Abstract
Background
Breast cancer (BC) screening can be performed in a screening program (BCSP) or in opportunistic screening. The existing reviews on the determinants of non-participation depend on self-reported data which may be biased. Furthermore, no distinction was made between the probably different determinants of both screening strategies.
Objective
To find the determinants of non-participation in BCSP by means of a meta-analysis.
Methods
PubMed, Embase, and Web of Science were searched for observational studies which quantified factors associated with non-participation in BCSP in a general population. Studies on opportunistic screening and studies using self-reported data were excluded. A random-effect model was used to calculate pooled odds ratios (ORs) and 95% confidence intervals (CIs). Potential sources of heterogeneity were explored by stratification of the results.
Results
Twenty-nine studies with in a total of 20,361,756 women were included. Low income (OR: 1.20, 95% CI: 1.10–1.30), low education (OR: 1.18, 95% CI: 1.05–1.32), living far from an assigned screening unit (OR: 1.15, 95% CI: 1.07–1.24), being immigrant (OR: 2.64, 95% CI: 2.48–2.82), and having a male family doctor (OR: 1.43, 95% CI: 1.20–1.61) was associated with higher non-participation in screening. Reminders sent to non-attenders and estimations of ORs (adjusted or not) partly explained substantial heterogeneity.
Conclusion
In this meta-analysis excluding studies on the non-participation in opportunistic screening, or with self-reported data on non-participation, the well-known determinants for non-participation are still significant, but less strong. This analysis only supports the relevance of meta-analysis of studies with registered non-participation in a BCSP.
Systematic Review Registration
PROSPERO, CRD42020154016.
Keywords: breast cancer, mammography, mass screening, participation, determinant
Introduction
Breast cancer (BC) is the most frequent cause of female cancer death (1) and accounts for an estimated 11.6% of the total cancer deaths worldwide in 2018 (2). The risk of BC death can be reduced by 20% when BCs are detected at early stages by mammography screening (3). A breast cancer screening program (BCSP) with mammography is therefore widely advised for early BC detection (4). Compared with opportunistic BC screening that provides mammography screening on request of women and depends on the healthcare insurance of women (5), a BCSP is population-based and characterized by actively inviting women to BC screening and comprehensive quality assurance activities such as training and audit of the program (6).
Sufficiently high participation is a crucial element for the success of a BCSP. To ensure the performance and the public health impact of the population-based BC screening program, a 70% participation rate is recommended as an acceptable level of participation by the European guidelines for quality assurance in breast cancer screening and diagnosis (6). While European countries had one of the earliest provided BCSP since 1986 (7, 8), the average level of screening participation in Europe was only 57.4% (range 27.4–82.6%) in 2016 (9). Outside Europe, BCSP has an even lower participation rate ranging from 18.1 to 55.3% in 2016 (9, 10).
There are several systematic reviews on determinants of non-participation in BC screening (5, 11–17). Main determinants for non-participation reported thus far are low income, low education, living in a rural area, being an immigrant, and comorbidity. However, these systematic reviews either combined results from BCSP and opportunistic screening settings, or included self-reported non-participation in BC screening. Studies showed that the self-reported non-participation tend to be over-reported by women (18, 19). Determinants of non-participation have not been reviewed and meta-analyzed specifically for registry data from BCSP. Therefore, we aimed to evaluate determinants of screening non-participation with registry based studies, namely, recent publications with meta-analysis.
Methods
We conducted a systematic review according to the guideline of the Cochrane Collaboration (20) and reported the results following the guideline of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (21). The protocol of this systematic review was registered on PROSPERO (record number CRD42020154016).
Search Strategy and Study Selection
Articles were identified in PubMed, Embase, and Web of Science. All databases were searched for studies published between January 1, 2010, and October 31, 2021. The search start year of 2010 was selected to balance the recency and efficiency as the screening guidelines and macro-social demographic factors changed over the last years. A detailed search strategy per database can be found in the Supplementary File. Additionally, the reference lists in the retrieved articles were searched to identify additional studies.
Inclusion and Exclusion Criteria
Observational studies were included if they examined the relationship between determinants and the non-participation of a BCSP with mammography and were published in English. The non-participation in a BCSP was defined as the proportion of women who did not participate in the mammography screening within a required screening interval of a BCSP among all invited women. Studies were excluded in one of the following cases: the non-participation in an opportunistic BC screening was studied, the screening participation data were collected through self-reporting of participants, and determinants of screening re-attendance were studied. Besides, case reports, letters, comments, editorials, reviews, and conference abstracts were excluded.
Two reviewers (LD, JW) independently conducted the screening of articles first based on title and abstract and then based on full text. Disagreements encountered were resolved through discussion or adjudicated by a third reviewer (GB).
Data Extraction and Quality Assessment
Two reviewers (LD, JW) independently extracted data regarding study characteristics (author, publication year, country, screening period and population size, determinants of non-participation, and non-participation rate), organizational characteristics of a BCSP (targeted age, screening interval, follow-up strategy and payment of screening), and odds ratio (OR) of the determinants of non-participation. In case the association represents determinants and screening participation, ORs were recalculated by 1/OR. The corresponding 95% confidence intervals (CIs) were recalculated likewise. If available, adjusted odds ratios (ORs) with 95% CIs were extracted. Otherwise, crude ORs and 95% CIs were extracted or calculated based on the number of screening non-attenders and attenders for each determinant (22). If multiple articles were published with data of the same study population, determinants in the article that reported the OR with the most adjusted model or with the largest sample size was selected. However, if the articles that were published from the same study reported multiple unique ORs for different determinants of screening non-participation, they were all included for the different determinants in the meta-analysis. The quality of the included studies was assessed with the critical Appraisal tool for Cross-Sectional Studies (AXIS) (23). The AXIS checklist intends to assess the validity and bias of cross-sectional studies with 20 questions in five domains, namely, study aim, methods, results, discussion, ethical approval, and funding (see Table S1).
