Abstract
Background
The effectiveness of messenger RNA coronavirus disease 2019 (COVID-19) vaccines in patients with atopic dermatitis (AD) is yet to be delineated. It remains largely unknown how AD-related immunosuppressive medications affect the development of vaccine-induced immunity.
Objective
We aimed to evaluate the prevalence of the BNT162b2 messenger RNA vaccine among patients with AD and to assess its effectiveness in protecting against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, COVID-19-associated hospitalization, and mortality. A specific analysis additionally examined whether AD-related immunosuppressive drugs influenced the effectiveness of the vaccine.
Methods
A population-based cohort study was performed using the database of Clalit Heath Services, Israel, to follow adult patients with AD. Multivariate Cox and logistic regression analyses were utilized to calculate the adjusted hazard ratio (HR) and odds ratio (OR) of the incident outcomes.
Results
As of 26 June, 2021, 58,582 (75.4%) out of 77,682 adult patients with AD completed two BNT162b2 vaccine doses in Israel. Adulthood-onset AD (adjusted OR, 1.34; 95% CI 1.28–1.40; p < 0.001) and moderate-to-severe AD (adjusted OR, 1.13; 95% CI 1.05–1.21; p = 0.001) predicted an increased vaccination rate. Vaccinated patients with AD demonstrated a significantly decreased risk of SARS-CoV-2 infection (adjusted HR, 0.20; 95% CI 0.16–0.26; p < 0.001), COVID-19-associated hospitalization (adjusted HR, 0.08; 95% CI 0.04–0.18; p < 0.001), and COVID-19-associated mortality (adjusted HR, 0.04; 95% CI 0.01–0.20; p < 0.001). Exposure to immunosuppressive drugs (n = 597; 0.8% of patients) did not impair the protection against SARS-CoV-2 infection after vaccination (adjusted HR, 0.95; 95% CI 0.13–6.81; p = 0.958).
Conclusions
In patients with AD, COVID-19 vaccination is highly effective for a wide range of COVID-19-related outcomes. Immunosuppressive drugs did not impair the effectiveness of the vaccine in preventing SARS-CoV-2 infection in this retrospective analysis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40257-022-00672-5.
Key Points
| Among patients with atopic dermatitis, messenger RNA coronavirus disease 2019 vaccination proved to be highly effective in protecting against coronavirus disease 2019 and its complications. |
| There is no indication that immunosuppressive drugs necessitate alternate vaccination strategies in patients with atopic dermatitis. |
| Messenger RNA coronavirus disease 2019 vaccination should be highly recommended for patients with atopic dermatitis. |
Introduction
While the current coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), still wreaks havoc on the entire globe, a new hope has arisen following the approval of messenger RNA (mRNA) vaccines against SARS-CoV-2. The two authorized mRNA COVID-19 vaccines, BNT162b2 and mRNA-1273, are chemically modified vaccines expressing the perfusion spike glycoprotein of SARS-CoV-2 packaged in lipid nanoparticles [1]. With more than 90% of individuals achieving a satisfactory humoral response, mRNA COVID-19 vaccines displayed promising immunogenicity and efficacy results among immunocompetent subjects [2–4]. However, the ability of patients with immune-mediated diseases to mount an immune response to these vaccines is yet to be established. Additionally, it remains largely unknown how immunosuppressive medications affect the development of vaccine-induced immunity.
Atopic dermatitis (AD) is a chronic immune-mediated skin disease affecting up to 20–30% of children and 2–10% of adults [5]. An increased burden of COVID-19-associated hospitalization and mortality was found in patients with AD with severe comorbidities and those who undergo prolonged systemic corticosteroid treatment [6]. The efficacy of mRNA COVID-19 vaccines among patients with AD remains to be investigated. In the current study, we aimed to evaluate the frequency, determinants, and efficacy of COVID-19 vaccination among patients with AD. The secondary endpoint was to estimate the influence of different AD-related treatment modalities on the utility of mRNA COVID-19 vaccines.
Methods
Study Design and Dataset
The current retrospective cohort study followed patients with AD for the occurrence of COVID-19 vaccination and COVID-19-associated outcomes. The study was approved by the Institutional Review Board of Clalit Health Services (CHS) in accordance with the Declaration of Helsinki (approval code: 0212-17-COM). The study was exempt from the requirement for informed consent.
