Abstract
This study was conducted with the objective of finding alternative management options for potato tuber sprouting during storage. Essential oils from Croton macrostachyus, Eucalyptus globulus, Allium sativum, Cymbopogon citratus, Cymbopogon martini, Rosmarinus officinalis and Thymus schimperi were applied in completely randomized design using two quantities (1 and 2 ml) and three frequencies (1, 2, 3). Essential oils from Cymbopogon citratus and Thymus schimperi showed the lowest percentage of weight loss relative to control tubers for varieties Gudene (8.07%) and Jalene (13.34%), respectively. Essential oils were applied in the form of vapour inside wooden box for 24 h without direct contact with tubers. For both varieties, the potato tuber weight loss found to be minimized using 2 ml essential oils. Similarly, lowest percentage of weight loss at one, two and three applications were found for variety Jalene from Cymbopogon martini, Eucalyptus globulus and Croton macrostachyus, respectively. On the other hand, lowest percentage of weight loss was maintained using Eucalyptus globulus for variety Gudene. The number and length of sprouts did not vary with quantities. Therefore, for variety Gudene, lower number of sprouts relative to control tuber (3 sprouts/tuber) was found on potato tuber treated with essential oils from Cymbopogon martini and Thymus schimperi (2.7 sprouts/tuber) whereas for variety Jalene, lower number of sprouts relative to control tuber (8.7 sprouts/tubers) was found on potato tubers treated with essential oils from Allium sativum and Rosmarinus officinalis (5.7 sprouts/tuber). The length of the longest sprouts on control tubers was 11.7 mm and 20 mm for varieties Gudene and Jalene, respectively. Longest and shortest length of sprout was found on potato tubers treated with Eucalyptus globulus and Rosmarinus officinalis, respectively, for both varieties. Therefore, essential oils used in the current study are promising alternatives to control potato sprouting during storage. However, seedlings of these species and other potential species must be made available to farmers with training on how to make extracts and apply them.
Keywords: Alternative management, Gudene, Jalene, Quantities, Frequencies, Weight loss
Alternative management; Gudene; Jalene; Quantities; Frequencies; Weight loss.
1. Introduction
Potato (Solanum tuberosum L.) is a major crop which enables smallholder farmers to achieve food security and household income generation in Ethiopia. The demand for potato tubers has been increased due to change in dietary habits following urbanization since part of potato produced continued to be used for production of potato for processing, chips, seed and fresh consumption (Tesfaye et al., 2010; Bond 2014). According to FAOSTAT 2020, around 743, 153 tons of potatoes were produced in 2018 in which the major part was used for consumption or sold for fresh consumption as well as a source of seed. Naturally, at harvest, potato tubers are in a state of endo-dormancy which cannot sprout even under suitable environmental conditions (Sonnewald and Sonnewald, 2014). Onsets of sprout are accompanied by biochemical changes following the release of dormancy (Campbell et al., 2008). Therefore, the importance of potato tuber sprouting depends on its final usage as fresh consumption, processing or use as seed.
Sprouted seed tubers with high growth vigour are not always available at each planting time due to dormancy. Consequently, the yield of potato is many times compromised due to delay in sprouting of seed potato tubers in the areas that have the potential to grow in two or more growing seasons per year (Teper-Bamnolker et al., 2010). To overcome these delays, some chemical enhancers like ethylene (Gamble et al., 2002), chlorohydrins, 1,2-dichloroethane and carbon tetrachloride are used extensively by seed producers to break potato seed tuber dormancy and to promote sprouting (Sharma, 2012). Moreover, increasing the humidity of the storage atmosphere and temperature variation (Fauconnier et al., 2002), application of thiourea (Perez and Lira, 2005), hydrogen peroxide (Bajji et al., 2007), nitrate (Bethke et al., 2007), storage methods (diffused light storage), damaging the tuber are used to induce sprouting. In contrast to potato tubers used for seed, sprouting causes softening, shrinkage, formation of toxic alkaloids and consequently leads to changes at nutritional and texture level for potato tubers used for fresh consumption and processing (Sorce et al., 2005). It also mobilizes starch and consumes a portion of the tubers biochemical reserves to grow sprout tissue which leads to elevated sugar content, weight loss and wilting of the tubers (Daniels-Lake and Prange, 2007). In turn, it results in reduction of the number of marketable tubers causing significant economic losses (Sonnewald and Sonnewald, 2014). Hence, for potato tubers intended for fresh consumption and processing, inhibition of sprout growth is critical for the maintenance of tuber nutrition and processing qualities during long-term storage (Mani et al., 2014). Among different methods to inhibit sprouting during storage, the most widely used sprout suppressants are isopropyl N-phenyl-carbamate (propham or IPC), isopropyl (N-3-chlorophenyl) carbamate (chloropham or CIPC) (Nurit et al., 1989), maleic hydazide (Weiss et al., 1980) and tecnazene (Dalziel and Duncan, 1980). Moreover, sprouting can be inhibited through low temperature storage (3–7 °C), but it results in undesirable tissue sweetening (Alamar et al., 2017). Temperature management could be useful for potato sprouting control during storage; however, it has its own limitations. On the one hand, storage of potato tubers at low temperature (2–4 °C) to inhibit sprouting induces cold-sweetening which results in substandard quality for potato processing industry (Sowokinos, 2007) since it results in dark colour during frying (Frazier et al., 2004). On the other hand, storage of potato tubers at higher temperature (15–20 °C) to enhance sprouting will shorten dormancy periods which in turn results in development of sprouts, leading to weight loss and quality reduction. Therefore, for quality of specific potato variety, its dormancy and sprouting behaviour are the major criteria that should be documented before releasing any potato variety for production (Virtanen et al., 2013).
Different methods have been used for management of sprouting in different parts of the world, but all have their own limitations. Firstly, the demand for more organic and chemical-free foods is increasing (Statista, 2018) since studies which described toxic and carcinogenic properties reported (Balaji et al., 2006; Sihtmae et al., 2010; Smith and Bucher, 2012; El-Awady et al., 2014; Mohammed et al., 2015). Secondly, using different temperatures during storage showed limitations as well. For these reasons, researchers continued searching technologies that control potato tuber sprouts during storage. Finger et al. (2018) reported that some natural components like eugenol, menthol can reduce the number of sprouts in refrigerated conditions. Eucalyptus (Eucalyptus globulus, Labill.) and coriander (Coriandrum sativum, L.) essential oil inhibit the sprouting of potato tuber in storage condition at 25 °C for 10 days (Gomez-Castillo et al., 2013). Moreover, S-carvone has been reported to inhibit sprouting in storage condition (4 °C) for 90 days (Xie et al., 2018). Therefore, essential oils have been mentioned as a potential alternative to control sprouting, however, there is no sufficient information about its doses and frequencies as well as ease of application to use at smallholder famers level in different parts of the world including Ethiopia. Studies related to essential oils in potatoes have been reported by different authors (Kleinkopf et al., 2003; Frazier et al., 2004; Costa et al., 2007; Gomez-Castillo et al., 2013; Suttle et al., 2016; Shukla et al., 2019).
In Ethiopia, farmers used to control sprouting using diffused light storage, dark storage and keeping potato tubers at home or in the ground until planting (Daniel et al., 2021). Storage of potato tubers on the floor, sacks or baskets result in poor and usually long sprouts that breaks easily during transportation and/or planting (Elmar, 2013). Gibberellic acid has been used for research purpose only to induce sprouting (Gemeda et al., 2017). Even if different authors reported the use of essential oils to control potato sprouting, there is insufficient information in Ethiopia about the use of essential oils to control potato sprouting during storage. Therefore, this study was conducted to see the effect of essential oils on sprouting ability of two potato varieties (Gudene and Jalene) with the objective of finding alternative safe natural products that would be effective for storage of potato tubers.
