Abstract
Somatosensory cortical activity is altered in individuals with cerebral palsy (CP). However, previous studies have focused on the lower extremities in children with CP and have given less attention to structural changes that may contribute to these alterations. We used a multimodal neuroimaging approach to investigate the relationship between somatosensory cortical activity and cortical thickness in 17 adults with CP (age = 32.8 ± 9.3 years) and 18 healthy adult controls (age = 30.7 ± 9.8 years). Participants performed a median nerve paired-pulse stimulation paradigm while undergoing magnetoencephalography (MEG) to investigate somatosensory cortical activity and sensory gating. Participants also underwent magnetic resonance imaging to evaluate cortical thickness within the area of the somatosensory cortex that generated the MEG response. We found that the somatosensory responses were attenuated in the adults with CP (P = 0.004). The adults with CP also hypergated the second stimulation (P = 0.030) and had decreased cortical thickness in the somatosensory cortex (P = 0.015). Finally, the strength of the somatosensory response was significantly correlated with the cortical thickness (P = 0.023). These findings demonstrate that the aberrant somatosensory cortical activity in adults with CP extends to the upper extremities and appears to be related to cortical thickness.
Keywords: gating, hand, magnetoencephalography, surface-based morphometry (SBM)
Introduction
Cerebral palsy (CP) is the most prevalent and costly pediatric movement disorder in the United States of America, and it is often accompanied by disturbances in hand motor actions (Strauss et al. 2008; Kirby et al. 2011; Christensen et al. 2014). These disturbances impact the ability of individuals with CP to perform a wide range of activities of daily living (i.e., buttoning shirt, grasping objects, writing, using utensils, etc.) (Burgess et al. 2020). Although these motor disturbances have traditionally been characterized as a deficiency in the musculoskeletal machinery, numerous clinical studies have illustrated that individuals with CP have notable deficits in tactile registration/ discrimination, proprioception, and stereognosis (Clayton et al. 2003; Wingert et al. 2008; Goble et al. 2009; Auld et al. 2012; Langan et al. 2014). Furthermore, the severity of such sensory deficits appears to be tightly related to the altered hand motor performance reported in the clinical literature (Auld et al. 2012). These clinical observations have sparked a series of studies that have been directed at uncovering the neurophysiological mechanisms that play a role in the altered hand motor actions and uncharacteristic somatosensory processing (Papadelis et al. 2014; Pihko et al. 2014; Kurz, Heinrichs-Graham, et al. 2015b; Kurz et al. 2020).
There are several neuroanatomical changes that occur throughout the lifespan that may contribute to the altered somatosensory processing seen in these individuals. The cortical gray matter thickness tends to increase during early childhood but then decreases in frontal and parietal lobes in adolescence and adulthood (Giedd et al. 1999; Sowell et al. 2002; Sowell et al. 2003; Lenroot and Giedd 2006; Wilke et al. 2007). Furthermore, synaptic density within gray matter tends to peak between 4 and 8 years of age and then declines thereafter, leveling off in the early 20s in many brain regions (Huttenlocher 1979; Goldman-Rakic 1987; Wilke et al. 2007; Pang 2011). The perinatal brain injuries incurred by children with CP might interrupt or accelerate the course of the regional changes in cortical gray matter density, which in turn might influence the strength of the somatosensory cortical activity seen in adults with CP. This premise is partly supported by several structural imaging studies, which have shown that early on youth with CP may have altered gray matter thickness across a number of cortical regions including sensory, motor, occipital, temporal, parietal, and insula areas (Scheck et al. 2014; Pagnozzi et al. 2016; Liu et al. 2019). Furthermore, it has been demonstrated that a reduction in the cortical volume of the primary somatosensory cortices is strongly associated with more impaired hand motor performance (Pagnozzi et al. 2016). However, a potential connection between reduced somatosensory cortical thickness and the weaker neural activity seen in individuals with CP has yet to be shown, despite the numerous magnetoencephalography (MEG) and electroencephalography studies that have illustrated that the somatosensory cortical activity is reduced in magnitude and latent in youth with CP in comparison with their age-matched peers (Kurz and Wilson 2011; Teflioudi et al. 2011; Maitre et al. 2012; Kurz et al. 2014; Papadelis et al. 2014; Kurz, Becker, et al. 2015a; Kurz, Heinrichs-Graham, et al. 2015b). Additionally, our recent experimental results have shown that the abnormal somatosensory responses seen in youth with CP become even more aberrant when they transition into adulthood (Trevarrow et al. 2020).