The computerized dataset of CHS was the source of the current study. Providing a broad spectrum of private and public healthcare services for 4,603,861 enrollees, CHS is the largest health maintenance organization in Israel. The computerized dataset of CHS retrieves clinical data from several sources covering general community clinics, referral centers, and inpatient healthcare facilities. Therefore, the CHS dataset features comprehensive access to clinical data and was proved compatible to generate valid and robust epidemiological data [7].
Study Population and Definition of COVID-19-Related and Vaccination-Related Variables
The computerized database of CHS was systematically screened for prevalent cases with a diagnosis of AD. The eligibility criteria of the study were: (1) documentation of an AD compatible diagnostic code by a board-certified dermatologist or by a discharge letter from inpatient dermatological wards, (2) being alive, and (3) aged 18 years and above at the beginning of the national COVID-19 vaccination campaign in Israel (20 December, 2020).
A diagnosis of COVID-19 was confirmed by a US Food and Drug Administration-approved SARS-CoV-2 polymerase chain reaction test. COVID-19-associated hospitalization was defined in COVID-19-confirmed patients admitted to intensive care units, internal medicine, or COVID-19-specific respiratory inpatient wards. COVID-19-associated mortality was defined in patients whose cause of death was ascribed to COVID-19 or its complications.
Access to a vaccine in Israel is a random event where all citizens have a consistent and equal probability of getting vaccinated. Fully vaccinated patients were those who had received two BNT162b2 (Comirnaty; Pfizer-BioNTech) mRNA vaccine doses during the period from 20 December, 2020 to 26 June, 2021. Study participants were vaccinated in accordance with the vaccination schedule specified in the BNT162b2 vaccine summary of product characteristics. When estimating the utility of COVID-19 vaccine among patients with AD, only those who were vaccinated by 28 February, 2021 were subject to inclusion. This aimed to provide a sufficient follow-up time enabling the capture of COVID-19-related outcomes. The reference group for this analysis comprised patients with AD who were not vaccinated until the end of the follow-up, whereas patients who were to be vaccinated after 28 February, 2021 were excluded from the reference group (as a subsequent vaccination might have conferred partial protection in this subgroup).
Follow-up began on the day in which the second dose of COVID-19 vaccine was given (in the vaccinated group) and on 28 February, 2021 (in the non-vaccinated group). For each study participant, the follow-up terminated at the earliest of the following events: fulfilling an outcome event, death unrelated to COVID-19, or the end of the follow-up (26 June, 2021). Participants’ date of death was determined by cross-linking the study cohort with the National Registry of Deaths Database.
Definition of AD-Related and Comorbidity Variables
Adult-onset AD was defined in patients presenting with the disease beyond the age of 18 years. As standardized severity scores of AD were not systematically available, the severity of AD was indirectly determined as moderate to severe in patients who were managed by the following systemic drugs at any timepoint throughout the course of the disease: (1) azathioprine, (2) mycophenolate mofetil, (3) methotrexate, (4) cyclosporine, (5) dupilumab, (6) extended courses of systemic corticosteroids [≥ 3 months]), or (7) phototherapeutic modalities (ultraviolet B, psoralen and ultraviolet A). Patients who were not managed by any of the aforementioned interventions were considered to have a mild disease.
Given the accumulation of data suggesting that patients undergoing immunosuppressive treatment mount a decreased antibody response to the COVID-19 vaccine [8, 9], we evaluated whether the intake of systemic drugs with immunosuppressive properties (azathioprine, mycophenolate mofetil, methotrexate, cyclosporine, dupilumab) during the pandemic influences the utility of the vaccine. Patients were considered to be exposed to a systemic drug when there were two prescriptions for more than 1 month during the pandemic.
Comorbidities of eligible patients were retrieved from the chronic diseases register of CHS. Metabolic syndrome was defined as the existence of at least three of the following conditions: type 2 diabetes mellitus, dyslipidemia, hypertension, or obesity [10–12]. Cardiovascular disease was designated when at least one of the following conditions were present: a history of myocardial infection, ischemic heart disease, congestive heart failure, or arrhythmia.
