that BMSCs has a strong phagocytic ability on nanoparticles. [43]; X [14].
Although CQ/TAM further boosted the anti-inflammation effect of the MSCs, inhibition of the MSCs proliferation by CQ/TAM combination was also observed. And the drug-loaded nanoparticles dramatically alleviated inhibition of the BMSCs proliferation relative to the free drugs especially at high concentrations according to the MTT assay (Fig. 6F). The survival rate of UMSCs after treatment of CQ-LPs (40 μM) and TAM-NPs (20 μM) was around 90%, which was significantly higher than that of free drug combination group (p < 0.05). The detoxification effect produced by the nanoparticles was still interesting and could be one of the reasons for the improvement in intracellular molecular action of the drugs. CQ-LPs and TAM-NPs used singly or jointly induced higher iNOS expression on BMSCs in vitro (Fig. 6E).
To further investigate the superiority of the nanoparticle combination, intracellular molecular regulation effect after endocytosis of the free drugs and the nanoparticles was studied. JAK/STAT is the canonical pathway that has been initially discovered to regulate IFNγ-triggered IFN stimulating gene (ISG) transcription and/or translation, followed by some non-canonical pathways discovered recently that constitute the mechanisms for interferon response. These pathways include Akt/mTOR pathways, ULK1 pathways, MAP kinase pathways, NF-κB pathways etc. [44] By screening the key molecule activation in canonical and non-canonical pathway including STAT1, Akt, ULK, ERK, p65 (Fig. 6G), phosphorylation of Akt and increase of STAT1 expression were detected after IFN-γ stimulation. There have been several studies about activation of phosphatidylinositol-3-kinase (PI3k) and its down-stream mediators including Akt/mTOR by IFNs [45]. And there are also some previous reports about positive regulation of Akt signaling pathway by both TAM and CQ [[15], [16], [17],19]. In this study, it was confirmed that both CQ and TAM promoted the phosphorylation of Akt instead of activating STAT1 signaling (Figure S8). Combination of IFN-γ and CQ/TAM synergistically enhanced Akt activation and up-regulated STAT1 expression. It is noteworthy that the nanoparticle combination group stimulated the IFN-γ signaling much better than the free drug combination group did, corroborated by the increase of Akt phosphorylation and STAT1 expression as well as the decrease of Akt protein level. It is speculated that rapid drug uptake and sustained drug release introduced by the nanoparticles facilitated the efficient intracellular transportation and persistent drug efficacy [46]; X [36]. (Fig. 6H).
Above all, nanoparticle encapsulated CQ/TAM is superior to the free drug combination for pretreatment of BMSCs in toxicity control and efficacy improvement.
4. Discussion
Among the key immune regulators, TSG-6 has been proved to be very important for MSC intervention of AP and SAP, while other molecules are rarely reported.(Z [47]. By using RNA interference, iNOS inhibitor and analysis of PIL, we confirmed the detrimental role of iNOS for intervention of immune cell recruitment by MSCs in AP. However, the exact signaling pathway involved needs further validation. Probably, NO production catalyzed by iNOS suppresses STAT5 phosphorylation of JAK-STAT signaling pathway and consequently leads to T-cell proliferation arrest [48]. It could be nitration of tyrosine residues in IRF5 by NO that suppresses M1 macrophage polarization and results in less severe inflammation.(G [28]. And NO-dependent regulation of CXCR2 on the neutrophil surface might be associated with failure of neutrophil migration to pancreas in AP mice with MSCs pretreatment [29]. The in-depth study of the mechanism will be carried out in the follow-up study.It has been reported that MSCs IDO activity is implicated in promoting the differentiation of monocytes into immunosuppressive M2-type macrophages, which will consequently suppress T cell proliferation and amplify the immunosuppressive effect generated by MSCs. [49]; J. G [50]. The increase of IDO stimulated by IFN-γ can inhibit the proliferation of T cells and induce the increase of T-reg.[[51], [52]] [[,52].