Sensory gating is a phenomenon that occurs when 2 identical stimuli are presented in short succession (e.g., a paired pulse), and the cortical response to the second stimulus is reduced in comparison with the first. Gating has been extensively studied within the somatosensory cortex of healthy individuals (Huttunen et al. 2008; Lenz et al. 2012; Stevenson et al. 2012; Spooner et al. 2018; Spooner, Wiesman, et al. 2020b; Wiesman and Wilson 2020; Casagrande et al. 2021; McCusker et al. 2021), and it is thought that the attenuation of the response to the second stimulus represents the filtering of redundant and/or irrelevant information to preserve resources for more task-relevant information (Cromwell et al. 2008; Hsiao et al. 2013). GABAergic inhibitory interneurons are thought to regulate this process of preattentive inhibitory control to limit sensory and perceptual overload (Croft et al. 2001), and these inhibitory interneurons are likely aberrant in youth with CP (Adler et al. 1982; Freedman et al. 1987; Jessen et al. 2001; Cromwell et al. 2008). Our prior research largely aligns with this premise, as we have shown that children with CP hypergate redundant somatosensory information in response to paired-pulse electrical stimulation of the foot (Kurz et al. 2018). In other words, children with CP appear to more completely filter the second stimulation relative to their age-matched peers. Whether similar hypergating is seen when paired stimulations are applied to the hand is unknown. Furthermore, it is unknown if such altered gating is connected with the reduced gray matter thickness (see prior paragraph) in the somatosensory cortices of individuals with CP. Addressing these gaps would provide key insight on the neurophysiological mechanisms responsible for the altered sensory processing reported in individuals with CP.
In the current multimodal neuroimaging study, we evaluate whether somatosensory gating is altered when somatosensations are applied to the hands of adults with CP and determine whether the strength of somatosensory cortical activity is linked with the local cortical structure. To this end, we used MEG brain imaging and a paired-pulse electrical stimulation paradigm to quantify somatosensory cortical activity and sensory gating. Secondarily, we used structural magnetic resonance imaging (MRI) and a surface-based morphometry protocol to quantify potential differences in cortical thickness within the somatosensory region generating the MEG responses. Overall, the results of our experimental work show that the somatosensory cortical activity is reduced in adults with CP, and these individuals also tended to hypergate the paired stimulations. Additionally, the altered somatosensory cortical responses were directly associated with the thickness of the cortical tissue generating the response. Hence, this illustrates that the functional somatosensory alterations seen in adults with CP may be at least partially attributable to structural changes in the somatosensory cortex that are sequelae of the early brain insult.
Methods
Participants
Seventeen adults with a diagnosis of spastic CP, gross motor function classification system levels between I and IV, and manual ability classification system levels between I and III completed this study (mean ± SD; age = 32.8 ± 9.3 years, females = 10). An additional cohort of 18 healthy adults (age = 30.7 ± 9.8 years, females = 9) served as a healthy control (HC) group. The groups did not significantly differ by age or sex (Ps > 0.5). The Institutional Review Board at the University of Nebraska Medical Center reviewed and approved this investigation. Informed consent was acquired from all participants.
MEG Acquisition and Experimental Paradigm
Throughout the somatosensory experiment, the participants were seated in a custom-made nonmagnetic chair with their head positioned within the MEG helmet-shaped sensor array and their eyes closed. Electrical stimulation was applied to the right median nerve using external cutaneous stimulators connected to a constant-current stimulator system (Digitimer Limited, Letchworth Garden City, UK). For each participant, we collected at least 80 paired-pulse trials with an interstimulation interval of 500 ms and an inter-pair interval that randomly varied between 4500 and 4800 ms (Spooner, Eastman, et al. 2020a). Each pulse generated a 0.2 ms constant-current square wave that was set to the motor threshold required to elicit a subtle twitch of the thumb.