Statistical Analysis
Comparison between different subgroups was performed using the chi-square test and t-test for categorical and continuous variables, respectively. The independent associations between demographic, disease-specific, and comorbidity variables and COVID-19 vaccination were assessed by a multivariable logistic regression analysis and reported as odds ratios (ORs) and 95% confidence intervals (CIs). Incidence rates of outcomes were calculated and expressed as the number of events per 1000 person-years. Hazard ratios (HRs) for the risk of incident outcomes were obtained by the use of the Cox regression model. Two-tailed p values less than 0.05 were considered statistically significant. SPSS software, version 25 (SPSS; IBM Corp, Armonk, NY, USA) was utilized to conduct all statistical analyses.
Results
Characteristics of the Study Population
The study population included 77,682 patients with AD, of whom 47,809 (61.5%) were female and 65,969 (84.9%) were of Jewish ancestry. The mean (standard deviation) age of eligible patients at the onset of the pandemic was 41.9 (18.6) years. Table 1 further delineates the clinical and demographic characteristics of the study participants.
Table 1.
Characteristics of the study population
| Characteristic | Patients with AD (N = 77,682) |
|---|---|
| Demographic and clinical variables | |
| Age at the onset of the pandemic, years | |
| Mean (SD) | 41.9 (18.6) |
| Median (range) | 37.2 (18.2–112.3) |
| Sex, n (%) | |
| Male | 29,873 (38.5) |
| Female | 47,809 (61.5) |
| Ethnicity, n (%) | |
| Jews | 65,969 (84.9) |
| Arabs | 11,713 (15.1) |
| Socioeconomic status, n (%) | |
| Low | 25,019 (32.2) |
| Intermediate | 32,255 (41.5) |
| High | 20,408 (26.3) |
| BMI, mean (SD), mg/kg2 | 25.4 (5.3) |
| Characteristics of AD | |
| Age at the onset of AD, years | |
| Mean (SD) | 34.2 (19.7) |
| Median (range) | 30.5 (0.3–101.2) |
| Adult-onset AD, n (%)a | 59,638 (76.8) |
| Severity of AD, n (%)b | |
| Mild | 71,986 (92.7) |
| Moderate to severe | 5696 (7.3) |
| Treatment modalities during the pandemic | |
| Immunosuppressive agents, n (%)c | 597 (0.8) |
| Azathioprine | 127 (0.2) |
| Mycophenolate mofetil | 71 (0.1) |
| Methotrexate | 71 (0.1) |
| Cyclosporine | 92 (0.1) |
| Dupilumab | 236 (0.3) |
| Phototherapy, n (%)d | 472 (0.6%) |
| Treatment modalities anytime during the course of the diseases | |
| Immunosuppressive agents, n (%)c | 1038 (1.3) |
| Phototherapy, n (%)d | 2163 (2.8) |
AD atopic dermatitis, BMI body mass index, SD standard deviation
aDefined when the onset of AD follows the age of 18 years
bModerate-to-severe cases of AD were defined in those who were managed by systemic drugs and/or phototherapeutic modalities anytime during the course of AD
cAzathioprine, mycophenolate mofetil, methotrexate, cyclosporine, dupilumab
dUltraviolet B or psoralen and ultraviolet A
The mean (standard deviation) age of the study participants at the onset of AD was 34.2 (19.7) years. The vast majority of patients featured a mild course of AD (n = 71,986; 92.7%), whereas 5696 (7.3%) patients had a moderate-to-severe disease course. During the pandemic, 597 (0.8%) and 472 (0.6%) patients were managed by immunosuppressive agents and phototherapy, respectively (Table 1).