2. Materials and methods
2.1. Experimental materials
The potato tubers of cultivars Gudene (CIP-386423.13) and Jalene (CIP-384321.19) were obtained from Holleta Agricultural Research Center, Ethiopia. The plant material for extraction of essential oils was collected from Ethio-AgriCEFT farms located at Holleta and West Gojam (Liebir farm). Leaves of rosemary (Rosmarinus officinalis) and thymus (Thymus schimperi) were collected from Ethio-Agriceft farm at Holleta while leaves of croton (Croton macrostachyus) and eucalyptus (Eucalyptus globulus) were collected near Holleta EthioAgriCEFT farm. Moreover, garlic (Allium sativum) bulbs were obtained from farmers at Holleta while leaves of lemon grass (Cymbopogon citratus) and palmarosa (Cymbopogon martini) were obtained from Liebir farm. Plants for sources of essential oil were selected based on the knowledge of their ethnobotanical uses and their previously demonstrated antioxidant properties (Mahmoudi et al., 2019; Pandey et al., 2019; Mohammed et al., 2020). The identities of these species were authenticated at the National Herbarium of Ethiopia using the taxonomic literature, authenticated specimens and through assistance of taxonomic experts.
Essential oils were extracted using the methods of hydrodistillation for 3 h from fresh leaves except for garlic while fresh bulbs were used in the case of garlic. Hydrodistillation is a conventional method which does not use organic solvents, but uses water and steam for extraction of the essential oils (Azmir et al., 2013). In the present study the extraction was undertaken by the method of hydrodistillation using water. Then, the essential oil was collected and stored at 4 °C in the refrigerator until used. The amounts of essential oils obtained per 1000 g of fresh plant material used were 12.0 ml, 11.0 ml, 1.3 ml, 6.7 ml, 6.5 ml, 5.4 ml and 8.5 ml for croton, eucalyptus, garlic, lemongrass, palmarosa, rosemary and thymus, respectively. These amounts were different since essential oil yield varied depending on the plant materials used. The total cost (in ETB) for extraction of 250 ml from croton, eucalyptus, garlic, lemongrass, palmarosa, rosemary and thymus were 416, 568, 13461, 1119, 1153, 2083, and 1176, respectively. According to the current exchange rate, 1 $ is equivalent to 47 ETB.
2.2. Experimental design
The experiments were designed with completely randomized designed in three replications. Application of the essential oils was based on the methods used by Vaughn and Spencer (1991) and Coleman et al. (2001). Harvested potato was allowed to suberize at warm temperatures for a week and then kept for three weeks prior to essential oils applications. Similar sized-tubers of Jalene and Gudene potato varieties were arranged and essential oils were applied in the form of vapour by putting three tubers per replication inside wooden boxes (plastic sheet was used to seal the box) using filter paper for 24 h at room temperature. Therefore, three boxes were used per treatment. The dimension of the box was 10 cm length, 10 cm width and 5 cm height. The filter paper with essential oils had no direct contact with tubers. A control experiment was set up in the same conditions without application of essential oils to compare the effect of treatments. Two quantities of essential oils (1 and 2 ml) were used to study their ability to induce and/or inhibit potato tuber sprouts. Another experiment was designed to study the effects of frequencies (one, two and three) using 1 ml essential oil per seven days interval. Therefore, the total amount of essential oil used to study essential oil quantities were 63 ml (1 ml x 3 replication x 7 plant species, 2 ml x 3 replications x 7 plant species) while the total amount of essential oil used to study essential oil frequencies were 126 ml (1 ml x 3 replications x 7 plant species x one time, 1 ml x 3 replications x 7 plant species x two times, 1 ml x 3 replications x 7 plant species x three times). After application of essential oils, tubers were placed at room temperature (21 °C–23 °C) and at relative humidity ranges from 75% to 80% for up to 30 weeks without application of essential oils. Effects of treatments were assessed via recording data on weight loss, number of sprouts and length of sprouts in comparison with control tubers. Furthermore, visual assessment of potato tubers’ quality was undertaken. Weight loss data were collected starting from prior to applications of essential oils and then it continued within a week interval up to 16 weeks. Weight loss was reported on a percentage of fresh weight bases using Eq. (1) as suggested by Srivastava and Tandon (1968).
| (1) |
where, initial weight refer weight prior to essential oil application, weight at ith Week refers weight at specified week.
2.3. Data analysis
The data collected were subjected to further analysis using analysis of variance (ANOVA) using SPSS software version 18.0. Types of varieties, essential oils and quantities of essential were analysed as main effects.
3. Results
Despite sources of essential oils, quantities and frequencies; weight loss showed a linear fashion throughout the storage period along with sprout development. Furthermore, effects of essential oils on the number and length of sprouts varied with potato varieties while there was no significant difference for quantities used (1 and 2 ml).
3.1. Effects of quantities of essential oils on potato tuber weight loss
Results indicated that there was a significant difference (p < 0.05) in percentage of weight loss between varieties during potato tubers storage up to 16 weeks (Table 1). Average initial weight of potato tubers prior to treatments were 54.4 g and 82.6 g for varieties Gudene and Jalene, respectively. The percentages of weight loss varied with sources of essential oils and varieties at 2 ml. For Gudene variety, the calculated percentages of weight losses for potato tubers treated with essential oils ranges from 8.07% up to 11.5% while it was 9.12% on untreated tuber (Table 2). Less percentage of weight loss relative to control was found on potato tubers treated with essential oils from lemongrass (8.07%) followed by eucalyptus (8.57%), garlic (8.77%) and thymus (8.95%). However, higher percentage of weight loss relative to control tubers was found on potato tubers treated with essential oils from croton (11.5%) followed by rosemary (9.97%) and palmarosa (9.40%). For Jalene variety, the calculated weight losses for potato tubers treated with essential oils ranged from 13.34% up to 37.49% while it was 15.67% for untreated potato tubers (Table 2). Less percentage of weight loss relative to control was found on potato tubers treated with essential oils from thymus (13.34%). However, higher percentage of weight loss relative to control was found on potato tubers treated with essential oils from garlic (37.49%) followed by croton (27.78%) and rosemary (23.07%). Therefore, percentage of weight loss showed linear trend as the days for storage increased with higher percentage of weight loss found on Jalene variety at both quantities. However, potato tubers treated with lemongrass and thymus showed lowest percentage of weight loss up to 16 weeks of storage for varieties Gudene and Jalene, respectively.
Table 1.
Tests of Between-Subjects Effects (Dependent Variable weight loss at week 16).
| Source | Type III Sum of Squares | df | Mean Square | F | Sig. |
|---|---|---|---|---|---|
| Corrected Model | 3825.494a | 9 | 425.055 | 3.142 | .014 |
| Intercept | 105369.461 | 1 | 105369.461 | 778.862 | .000 |
| Source of Essential oils | 1245.777 | 7 | 177.968 | 1.315 | .289 |
| Varieties | 2388.836 | 1 | 2388.836 | 17.658 | .000 |
| Quantity | 190.881 | 1 | 190.881 | 1.411 | .248 |
| Error | 2976.300 | 22 | 135.286 | ||
| Total | 112171.256 | 32 | |||
| Corrected Total | 6801.794 | 31 |
R Squared = .562 (Adjusted R Squared = .383).
Table 2.