Statistical Analysis
Determinants reported as categorical variables were dichotomized, in which the reference category applied in the study was tested against the other categories combined. OR and the corresponding 95% CI between the reference group and combined category was calculated (22). Estimates of continuous variables were included or if needed, transformed from regression coefficients to ORs and 95% CIs. The inconsistency (I2) test was used to measure heterogeneity. Under the assumption of heterogeneity, a meta-analysis using a random-effects model was performed for each determinant for which at least three studies were available. For each determinant, a stratified analysis was performed to explore the sources of heterogeneity. Based on the published studies, the factors that were related to the heterogeneity of non-participation were considered as stratified factors which included the type of invitation (any invitation or the first invitation), the interval of screening (24 months or 36 months), study region (North America, Europe or Asia), payment of screening (free or co-payment), reminders for non-attenders (yes or no) and estimations of ORs (adjusted or not). For the dichotomized determinants, the heterogeneity caused by the different categorization of determinants was also explored in the stratified analyses in which studies applied different categorizations were pooled separately. A sensitivity analysis was performed to evaluate the robustness of the pooled estimates by sequentially removing each study (24). Publication bias was estimated using a funnel plot and assessed formally with Begg’s test. All statistical analyses were performed with Stata 14 (StataCorp LP, College Station, TX, USA).
Results
Characteristics of the Included Studies
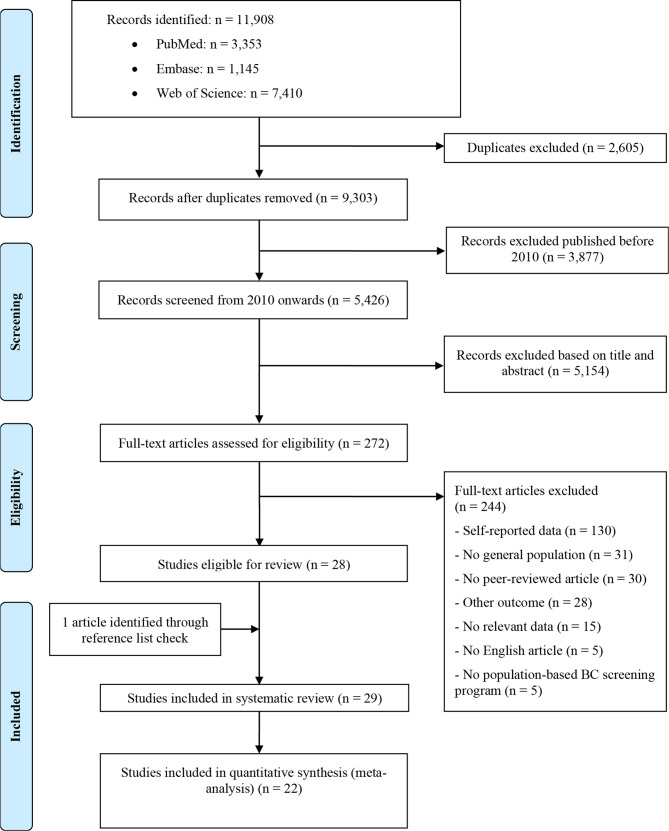
A total of 11,239 studies were identified in the search. A review of 5,299 titles and abstracts and 272 full texts resulted in 29 studies for the systematic review (Figure 1). Studies were from 11 countries where a BCSP was established (Canada, Denmark, Sweden, Norway, the United Kingdom (UK), France, Germany, the Netherlands, Israel, South Korea, and Australia). The total number of women in the included studies was 20,361,756, of which 14,944,899 were included in the meta-analysis. Three large studies from Asian countries (Korea, Israel, and Australia) took half of the total population size. The rest of the included women were of European or Canadian origin. The characteristics of the included studies are summarized in Table 1 (25–53). Twenty-two studies were included in the meta-analysis (Table S3) (26–28, 31–41, 44, 46, 47, 49–53).
Figure 1.
Flow chart of the study selection.
Table 1.
Characteristics of the included studies.
| Author, year | Country, screening year | Data source | Number of women | Target screening age, years | Screening interval, month | Fully subsidized | Reminder for all non-attenders | Non-participation % | Meta-analyzed determinants* |
|---|---|---|---|---|---|---|---|---|---|
| Hellmann (25) | Denmark, 1993–1999 | Copenhagen mammographic screening register; Danish Diet, Cancer, and Health cohort baseline data | 5,134 | 50–64 | 24 | yes | yes | 10.8 | – |
| Vahabi (26) | Canada, 2010–2012 | Citizenship and Immigration Canada database; Ontario Cancer Registry; Ontario BC Screening Program database | 1,407,060 | 50–69 | 24 | yes | no | 36.0 | Income level, place of residence, gender of family physician |
| Jack (27) | UK, 2006–2009 | London Quality Assurance Reference Centre database | 159,078 | 50–52 | 36 | yes | no | 39.0 | Income level |
| Woods (28) | Canada, 2013–2014 | Screening Mammography Program of British Columbia database; BC Cancer Registry database; Medical Services Plan physician payment file; Citizenship and Immigration Canada database | 537,783 | 50–69 | 24 | yes | yes | 49.7 | Age of women, income level, number of comorbidities |
| Woodhead (29) | UK, 2010–2013 | Clinical Record Interactive Search Lambeth DataNet | 26,010 | 50–70 | 36 | yes | no | 44.2 | – |
| Price (30) | UK, 2000–2002 | Warwickshire, Solihull and Coventry Breast Screening Service database | 18,730 | 50–70 | 36 | yes | no | 20.7 | – |
| Guillaume (31) | France, 2003–2012 | French cancer screening management database | 64,102 | 50–74 | 24 | yes | yes | 49.9 | Age of women, income level, distance to an assigned screening unit |
| Vigod (32) | Canada, 2002–2004 | Ontario Breast Screening Program; Ontario Health Insurance Plan; Ontario Cancer Registry; Canadian Community Health Survey database | 1,403 | 50–68 | 24 | yes | no | 39.2 | Education level, number of comorbidities, marital status |
| Renshaw (33) | UK, 2004–2007 | London Quality Assurance Reference Centre database | 742,786 | 50–70 | 36 | yes | no | 37.9 | Age of women, income level |
| Ouédraogo (34) | France, 2010–2011 | French cancer screening management database | 13,565 | 50–74 | 24 | yes | yes | 47.5 | Age of women, income level, place of residence |
| St-Jacques (35) | Canada, 2006–2008 | Information system of the Quebec BC Screening Program; comprehensive Quebec Health Insurance Plan database | 833,856 | 50–69 | 24 | yes | yes | 47.9 | Age of women, income level, place of residence, distance to an assigned screening unit |