Frequency and Predictors of SARS-CoV-2 Vaccination Among Patients with AD
As of 26 June, 2021, 58,582 (75.4%) patients completed two BNT162b2 vaccine doses. We then evaluated the determinants of fully vaccinated patients with AD as compared to their non-vaccinated counterparts (Table 2). In a multivariate model adjusting for age, sex, and ethnicity, older age (≥ 33.2 years; adjusted OR, 2.29; 95% CI 2.21–2.37; p < 0.001), Jewish ethnicity (adjusted OR, 1.60; 95% CI 1.53–1.67; p < 0.001), high socioeconomic status (adjusted OR, 1.32; 95% CI 1.30–1.35; p < 0.001), adulthood-onset AD (adjusted OR, 1.34; 95% CI 1.28–1.40; p < 0.001), and moderate-to-severe AD (adjusted OR, 1.13; 95% CI 1.05–1.21; p = 0.001) were all found to predict an increased vaccination rate. Of interest, patients with AD managed by immunosuppressive agents did not exhibit an increased prevalence of vaccination (adjusted OR, 1.16; 95% CI 0.94–1.42; p = 0.171).
Table 2.
Determinants of COVID-19 vaccination among patients with AD
| Prevalence in fully vaccinated AD patients, n (%); (N = 58,582) | Prevalence in non-fully vaccinated AD patients, n (%); (N = 19,100) | Univariate OR | 95% confidence interval | p value | Multivariate ORd | 95% confidence interval | p value | |
|---|---|---|---|---|---|---|---|---|
| Age at the onset of pandemic ≥ 33.2 yearsa | 37,544 (64.1) | 8095 (42.4) | 2.43 | 2.35–2.51 | < 0.001 | 2.29 | 2.21–2.37 | < 0.001 |
| Male sex | 22,518 (38.4) | 7355 (38.5) | 0.99 | 0.96–1.03 | 0.864 | 1.01 | 0.98–1.05 | 0.454 |
| Jewish ethnicity (vs Arab ethnicity) | 51,074 (87.2) | 14,895 (78.0) | 1.92 | 1.84–2.00 | < 0.001 | 1.60 | 1.53–1.67 | < 0.001 |
| High SES (vs low and intermediate) | 17,057 (29.1) | 3351 (17.5) | 1.93 | 1.85–2.01 | < 0.001 | 1.32 | 1.30–1.35 | < 0.001 |
| Adult-onset AD (vs childhood-onset AD) | 47,535 (81.1) | 12,103 (63.4) | 2.49 | 2.40–2.58 | < 0.001 | 1.34 | 1.28–1.40 | < 0.001 |
| Moderate-to-severe AD (vs mild AD)b | 4573 (7.8) | 1123 (5.9) | 1.36 | 1.27–1.45 | < 0.001 | 1.13 | 1.05–1.21 | 0.001 |
| Immunosuppressive agents during the pandemicc | 479 (0.8) | 118 (0.6) | 1.33 | 1.08–1.62 | 0.006 | 1.16 | 0.94–1.42 | 0.171 |
| Phototherapy during the pandemic | 373 (0.6) | 99 (0.5) | 1.23 | 0.99–1.54 | 0.067 | 1.10 | 0.87–1.37 | 0.433 |
| Cardiovascular diseases | 4,667 (8.0) | 822 (4.3) | 1.93 | 1.78–2.08 | < 0.001 | 1.32 | 1.22–1.43 | < 0.001 |
| Metabolic syndrome | 5753 (9.8) | 973 (5.1) | 2.03 | 1.89–2.18 | < 0.001 | 1.36 | 1.27–1.47 | < 0.001 |
| COPD | 1372 (2.3) | 261 (1.4) | 1.73 | 1.52–1.98 | < 0.001 | 1.17 | 1.02–1.34 | 0.024 |
| Asthma | 8956 (15.3) | 2945 (15.4) | 0.99 | 0.95–1.04 | 0.663 | 1.00 | 0.96–1.05 | 0.923 |
| Smoking | 18,745 (32.0) | 5063 (26.5) | 1.31 | 1.26–1.35 | < 0.001 | 1.08 | 1.04–1.12 | < 0.001 |
| Cirrhosis | 126 (0.2) | 27 (0.1) | 1.52 | 1.00–2.31 | 0.046 | 1.05 | 0.69–1.60 | 0.802 |
| Chronic renal failure | 1287 (2.2) | 246 (1.3) | 1.72 | 1.50–1.98 | < 0.001 | 1.19 | 1.03–1.37 | 0.016 |
| Malignancy | 4369 (7.5) | 743 (3.9) | 1.99 | 1.84–2.16 | < 0.001 | 1.41 | 1.30–1.53 | < 0.001 |
| Depression | 3872 (6.6) | 946 (5.0) | 1.36 | 1.26–1.46 | < 0.001 | 0.99 | 0.92–1.07 | 0.811 |
Bold: significant values