Weight loss (%) per tubers for both potato varieties using 2 ml essential oils.
| Source of Essential oils |
Gudene |
Jalene |
||||||
|---|---|---|---|---|---|---|---|---|
| Weeks during storage | ||||||||
| 4 | 8 | 12 | 16 | 4 | 8 | 12 | 16 | |
| Croton | 2.37 | 5.34 | 8.51 | 11.50 | 9.63 | 19.66 | 22.78 | 27.78 |
| Eucalyptus | 2.11 | 3.79 | 5.66 | 8.57 | 2.90 | 8.80 | 14.35 | 20.54 |
| Garlic | 2.07 | 3.82 | 5.76 | 8.77 | 5.75 | 22.72 | 30.44 | 37.49 |
| Lemongrass | 1.98 | 3.62 | 5.52 | 8.07 | 3.44 | 6.12 | 10.46 | 15.65 |
| Palmarosa | 2.35 | 4.26 | 6.32 | 9.40 | 3.95 | 7.89 | 12.34 | 17.94 |
| Rosemary | 2.57 | 4.54 | 6.69 | 9.97 | 3.63 | 15.07 | 18.79 | 23.07 |
| Thymus | 2.14 | 3.79 | 5.47 | 8.95 | 2.68 | 5.42 | 8.92 | 13.34 |
| Control | 2.07 | 3.68 | 5.68 | 9.12 | 3.15 | 6.52 | 10.39 | 15.67 |
| Mean (SE) | 2.21 0.07 |
4.11 0.21 |
6.20 0.36 |
9.29 0.37 |
4.39 0.82 |
11.53 2.38 |
16.06 2.64 |
21.44 2.82 |
The values in bold showed the lowest weight loss (%) found per potato variety.
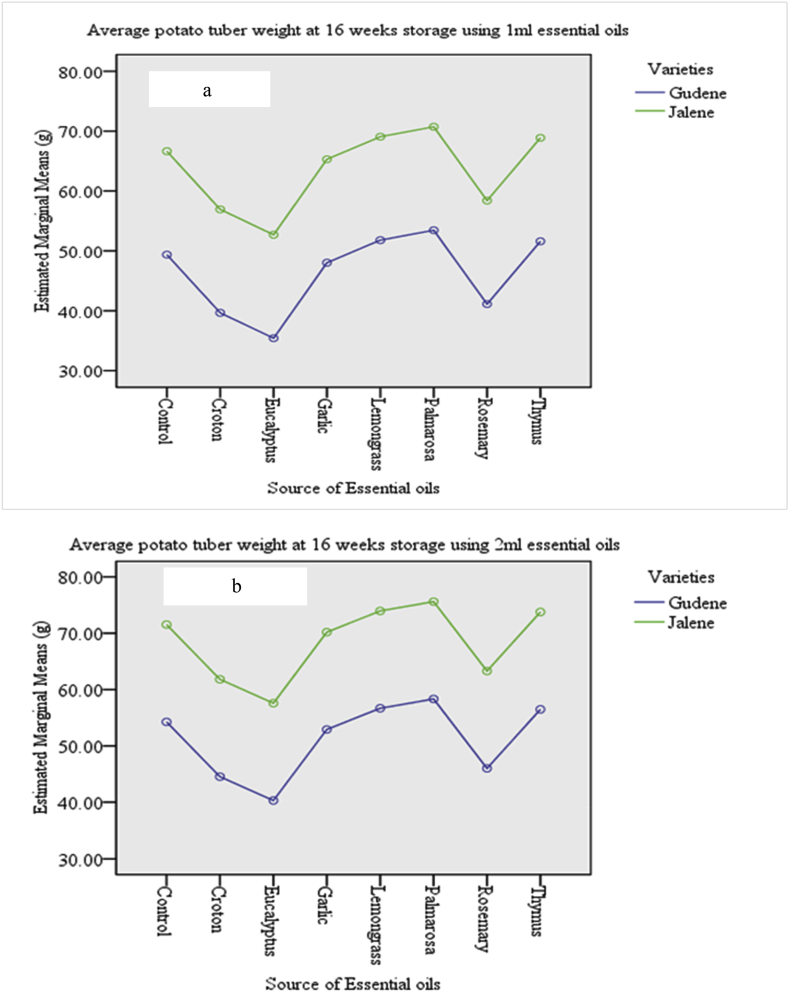
Considering the initial average weight as bases for comparison relative to control tubers, the average weights of potato tubers were better maintained up to 16 weeks of storage using essential oils of palmarosa, lemongrass, thymus and garlic for both varieties at both quantities used (Figure 1a and b). However, the lowest weight was obtained on potato tubers treated with eucalyptus for both varieties using 1 and 2 ml essential oils. Moreover, for both varieties, the potato tuber weight loss was less using 2 ml essential oils.
Figure 1.
a and b. Effects of sources of essential oils on Gudene and Jalene potato varieties tubers weight loss at 16 weeks of storage at room temperature. Average initial weight of potato tubers were 54.4 g and 82.6 g for Gudene and Jalene varieties, respectively.
3.2. Essential oils frequencies on potato tuber weight loss
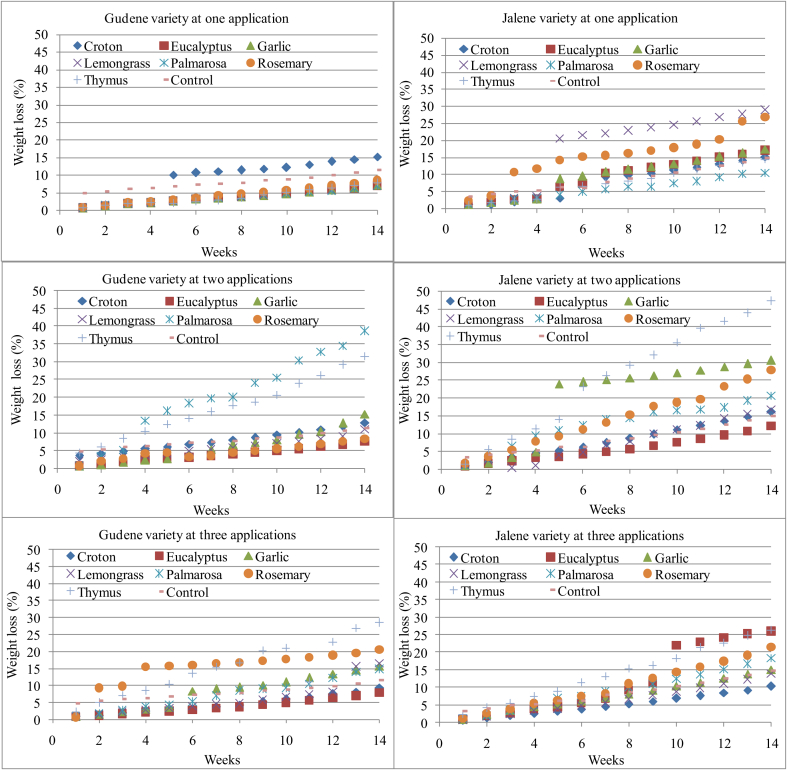
Average initial weight of potato tuber prior to essential oils application was 46.24 g and 68.52 g for Gudene and Jalene variety, respectively. Results indicated that percentage of weight loss varied between varieties using 1 ml essential oils at three frequencies (one, two and three) during potato tubers storage up to 14 weeks (Figure 2). Analysis of variance indicated that there was significant difference (p < 0.05) between varieties and essential oils frequencies. Effects of essential oils frequencies on percentage of weight loss were compared with control potato tubers for both varieties. At one time essential oils application, for Gudene variety, the highest percentage of weight loss was found on potato tubers treated with croton (15.2%) while the remaining essential oils maintained the percentage of weight loss below the control potato tubers (11.53%). At two times essential oils applications, the highest percentage of weight loss was found in the case of potato tubers treated with palmarosa (38.64%) followed by thymus (31.47%) while the lowest percentage of weight loss was found on potato tubers treated with eucalyptus (7.56%) essential oil followed by rosemary (8.37%) and lemongrass (10.99%). At three times applications, the highest percentage of weight loss was found on potato tubers treated with thymus (28.46%) essential oil followed by rosemary (20.41%) while the lowest percentage of weight loss was found on potato tubers treated with eucalyptus (7.78%) followed by croton (9.34%). On the other hand, for variety Jalene at one time application, the highest percentage of weight loss was found on potato tubers treated with lemongrass (28.90%) followed by rosemary (26.71%) while the lowest percentage of weight loss was found on potato tubers treated with palmarosa (10.36%) as compared to control potato tubers (14.87%). At two times applications, the highest percentage of weight loss was found on potato tubers treated with thymus (47.23%) followed by garlic (30.60%), rosemary (27.81%) and croton (16.27%) while the lowest percentage of weight loss was found on potato tubers treated with eucalyptus (12.06%). At three time applications, the highest percentage of weight loss was found on potato tubers treated with thymus (26.37%) followed by eucalyptus (25.95%) and rosemary (21.39%) while the lowest percentage of weight loss was found on potato tubers treated with croton (10.26%) followed by lemongrass (13.78%). Therefore, results indicated that effects of essential oils frequencies on percentage of weight loss varied between varieties. For variety Gudene, lowest percentage of weight loss was found on potato tubers treated with eucalyptus essential oil at all frequencies used. Moreover, lower percentage of weight loss relative to control was found on all essential oils except croton at one application. On the other hand, for variety Jalene, the lowest percentage of weight loss relative to control found at one, two and three applications on potato tuber treated with essential oils from palmarosa, eucalyptus and croton, respectively.