The interferons (IFNs) have been conserved through evolution as elements with very important immune modulatory effect. The binding of IFNs to their receptors would trigger the transcription and/or translation of several IFN-stimulating genes (ISGs), which attributes to very important biological process like cell cycle progression, proliferation, apoptosis, differentiation, migration and survival [53,54]. And iNOS and IDO are IFN-responsive genes. JAK/STAT is the canonical pathway that has been initially discovered, followed by some non-canonical pathways discovered recently that constitute the mechanisms for interferon response (Figure S8). Both the canonical pathway and the non-canonical pathway are important for interferon responses, and these pathways might be activated in parallel [55]. Our study reveals that some non-canonical pathway activators, without interferon signal activation ability themselves, would markedly promote IFN-γ related signaling. Based on this research, our follow-up study will focus on how the connection between the two pathways happens, which would provide basis for development of therapeutic approaches incorporating IFNs and adjuvants.Free compounds are usually used for pretreating MSCs as reported previously.(G [14]. However, in the light of our experiments, the inhibition of MSCs proliferation by both TAM and CQ is very significant especially at high concentrations. To overcome this drawback, nanoparticles or liposomes encapsulated drugs were used instead, which perfectly avoided the problem mentioned above. We infer that nanoparticle encapsulation reduced instantaneous exposure of MSC to drugs and thus avoid the toxicity of the drugs. In addition to this protection mechanism, it may also be a compensation mechanism. It has been reported that gold nanoparticles could promote MSCs proliferation as osteoblast through up-regulation of proliferation inducer expression like TGF-β and Smad3.(J. J. [40]; S [56].
In conclusion, the main findings of this study are as follows. Firstly, BMSCs ameliorated AP severity by decreasing pancreatic recruitment of inflammatory cells including neutrophils, macrophages as well as CD4+T cells. Then the key molecule that reduced the recruitment of these cells turned out to be iNOS. Moreover, CQ in combination with TAM synergistically promoted iNOS/IDO expression in MSCs and enhanced its intervention in AP and SAP. Finally, MSCs with the pretreatment of the drug-loaded nanoparticle combination (CQ-LPs/TAM-NPs) dramatically enhanced the therapeutic effect of MSCs and provides essential insights to devise alternative regimens for AP and SAP. (see Table 1)
Table 1.
Primer sequences.
| TARGET | FORWARD PRIMER | REVERSE PRIMER |
|---|---|---|
| RACTIN | AGCCATGTACGTAGCCATCC | CTCTCAGCTGTGGTGGTGAA |
| RINOS | GGAGAGATTTTTCACGACACCC | CCATGCATAATTTGGACTTGCA |
| RIDO | TGGTGGGGACTGCGATAAAG | CTTCTCCAGTGCTTTCGGGT |
| RCOX2 | CTCTGCGATGCTCTTCCGAG | AAGGATTTGCTGCATGGCAG |
| RHO-1 | CGACAGCATGTCCCAGGATT | TCGCTCTATCTCCTCTTCCAGG |
| RIL-6 | TCCTACCCCAACTTCCAATGCTC | TTGGATGGTCTTGGTCCTTAGCC |
| RPD-L1 | TTATAGTCACAGCCTGCAGTCACG | ATCGTGACATTGCTGCCATACTC |
| RTGF-Β | CCTGCAAGACCATCGACATG | ACAGGATCTGGCCACGGAT |
| HIDO | TTGCTAAAGGCGCTGTTGGA | GTCTGATAGCTGGGGGTTGC |
| R-SI-INOS | GGUCAAAGACAAGAGGCUUTT | AAGCCUCUUGUCUUUGACCTT |
| MACTIN | CGTGAAAAGATGACCCAGATCA | CACAGCCTGGATGGCTACGT |
| MFASL | GACAATGCAGAGGCACAGAGAA | CCTCTGTGAGGTAGTAAGTAGA |
| MIL-1Β | AAGCCTCGTGCTGTCGGACC | TGAGGCCCAAGGCCACAGGT |
| MIL-6 | CACAGAGGATACCACTCCCAACA | TCCACGATTTCCCAGAGAACA |
| MTNFΑ | CATCTTCTCAAAATTCGAGTGACAA | CCAGCTGCTCCTCCACTTG |
Author contribution statement
Yu Zheng was in charge of the conceptualization, supervision and project administration. Yu Zheng,Huimin Lu and Weiming Hu were responsible for the funding acquisition together. Huimin Liu, Simeng Liu and Xiaoshuang Song completed methodology and investigation. Ailing Jiang, Yu Zou, Yuchuan Deng, Zhenlu Li, Dujiang Yang, and Chao Yue fulfilled validation and formal analysis. Dan Sun, Chengli Yang, Mao Li, Fan Yang and Kun Jiang finished data curation, visualization and writing. All authors read and approved the final manuscript.