AD atopic dermatitis, COPD chronic obstructive pulmonary disease, COVID-19 coronavirus disease 2019, OR odds ratio, SES socioeconomic status
aDichotomy of this continuous variable was based on its median value
bDisease severity and intake of oral systemic agents during the pandemic were collapsed as a single variable in the regression model because of significant collinearity between the two variables
cAzathioprine, mycophenolate mofetil, methotrexate, cyclosporine, dupilumab
dAdjusted for age, sex, and ethnicity
A greater vaccination rate was also observed among patients with AD with the following comorbidities: cardiovascular disease (adjusted OR, 1.32; 95% CI 1.22–1.43; p < 0.001), metabolic syndrome (adjusted OR, 1.36; 95% CI 1.27–1.47; p < 0.001), chronic obstructive pulmonary disease (adjusted OR, 1.17; 95% CI 1.02–1.34; p = 0.024), chronic renal failure (adjusted OR, 1.19; 95% CI 1.03–1.37; p = 0.016), and malignancy (adjusted OR, 1.41; 95% CI 1.30–1.53; p < 0.001; Table 2).
Efficacy of COVID-19 Vaccination in Patients with AD
The incidence rate of SARS-CoV-2 infection, COVID-19-associated hospitalization, and COVID-19-associated mortality in vaccinated patients was estimated at 6.2 (95% CI 5.1–7.5), 0.5 (95% CI 0.3–1.0), and 0.1 (95% CI 0.02–0.4), per 1000 person-years, respectively. The corresponding incidence rates in non-vaccinated patients were 34.3 (95% CI 29.9–39.2), 6.7 (95% CI 4.8–8.9), and 1.5 (95% CI 0.7–2.7) per 1000 person-years, respectively (Table 3). Vaccinated patients with AD demonstrated a significantly decreased risk of SARS-CoV-2 infection (fully adjusted HR, 0.20; 95% CI 0.16–0.26; p < 0.001), COVID-19-associated hospitalization (fully adjusted HR, 0.08; 95% CI 0.04–0.18; p < 0.001), and COVID-19-associated mortality (HR, 0.04; 95% CI 0.01–0.20; p < 0.001; Table 3).
Table 3.
Risk of COVID-19 and its complications among fully vaccinated patients with AD compared to non-vaccinated patients with AD
| COVID-19 infection | COVID-19-associated hospitalization | COVID-19-associated mortality | ||||
|---|---|---|---|---|---|---|
| Fully vaccinated until February/2021 (N = 42,433) | Non-vaccinated until February/2021 (N = 19,100) | Fully vaccinated until February/2021 (N = 42,433) | Non-vaccinated until February/2021 (N = 19,100) | Fully vaccinated until February/2021 (N = 42,433) | Non-vaccinated until February/2021 (N = 19,100) | |
| Follow-up time, PY | 16,714.6 | 6,116.4 | 16,744.8 | 6,163.1 | 16,747.0 | 6,172.3 |
| Median follow-up time, months (range) | 4.8 (0.2–5.5) | 3.9 (0.2–3.9) | 4.8 (0.2–5.5) | 3.9 (0.2–3.9) | 4.8 (0.7–5.5) | 3.9 (0.1–3.9) |
| Number of events | 104 | 210 | 9 | 41 | 2 | 9 |
| Incidence rate/1000 PY (95% CI) | 6.2 (5.1–7.5) | 34.3 (29.9–39.2) | 0.5 (0.3–1.0) | 6.7 (4.8–8.9) | 0.1 (0.02–0.4) | 1.5 (0.7–2.7) |
| Unadjusted HR (95% CI) [p value] | 0.21 (0.17–0.27) [< 0.001] | Reference | 0.14 (0.06–0.28) [< 0.001] | Reference | 0.10 (0.02–0.46) [0.003] | Reference |
| Age-adjusted and sex-adjusted HR (95% CI) [p value] | 0.19 (0.15–0.24) [< 0.001] | Reference | 0.08 (0.04–0.17) [< 0.001] | Reference | 0.04 (0.01–0.17) [< 0.001] | Reference |
| Fully adjusted HR (95% CI) [p value]a | 0.20 (0.16–0.26) [< 0.001] | Reference | 0.08 (0.04–0.18) [< 0.001] | Reference | 0.04 (0.01–0.20) [< 0.001] | Reference |
Bold: significant values