Figure 2.
Percentage of weight loss during room temperature storage for Gudene and Jalene potato tubers treated with 1 ml essential oils from croton, eucalyptus, garlic, lemongrass, palmarosa, rosemary, thymus and control at three applications (one, two and three).
3.3. Effects of essential oils on potato tuber sprouting number and length
Essential oils were evaluated for their ability to induce and/or inhibit potato tuber sprouting during storage at room temperature using two quantities (1 and 2 ml) and three frequencies (1, 2 and 3). Results indicated that sources of essential oils influenced the number and length of sprouts significantly while there was no significant difference between quantities. Therefore, commencement of tuber sprouting in the current study depends on potato varieties and sources of essential oils used. At 12 weeks of storage, there was induction of sprouts observed for the variety Jalene treated with croton and rosemary at 2 ml essential oils while for Gudene variety no sprout was observed on potato tubers treated with essential oils (Figures 3a and b, 4a and b). However, rotted potato tubers were observed at 2 ml eucalyptus and lemongrass as well as at 1 ml of croton and palmarosa on variety Jalene.
Figure 3.
a. Potato tubers sprouting following essential oils application for Jalene. Photo by Daniel Wondimu, Ethio-AgriCeft Laboratory, Ethiopia, 2021. b. Potato tubers sprouting following essential oils application for Jalene. Photo by Daniel Wondimu, Ethio-AgriCeft Laboratory, Ethiopia, 2021.
Figure 4.
a. Potato tubers sprouting following essential oils application for Gudene. Photo by Daniel Wondimu, Ethio-AgriCeft Laboratory, Ethiopia, 2021. b. Potato tubers sprouting following essential oils application for Gudene. Photo by Daniel Wondimu, Ethio-AgriCeft Laboratory, Ethiopia, 2021.
3.4. Essential oils quantities on the number of sprouts
Effects of essential oils quantities on the number of sprouts per tuber varied with varieties. However, there was no significant difference between essential oils quantities. For the variety Gudene using 2 ml essential oils, the highest number of sprout at week 16 was found on potato tubers treated with lemongrass (5.3 sprouts/tuber) followed by rosemary (4.7 sprouts/tuber) while the lowest number of sprouts was found on potato tubers treated with both palmarosa and thymus (2.7 sprouts/tuber) followed by potato tubers treated with eucalyptus and untreated (control) potato tubers (3 sprouts/tuber) (Table 3). For variety Jalene using 2 ml essential oils, the highest number of sprout at week 16 was found on untreated potato tubers (8.7 sprouts/tuber) followed by both eucalyptus and lemongrass (7.3 sprouts/tuber) while the lowest number of sprouts was found on potato tubers treated with both garlic and rosemary (5.7 sprouts/tuber) followed by croton (6 sprouts/tuber) (Table 3). The variety Jalene showed earlier sprouting regardless of essential oils used along with higher number of sprouts.
Table 3.
Number of sprouts and length of the longest sprout per potato tuber during storage.
| Source of Essential oils (2 ml) |
Gudene |
Jalene |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Sprout numbers |
Length of longest sprout (mm) |
Sprout numbers |
Length of longest sprout (mm) |
||||||
| Weeks during storage | |||||||||
| 16 | 24 | 28 | 30 | 16 | 24 | 28 | 30 | ||
| Croton | 3.7 | 8.0 | 12.3 | 12.3 | 6.0 | 13.3 | 19.0 | 21.0 | |
| Eucalyptus | 3.0 | 6.7 | 10.3 | 14.0 | 7.3 | 10.5 | 20.0 | 22.0 | |
| Garlic | 3.7 | 9.3 | 12.0 | 13.7 | 5.7 | 15.0 | 19.3 | 21.0 | |
| Lemongrass | 5.3 | 7.0 | 10.7 | 11.0 | 7.3 | 11.0 | 17.0 | 19.0 | |
| Palmarosa | 2.7 | 8.7 | 12.7 | 13.0 | 7.0 | 12.0 | 19.0 | 21.0 | |
| Rosemary | 4.7 | 6.0 | 6.7 | 10.0 | 5.7 | 10.7 | 16.0 | 17.0 | |
| Thymus | 2.7 | 5.0 | 10.3 | 10.3 | 6.7 | 10.3 | 18.0 | 20.0 | |
| Control | 3.0 | 5.7 | 11.7 | 11.7 | 8.7 | 9.7 | 16.7 | 20.0 | |
| Mean (SE) | 3.6 (0.34) | 7.1 (0.53) | 10.8 (0.67) | 12.0 (0.53) | 6.8 (0.36) | 11.6 (0.63) | 18.1 (0.50) | 20.1 (0.55) | |
The values in bold showed the maximum values for the specified variable.
3.5. Essential oils quantities on length of the longest sprouts
The length of the longest sprout also varied with varieties and sources of essential oils. In both varieties at 2 ml essential oils, tubers producing longest sprouts had also thicker sprouts at 30 weeks of storage. The length of longest sprouts on control potato tubers was 11.7 mm and 20 mm for varieties Gudene and Jalene, respectively (Table 3). In comparison to control tubers, these tubers treated with essential oils showed longer and shorter sprouts depending on the sources of essential oils for both varieties. For variety Gudene, potato tubers treated with essential oil from eucalyptus showed the longest sprouts (14 mm) followed by potato tubers treated with garlic (13.7 mm), whereas potato tubers treated with rosemary produced shorter sprouts (10 mm). For variety Jalene, longer sprouts (22 mm) were obtained on potato tubers treated with eucalyptus essential oil followed by garlic (21 mm) while shorter sprouts (17 mm) were found on tubers treated with rosemary essential oil. The longest and the shortest sprouts were obtained for both varieties on potato tuber treated with eucalyptus and rosemary essential oils, respectively.