| Jensen (36) | Denmark, 2008–2009 | Central Denmark regional cancer screening administrative database; Danish Cancer Registry; Statistics Denmark | 144,264 | 50–69 | 24 | yes | no | 21.1 | Income level, distance to an assigned screening unit, immigration status |
| Le (37) | Norway, 1996–2015 | Cancer Registry of Norway’s databases; Statistics Norway | 885,979 | 50–69 | 24 | no | yes | 26.0 | Age of women, income level, education level, marital status, immigration status, |
| Zidar (38) | Sweden, 2011–2012 | Radiological Information System; Statistics Sweden; Public Health Agency of Sweden; National Board of Health and Welfare; Swedish Social Insurance Agency | 52,541 | 50–74 | 24 | no | no | 19.0 | Age of women, distance to an assigned screening unit |
| Jensen (39) | Denmark, 2008–2009 | Central Denmark regional cancer screening administrative database; Danish Cancer Registry; Statistics Denmark; Danish National Patient Registry; Danish Psychiatric Central Research Register | 144,264 | 50–69 | 24 | yes | no | 21.1 | Age of women, education level, number of comorbidities, marital status |
| McDonald (40) | Canada, 1996–2011 | Medicare Decision Support System; BC screening service database; Provincial Cancer Registry; Vital Statistics database of the Province of New Brunswick, Canada | 91,917 | 50–69 | 24 | yes | yes | 45.0 | Income level, place of residence, distance to an assigned screening unit, education level, marital status |
| Berens (41) | Germany, 2010–2011 | Routine data from screening units and population registries in Duisburg, Bielefeld, Paderborn, Hamburg, and Berlin, Germany | 423,649 | 50–69 | 24 | yes | no | 50.8 | Age of women |
| Jensen (42) | Denmark, 2008–2009 | Central Denmark regional cancer screening administrative database; Danish Cancer Registry; Statistics Denmark; Danish National Patient Registry; Danish Psychiatric Central Research Register | 4,512 | 50–69 | 24 | yes | no | 14.9 | – |
| Jensen (43) | Denmark, 2008–2009 | Central Denmark regional cancer screening administrative database; Danish Cancer Registry; Statistics Denmark; Health Survey database in the Central Denmark Region | 4,512 | 50–69 | 24 | yes | no | 14.9 | – |
| Pornet (44) | France, 2004–2006 | Database of the Association Mathilde, in charge of organizing BCS in Calvados; health insurance organizations database | 4,865 | 50–74 | 24 | yes | yes | 44.3 | Age of women, income level, |
| Larsen (45) | Denmark, 2008–2009 | Central Denmark regional cancer screening administrative database; Danish Cancer Registry; Statistics Denmark; National Patient Register; National Pathology Data Bank | 91,787 | 50–64 | 24 | yes | no | 20.2 | – |
| Jensen (46) | Denmark, 2008–2009 | Department for Public Health Programs database, Central Denmark Region; Statistics Denmark; Danish National Board of Health | 13,288 | 50–69 | 24 | yes | no | 19.0 | Gender of family physician |
| Wilf-Miron (47) | Israeli, 2006–2008 | Maccabi Healthcare Services (MHS) computerized billing system; MHS computerized Performance Measurement System; Israeli Census for data on socio-economic status ranks and ethnicity | 157,928 | 50–74 | 24 | yes | yes | 31.2 | Age of women, income level |
| Roder (48) | Australia, 2001–2005 | Australian BreastScreen program database; Australian Institute of Health and Welfare database | 5,366,983 | 50–69 | 24 | yes | no | 44.9 | – |
| Tavasoli (49) | Canada, 2013–2015 | Integrated Client Management System database for cancer screening program; Ontario Health Insurance Plan’s Claims History databases; Ontario Cancer Registry and Pathology Information Management System; Client Agency Program Enrolment database and Corporate Providers Database; Canadian Institute of Health Information Discharge Abstract Database and National Ambulatory Care Reporting System | 1,173,456 | 52–69 | 24 | yes | no | 47.6 | Age of women, income level, place of residence, family number of comorbidities, gender of physician |
| Vermeer (50) | The Netherlands, 2007–2008 | Database of regional screening organizations | 1,279,982 | 50–75 | 24 | yes | yes | 18.0 | Immigration status |
| O’Reilly (51) | UK, 2001–2004 | Northern Ireland Quality Assurance Reference Centre; database of the Northern Ireland Longitudinal Study | 37,059 | 48–64 | 36 | yes | no | 24.9 | Age of women, place of residence, education level, number of comorbidities, marital status |
| Shin (52) | Korea, 2014–2015 | Korean National Health Information Database | 6,283,623 | ≥40 | 24 | no | no | 40.9 | Income level, place of residence |
| Viuff (53) | Denmark, 2007–2010 | The Danish Quality Database for Mammography Screening; The Danish National Patient Registry | 650,003 | 50–69 | 24 | yes | no | 20.2 | Age of women, number of comorbidities |
*A full list of all the determinants reported by each study is shown in Table S2.
The risk of bias of the included studies is presented in Table S2. Requirements that were not satisfied were found for: sample size justification was unclear or missing in 10.3% of the studies; no measures were undertaken to address non-responders in 6.8% of the studies; basic data were not adequately described in 20.7% of the studies; limitations of the study were not discussed in 20.7% of the studies; sources of funding and conflicts of interest were not indicated in 6.8% of the studies, and ethical approval or consent of participants was not indicated in 17.2% of the studies.