AD atopic dermatitis, CI confidence interval, COVID-19 coronavirus disease 2019, HR hazard ratio, NA non-applicable, PY person-years
aMulti-variate logistic regression model adjusting for age, sex, ethnicity, cardiovascular diseases, metabolic syndrome, chronic obstructive pulmonary disorder, chronic renal failure, malignancy, and smoking
We then evaluated the influence of immunosuppressive drugs and phototherapeutic modalities on the effectiveness of the BNT162b2 vaccine in preventing SARS-CoV-2 infection (Fig. 1). The risk of SARS-CoV-2 infection was not significantly different among vaccinated patients with AD managed by immunosuppressive drugs (adjusted HR, 0.95; 95% CI 0.13–6.81; p = 0.958) or phototherapy (adjusted HR, 1.28; 95% CI 0.18–9.18; p = 0.807) during the pandemic. In a sensitivity analysis, we included all patients with AD managed by immunosuppressants and phototherapy anytime during the course of the disease. Neither anytime exposure to immunosuppressive drugs (adjusted HR, 1.80; 95% CI 0.57–5.69; p = 0.315) nor to phototherapy (adjusted HR, 0.81; 95% CI 0.26–2.56; p = 0.718) influenced the utility of the vaccine in protecting against SARS-CoV-2 infection (Fig. 1).
Fig. 1.
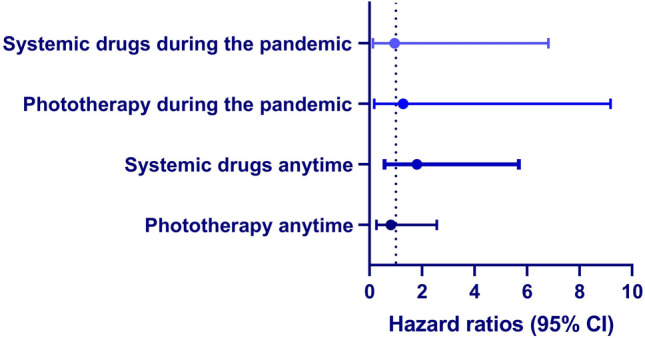
Fully adjusted hazard ratios of severe acute respiratory syndrome coronavirus 2 infection in patients with atopic dermatitis treated with immunosuppressive drugs and phototherapy. CI confidence interval
Discussion
The current population-based study demonstrated that the vast majority of patients with AD in Israel were vaccinated against SARS-CoV-2 during the national mass vaccination program. Patients with adult-onset and moderate-to-severe AD were more likely to get vaccinated. Older age, as well as the presence of comorbid cardiovascular diseases, metabolic syndrome, chronic obstructive pulmonary disorder, chronic renal failure, and malignancy projected an increased frequency of vaccination. Among patients with AD, the BNT162b2 vaccine conferred an 80%, 92%, and 96% protection against SARS-CoV-2 infection, COVID-19-associated hospitalization, and COVID-19-associated mortality, respectively. Exposure to immunosuppressive agents and phototherapy was not associated with an increased risk of SARS-CoV-2 infection after vaccination.
In view of the data provided by Israel’s Ministry of Health, 77.2% of the adult population of Israel was fully vaccinated against SARS-CoV-2 until 26 June, 2021 (https://datadashboard.health.gov.il/COVID-19/general). The current study denotes that the vaccination rate among adults with AD (75.4%) was comparable to the general population. Our findings do not thoroughly align with a recent study displaying a limited SARS-CoV-2 vaccine willingness in patients with autoimmune and inflammatory rheumatic diseases [13]. Interpretation for this discrepancy should be further delineated. However, recruiting individuals with a self-reported diagnosis of these diseases (captured via social media), the aforementioned study was prone to a selection bias that might have accounted for the discordant findings. These patients have supposedly more severe disease and do not represent the entire repertoire of patients with autoimmune and inflammatory rheumatic diseases [13].