Data availability statement
All the raw data to reproduce these findings are available.
Funding statement
This work was supported by Science and Technology Project of Sichuan Province (21YYJC2789); Sichuan Provincial Department of Science and Technology Supporting Project (2018SZ0381); Sichuan Science and Technology Program (2021YFS0234); 1.3.5 Project for Disciplines of Excellence of West China Hospital, Sichuan University (ZYJC18027).
Ethics approval statement
All animal experiments involved in the present study were approved by the Animal Care and Use Committee of Sichuan University (20190509023, Chengdu, Sichuan, China).
Ethic statement
All human and animal experiments conducted were compliant with Ethics Committee of Sichuan University.
Data availability statement
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by Science and Technology Project of Sichuan Province (21YYJC2789); Sichuan Provincial Department of Science and Technology Supporting Project (2018SZ0381); Sichuan Science and Technology Program (2021YFS0234); 1.3.5 Project for Disciplines of Excellence of West China Hospital, Sichuan University (ZYJC18027).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mtbio.2022.100226.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Barreto S.G., Habtezion A. vol. 70. 2021. pp. 194–203. (Critical Thresholds: Key to Unlocking the Door to the Prevention and Specific Treatments for Acute Pancreatitis). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moggia E., Koti R., Belgaumkar A.P., Fazio F., Pereira S.P., Davidson B.R., Gurusamy K.S. Pharmacological interventions for acute pancreatitis. Cochrane Database Syst. Rev. 2017;4:Cd011384. doi: 10.1002/14651858.CD011384.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hines O.J., Pandol S.J. Management of severe acute pancreatitis. Bmj. 2019;367:l6227. doi: 10.1136/bmj.l6227. [DOI] [PubMed] [Google Scholar]
- 4.Shi Y., Wang Y., Li Q., Liu K., Hou J., Shao C., Wang Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat. Rev. Nephrol. 2018;14:493–507. doi: 10.1038/s41581-018-0023-5. [DOI] [PubMed] [Google Scholar]
- 5.Ellis C., Ramzy A., Kieffer T.J. Regenerative medicine and cell-based approaches to restore pancreatic function. Nat. Rev. Gastroenterol. Hepatol. 2017;14:612–628. doi: 10.1038/nrgastro.2017.93. [DOI] [PubMed] [Google Scholar]
- 6.Jung K.H., Song S.U., Yi T., Jeon M.S., Hong S.W., Zheng H.M., Lee H.S., Choi M.J., Lee D.H., Hong S.S. Human bone marrow-derived clonal mesenchymal stem cells inhibit inflammation and reduce acute pancreatitis in rats. Gastroenterology. 2011;140:998–1008. doi: 10.1053/j.gastro.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 7.Yang J., Su J., Xi S.S., Ke X.F., Zhu Y., Lin H.P., Zeng X.K., Liu B.W., Zhu M.L., Dai W.Y., Hu W. Human umbilical cord mesenchymal stem cells pretreated with Angiotensin-II attenuate pancreas injury of rats with severe acute pancreatitis. Biomed. Pharmacother. 2019;117:109052. doi: 10.1016/j.biopha.2019.109052. [DOI] [PubMed] [Google Scholar]
- 8.Li L., Zhang Y., Mu J., Chen J., Zhang C., Cao H., Gao J. vol. 20. 2020. pp. 4298–4305. (Transplantation of Human Mesenchymal Stem-Cell-Derived Exosomes Immobilized in an Adhesive Hydrogel for Effective Treatment of Spinal Cord Injury). [DOI] [PubMed] [Google Scholar]