All recordings were conducted in a 1-layer magnetically shielded room with active shielding engaged for advanced environmental noise compensation. During data acquisition, participants were monitored via real-time audio–video feeds from inside the shielded room. With an acquisition bandwidth of 0.1–330 Hz, neuromagnetic responses were sampled continuously at 1 kHz using an Elekta MEG system (Helsinki, Finland) with 306 sensors, including 204 planar gradiometers and 102 magnetometers. Each MEG data set was individually corrected for head motion during task performance and subjected to noise reduction using the signal space separation method with a temporal extension (Taulu and Simola 2006).
MEG Coregistration and Structural MRI Processing
Four coils were affixed to the head of the participant for continuous head localization during the experiment. Prior to the experiment, the location of these coils, 3 fiducial points, and the scalp surface were digitized to determine their 3-dimensional position (Fastrak 3SF0002, Polhemus Navigator Sciences, Colchester, VT). Once the participant was positioned for the MEG recording, an electric current with a unique frequency label (e.g., 322 Hz) was fed to each of the 4 coils. This induced a measurable magnetic field and allowed each coil to be localized in reference to the sensors throughout the recording session. Since the coil locations were also known in head coordinates, all MEG measurements could be transformed into a common coordinate system. With this coordinate system (including the scalp surface points), each participant’s MEG data were coregistered with structural T1-weighted MRI data prior to source reconstruction. Structural MRI data were aligned parallel to the anterior and posterior commissures and transformed into standardized space. Structural MRI data were acquired using a Siemens Skyra 3T scanner. High-resolution T1-weighted sagittal images were obtained with a 32-channel head coil using a 3D fast field echo sequence with the following parameters: time repetition: 2400 ms; time echo: 1.94 ms; flip angle = 8 deg; field of view: 256 mm; slice thickness: 1 mm slice with no gap; in-plane resolution: 1.0 mm3.
MEG Preprocessing
Cardiac artifacts were removed from the data using signal-space projection, which was accounted for during source reconstruction (Uusitalo and Ilmoniemi 1997). The continuous magnetic time series was divided into epochs of 3700 ms duration, from −800 to 2900 ms with the baseline being defined as −700 to −300 ms and 0.0 ms being the first stimulation onset. Epochs containing artifacts (e.g., eye blinks, muscle artifacts, etc.) were rejected based on a fixed-threshold method using individual amplitude and gradient thresholds, supplemented with visual inspection. An independent samples t-test revealed that the number of trials accepted between groups was not significantly different (CP = 74.65 ± 2.32, HC = 75.78 ± 4.92, P = 0.375).
Sensor-Level Analysis
The artifact-free epochs were next averaged across trials to generate a mean time series per sensor, and the specific time windows used for subsequent source analysis were determined by statistical analysis of the sensor-level time series across both groups. Each data point in the time series was initially evaluated using a mass univariate approach based on the general linear model. To reduce the risk of false-positive results while maintaining reasonable sensitivity, a 2-stage procedure was followed to control for Type 1 error. In the first stage, paired-sample t-tests were conducted to test for differences from baseline at each data point and the output time series of t-values was threshold at P < 0.05 to define time bins containing potentially significant responses across all participants. In stage 2, the time points that survived the threshold were clustered with temporally neighboring bins that were also above the threshold (P < 0.05), and a cluster value was derived by summing all of the t-values of all data points in the cluster. Nonparametric permutation testing was then used to derive a distribution of cluster values, and the significance level of the observed clusters (from stage one) was tested directly using this distribution (Maris and Oostenveld 2007). For each comparison, 1000 permutations were computed to build a distribution of cluster values. Based on these analyses, the time windows that contained significant neural events across all participants were used to guide subsequent time domain source level analysis.