3.6. Essential oils frequencies on the number of sprouts
Results also indicated that effects of essential oils frequencies on number of sprouts were varied between varieties (Table 4). For varieties Gudene and Jalene the number of sprouts at week 16 was from 1.7 to 6.7 sprouts/tuber and from 3 to 9.3 sprouts/tuber, respectively. However, the number of sprouts per tuber for control potato tubers was 3 sprouts/tuber and 8.7 sprouts/tuber for varieties Gudene and Jalene, respectively. Therefore, in comparison to control tubers, less number of sprouts was found on Gudene potato tubers treated with croton (1 sprout/tuber) followed by garlic (2 sprouts/tuber) at one application; rosemary (1 sprout/tuber) followed by both lemongrass and palmarosa (2 sprouts/tuber) at two applications; garlic (1 sprout/tuber) followed by palmarosa (2 sprouts/tuber) at three applications. However, for variety Jalene, less number of sprouts was found on potato tubers treated with all essential oils at all frequencies relative to control tubers except on potato tubers treated with garlic (9.3 sprouts/tuber) at two applications. Moreover, in one and two applications of rosemary essential oil, the number of sprouts increased for variety Gudene while the number of sprouts decreased for variety Jalene relative to control.
Table 4.
Number of sprouts and length of the longest sprout per potato tuber during storage.
| Source of Essential oils (1 ml) | Frequencies |
Gudene |
Jalene |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Sprout numbers |
Length of longest sprout (mm) |
Sprout numbers |
Length of longest sprout (mm) |
||||||
| Weeks during storage | |||||||||
| 16 | 24 | 28 | 30 | 16 | 24 | 28 | 30 | ||
| Croton | 1 | 4.0 | 6.7 | 11.3 | 11.7 | 7.7 | 7.0 | 18.5 | 22.0 |
| 2 | 3.7 | 6.0 | 11.3 | 13.3 | 8.0 | 10.3 | 16.7 | 19.0 | |
| 3 | 6.0 | 8.3 | 10.3 | 12.3 | 6.3 | 13.0 | 21.0 | 24.0 | |
| Eucalyptus | 1 | 1.7 | 7.7 | 14.3 | 17.3 | 7.3 | 9.3 | 13.3 | 16.0 |
| 2 | 5.0 | 4.0 | 12.3 | 13.0 | 5.5 | 8.5 | 15.5 | 18.0 | |
| 3 | 5.0 | 11.0 | 13.0 | 15.0 | 5.7 | 11.3 | 19.3 | 22.0 | |
| Garlic | 1 | 3.3 | 8.3 | 14.3 | 15.0 | 8.0 | 12.0 | 16.0 | 18.0 |
| 2 | 3.0 | 6.3 | 9.7 | 12.3 | 9.3 | 12.3 | 19.0 | 21.0 | |
| 3 | 6.7 | 7.7 | 10.7 | 13.0 | 5.5 | 11.0 | 14.5 | 17.0 | |
| Lemongrass | 1 | 2.7 | 5.7 | 11.3 | 12.7 | 7.7 | 12.7 | 17.3 | 20.0 |
| 2 | 5.7 | 6.3 | 11.3 | 13.3 | 6.5 | 11.0 | 20.0 | 23.0 | |
| 3 | 4.5 | 6.5 | 10.0 | 12.0 | 6.7 | 10.0 | 16.7 | 20.0 | |
| Palmarosa | 1 | 2.0 | 6.3 | 9.3 | 11.3 | 6.3 | 8.3 | 21.0 | 23.0 |
| 2 | 5.7 | 7.7 | 15.0 | 16.7 | 4.3 | 14.3 | 18.7 | 21.0 | |
| 3 | 6.5 | 4.0 | 10.0 | 10.0 | 5.7 | 10.3 | 18.5 | 22.0 | |
| Rosemary | 1 | 3.0 | 7.3 | 12.3 | 14.3 | 6.0 | 7.3 | 18.5 | 22.0 |
| 2 | 6.0 | 6.7 | 12.7 | 16.7 | 3.0 | 10.0 | 10.0 | 10.0 | |
| 3 | 6.3 | 7.7 | 11.7 | 11.7 | 4.7 | 8.0 | 10.0 | 10.0 | |
| Thymus | 1 | 2.3 | 4.3 | 9.3 | 12.0 | 5.3 | 10.7 | 15.7 | 18.0 |
| 2 | 3.0 | 5.7 | 9.0 | 14.0 | 8.3 | 10.0 | 17.7 | 20.0 | |
| 3 | 5.0 | 8.0 | 15.0 | 16.0 | 7.5 | 10.0 | 17.3 | 20.0 | |
| Control | 3.0 | 5.7 | 11.7 | 11.7 | 8.7 | 9.7 | 16.7 | 20.0 | |
| Mean (SE) | 4.3 (0.34) | 6.7 (0.34) | 11.6 (0.39) | 13.4 (0.42) | 6.5 (0.33) | 14.9 (0.40) | 16.9 (0.63) | 19.4 (0.78) | |
3.7. Essential oils frequencies on the length of longest sprouts
Results indicated effects of essential oils (1 ml) using three frequencies on the length of longest sprout varied between varieties and among the sources of essential oils at 30 weeks of storage. For variety Gudene at one application, the longest sprout (17.3 mm) was found on potato tubers treated with eucalyptus while the shortest sprout (10 mm) was found on potato tubers treated with palmarosa. For variety Jalene, the longest sprout was obtained on potato tubers treated with garlic (24 mm) at three applications while the shortest sprout was found on potato tubers treated with rosemary (10 mm) at two and three applications. Furthermore, longer sprouts found on Gudene potato tubers relative to control potato tuber sprout length (11.7 mm) at all frequencies except for potato tubers treated with palmarosa at one application (11.3 mm) and three applications (10 mm). Whereas, for Jalene variety, longer sprouts found on potato tubers treated with croton, palmarosa and rosemary at one application relative to control potato tuber (20 mm); treated with garlic, lemongrass and palmarosa at two applications; treated with croton, eucalyptus and palmarosa at three applications. Therefore, in the current study, plants used as sources of essential oils showed the ability to control potato tuber sprouting during storage. Even the same essential oils showed the ability to increasing and decreasing the length of the longest sprouts relative to control tubers (Table 4).
4. Discussion
Usually, most of the harvested potato tubers are stored for short or long periods depending on the market demand (Ghazavi and Houshmand, 2010). Therefore, harvested potato tubers require controlled storage conditions to maintain tuber quality (Eltawil et al., 2006). If potato tubers in storage are unmanaged, losses of harvested potato tuber would result from sprouting, weight loss and rotting, among others. Therefore, sprouting control in stored potato is crucial since it leads to reduction of weight, increase in transpiration and respiration, changes in textural and nutritional quality (Suttle et al., 2016). The current study was focusing in finding alternative eco-friendly botanical management options for potato tuber sprouting during storage. Seven essential oils were evaluated for their ability to induce and/or inhibit potato tuber sprouting at room temperature storage.