Pooled Estimates of the Determinants of Screening Non-Participation
Of all the 24 identified determinants (Tables 1 and S3), nine were included for the meta-analysis. The other determinants were reported by less than three studies or had inconsistent definition were not meta-analyzed. The characteristics of the studies that reported these determinants are described in Table 1. All the determinates reported by the included studies are reported in Table S3. Having low income (OR: 1.20, 95% CI: 1.10–1.30), being in younger age (OR: 1.09, 95% CI: 1.01–1.18), having low education (OR: 1.18, 95% CI: 1.05–1.32), living far from an assigned screening unit (OR: 1.15, 95%CI: 1.07-1.24), being unmarried (OR: 1.68, 95% CI: 1.32–2.14), being an immigrant (OR: 2.64, 95% CI: 2.48–2.82), and having a male family physician (OR: 1.43, 95% CI: 1.20–1.61) was associated with a higher non-participation in screening (Table 2 and Figures S1–S5).
Table 2.
Summary of determinants of screening non-participation in breast cancer screening programs.
| Determinantsa | Number of studies | Number of womenb | Non-participation % | Odds ratio | 95% CI | I2% |
|---|---|---|---|---|---|---|
| Income level | 14 | 12,500,262 | 32.7 | 99.6 | ||
| highc | 1,42,962 | 11.9–49.7 | 1.00 | – | ||
| Low | 4,804,875 | 12.0–51.1 | 1.20 | 1.10–1.30 | ||
| Age of women | 14 | 5,721,776 | 31.5 | 99.8 | ||
| old | 1,060,746 | 8.0–53.3 | 1.00 | – | ||
| youngd | 4,545,696 | 12.6–52.0 | 1.09 | 1.01–1.18 | ||
| Place of residence | 7 | 9,342,846 | 27.9 | 99.5 | ||
| rurale | 528,624 | 12.4–51.3 | 1.00 | – | ||
| urban | 2,545,607 | 11.9–45.9 | 1.01 | 0.90–1.12 | ||
| Number of comorbidities | 6 | 2,412,969 | 22.6 | 99.5 | ||
| Zero | 2,101,610 | 12.0–51.7 | 1.00 | – | ||
| at least one | 423,951 | 11.0–46.4 | 1.04 | 0.84–1.28 | ||
| Education level | 5 | 1,160,622 | 24.6 | 90.6 | ||
| high | 73,651 | 19.8–29.0 | 1.00 | – | ||
| low f | 951,464 | 21.1–25.1 | 1.18 | 1.05–1.32 | ||
| Distance to an assigned screening unit | 5 | 1,186,680 | 43.6 | 94.5 | ||
| smallg | 549,621 | 18.0–54.0 | 1.00 | – | ||
| large | 538,237 | 20.1–47.6 | 1.15 | 1.07–1.24 | ||
| Marital status | 5 | 1,160,622 | 23.5 | 99.4 | ||
| marriedh | 620,694 | 17.3–22.0 | 1.00 | – | ||
| unmarried | 134,188 | 31.1–35.0 | 1.68 | 1.32–2.14 | ||
| Immigration status | 3 | 2,310,177 | 20.5 | 95.9 | ||
| non-immigrants | 2,210,697 | 15.7–25.0 | 1.00 | – | ||
| immigrantsi | 99,480 | 34.3–49.0 | 2.64 | 2.48–2.82 | ||
| Gender of family physician | 3 | 2,272,225 | 24.9 | 98.6 | ||
| Female | 949,434 | 12.7–29.0 | 1.00 | – | ||
| Male | 1,322,791 | 11.4–37.0 | 1.43 | 1.20–1.61 |
The first group of each determinant was the reference group.
For each determinant, the total number of women is larger than the sum of women in the stratified groups, because there are studies that only provided the effect size of a determinant without the cross-tables behind it.
The definition of high-income level varied in the included studies: “Most affluent 20%”, “most affluent 30%” and “most affluent 50% and above” was applied in 8, 2, and 4 studies, respectively. The heterogeneity related to the different definition of high income was explored in the stratified analyses.
The definition of old age varied in the included studies: “60–64”, “60–69”, “67–69”, “65–70” and “70–74” was applied in 1, 1, 1, 6. and 5 studies, respectively. The heterogeneity related to the different definition of old age was explored in the stratified analyses.
The definition of urban area was based on the population size in which the rural area was defined as area with less than 2,250 population in studies from UK. While the specific population size was not reported in studies from Canada and South Korea, the heterogeneity related to the different definition of rural area was explored in the stratified analyses.
The definition of low education level varied in the included studies: “<Secondary graduate”, “≤10 years education” and “<University graduate” were applied in 1, 2, and 2 studies, respectively. The heterogeneity related to the different definition of low education was explored in the stratified analyses.
The definition of small distance varied in included studies: “≤2.5 km”,” ≤5 km”,: “≤10 km”, and “≤20 km”, were applied in 1, 1, 1, and 2 studies, respectively. The heterogeneity related to the different definition of small distance was explored in the stratified analyses.
Married woman was defined as woman married or living with a partner.
Immigrant were defined as woman born abroad and both her two parents and four grandparents were born abroad.
Stratified Analysis and Source of Heterogeneity
Substantial heterogeneity was found among the studies that reported the above-noted nine determinants. The Index of Inconsistency (I2) ranged from 90.6 to 99.8% for the studies which reported the education level and reported the age of women, respectively (Table 2).
In the stratified analysis the heterogeneity decreased for the resident place when stratified by whether or not a reminder was sent to non-attendees. When there was no reminder for non-attendees, women living in an urban area showed a higher non-participation than those living in a rural area (OR: 1.14, 95% CI: 1.03–1.26). However, when a reminder was sent, women living in an urban area showed a lower non-participation than those living in a rural area (OR: 0.83, 95% CI: 0.82–0.84) (Table S4 and Figure S6). For education level, distance to an assigned screening unit, and marital status, whether a reminder was sent to non-attendees or not partly explained the heterogeneity across the studies, where the heterogeneity decreased in the stratified analysis (Table S4).