Source Imaging (sLORETA)
Time domain source images were computed using standardized low resolution brain electromagnetic tomography (sLORETA) (Pascual-Marqui 2002). The resulting whole-brain maps were 4-dimensional estimates of current density per voxel, per time sample, across the experimental epoch. These data were normalized to the sum of the noise covariance and theoretical signal covariance, and thus, the units are arbitrary. These maps were then averaged temporally over the time windows identified in the sensor-level analysis (see above). The resulting maps were then grand-averaged across the participants to determine the location of the peak voxel of the time domain neural response to the stimuli across participants. The neural time course was then extracted from this peak voxel; note that this time course was extracted per participant, once the coordinates of interest were known from the grand-averaged image. The peak amplitude within each of the windows identified at the sensor level was then determined for each participant. The gating ratio was subsequently calculated as the maximum response to the second pulse divided by the maximum response to the first pulse. All imaging procedures were done with the Brain Electrical Source Analysis (BESA) software (BESA v7.0; Grafelfing, Germany).
Structural MRI Acquisition and Processing
The structural MRI data were processed using a standard pipeline in the CAT12 toolbox (http://dbm.neuro.uni-jena.de/cat/, version 12.6) at a resolution of 1 mm3 within SPM12 (Wellcome Trust Center for Neuroimaging, http://www.fil.ion.ucl.ac.uk/spm/) using MATLAB (2017b) software (MathWorks, Natick, MA). The surface-based morphometry pipeline in CAT12 is fully automated and utilizes a projection-based thickness (PBT) approach to estimate cortical thickness and reconstruct the central surface in one step (Dahnke et al. 2013). Essentially, following tissue segmentation (Ashburner and Friston 2005), the white matter (WM) distance is estimated, and the local maxima (which is equal to the cortical thickness) are projected onto other gray matter voxels using a neighboring relationship described by the WM distance. PBT accounts for partial volume correction, sulcal blurring, and sulcal asymmetries without sulcus reconstruction. To rectify topological defects, a correction based on spherical harmonics was employed (Yotter et al. 2011), and the cortical surface mesh was reparameterized into a common coordinate system via an algorithm that reduces area distortion. Finally, the resulting maps were resampled and smoothed using a 15 mm FWHM Gaussian kernel.
For quality assurance, a 2-step process was adopted. First, prior to segmentation, data were visually inspected for artifacts. Second, the quality control measures incorporated in the CAT12 processing pipeline were utilized to identify the most deviant data following segmentation. These data were inspected further for the presence of newly introduced artifacts.
Region of Interest Analysis
Utilizing the peak voxel coordinates identified in the grand-averaged sLORETA images, a mask was constructed for the cortical surface mesh. Specifically, a 4 mm cortically constrained sphere centered on the peak voxel coordinates was generated using the WFU Pickatlas (version 3.0) (Maldjian et al. 2003; Maldjian et al. 2004). A 4 mm sphere was selected since it aligned with the 4 mm native resolution of the sLORETA source images. This mask was spatially resampled to 1 mm isotropic voxels to align with the processed structural MRI and MEG data.
Finally, the normalized volume mask was transformed into the surface template space using the transform provided in CAT12. Cortical thickness values were then extracted per participant utilizing this ROI mask. Thus, the attained values reflect the average cortical thickness within the estimated group-wise peak voxel of the somatosensory responses. Of note, the same procedure was applied to derive the average cortical thickness across spheres of varying sizes (4, 8, and 12 mm) to ensure the robustness of any statistically significant findings seen at the 4 mm ROI (Proskovec et al. 2020).
Statistical Analysis
The data were tested for normality using the Shapiro–Wilk test, and any data that failed the test were log transformed for statistical analysis. A mixed model ANOVA (group × stimulation) was used to test if there were differences in the somatosensory cortical responses. The gating ratios and cortical thickness at the extracted ROI for the respective groups were compared using separate independent samples t-tests. Lastly, Pearson correlation coefficients were calculated between the extracted cortical thickness values and the magnitude of the somatosensory cortical responses and gating ratios to identify potential structure–function relationships. Neuroimaging results are presented as the mean ± standard error of the mean. Statistical analyses were conducted with JASP (Version 12.2.0) using a 0.05 alpha level.
Results
MEG Imaging
Permutation testing of the sensor-level data revealed that the somatosensory cortical response was significantly different from baseline during the 32–136 ms time window for the first stimulus and 532—636 ms for the second stimulus (P’s < 0.001, Fig. 1A); thus, source activity estimates were averaged across these time windows and then across all participants to derive the peak voxel. The peak voxel was the same for both the first stimulation and second stimulation. Not surprisingly, the resulting grand-averaged sLORETA data revealed that the peak neural response emanated from the hand region of the contralateral (left) somatosensory cortices (Fig. 1B).