- 9.Yu C., Ding S. Therapeutic strategies targeting somatic stem cells: chemical approaches. Bioorg. Med. Chem. 2020;28:115824. doi: 10.1016/j.bmc.2020.115824. [DOI] [PubMed] [Google Scholar]
- 10.Ren G., Zhang L., Zhao X., Xu G., Zhang Y., Roberts A.I., Zhao R.C., Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Abdolmohammadi K., Mahmoudi T., Nojehdehi S., Tayebi L., Hashemi S.M. vol. 10. 2020. pp. 297–306. (Effect of Hypoxia Preconditioned Adipose-Derived Mesenchymal Stem Cell Conditioned Medium on Cerulein-Induced Acute Pancreatitis in Mice). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vigo T., Procaccini C., Ferrara G., Baranzini S., Oksenberg J.R., Matarese G., Diaspro A., Kerlero de Rosbo N., Uccelli A. IFN-γ orchestrates mesenchymal stem cell plasticity through the signal transducer and activator of transcription 1 and 3 and mammalian target of rapamycin pathways. J. Allergy Clin. Immunol. 2017;139:1667–1676. doi: 10.1016/j.jaci.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Rossi F., Noren H., Sarria L., Schiller P.C., Nathanson L., Beljanski V. vol. 10. 2019. p. 395. (Combination Therapies Enhance Immunoregulatory Properties of MIAMI Cells). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang G., Cao K., Liu K., Xue Y., Roberts A.I., Li F., Han Y., Rabson A.B., Wang Y., Shi Y. vol. 25. 2018. pp. 1209–1223. (Kynurenic Acid, an Ido Metabolite, Controls TSG-6-Mediated Immunosuppression of Human Mesenchymal Stem Cells). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietze E.C., Troch M.M., Bean G.R., Heffner J.B., Bowie M.L., Rosenberg P., Ratliff B., Seewaldt V.L. Tamoxifen and tamoxifen ethyl bromide induce apoptosis in acutely damaged mammary epithelial cells through modulation of AKT activity. Oncogene. 2004;23:3851–3862. doi: 10.1038/sj.onc.1207480. [DOI] [PubMed] [Google Scholar]
- 16.Halaby M.J., Kastein B.K., Yang D.Q. Chloroquine stimulates glucose uptake and glycogen synthase in muscle cells through activation of Akt. Biochem. Biophys. Res. Commun. 2013;435:708–713. doi: 10.1016/j.bbrc.2013.05.047. [DOI] [PubMed] [Google Scholar]
- 17.Hamadneh L., Abuarqoub R., Alhusban A., Bahader M. Upregulation of PI3K/AKT/PTEN pathway is correlated with glucose and glutamine metabolic dysfunction during tamoxifen resistance development in MCF-7 cells. Sci. Rep. 2020;10:21933. doi: 10.1038/s41598-020-78833-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGlynn L.M., Kirkegaard T., Edwards J., Tovey S., Cameron D., Twelves C., Bartlett J.M., Cooke T.G. Ras/Raf-1/MAPK pathway mediates response to tamoxifen but not chemotherapy in breast cancer patients. Clin. Cancer Res. 2009;15:1487–1495. doi: 10.1158/1078-0432.CCR-07-4967. [DOI] [PubMed] [Google Scholar]
- 19.Spears L.D., Tran A.V., Qin C.Y., Hobbs S.B., Burns C.A., Royer N.K., Zhang Z., Ralston L., Fisher J.S. Chloroquine increases phosphorylation of AMPK and Akt in myotubes. Heliyon. 2016;2 doi: 10.1016/j.heliyon.2016.e00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H., Xu G.W., Wang Y.F., Zhao H.S., Xiong S., Wu Y., Heng B.C., An C.R., Zhu G.H., Xie D.H. Composite scaffolds of nano-hydroxyapatite and silk fibroin enhance mesenchymal stem cell-based bone regeneration via the interleukin 1 alpha autocrine/paracrine signaling loop. Biomaterials. 2015;49:103–112. doi: 10.1016/j.biomaterials.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Palestino-Dominguez M., Pelaez-Luna M., Lazzarini-Lechuga R., Rodriguez-Ochoa I., Souza V., Miranda R.U., Perez-Aguilar B., Bucio L., Marquardt J.U., Gomez-Quiroz L.E., Gutierrez-Ruiz M.C. vol. 233. 2018. pp. 9354–9364. (Recombinant Human Hepatocyte Growth Factor Provides Protective Effects in Cerulein-Induced Acute Pancreatitis in Mice). [DOI] [PubMed] [Google Scholar]