For income level, number of comorbidities, and marital status reporting adjusted estimate or not in the included studies partly explained the heterogeneity across the studies, where in these stratified groups the heterogeneity decreased (Table S4). The heterogeneity of the dichotomized determinants: age of women, education level, and distance to an assigned screening unit were partly explained by the different categorization of determinants. For example, the heterogeneity of the education level decreased from 90.6% for the overall estimate to 78.6% in the stratified group that defined ≤10 years education as low education (Tables 2 and S4). However, the heterogeneity in almost all stratified groups with different categorization of determinants remained above a substantial level (I2 >50%).
Sensitivity Analysis and Publication Bias
The pooled estimates of the determinants of screening participation were robust in the sensitivity analysis. The direction of the pooled estimates did not change when a single study was excluded sequentially (Figure S7). Publication bias was assessed for income and age of women. The Begg’s test of the asymmetry of the funnel plot did not reach statistical significance P = 0.743 and 0.661, respectively (Figures S8, S9).
Discussion
Main Results of This Review
In this meta-analysis excluding studies with self-reported data on non-participation in screening and/or studies on the non-participation in opportunistic screening, we found that lower income, younger age, lower education, living at a larger distance from an assigned screening unit, being unmarried, being an immigrant, and having a male family physician were associated with a higher non-participation in BCSPs. Women living in urban areas have higher non-participation in screening than women living in rural areas; however women living in urban areas have lower non-participation in screening when a reminder was sent to non-attenders. The heterogeneity of the pooled estimates was partially explained by whether or not a reminder was sent to non-attenders and whether or not the adjusted estimates were used.
Comparison With Published Studies
Compared with other meta-analyses that included non-participation data from opportunistic screening and/or self-reported data, we found significant yet less strong association estimates with a narrower 95% CI for the well-known determinants of non-participation in screening. In our study, low-income women were more likely to not participate in a BCSP than high-income women (OR: 1.20, 95% CI: 1.10–1.30), whereas a meta-analysis reported a larger effect size with a wider 95% CI of low-income on non-participation in screening (OR: 1.35, 95% CI: 1.22–1.49) (12). Low educated women were more likely to not participate in a BCSP than high educated women. The effect size of low education on non-participation in screening was larger in a meta-analysis (OR: 1.61, 95% CI: 1.36–1.91) than our study (OR: 1.18, 95% CI: 1.05–1.32) (11), and the 95% CI was wider than our study. Immigrants were more likely to not participate in a BCSP than non-immigrants. The effect size of immigrant status on non-participation in screening was smaller in a meta-analysis (OR: 1.85, 95% CI: 1.27–2.70) than our study (OR: 2.64, 95% CI: 2.48–2.82) (12), but the 95% CI was wider than our study.
The main possible reasons for the difference between our estimates and the published meta-analyses are two-fold. First, the registry and self-reported data were mixed and pooled together in these reviews published thus far. As women tend to over-report the utilization of BC screening, the estimates in these reviews can be influenced by recall bias (18). Second, determinants of screening participation of a BCSP were not studied separately from an opportunistic screening in these reviews. However, a BCSP and an opportunistic screening have different implementation strategies (4), and can cover different women groups in a population (54), and have different determinants of non-participation in screening (55). We, however, focused on population-based BC screening programs with registry data, which can avoid the recall bias. The smaller 95% CIs indicate that we provided more accurate estimates.
Interestingly, when a reminder was not applied, women living in an urban area were more likely to not participate in screening than women living in a rural area (OR: 1.14, 95% CI: 1.03–1.26). However, when a reminder was sent, women living in an urban area were related to lower non-participate in screening (OR: 0.83, 95% CI: 0.82–0.84) than women living in rural area. A meta-analysis has shown that a reminder is effective in motivating more women to participate in a screening program (56). Our findings further suggest that the positive effect of a reminder plays a more important role in motivating women living in an urban area than women living in a rural area to attend a BCSP.
The pooled estimates for all the meta-analyzed determinants of non-participation in screening had substantial heterogeneity. Such heterogeneous estimates were also seen in other meta-analyses. For example, the I2 of two reviews on the effect of living in a rural area and comorbidity on BC screening participation was 95 and 99%, respectively (13, 57). In our stratified analysis a reminder sent to non-attendees or not and reporting adjusted estimate or not in the included studies partly explained the heterogeneity across the studies. Moreover, for the dichotomized determinants, since the contents/definitions of these determinants vary between the studies, pooled estimates are likely to be heterogeneous. In the stratified analyses, we found that the heterogeneity decreased slightly when studies were stratified based on the different categorization. As the results of the meta-analysis resembled that of the original studies, it suggest that despite of wide variation in the categorization of determinants, their impact to non-participation was similar in each study. However, we were not able to fully explain the heterogeneity. Other potential explanations could be the differences in study settings and methodologies of the included studies such as the different confounders that were adjusted for by different studies.
The study also has some limitations. First, only studies published in the English language were included; however, the publication bias was not statistically significant for the determinant income and age of women on screening non-participation. We would not expect a large difference between English or non-English publications for other determinants. Second, not all studies evaluated all nine determinants. Some determinants such as gender of family physician gender were only included in three studies. When a smaller number of studies are available, wider confidence intervals can be expected. Third, all the included studies were published from high-income countries where an organized breast cancer screening program was implemented. Moreover, half of the women included in the meta-analysis were of European or Canadian origin. Therefore, the results in this meta-analysis are less applicable to breast cancer screening globally. Lastly, the meta-analysis was based on data from the observational studies and most of the pooled ORs of the meta-analyzed determinants of non-participation in BC screening were below 2. Therefore, the determinants in our meta-analysis are less likely to be causally related to non-participation in BC screening.