Figure 1.
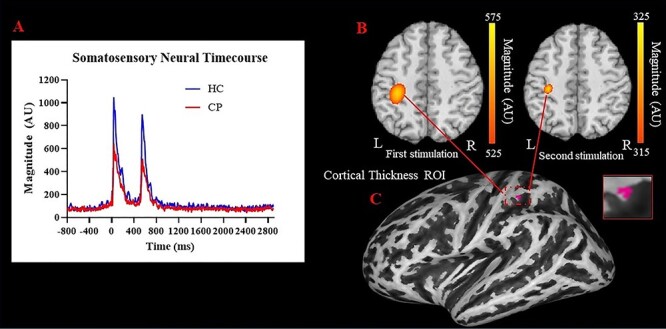
(A) Neural time course of somatosensory cortical responses during the paired-pulse stimulation paradigm. As depicted, the individuals with CP had weaker responses overall and hypergated the response to the second stimulation relative to the healthy adult controls. (B) Group-averaged sLORETA images of the somatosensory-evoked cortical activity emanating from the contralateral (left) somatosensory cortex for the first (32–136 ms, left) and the second stimulation (532–636 ms, right). As shown, the strength of the response was significantly weaker following the second stimulation, indicating a gating effect (P = 0.001). (C) The coordinates of the peak voxel from the grand-averaged sLORETA image were used to generate a mask around the somatosensory hand area from which the cortical thickness estimates were generated.
Our mixed model ANOVA revealed that there were main effects for stimulation (P = 0.001) and group (P = 0.004). Hence, this indicates that the second stimulation was reduced for both groups (Stim 1 = 1100.85 ± 91.68 AU; Stim 2 = 886.62 ± 71.23 AU; P = 0.001) and that the primary somatosensory cortical responses were weaker in the adults with CP in comparison with the HCs (CP = 796.73 ± 92.01 AU; HC = 1206.979 ± 121.43 AU; P = 0.004; Fig. 2A). These effects are clearly discernable from the neural time course data shown in Figure 1A. Furthermore, the gating ratio was significantly smaller in the adults with CP in comparison with the controls (CP = 0.79 ± 0.04 AU; HC = 0.91 ± 0.04 AU; P = 0.030; Fig. 2B), indicating that the adults with CP hypergated the second stimulation.
Figure 2.
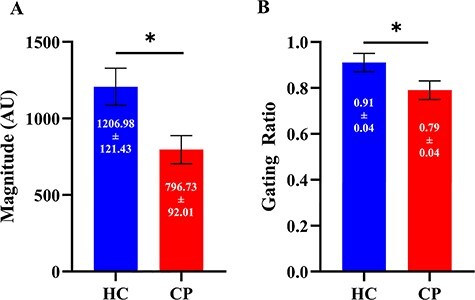
(A) Somatosensory cortical activity across both stimulations was sharply reduced in the adults with CP compared to the healthy adult controls (P = 0.004). (B) The gating ratio was significantly smaller in the adults with CP, indicating stronger gating (i.e., hypergating) and abnormal inhibition within the somatosensory cortices of those with CP (P = 0.03). Results are presented as mean ± SEM. Note: * indicates significance at P < 0.05.
Cortical Thickness Is Associated with Somatosensory Cortical Responses
The coordinates from the peak voxel of the somatosensory responses were subsequently used to generate a cortically constrained sphere (Fig. 1C). Our results indicated that the cortical thickness within this ROI was reduced in the adults with CP relative to the controls within the 4 mm ROI (CP = 2.471 ± 0.023 mm, HC = 2.554 ± 0.22 mm, P = 0.015, Fig. 3A), as well as the 8 mm ROI (CP = 2.217 ± 0.031 mm, HC = 2.419 ± 0.040 mm, P < 0.001) and the 12 mm ROI (CP = 2.206 ± 0.030 mm, HC = 2.389 ± 0.035 mm, P < 0.001). Thus, this indicates that the size of the sphere did not have a major impact on the results.
Figure 3.