4.1. Effects of essential oils on potato tuber weight loss
In the current studies, essential oils exhibited the ability to reduce weight loss during storage. Presumably, the antioxidant property of essential oils may be involved in reduction of biochemical activities which in turn minimizes water losses from potato tuber surfaces (El-Awady et al., 2014). Moreover, the process of respiration and water loss reduced due to essential oils applications (Etemadipoor et al., 2019; Shehata et al., 2020). Tuber weight loss during storage primarily results from the process of respiration and transpiration in un-sprouted tubers (Azad et al., 2017). However, as the duration of storage increases weight loss is likely due to tuber sprouting. It leads to growth of visible buds and shoots which are very vulnerable to transpiration (water loss from tuber surface) (Pande et al., 2007). When there is sprouting, it is accompanied by higher respiration rate which leads to higher weight loss and biochemical changes (Edmunds and Holmes, 2008). Potato tubers develop wilting (shrivelling) symptoms which in turn resulted in poor quality of potato tuber. The current findings revealed the ability of essential oils to inhibit and/or induce potato sprouting depending on the sources of essential oils and frequencies of applications. Therefore, these essential oils which showed the ability to inhibit tuber sprouting could be used to reduce weight loss and maintain quality of potato tubers used for fresh consumption and processing. Hence, the current study exhibited essential oils from lemongrass and thymus was most effective to minimize weight loss for both varieties. Different authors reported citral and carvacrol as the major components of essential oil from lemongrass and thymus, respectively (Majewska et al., 2019). Hence, these compounds play a role in reduction of weight loss in stored potato tuber. Therefore, depending on the frequencies used, these essential oils could be used to reduce the weight loss especially for potato tubers used for fresh consumption.
4.2. Effects of essential oils on potato tuber sprouting
Potato tubers that can be used for fresh consumption and processing requires the use of sprout inhibitors, whereas sprouting induction is required for potato tubers used as source of seed (Cheema et al., 2010, 2013; Hu et al., 2011; Sheibani et al., 2012; Pankomera et al., 2016). The current findings indicated that essential oils showed the ability to inhibit/induce sprouting depending on potato varieties, source of essential oils and frequencies used. However, the rate of sprouting varied between potato varieties which may be due to their genetic variation (Frazier et al., 2004). In contrary to seed potato tubers, sprouting increase the conversion of starch which leads to higher reducing sugars that affects the quality of processing tubers (Bhattacharjee et al., 2014). Therefore, sprouting reduces tuber weight, nutritional and processing quality of tubers as well as the number of marketable potatoes, being responsible for important economic losses during potato storage for potato tubers used as fresh consumption and processing (Delaplace et al., 2008; Sonnewald and Sonnewald, 2014; Chala et al., 2019). Therefore, researchers continue searching alternative management options to inhibit potato sprouting. Previous research findings in Ethiopia indicated that the potential of essential oils from eucalyptus and dill weed to delay potato sprouting for the variety known as Gera (Biruk et al., 2015). Similarly, essential oils from palmarosa and thymus for variety Gudene while essential oils from garlic and rosemary for variety Jalene exhibited inhibitory effects of potato tuber sprouting. Presumably, essential oil inhibits sprouting due to the capacity of main components to restrict the activities of meristematic cell division similar to the effects of auxins and cytokinins (Pierre-Jerome et al., 2018). Therefore, Shukla et al. (2019) reported inhibition of potato tuber sprouts using essential oils from palmarosa and ajwain. Moreover, essential oils from spearmint and peppermint used to prevent sprouting in stored potatoes for longer period due to the presence of S-carvone and eugenol (Kleinkopf et al., 2003; Cheema et al., 2010). Hence, it is reported that monoterpene rich essential oils strongly inhibited germination, seedling, roots and shoot growth (Zhang et al., 2014). Thus, it could be concluded that sprouting inhibition probably delays tuber weight loss, as previously reported by Teper-Bamnolker et al. (2010).
On the other hand, some essential oils exhibited the ability to induce potato sprouting due to increasing of reducing sugar (Mani et al., 2014; Shukla et al., 2019); this is probably as a result of increasing rate of respiration due to essential oil applications. Sprout induction in potato tuber is believed to be regulated primarily by endogenous abscisic acid, the presence of antioxidant and sucrose (Claassens and Vreugdenhil, 2000; Suttle, 2004; Sonnewald and Sonnewald, 2014). In the current findings, for variety Gudene, essential oils from lemongrass and rosemary and all essential oils for variety Jalene exhibited promoting of potato tuber sprouting. Similarly, Shukla et al. (2019) reported induction of potato tuber sprouts using essential oils from lemongrass and clove, respectively. Hence, essential oils that showed the ability to induce sprouting can be used to break dormancy in areas which have more than one growing seasons including Ethiopia.
According to Shukla et al. (2019), the process of potato tuber sprouting is affected by essential oils since they have a role in changing accumulated reducing sugars, ethylene production and expression of key genes recognized to be involved in the processes of potato tuber sprouting. Therefore, based on the current studies, potato tubers can be stored at room temperature using essential oils. Smallholder farmers can store their potato tubers at room temperature without additional storage facilities.
5. Conclusion
The ability of seven essential oils to control potato tuber sprouting during storage was investigated. Essential oils from eucalyptus, lemongrass and rosemary were found to induce sprouting while essential oils from garlic, palmarosa, rosemary and thymus inhibited potato tuber sprouting during storage. Therefore, it can be concluded that the current findings revealed the potential of essential oils as alternative eco-friendly botanical management options for potato tuber sprouting during storage. Essential oils from species (eucalyptus, lemongrass and rosemary) induce sprouting and essential oils from other species (garlic, palmarosa, rosemary and thymus) inhibit sprouting. However, the effect of essential oils on potato tuber nutritional quality, cooking quality and chips quality should be studied. Since Ethiopia has enormous plant species (the Lamiaceae being one of the largest families in the Flora with about 44 genera and 170 species as given in the Flora of Ethiopia and Eritrea, volume 5, 2006) that can be used as a source of essential oils, the untapped potential of existing plant species should be explored for management of potato sprouting. Thus, alternative management options for potato tuber sprouting will be available to provide quality potato tubers based on the intended purpose.
Declarations
Author contribution statement
Daniel Wondimu Belay: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Zemede Asfaw; Ermias Lulekal; Bekele Kassa: Analyzed and interpreted the data; Wrote the paper.
Habtamu Kifele: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to thank the Department of Plant Biology and Biodiversity Management, Addis Ababa University for facilitating the research. We also would like to thank Ethio-AgriCEFT that kindly provided the equipment and plant materials for essential oil extraction, providing laboratory access to conduct storage experiments. We also would like to thank Holleta Agricultural Research Center for providing the potato tubers.