- 22.Kusske A.M., Rongione A.J., Reber H.A. Cytokines and acute pancreatitis. Gastroenterology. 1996;110:639–642. doi: 10.1053/gast.1996.v110.agast960639. [DOI] [PubMed] [Google Scholar]
- 23.Lu F., Wang F., Chen Z., Huang H. Effect of mesenchymal stem cells on small intestinal injury in a rat model of acute necrotizing pancreatitis. Stem Cell Res. Ther. 2017;8:12. doi: 10.1186/s13287-017-0471-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao H., He Z. 2016. Infusion of Bone Marrow Mesenchymal Stem Cells Attenuates Experimental Severe Acute Pancreatitis in Rats; p. 7174319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demols A., Le Moine O., Desalle F., Quertinmont E., Van Laethem J.L., Devière J. CD4(+)T cells play an important role in acute experimental pancreatitis in mice. Gastroenterology. 2000;118:582–590. doi: 10.1016/s0016-5085(00)70265-4. [DOI] [PubMed] [Google Scholar]
- 26.Saeki K., Kanai T., Nakano M., Nakamura Y., Miyata N., Sujino T., Yamagishi Y., Ebinuma H., Takaishi H., Ono Y., Takeda K., Hozawa S., Yoshimura A., Hibi T. CCL2-induced migration and SOCS3-mediated activation of macrophages are involved in cerulein-induced pancreatitis in mice. Gastroenterology. 2012;142:1010–1020. doi: 10.1053/j.gastro.2011.12.054. e1019. [DOI] [PubMed] [Google Scholar]
- 27.García-Ortiz A., Serrador J.M. Nitric oxide signaling in T cell-mediated immunity. Trends Mol. Med. 2018;24:412–427. doi: 10.1016/j.molmed.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Lu G., Zhang R., Geng S., Peng L., Jayaraman P., Chen C., Xu F., Yang J., Li Q., Zheng H., Shen K., Wang J., Liu X., Wang W., Zheng Z., Qi C.F., Si C., He J.C., Liu K., Lira S.A., Sikora A.G., Li L. vol. 6. 2015. p. 6676. (Myeloid Cell-Derived Inducible Nitric Oxide Synthase Suppresses M1 Macrophage Polarization). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rios-Santos F., Alves-Filho J.C., Souto F.O., Spiller F., Freitas A., Lotufo C.M., Soares M.B., Dos Santos R.R., Teixeira M.M., Cunha F.Q. Down-regulation of CXCR2 on neutrophils in severe sepsis is mediated by inducible nitric oxide synthase-derived nitric oxide. Am. J. Respir. Crit. Care Med. 2007;175:490–497. doi: 10.1164/rccm.200601-103OC. [DOI] [PubMed] [Google Scholar]
- 30.Zhou H.F., Yan H., Hu Y., Springer L.E., Yang X., Wickline S.A., Pan D., Lanza G.M., Pham C.T. Fumagillin prodrug nanotherapy suppresses macrophage inflammatory response via endothelial nitric oxide. ACS Nano. 2014;8:7305–7317. doi: 10.1021/nn502372n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sotiropoulou P.A., Perez S.A., Gritzapis A.D., Baxevanis C.N., Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cell. 2006;24:74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 32.Weiss A.R.R., Dahlke M.H. Immunomodulation by mesenchymal stem cells (MSCs): mechanisms of action of living, apoptotic, and dead MSCs. Front. Immunol. 