The increased frequency of vaccination among older patients and those with severe comorbidities is conceivable as these patients have been disproportionately affected by COVID-19 and therefore have been prioritized for vaccination. Patients with moderate-to-severe AD were found to be vaccinated more often. This finding might actually mirror, at least in part, the fact that patients taking immunosuppressive drugs are prioritized for vaccination. The propensity of patients with a high socioeconomic status to be vaccinated might arise from the association of vaccine willingness with a greater formal education [14].
Our data indicated that the protection conferred by mRNA vaccines against SARS-CoV-2 infection was not hampered in patients managed by immunomodulatory agents. This finding lends credibility to a German study examining the immunogenicity of SARS-CoV-2 mRNA vaccines in 26 patients with chronic inflammatory diseases receiving immunosuppressive therapy relative to 40 healthy controls [15]. In the latter study, vaccines induced production of anti-SARS-CoV-2 IgG and neutralizing antibodies in all eligible iatrogenically immunosuppressed patients [15]. Correspondingly, a recent study demonstrated that BNT162b2 mRNA vaccines induced adequately increased levels of SARS-CoV-2 spike-specific IgG in more than 90% of patients with immune-mediated inflammatory diseases managed by biologic treatments [9]. Nevertheless, only 62.6% of patients taking methotrexate developed immunogenicity following vaccination [9]. Given the small sample size of exposed patients, our study was underpowered to analyze the influence of methotrexate on the efficacy of mRNA vaccines among patients with AD.
Some concerns were raised about vaccine-induced Th2 immunopathology, in which a faulty T-cell response triggers allergic inflammation that potentially damages the airways and leads to a more severe COVID-19 infection [16, 17]. This concern might be of further relevance in a Th2-predominant disease like AD. Notwithstanding, our results revealed a satisfactory efficacy of mRNA COVID-19 vaccination in line with data accumulated from other vaccines [18].
The current study conveys important population-based insights about the determinants and efficacy of SARS-CoV-2 mRNA vaccinations among patients with AD. The large sample size and the comprehensive nature of the study population enabled us to explore the impact of vaccinations on COVID-19-related prognostic outcomes and to provide a representative view of this important topic. The rapid pace and high uptake of the COVID-19 vaccine in Israel enabled us to evaluate the effectiveness of the BNT162b2 vaccine in a practical application. The current study has several limitations to acknowledge. The low number of patients with COVID-19-associated hospitalization and mortality interfered with the evaluation of the influence of different treatment regimens on these outcomes. Lack of documentation regarding the standardized severity scores necessitated the utilization of indirect measurements to evaluate disease severity. Although the study relied on diagnostic codes registered by certified dermatologists, misclassification and inclusion of other types of dermatitis could not be thoroughly excluded. The different demographic and clinical features of the fully vaccinated group relative to the non-vaccinated groups embody another limitation of the current study. The definition of drug exposure does not thoroughly guarantee that the patients received continuous treatment. The low number of patients undergoing immunosuppressant and phototherapy treatment is another limitation of the study. The low number of positive outcomes interfered with the conduct of a meaningful stratified drug-specific analysis.
Conclusions
Patients with AD displayed a very high vaccination rate. Patients with adult-onset and moderate-to-severe AD, as well as those with older age and severe comorbid conditions, were more likely to get vaccinated. The BNT162b2 vaccine decreased the risk of SARS-CoV-2 infection, COVID-19-associated hospitalization, and mortality by 80, 92, and 96%, respectively. In the small percentage of patients taking immunosuppressive drugs or undergoing phototherapy, these treatments did not hamper the effectiveness of the vaccine in protecting against SARS-CoV-2 infection.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
Open Access funding enabled and organized by Projekt DEAL. No sources of funding were received for the conduct of this study of the preparation of this article.
Conflict of interest
ADC served as an advisor, investigator, or speaker for AbbVie, BI, Dexcel Pharma, Janssen, Novartis, Perrigo, Pfizer, and Rafa. None of the other authors has any conflicts of interest to declare.