References
- Alamar M.C., Tosetti R., Landahl S., Bermejo A., Terry L.A. Assuring potato tuber quality during storage: a future perspective. Front. Plant Sci. 2017;8:2034. doi: 10.3389/fpls.2017.02034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad A., Kabir H., Eaton T., Binod Soren E. Storage potetialities of some exotic potato varieties at farmers’ conditions in Bangladesh. Agric. Sci. 2017;8:183–193. [Google Scholar]
- Azmir J., Zaidul I.S.M., Rahman M.M., Sharif K.M., Mohamed A., Sahena F., Jahurul M.H.A., Ghafoor K., Norulaini N.A.N., Omar A.K.M. Techniques for extraction of bioactive compounds from plant materials: a review. J. Food Eng. 2013;117:426–436. [Google Scholar]
- Bajji M., Mhamdi M., Castiny F., Rojas-Beltran J., Du Jardin P. Catalase inhibition accelerates dormancy release and sprouting in potato (Solanum tuberosum L.) Biotechnol. Agron. Soc. Environ. 2007;11(2):121–131. [Google Scholar]
- Balaji V., Chandra S., Goswami D.A., Das S.K., Mandal T.K., Chakraborty A.K., Bhattacharyya A. Toxicokinetics, metabolism and microsomal studies of chloropham in rats. J. Toxicol. Environ. Chem. 2006;88:527–539. [Google Scholar]
- Bethke P., Libourel I., Aoyama N., Chung Y., Still D., Jones R. The Arabidopsis aleurone layer responds to nitric oxide, gibberellins and abscisic acid and is sufficient and necessary for seed dormancy. Plant Physiol. 2007;143:1173–1388. doi: 10.1104/pp.106.093435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee A., Roy T.S., Haque N., Pulok A.I. Changes of sugars and starch levels in ambient stored potato derived from TPS. Int. J. Scient. Res. Publ. 2014;41(11):1–5. [Google Scholar]
- Biruk M., Nigussie D., Yibekal A., Bekele A., Tamado T. Influence of treatments of seed potato tubers with plant crude essential oil extracts on performance of the crop. Afr. Crop Sci. J. 2015;2(3):295–304. [Google Scholar]
- Bond J.K. In: The Potato Botany, Production And Uses. Navarre R., Pavek M., editors. CABI; 2014. Potato utilization and markets; pp. 29–44. [Google Scholar]
- Campbell M., Segear E., Beers L., Knauber D., Suttle J. Dormancy in potato tubers meristems: chemically induced cessation in dormancy matches the natural process based on transcript profiles. Funct. Integr. Genom. 2008;8(4):317–328. doi: 10.1007/s10142-008-0079-6. [DOI] [PubMed] [Google Scholar]
- Chala G., Yetenyet B., Gemechu G. Study on post-harvest quantitative and qualitative losses of potato tubers from two different road access districts of Jimma zone, South West Ethiopia. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02272. 1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheema M., Rees D., Westby A., Taylor M. Hormonal control of sweet potatoes in storage. Acta Hortic. 2010;858:173–177. [Google Scholar]
- Cheema M., Rees D., Colgan R., Taylor M., Westby A. The effects of ethylene, 1-MCP and AVG on sprouting in sweet potato roots. Postharvest Biol. Technol. 2013;85:89–93. [Google Scholar]
- Claassens M.M.J., Vreugdenhil D. Is dormancy breaking of potato tubers the reverse of tuber initiation? Potato Res. 2000;43:347–369. [Google Scholar]
- Coleman W.K., Lonergan G., Silk P. Potato sprout growth suppression by menthone and neomenthol, volatile oil components of minthostachys, satureja, by stropogon and menthe species. Am. J. Potato Res. 2001;78:345–354. [Google Scholar]
- Costa E.-S., Galhano C.I.C., Moreira Da Silva A.M.G. A new sprout inhibitor of potato tuber based on carvon/B-cyclodextrin inclusion compound. J. Inclusion Phenom. Macrocycl. Chem. 2007;57:121–124. [Google Scholar]
- Dalziel J., Duncan H.J. Studies on potato suppressants. 4. The distribution of tecnazene in potato tubers and the effect of processing on residue levels. Potato Res. 1980;23:405–411. [Google Scholar]
- Daniel W.B., Zemede A., Ermias L., Bekele K. Farmers’ management of potato (Solanum tuberosum L.) late blight (Phytophtora infestans (Mont.) de Bary) sprouting in shashemene and West Shewa districts, Ethiopia. J. Cogent Agricul. 2021;7(1):1925432. [Google Scholar]
- Daniels-Lake B.J., Prange R.K. The canon of potato science 41. Sprout. Potato Res. 2007;50:379–382. [Google Scholar]
- Delaplace P., Brostaux, Fauconnier M.L., Du Jardin P. Potato (Solanum tuberosum L.) tuber physiological age index is a valid reference frame in postharvest ageing studies. Postharvest Biol. Technol. 2008;50:103–104. [Google Scholar]
- Edmunds B.A., Holmes G.J. Susceptibility of sweet potato table stock and high dry matter cultigens to Rhizopus soft rot plant diseases. Manag. Rep. 2008;2:146. [Google Scholar]
- El-Awady A.A., Moghazy A.M., Gouda A.E., Elshatoury R.S. Inhibition of sprout growth and increase storability of processing potato by antisprouting agent. Trends Horticult. Res. 2014;4:31–40. [Google Scholar]
- Elmar Schulte-Geldermann. In: Seed Potato Tuber Production and Dissimentaion: Experiences, Challenges and Prospects. Proceedings Of the National Workshop On Seed Potato Tuber Production And Dissemination, 12-14 March 2012, Bahir Dar, Ethiopia. Woldegiorgis Gebremedhin, Steffen S., Berahnu Baye., editors. 2013. Tackling low potato yields in eastern Africa: an overview of constraints and potential strategies. [Google Scholar]
- Eltawil M.A., Samuel D.V.K., Singhal O.P. Potato storage technology and store design Aspects. Agric. Eng. Int.: CIGRE J. Invited Overv. 2006;8(11) [Google Scholar]
- Etemadipoor R., Ramezanian A., Dastjerdi A.M., Shamili M. The potential of gum Arabic enriched with cinnamon essential oil for improving the quality characteristics and storability of guava (Psidium guajava L.) fruit. Sci. Hortic. 2019;251:101–107. [Google Scholar]
- FAOSTAT . 2020. World Food and Agricultural Organization Data of Statistics. Rome, Italy. [Google Scholar]
- Fauconnier M., Rojas-Beltran J., Dejaeghere F., Marlier M., Du Jardin P. Lipooxygenease pathway and membrane permeability and composition during storage of potato tubers (Solanum tuberosum L. cv. Bintje and Desiree) in different conditions. Plant Biol. 2002;4:77–85. [Google Scholar]
- Finger F.L., Mayana M., Santos D.S., Araujo F.F., Cristina P., Lima C., Queiroz C. Action of essential oils on sprouting of non-dormant potato tubers. Braz. Arch. Biol. Technol. 2018;61(3):1–10. [Google Scholar]
- Frazier M.J., Olsen N., Kleinkopf G. Vol. 2004. University of Idaho, College of Agriculture and Life Sciences; Idaho, USA: 2004. Organic and Alternative Methods for Potato Sprout Control in Storage, CIS 1120. [Google Scholar]
- Gamble R., Qu X., Schaller G. Mutational analysis of the ethylene receptor ETR 1. Role of histidine kinase domain in dominant ethylene insensitivity. Plant Physiol. 2002;128:1428–1438. doi: 10.1104/pp.010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemeda M., Wassu M., Nigussie D., Dandena G. 2017. Effects of Different Dormancy-Breaking and Storage Methods on Seed Tuber Sprouting and Subsequent Yield of Two Potato (Solanum tuberosum L.) Varieties. [Google Scholar]
- Ghazavi M.A., Houshmand S. Effects of mechanical damage and temperature on potato respiration rate and weight loss. World Appl. Sci. J. 2010;8:647–652. [Google Scholar]