Conclusions
In this meta-analysis excluding studies focusing on opportunistic screening, or using self-reported data, women who were characterized by low income, younger age, low education, living at a large distance to an assigned screening unit, being unmarried, being an immigrant, and having a male family physician were associated with a high non-participation in a BCSP. Interventions to improve the participation of BCSP need to pay more attention to women that are characterized by the above-noted determinants. The association between these determinants and non-participation in BCSP screening was significant but less strong than the report from the reviews, namely, studies on the non-participation in opportunistic screening or with self-reported data on non-participation. This might be explained by a tendency of over-reporting screening utilization collected using a self-reporting method. This analysis only supports the relevance of studies with registry data of the non-participation in BCSP.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
LD: Conceptualization, systematic search strategy construction, paper selection and data extraction, data analysis, the original draft of manuscript writing and revision. JW: Second reader for paper selection and data extraction, manuscript reviewing, and editing. MJWG: Conceptualization, Methodology, Writing—Reviewing, Editing, and Validation. GHd: Supervision, Conceptualization, Methodology, Writing—Reviewing, Editing, and Validation. MG: Writing—Reviewing and Editing, Validation. GV: Conceptualization, Methodology, Writing—Reviewing, Editing, and Validation. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to give our sincere acknowledgment to Dr. S. (Sjoukje) van der Werf, who works as a Medical Information Specialist in the Central Medical Library of the University of Groningen, University Medical Center Groningen, Groningen, the Netherlands for her help in the construction and refinement of the systematic search strategy of this study. LD is supported by the China Scholarship Council (CSC) Ph.D. scholarship (file No. 201808320439) for his research and study at the University of Groningen, University Medical Center Groningen, Groningen, The Netherlands. The scholarship had no role in study design, data analysis, and interpretation, the writing of the manuscript, and the decision to submit the article for publication.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.817222/full#supplementary-material
References
- 1. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the Global Cancer Incidence and Mortality in 2018: GLOBOCAN Sources and Methods. Int J Cancer (2019) 144:1941–53. doi: 10.1002/ijc.31937 [DOI] [PubMed] [Google Scholar]
- 2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, et al. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 3. Myers ER, Moorman P, Gierisch JM, Havrilesky LJ, Grimm LJ, Ghate S, et al. Benefits and Harms of Breast Cancer Screening: A Systematic Review. JAMA J Am Med Assoc (2015) 314:1615–34. doi: 10.1001/jama.2015.13183 [DOI] [PubMed] [Google Scholar]
- 4. Lauby-Secretan B, Scoccianti C, Loomis D, Benbrahim-Tallaa L, Bouvard V, Bianchini F, et al. Breast-Cancer Screening — Viewpoint of the IARC Working Group. N Engl J Med (2015) 372:2353–8. doi: 10.1056/NEJMsr1504363 [DOI] [PubMed] [Google Scholar]
- 5. Bhargava S, Moen K, Qureshi SA, Hofvind S. Mammographic Screening Attendance Among Immigrant and Minority Women: A Systematic Review and Meta-Analysis. Acta Radiol (2018) 59:1285–91. doi: 10.1177/0284185118758132 [DOI] [PubMed] [Google Scholar]
- 6. Perry N, Broeders M, de Wolf C, Törnberg S, Holland R, von Karsa L, et al. European Guidelines for Quality Assurance in Breast Cancer Screening and Diagnosis. Fourth Edition - Summary Document. Ann Oncol (2008) 19:614–22. doi: 10.1093/annonc/mdm481 [DOI] [PubMed] [Google Scholar]
- 7. Autier P, Koechlin A, Smans M, Vatten L, Boniol M. Mammography Screening and Breast Cancer Mortality in Sweden. J Natl Cancer Inst (2012) 104:1080–93. doi: 10.1093/jnci/djs272 [DOI] [PubMed] [Google Scholar]
- 8. Giordano L, Karsa LV, Tomatis M, Majek O, Wolf CD, Lancucki L, et al. Mammographic Screening Programmes in Europe: Organization, Coverage and Participation. J Med Screen (2012) 19:72–82. doi: 10.1258/jms.2012.012085 [DOI] [PubMed] [Google Scholar]
- 9. Health Care Utilisation . Available at: https://stats.oecd.org/Index.aspx?DataSetCode=HEALTH_PROC (Accessed Accessed: 5th January 2021).
- 10. BreastScreen Australia Monitoring Report 2018, Summary - Australian Institute of Health and Welfare. Available at: https://www.aihw.gov.au/reports/cancer/breastscreen-australia-monitoring-report-2018/contents/summary (Accessed Accessed: 5th January 2021).
- 11. Damiani G, Basso D, Acampora A, Bianchi C, Silvestrini G, Frisicale EM, et al. The Impact of Level of Education on Adherence to Breast and Cervical Cancer Screening: Evidence From a Systematic Review and Meta-Analysis. Prev Med (2015) 81:281–9. doi: 10.1016/j.ypmed.2015.09.011 [DOI] [PubMed] [Google Scholar]
- 12. Schueler KM, Chu PW, Smith-Bindman R. Factors Associated With Mammography Utilization: A Systematic Quantitative Review of the Literature. J Women’s Health (2008) 17:1477–98. doi: 10.1089/jwh.2007.0603 [DOI] [PubMed] [Google Scholar]
- 13. Leung J, McKenzie S, Martin J, McLaughlin D. Effect of Rurality on Screening for Breast Cancer: A Systematic Review and Meta-Analysis Comparing Mammography. Rural Remote Health (2014) 14:2730. doi: 10.22605/RRH2730 [DOI] [PubMed] [Google Scholar]
- 14. Sarma EA. Barriers to Screening Mammography. Health Psychol Rev (2015) 9:42–62. doi: 10.1080/17437199.2013.766831 [DOI] [PubMed] [Google Scholar]
- 15. Smith D, Thomson K, Bambra C, Todd A. The Breast Cancer Paradox: A Systematic Review of the Association Between Area-Level Deprivation and Breast Cancer Screening Uptake in Europe. Cancer Epidemiol (2019) 60:77–85. doi: 10.1016/j.canep.2019.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zha N, Alabousi M, Patel BK, Patlas MN. Beyond Universal Health Care: Barriers to Breast Cancer Screening Participation in Canada. J Am Coll Radiol (2019) 16:570–9. doi: 10.1016/j.jacr.2019.02.044 [DOI] [PubMed] [Google Scholar]
- 17. Demb J, Akinyemiju T, Allen I, Onega T, Hiatt RA, Braithwaite D, et al. Screening Mammography Use in Older Women According to Health Status: A Systematic Review and Meta-Analysis. Clin Interventions Aging (2018) 13:1987–97. doi: 10.2147/CIA.S171739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Howard M, Agarwal G, Lytwyn A. Accuracy of Self-Reports of Pap and Mammography Screening Compared to Medical Record: A Meta-Analysis. Cancer Causes Control (2009) 20:1–13. doi: 10.1007/s10552-008-9228-4 [DOI] [PubMed] [Google Scholar]
- 19. Rauscher GH, Johnson TP, Young IC, Walk JA. Accuracy of Self-Reported Cancer-Screening Histories: A Meta-Analysis. Cancer Epidemiol Biomarkers Prev (2008) 17:748–57. doi: 10.1158/1055-9965.EPI-07-2629 [DOI] [PubMed] [Google Scholar]
- 20. Cochrane Handbook for Systematic Reviews of Interventions | Cochrane Training. Available at: https://training.cochrane.org/handbook/current (Accessed Accessed: 5th January 2021).