Ethics approval
The study was approved by the institutional review board in accordance with the Declaration of Helsinki (approval code: 0212-17-COM).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Author contributions
KK and ADC had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: KK and ADC. Acquisition, analysis, and interpretation of data: KK, DTB, and TL. Drafting of the manuscript: KK and YS. Critical revision of the manuscript for important intellectual content: KK, ADC, DTB, EO, TL. Statistical analysis: OW and YS. Obtained funding: None. Administrative, technical, or material support: ADC, EO, and OW. Study supervision: KK and ADC.
Footnotes
Orly Weinstein and Arnon D. Cohen contributed equally to the article.
References
- 1.Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson L, Anderson E, Rouphael N, Roberts P, Makhene M, Coler R, et al. An mRNA vaccine against SARS-CoV-2: preliminary report. N Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack F, Thomas S, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stefanovic N, Flohr C, Irvine AD. The exposome in atopic dermatitis. Allergy. 2020;75:63–74. doi: 10.1111/all.13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kridin K, Schonmann Y, Tzur Bitan D, Damiani G, Weinstein O, Cohen A. The burden of coronavirus disease 2019 and its complications in patients with atopic dermatitis: a nested case–control study. Dermatitis. 2021;32:S45–52. doi: 10.1097/DER.0000000000000772. [DOI] [PubMed] [Google Scholar]
- 7.Cohen AD, Dreiher J, Regev-Rosenberg S, Yakovson O, Lieberman N, Goldfracht M, et al. The quality indigators program in Clalit Health Services: the first decade. Harefuah. 2010;149(204–9):265. [PubMed] [Google Scholar]
- 8.Deepak P, Kim W, Paley MA, Yang M, Carvidi AB, El-Qunni AA, et al. Glucocorticoids and B cell depleting agents substantially impair immunogenicity of mRNA vaccines to SARS-CoV-2. medRxiv. 2021.
- 9.Haberman RH, Herati R, Simon D, Samanovic M, Blank RB, Tuen M, et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis. 2021;26:593. doi: 10.1136/annrheumdis-2021-220597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cleeman JI. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) J Am Med Assoc. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 11.Alberti KGMM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 12.Kridin K, Solomon A, Tzur-Bitan D, Damiani G, Comaneshter D, Cohen AD. Acne keloidalis nuchae and the metabolic syndrome: a population-based study. Am J Clin Dermatol. 2020;21:733–739. doi: 10.1007/s40257-020-00541-z. [DOI] [PubMed] [Google Scholar]
- 13.Felten R, Dubois M, Ugarte-Gil MF, Chaudier A, Kawka L, Bergier H, et al. Vaccination against COVID-19: expectations and concerns of patients with autoimmune and rheumatic diseases. Lancet Rheumatol. 2021;3:e243–e245. doi: 10.1016/S2665-9913(21)00039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai R, Hervey J, Hoffman KD, Wood J, Novack J, Johnson J, et al. COVID-19 vaccine hesitancy among individuals with cancer, autoimmune diseases, and other serious comorbid conditions. medRxiv. 2021. [DOI] [PMC free article] [PubMed]
- 15.Geisen UM, Berner DK, Tran F, Sümbül M, Vullriede L, Ciripoi M, et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis. 2021;80:1306–1311. doi: 10.1136/annrheumdis-2021-220272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tirado SMC, Yoon KJ. Antibody-dependent enhancement of virus infection and disease. Viral Immunol. 2003;16:69–86. doi: 10.1089/088282403763635465. [DOI] [PubMed] [Google Scholar]
- 17.Bolles M, Deming D, Long K, Agnihothram S, Whitmore A, Ferris M, et al. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J Virol. 2011;85:12201–12215. doi: 10.1128/JVI.06048-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blauvelt A, Simpson EL, Tyring SK, Purcell LA, Shumel B, Petro CD, et al. Dupilumab does not affect correlates of vaccine-induced immunity: a randomized, placebo-controlled trial in adults with moderate-to-severe atopic dermatitis. J Am Acad Dermatol. 2019;80:158–67.e1. doi: 10.1016/j.jaad.2018.07.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