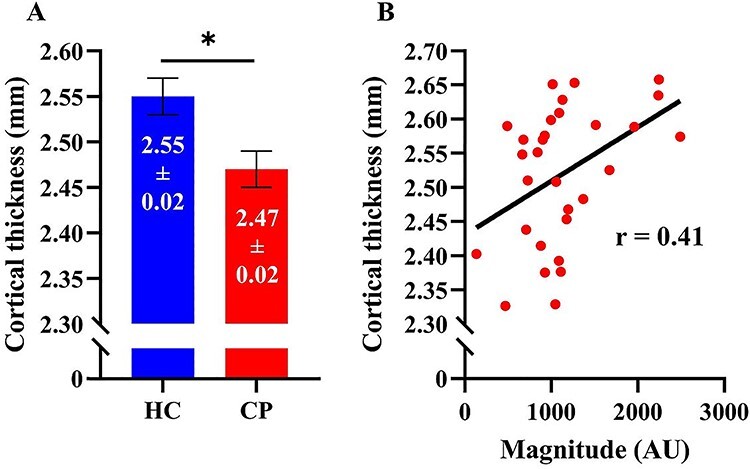
(A) Cortical thickness in the hand region of the somatosensory cortex. Cortical thickness was significantly decreased in the adults with CP in comparison with the healthy adult controls (P = 0.015). (B) Scatterplot depicting the relationship between cortical thickness and somatosensory cortical activity in response to the first stimulus. Individuals with increased cortical thickness in the hand region also tended to have greater somatosensory cortical activity (P = 0.023). Results are presented as mean ± SEM. Note that the y-axes do not start at zero. Note: * indicates significance at P < 0.05.
Interestingly, Pearson correlation showed that the strength of the somatosensory response to the first stimulation was positively correlated with the cortical thickness seen in the 4 mm ROI (r = 0.41, P = 0.023, Fig. 3B), the 8 mm ROI (r = 0.46, P = 0.013), and 12 mm ROI (r = 0.49, P = 0.006). Similarly, the strength of the somatosensory response to the second stimulation was positively correlated with the cortical thickness seen in the 4 mm ROI (r = 0.36, P = 0.04), the 8 mm ROI (r = 0.44, P = 0.014), and 12 mm ROI (r = 0.47, P = 0.008). Overall, these correlations indicate that stronger somatosensory cortical responses are associated with greater cortical thickness. In contrast, cortical thickness was not significantly associated with the gating ratio.
We performed a follow-up analysis on the significant relationships found between cortical thickness and somatosensory cortical activity to ensure that this relationship was not a result of differences in the respective variables for the groups. This follow-up consisted of performing Fisher’s Z transformations to determine whether this relationship differed when calculated separately for the adults with CP and the controls. We found that the relationship between the somatosensory responses and cortical thickness for the 4 mm (Z = 0.08, P = 0.936), 8 mm (Z = −0.15, P = 0.881), and 12 mm (Z = −0.18, P = 0.857) regions of interest were each similar between the groups. Thus, this follow-up analysis confirmed that the group differences in cortical thickness and somatosensory responses cannot solely explain the association between these variables.
Discussion
Our results build upon the framework that adults with CP have decreased somatosensory responses and tend to hypergate repeated somatosensations of the hand. We also determined that the hand region of the somatosensory cortices is thinner in adults with CP. Finally, our results show that the strength of somatosensory cortical responses is linked with the local cortical thickness. This connection implies that the functional alterations seen in the somatosensory cortices of adults with CP could be partially attributable to structural changes, which could be a downstream effect of the early brain insult. Further implications of these novel experimental results are discussed below.
Our results corroborate numerous studies that have shown that somatosensory cortical activity is reduced in individuals with CP compared with neurotypical controls (Teflioudi et al. 2011; Maitre et al. 2012; Papadelis et al. 2014; Kurz, Becker, et al. 2015a; Trevarrow et al. 2020). However, this literature on the somatosensory processing deficits has predominately been focused on youth with CP. Our experimental work has begun to address this critical knowledge gap by initially showing that the somatosensory cortical activity is aberrant when a stimulation is applied to the tibial nerve of adults with CP (Trevarrow et al. 2020). The results shown here add to these findings by showing that the somatosensory cortical responses for the hand are also uncharacteristic for adults with CP. Altogether these results imply that the perinatal brain insults have widespread and long-term effects on the somatosensory cortical processing of individuals with CP. We propose that such alterations in the somatosensory cortical responses are likely contributing to the progressively declining motor actions reported in adults with CP (Hanna et al. 2009).