- 21. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PloS Med (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuiper JS, Zuidersma M, Zuidema SU, Burgerhof JGM, Stolk RP, Oude V, et al. Social Relationships and Cognitive Decline: A Systematic Review and Meta-Analysis of Longitudinal Cohort Studies. Int J Epidemiol (2016) 45:1169–206. doi: 10.1093/ije/dyw089 [DOI] [PubMed] [Google Scholar]
- 23. Downes MJ, Brennan ML, Williams HC, Dean RS. Development of a Critical Appraisal Tool to Assess the Quality of Cross-Sectional Studies (AXIS). BMJ Open (2016) 6:1–7. doi: 10.1136/bmjopen-2016-011458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Partin MR, Casey-Paal AL, Slater JS, Korn JE. Measuring Mammography Compliance: Lessons Learned From a Survival Analysis of Screening Behavior. Cancer Epidemiol Prev Biomarkers (1998) 7:681–7. [PubMed] [Google Scholar]
- 25. Hellmann SS, Njor SH, Lynge E, von Euler-Chelpin M, Olsen A, Tjønneland A, et al. Body Mass Index and Participation in Organized Mammographic Screening: A Prospective Cohort Study. BMC Cancer (2015) 15. doi: 10.1186/s12885-015-1296-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vahabi M, Lofters A, Kumar M, Glazier RH. Breast Cancer Screening Disparities Among Urban Immigrants: A Population-Based Study in Ontario, Canada Health Behavior, Health Promotion and Society. BMC Public Health (2015) 15:1–12. doi: 10.1186/s12889-015-2050-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jack RH, Møller H, Robson T, Davies EA. Breast Cancer Screening Uptake Among Women From Different Ethnic Groups in London: A Population-Based Cohort Study. BMJ Open (2014) 4:1–9. doi: 10.1136/bmjopen-2014-005586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Woods RR, McGrail KM, Kliewer EV, Kazanjian A, Mar C, Kan L, et al. Breast Screening Participation and Retention Among Immigrants and Nonimmigrants in British Columbia: A Population-Based Study. Cancer Med (2018) 7:4044–67. doi: 10.1002/cam4.1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Woodhead C, Cunningham R, Ashworth M, Barley E Stewart RJ, Henderson MJ, et al. Cervical and Breast Cancer Screening Uptake Among Women With Serious Mental Illness: A Data Linkage Study. BMC Cancer (2016) 16:1–9. doi: 10.1186/s12885-016-2842-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Price CL, Szczepura AK, Gumber AK, Patnick J. Comparison of Breast and Bowel Cancer Screening Uptake Patterns in a Common Cohort of South Asian Women in England. BMC Health Serv Res (2010) 10:1–9. doi: 10.1186/1472-6963-10-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guillaume E, Launay L, Dejardin O, Bouvier V, Guittet L, Déan P, et al. Could Mobile Mammography Reduce Social and Geographic Inequalities in Breast Cancer Screening Participation? Prev Med (Baltim) (2017) 100:84–8. doi: 10.1016/j.ypmed.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 32. Vigod SN, Kurdyak PA, Stewart DE, Gnam WH, Goering PN. Depressive Symptoms as a Determinant of Breast and Cervical Cancer Screening in Women: A Population-Based Study in Ontario, Canada. Arch Womens Ment Health (2011) 14:159–68. doi: 10.1007/s00737-011-0210-x [DOI] [PubMed] [Google Scholar]
- 33. Renshaw C, Jack RH, Dixon S, Møller H, Davies EA. Estimating Attendance for Breast Cancer Screening in Ethnic Groups in London. BMC Public Health (2010) 10:1–8. doi: 10.1186/1471-2458-10-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ouédraogo S, Dabakuyo-Yonli TS, Roussot A, Pornet C, Sarlin N, Lunaud P, et al. European Transnational Ecological Deprivation Index and Participation in Population-Based Breast Cancer Screening Programmes in France. Prev Med (Baltim) (2014) 63:103–8. doi: 10.1016/j.ypmed.2013.12.007 [DOI] [PubMed] [Google Scholar]
- 35. St-Jacques S, Philibert MD, Langlois A, Daigle JM, Pelletier E, Major D, et al. Geographic Access to Mammography Screening Centre and Participation of Women in the Quebec Breast Cancer Screening Programme. J Epidemiol Community Health (2013) 67:861–7. doi: 10.1136/jech-2013-202614 [DOI] [PubMed] [Google Scholar]
- 36. Jensen LF, Pedersen AF, Andersen B, Vedsted P. Identifying Specific Non-Attending Groups in Breast Cancer Screening - Population-Based Registry Study of Participation and Socio-Demography. BMC Cancer (2012) 12:1–9. doi: 10.1186/1471-2407-12-518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Le M, Hofvind S, Tsuruda K, Braaten T, Bhargava S. Lower Attendance Rates in BreastScreen Norway Among Immigrants Across All Levels of Socio-Demographic Factors: A Population-Based Study. J Public Heal (2019) 27:229–40. doi: 10.1007/s10389-018-0937-1 [DOI] [Google Scholar]