2019;10:1191. doi: 10.3389/fimmu.2019.01191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su J., Chen X., Huang Y., Li W., Li J., Cao K., Cao G., Zhang L., Li F., Roberts A.I., Kang H., Yu P., Ren G., Ji W., Wang Y., Shi Y. Phylogenetic distinction of iNOS and Ido function in mesenchymal stem cell-mediated immunosuppression in mammalian species. Cell Death Differ. 2014;21:388–396. doi: 10.1038/cdd.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding D.C., Chang Y.H., Shyu W.C., Lin S.Z. Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant. 2015;24:339–347. doi: 10.3727/096368915X686841. [DOI] [PubMed] [Google Scholar]
- 35.Kimura Y., Hirota M., Okabe A., Inoue K., Kuwata K., Ohmuraya M., Ogawa M. Dynamic aspects of granulocyte activation in rat severe acute pancreatitis. Pancreas. 2003;27:127–132. doi: 10.1097/00006676-200308000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Zhou X., Liu Z., Cheng X., Zheng Y., Zeng F., He Y. Socs1 and Socs3 degrades Traf6 via polyubiquitination in LPS-induced acute necrotizing pancreatitis. Cell Death Dis. 2015;6 doi: 10.1038/cddis.2015.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saluja A.K., Lerch M.M., Phillips P.A., Dudeja V. Why does pancreatic overstimulation cause pancreatitis? Annu. Rev. Physiol. 2007;69:249–269. doi: 10.1146/annurev.physiol.69.031905.161253. [DOI] [PubMed] [Google Scholar]
- 38.Corradetti B., Ferrari M. Nanotechnology for mesenchymal stem cell therapies. J. Contr. Release. 2016;240:242–250. doi: 10.1016/j.jconrel.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 39.Jeon S.Y., Park J.S., Yang H.N., Lim H.J., Yi S.W., Park H., Park K.H. Co-delivery of Cbfa-1-targeting siRNA and SOX9 protein using PLGA nanoparticles to induce chondrogenesis of human mesenchymal stem cells. Biomaterials. 2014;35:8236–8248. doi: 10.1016/j.biomaterials.2014.05.092. [DOI] [PubMed] [Google Scholar]
- 40.Li J.J., Kawazoe N., Chen G. Gold nanoparticles with different charge and moiety induce differential cell response on mesenchymal stem cell osteogenesis. Biomaterials. 2015;54:226–236. doi: 10.1016/j.biomaterials.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 41.Zhang T.Y., Huang B., Wu H.B., Wu J.H., Li L.M., Li Y.X., Hu Y.L., Han M., Shen Y.Q., Tabata Y., Gao J.Q. Synergistic effects of co-administration of suicide gene expressing mesenchymal stem cells and prodrug-encapsulated liposome on aggressive lung melanoma metastases in mice. J. Contr. Release. 2015;209:260–271. doi: 10.1016/j.jconrel.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Yang C., Hu T., Cao H., Zhang L., Zhou P., He G., Song X., Tong A., Guo G., Yang F., Zhang X., Qian Z., Qi X., Zhou L., Zheng Y. Facile construction of chloroquine containing PLGA-based pDNA delivery system for efficient tumor and pancreatitis targeting in vitro and in vivo. Mol. Pharm. 2015;12:2167–2179. doi: 10.1021/acs.molpharmaceut.5b00155. [DOI] [PubMed] [Google Scholar]