Our results also showed that adults with CP hypergate repeated somatosensations applied to the hand, which is remarkable since the somatosensory cortical responses were already reduced across both stimulations. It is likely that the hypergating of subsequent somatosensations may even further diminish the processing of important peripheral information. These results are also aligned with our prior study that showed youth with CP hypergate repeated and similar somatosensations that are applied to the feet (Kurz et al. 2018). Thus, the ability to properly discriminate salient incoming information seems to be impaired for both the lower and upper extremities in individuals with CP, which may lead to difficulties in sensorimotor integration and in turn contribute to the impairments of both the lower and upper extremities. Sensory gating is reflective of preattentive inhibition and has been used as a marker to assess inhibitory functioning within the somatosensory cortices (Thoma et al. 2007; Huttunen et al. 2008; Lenz et al. 2012; Stevenson et al. 2012). Thus, the hypergating effect seen in the respective studies is likely indicative of heightened activity within cortical GABAergic inhibitory interneurons. Previous Hoffmann reflex studies have demonstrated hyperexcitability in the spinal cord of individuals with CP (Hodapp, Klisch, Berger, et al. 2007a; Hodapp, Klisch, Mall, et al. 2007b). This hyperexcitability may result in an overall increase in afferent information, albeit noise, that ascends from the spinal cord through the thalamocortical tracts, heightening the activity of GABAergic interneurons in the cortex meant to filter the incoming information. Damage to the structural integrity of the thalamocortical tracts seen in CP (Rose et al. 2007; Trivedi et al. 2008, 2010; Hoon et al. 2009) may result in an even further increase in noise within the pathways transmitting somatosensory information to the cortex, resulting in added increases in GABAergic inhibitory activity. Altogether, this increase in signals ascending from the spinal cord and thalamocortical tracts may create difficulty for adults with CP in deciphering the salience of incoming information, resulting in the second stimulus being interpreted as noise and thus being hypergated.
Our structural imaging results showed that the cortical thickness in the somatosensory hand region was significantly decreased in the adults with CP. These results are aligned with several studies that have shown that youth with CP may have altered gray matter thickness in the somatosensory cortical area (Scheck et al. 2014; Pagnozzi et al. 2016; Liu et al. 2019). Healthy aging studies have shown that there is age-dependent cortical thinning in this region (Peters 2006), and these changes have been largely attributed to both neuronal cell death and a reduction in synaptic density and dendritic arborization. Thus, there are likely fewer neuronal cell bodies and synapses within the somatosensory cortex of the adults with CP compared to their youth counterparts, and this could contribute to the aberrant processing of incoming sensory information that appears to extend from youth through adulthood. That being said, it still remains unknown if the cortical thinning seen in the older population with CP reflects what is seen in the healthy aging population or if it represents an accelerated/altered trajectory.
Our results are the first to show that the strength of somatosensory-evoked cortical activity is associated with the thickness of the somatosensory cortices in those with CP. This relationship suggests that the individuals with more gray matter tend to have greater somatosensory cortical activity. MEG primarily detects cortical activity generated by the underlying postsynaptic potentials of pyramidal neuronal populations. Thus, if there are fewer cell bodies and/or a reduction in dendritic arborization within a given neuronal population, then there will be fewer synapses contributing to the underlying electrical activity generating the neural response. This would in turn be reflected by a reduction in magnitude of neural activity in response to a given stimulus, which aligns with the relationship between structure and function that we have identified in the somatosensory cortex. While this was a moderate correlation, the strength of the relationship may become more prominent with a larger sample size. Alternatively, the variability of individual responses in CP may affect the strength of this relationship. Future studies should explore this relationship further to determine if it is potentially stronger with a larger sample size. Contrary to our expectation, the somatosensory gating was not related to the cortical thickness. This implies that the gating ratio may provide a normalized metric that more accurately quantifies the extent of the cortical activation because it is not dependent on the number of neurons that are activated in the cortical region of interest.