- Gomez-Castillo D., Cruz E., Iguaz A., Arroqui C., Virseda P. Effect of essential oils on sprout suppression and quality of potato cultivars. Postharvest Biol. Technol. 2013;82:15–21. [Google Scholar]
- Hu W., Jiang A., Jin L., Liu C., Tian M., Wang Y. Effect of heat treatment on quality, thermal and pasting properties of sweet potato starch during yearlong storage. J. Food Sci. Agric. 2011;91:1499–1504. doi: 10.1002/jsfa.4340. [DOI] [PubMed] [Google Scholar]
- Kleinkopf G.E., Oberg N.A., Oslen N.L. Sprout Inhibition in storage: current Status, new chemistries and natural compounds. Am. J. Potato Res. 2003;80:317–327. [Google Scholar]
- Mahmoudi R., Kaboudari A., Pakbin B. 2019. Applications of Medicinal Herbs and Essential Oils in Food Safety. [Google Scholar]
- Majewska E., Koziwoska M., Gruczynska-Sekowska E., Kowalska D., Tarnowska K. Lemongrass (Cymbopogon citratus) essential oil: extraction, composition, bioactivity and uses for food preservations-a review. Pol. J. Food Nutr. Sci. 2019;69(4):327–341. [Google Scholar]
- Mani F., Bettaieb T., Doudech N., Hannachi C. Physiological mechanisms for potato dormancy release and sprouting: a review. Afr. Crop Sci. J. 2014;22(2):155–174. [Google Scholar]
- Mohammed N.S., Flowers T.H., Duncan H.J. HPLC-UV method for the analysis of potato sprout inhibitor chlorpropham and its metabolite 3-chloroaniline in potatoes. IOSR. J. Environ. Sci. Toxicol. Food Sci. 2015;9:78–85. [Google Scholar]
- Mohammed F.S., Şabik A.E., Sevindik E., Pehlivan M., Sevindik M. Determination of antioxidant and oxidant potentials of Thymbra spicata collected from Duhok-Iraq. Turkish J. Agricult. Food Sci. Technol. 2020;8(5):1171–1173. [Google Scholar]
- Nurit F., Gomes De Melo E., Ravanel P., Tissut M. Specific inhibition of mitosis in cell suspension cultures by a N-phenylcarbamate series. Pestic. Biochem. Physiol. 1989;35:203–210. [Google Scholar]
- Pande P.C., Singh S.V., Pandey S.K., Singh B. Dormancy, sprouting behavior and weight loss in Indian potato (Solanum tuberosum) varieties. Indian J. Agric. Sci. 2007;77(11):715–720. [Google Scholar]
- Pandey A., Belwal T., Tamta S., Bhatt I.D., Rawal R.S. Phenolic compounds, antioxidant capacity and antimutagenic activity in different growth stages of in vitro raised plants of Origanum vulgare L. Mol. Biol. Rep. 2019;46(2):2231–2241. doi: 10.1007/s11033-019-04678-x. [DOI] [PubMed] [Google Scholar]
- Pankomera P., Heyes J., Lewthwaite S., Roskruge N. Effects of ethylene and 1-methylcyclopropene on sweet potato storage root quality. Acta Hortic. 2016:163–170. [Google Scholar]
- Perez F., Lira W. Possible role of catalse in post-dormancy bud break in grapevines. J. Plant Physiol. 2005;162:301–308. doi: 10.1016/j.jplph.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Sharma A. Essential oil as organic and alternative methods for potato. Int. J. Eng. Math. Sci. 2012;1:34–39. [Google Scholar]
- Shehata S.A., Abdeldaym E.A., Ali M.R., Mohamed R.M., Bob R.I., Abdelgawad K.F. Effect of some citrus essential oils on postharvest shelf life and physicochemical quality of strawberries during cold storage. Agronomy. 2020;10(10):1466. [Google Scholar]
- Sheibani E., Kim T., Wang D.S., Silva J., Arancibia R., Matta F.B., Picha D. Optimaization of hot water treatment for sprout and spoilage inhibition of cured sweet potato. J. Food Process. Preserv. 2012;38:493–498. [Google Scholar]
- Shukla S., Pandey S.S., Chandra M., Pandey A., Bharti N., Barnawal D., Chanotiya C.S., Tandon S., Darokar M.P., Kalra A. Application of essential oils as a natural and alternative method for inhibiting and inducing the sprouting of potato tubers. Food Chem. 2019;284:171–179. doi: 10.1016/j.foodchem.2019.01.079. [DOI] [PubMed] [Google Scholar]
- Sihtmae M.J., Mortimer M., Kahru A., Blinova I. Toxicity of five anilines to crustaceans, protozoa and bacteria. J. Serb. Chem. Soc. 2010;75(9):1291–1302. [Google Scholar]
- Smith M.J., Bucher G. Tools to study the degradation and loss of the N-phenyl carbamate chloropham-A comprehensive review. Environ. Int. 2012;49:38–50. doi: 10.1016/j.envint.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Sonnewald S., Sonnewald U. Regulation of potato tuber sprouting. Planta. 2014;239:27–38. doi: 10.1007/s00425-013-1968-z. [DOI] [PubMed] [Google Scholar]
- Sorce C., Lorenzi R., Parisi B., Ranalli P. Physiological mechanisms involved in potato (Solanum tuberosum) tuber dormancy and the control of sprouting by chemical supperessants. Acta Hortic. 2005:177–186. [Google Scholar]
- Sowokinos J.R. The canon of potato science: 38. Carbohyd. Metabol. 2007;50:367–370. Potato Research. [Google Scholar]
- Srivastava M.P., Tandon R.N. Influence of temperature in Botrydiplodia rots of citrus and sapodilla. Indian Phytopathol. 1968;21:195–197. [Google Scholar]
- Statista . 2018. Organic Food Sales Growth in the U.S., 2017.https://www.staista.com/statistics/196962/organic-food-sales-growth-in-the-us-since-2000/ [WWW document]. Stat. Portal.URL. [Google Scholar]
- Suttle J. Physiological regulation of potato tuber dormancy. Am. J. Potato Res. 2004;81:253–262. [Google Scholar]
- Suttle J.C., Campbell M.A., Olsen N.L. In: Postharvest Ripening Physiology of Crops 449-476. Pareek S., editor. CRC Press; Boca raton, Fl: 2016. Potato tuber dormancy and postharvest sprout control. [Google Scholar]
- Teper-Bamnolker P., Dudai N., Fischer R., Belausov E., Zemach H., Shoseyov O., Eshel D. Mint essential oil can induce or inhibit potato sprouting by differential alteration of apical meristem. Planta. 2010;232:179–186. doi: 10.1007/s00425-010-1154-5. [DOI] [PubMed] [Google Scholar]
- Tesfaye Abebe, Lemega Berega, Mwakasendo J.A., Nzohbonayoz Z., Mutware J., Wando K.Y., Kinyae P.M., Ortiz O., Crissman C., Thiele G. CIP; Burundi and Uganda, Lima: 2010. Markets for Fresh and Frozen Potato Chips in the ASARECA Region and the Potential for Regional Trade: Ethiopia, Tanzania, Rwands, Kenya. [Google Scholar]
- Vaughn S.F., Spencer G.F. Volatile monoterpenes inhibit potato tuber sprouting. Am. J. Potato Res. 1991;68(12):82–831. [Google Scholar]
- Virtanen E., Haggman H., Degefu Y., Valimaa A., Seppanen M. Effects of production history and Gibberellic Acid on seed potatoes. J. Agric. Sci. 2013;5(12) Published by Canadian Center of Science and Education. [Google Scholar]
- Weiss G.G., Schoeneman J.A., Groskopp D.H. Influence of time of application of maleic hydazide on the yield and quality of Russett Burbank potatoes. Am. Potato J. 1980;57:197–204. [Google Scholar]
- Xie Y., Onlik J., Hu X., Duan Y., Lin Q. Effects of (S)-carvone and gibberellins on sugar accumulation in potato during low temperature storage. Molecules. 2018;23(12):3118. doi: 10.3390/molecules23123118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., An M., Wu H., Li Lue D., Stanton R. Chemical composition of essential oils of four Eucalyptus species and their phytotoxicity on silverleaf nightshade (Solanum elaeagnifolium Cav) in Australia. Plant Growth Regul. 2014;68:231–237. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.