- 38. Zidar MN, Larm P, Tillgren P, Akhavan S. Non-Attendance of Mammographic Screening: The Roles of Age and Municipality in a Population-Based Swedish Sample. Int J Equity Health (2015) 14:1–11. doi: 10.1186/s12939-015-0291-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jensen LF, Pedersen AF, Andersen B, Vestergaard M, Vedsted P. Non-Participation in Breast Cancer Screening for Women With Chronic Diseases and Multimorbidity: A Population-Based Cohort Study. BMC Cancer (2015) 15. doi: 10.1186/s12885-015-1829-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McDonald JT, Wang Y, Liu Z. Participation and Retention in the Breast Cancer Screening Program in New Brunswick Canada. Prev Med Rep (2017) 6:214–20. doi: 10.1016/j.pmedr.2017.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Berens EM, Stahl L, Yilmaz-Aslan Y, Sauzet O, Spallek J, Razum O, et al. Participation in Breast Cancer Screening Among Women of Turkish Origin in Germany - a Register-Based Study. BMC Womens Health (2014) 14:1–6. doi: 10.1186/1472-6874-14-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jensen LF, Pedersen AF, Andersen B, Vedsted P. Self-Assessed Health, Perceived Stress and Non-Participation in Breast Cancer Screening: A Danish Cohort Study. Prev Med (Baltim) (2015) 81:392–8. doi: 10.1016/j.ypmed.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 43. Jensen LF, Pedersen AF, Andersen B, Vedsted P. Social Support and Non-Participation in Breast Cancer Screening: A Danish Cohort Study. J Public Heal (United Kingdom) (2016) 38:335–42. doi: 10.1093/pubmed/fdv051 [DOI] [PubMed] [Google Scholar]
- 44. Pornet C, Dejardin O, Morlais F, Bouvier V, Launoy G. Socioeconomic and Healthcare Supply Statistical Determinants of Compliance to Mammography Screening Programs: A Multilevel Analysis in Calvados, France. Cancer Epidemiol (2010) 34:309–15. doi: 10.1016/j.canep.2010.03.010 [DOI] [PubMed] [Google Scholar]
- 45. Larsen SH, Virgilsen LF, Kristiansen BK, Andersen B, Vedsted P. Strong Association Between Cervical and Breast Cancer Screening Behaviour Among Danish Women; A Register-Based Cohort Study. Prev Med Rep (2018) 12:349–54. doi: 10.1016/j.pmedr.2018.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jensen LF, Mukai TO, Andersen B, Vedsted P. The Association Between General Practitioners’ Attitudes Towards Breast Cancer Screening and Women’s Screening Participation. BMC Cancer (2012) 12:1–6. doi: 10.1186/1471-2407-12-254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wilf-Miron R, Peled R, Yaari E, Vainer A, Porath A, Kokia E, et al. The Association Between Socio-Demographic Characteristics and Adherence to Breast and Colorectal Cancer Screening: Analysis of Large Sub Populations. BMC Cancer (2011) 11:1–8. doi: 10.1186/1471-2407-11-376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Roder D, Webster F, Zorbas H, Sinclair S. Breast Screening and Breast Cancer Survival in Aboriginal and Torres Strait Islander Women of Australia. Asian Pacific J Cancer Prev (2012) 13:147–55. doi: 10.7314/APJCP.2012.13.1.147 [DOI] [PubMed] [Google Scholar]
- 49. Tavasoli SM, Kane E, Chiarelli AM, Kupets R. Women’s Behaviors Toward Mammogram and Pap Test: Opportunities to Increase Cervical Cancer Screening Participation Rates Among Older Women. Women’s Heal Issues (2018) 28:42–50. doi: 10.1016/j.whi.2017.10.010 [DOI] [PubMed] [Google Scholar]
- 50. Vermeer B, Van Den Muijsenbergh METC. The Attendance of Migrant Women at the National Breast Cancer Screening in the Netherlands 1997-2008. Eur J Cancer Prev (2010) 19:195–8. doi: 10.1097/CEJ.0b013e328337214c [DOI] [PubMed] [Google Scholar]
- 51. Oreilly D, Kinnear H, Rosato M, Mairs A, Hall C. Using Record Linkage to Monitor Equity and Variation in Screening Programmes. BMC Med Res Methodol (2012) 12:1–6. doi: 10.1186/1471-2288-12-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shin DW, Yu J, Cho J, Lee SK, Jung JH, Han K, et al. Breast Cancer Screening Disparities Between Women With and Without Disabilities: A National Database Study in South Korea. Cancer (2020) 126:1522–9. doi: 10.1002/cncr.32693 [DOI] [PubMed] [Google Scholar]
- 53. Viuff JH, Vejborg I, Schwartz W, Bak M, Mikkelsen EM. Morbidity as a Predictor for Participation in the Danish National Mammography Screening Program: A Cross-Sectional Study. Clin Epidemiol (2020) 12:509–18. doi: 10.2147/CLEP.S250418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Duport N. Characteristics of Women Using Organized or Opportunistic Breast Cancer Screening in France. Analysis of the 2006 French Health, Health Care and Insurance Survey. Rev Epidemiol Sante Publique (2012) 60:421–30. doi: 10.1016/j.respe.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 55. Eichholzer M, Richard A, Rohrmann S, Schmid SM, Leo C, Huang DJ, et al. Breast Cancer Screening Attendance in Two Swiss Regions Dominated by Opportunistic or Organized Screening. BMC Health Serv Res (2016) 16:1–10. doi: 10.1186/s12913-016-1760-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wagner TH. The Effectiveness of Mailed Patient Reminders on Mammography Screening: A Meta-Analysis. Am J Prev Med (1998) 14:64–70. doi: 10.1016/S0749-3797(97)00003-2 [DOI] [PubMed] [Google Scholar]
- 57. Diaz A, Kang J, Moore SP, Baade P, Langbecker D, Condon J, et al. Association Between Comorbidity and Participation in Breast and Cervical Cancer Screening: A Systematic Review and Meta-Analysis. Cancer Epidemiol (2017) 47:7–19. doi: 10.1016/j.canep.2016.12.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.