- 43.Wang X., Chen H., Zeng X., Guo W., Jin Y., Wang S., Tian R., Han Y., Guo L., Han J., Wu Y., Mei L. Efficient lung cancer-targeted drug delivery via a nanoparticle/MSC system. Acta Pharm. Sin. B. 2019;9:167–176. doi: 10.1016/j.apsb.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kroczynska B., Mehrotra S., Arslan A.D., Kaur S., Platanias L.C. Regulation of interferon-dependent mRNA translation of target genes. J. Interferon Cytokine Res. 2014;34:289–296. doi: 10.1089/jir.2013.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goncharova E.A., Lim P.N., Chisolm A., Fogle H.W., 3rd, Taylor J.H., Goncharov D.A., Eszterhas A., Panettieri R.A., Jr., Krymskaya V.P. Interferons modulate mitogen-induced protein synthesis in airway smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 2010;299:L25–L35. doi: 10.1152/ajplung.00228.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Irvine D.J., Dane E.L. vol. 20. 2020. pp. 321–334. (Enhancing Cancer Immunotherapy with Nanomedicine). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He Z., Hua J., Qian D., Gong J., Lin S., Xu C., Wei G., Meng H., Yang T., Zhou B., Song Z. Intravenous hMSCs ameliorate acute pancreatitis in mice via secretion of tumor necrosis factor-α stimulated gene/protein 6. Sci. Rep. 2016;6:38438. doi: 10.1038/srep38438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sato K., Ozaki K., Oh I., Meguro A., Hatanaka K., Nagai T., Muroi K., Ozawa K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109:228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 49.François M., Romieu-Mourez R., Li M., Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol. Ther. 2012;20:187–195. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]
- 50.He J.G., Li B.B., Zhou L., Yan D., Xie Q.L., Zhao W. vol. 68. 2020. pp. 728–737. (Indoleamine 2,3-Dioxgenase-Transfected Mesenchymal Stem Cells Suppress Heart Allograft Rejection by Increasing the Production and Activity of Dendritic Cells and Regulatory T Cells). [DOI] [PubMed] [Google Scholar]
- 51.Ling W., Zhang J., Yuan Z., Ren G., Zhang L., Chen X., Rabson A.B., Roberts A.I., Wang Y., Shi Y. Mesenchymal stem cells use Ido to regulate immunity in tumor microenvironment. Cancer Res. 2014;74:1576–1587. doi: 10.1158/0008-5472.CAN-13-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meisel R., Zibert A., Laryea M., Göbel U., Däubener W., Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 53.Sacks D., Baxter B., Campbell B.C.V., Carpenter J.S., Cognard C., Dippel D., Eesa M., Fischer U., Hausegger K., Hirsch J.A., Shazam Hussain M., Jansen O., Jayaraman M.V., Khalessi A.A., Kluck B.W., Lavine S., Meyers P.M., Ramee S., Rüfenacht D.A., Schirmer C.M., Vorwerk D. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int. J. Stroke. 2018;13:612–632. doi: 10.1177/1747493018778713. [DOI] [PubMed] [Google Scholar]
- 54.Saleiro D., Platanias L.C. Interferon signaling in cancer. Non-canonical pathways and control of intracellular immune checkpoints. Semin. Immunol. 2019;43:101299. doi: 10.1016/j.smim.2019.101299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazewski C., Perez R.E., Fish E.N., Platanias L.C. Type I interferon (IFN)-Regulated activation of canonical and non-canonical signaling pathways. Front. Immunol. 2020;11:606456. doi: 10.3389/fimmu.2020.606456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou S., Pan Y., Zhang J., Li Y., Neumann F., Schwerdtle T., Li W., Haag R. Dendritic polyglycerol-conjugated gold nanostars with different densities of functional groups to regulate osteogenesis in human mesenchymal stem cells. Nanoscale. 2020;12:24006–24019. doi: 10.1039/d0nr06570f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the raw data to reproduce these findings are available.
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.