The source of this reduction in the somatosensory cortical gray matter and altered somatosensory activity seen in adults with CP could be a result of a lack of enriched somatosensory experiences. We suspect that the greater sedentary behavior and reduced social/environmental experiences often reported for individuals with CP would inherently lead to fewer somatosensory experiences (Carlon et al. 2013). With a lack of input to the somatosensory system, there is less capacity for neuroplastic change within the somatosensory cortices, which would result in alterations in the synaptic connections that are involved in processing incoming information. The emerging clinical question is how the somatosensory cortical processing deficits reported here and across several other studies can be overcome and if a lack of enriched experiences play a central role in the altered somatosensory processing we have seen in those with CP. A few studies have shown that clinical assessments of proprioception and tactile acuity can improve after undergoing a sensory-based training protocol or hand-arm bimanual intensive therapy (Langan et al. 2014; Rich et al. 2017; Kuo et al. 2018). Albeit for the lower extremity, a preliminary study we conducted also suggested that the somatosensory cortical activity might be enhanced after youth with CP undergo an intensive gait training protocol (Kurz et al. 2012). Further testing of these therapeutic concepts would be laudable and may have the potential to alter the treatment strategies currently being used to improve the motor actions of individuals with CP.
In conclusion, we have demonstrated that the altered somatosensory cortical activity and gating seen in children with CP persists into adulthood. Potentially, increased noise from a hyperexcitable spinal cord and damaged thalamocortical tracts results in overactivity of GABAergic inhibitory interneurons that are meant to suppress incoming redundant information. We have expanded on these findings by showing that the cortical thickness within the area of the cortex generating this response is reduced in adults with CP and is directly associated with the strength of the somatosensory cortical activity. This indicates that adults with CP likely have decreased cell number and synaptic density within their somatosensory cortices, likely stemming from abnormal development of the somatosensory system or perhaps an accelerated aging effect, which ultimately contributes to their clinical sensorimotor impairments.
Funding
National Institutes of Health (1R01-HD086245, 1R01-HD101833, R21-HD096390).
Notes
Conflict of Interest. None declared.
References
- Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, Freedman R. 1982. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry. 17:639–654. [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. 2005. Unified segmentation. Neuroimage. 26:839–851. [DOI] [PubMed] [Google Scholar]
- Auld ML, Boyd RN, Moseley GL, Ware RS, Johnston LM. 2012. Impact of tactile dysfunction on upper-limb motor performance in children with unilateral cerebral palsy. Arch Phys Med Rehabil. 93:696–702. [DOI] [PubMed] [Google Scholar]
- Burgess A, Boyd RN, Chatfield MD, Ziviani J, Sakzewski L. 2020. Self-care performance in children with cerebral palsy: a longitudinal study. Dev Med Child Neurol. 62:1061–1067. [DOI] [PubMed] [Google Scholar]
- Carlon SL, Taylor NF, Dodd KJ, Shields N. 2013. Differences in habitual physical activity levels of young people with cerebral palsy and their typically developing peers: a systematic review. Disabil Rehabil. 35:647–655. [DOI] [PubMed] [Google Scholar]
- Casagrande CC, Lew BJ, Taylor BK, Schantell M, O'Neill J, May PE, Swindells S, Wilson TW. 2021. Impact of HIV-infection on human somatosensory processing, spontaneous cortical activity, and cortical thickness: a multimodal neuroimaging approach. Hum Brain Mapp. 42:2851–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen D, Van Naarden BK, Doernberg NS, Maenner MJ, Arneson CL, Durkin MS, Benedict RE, Kirby RS, Wingate MS, Fitzgerald R, et al. 2014. Prevalence of cerebral palsy, co-occurring autism spectrum disorders, and motor functioning - Autism and Developmental Disabilities Monitoring Network, USA, 2008. Dev Med Child Neurol. 56:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton K, Fleming JM, Copley J. 2003. Behavioral responses to tactile stimuli in children with cerebral palsy. Phys Occup Ther Pediatr. 23:43–62. [PubMed] [Google Scholar]
- Croft RJ, Lee A, Bertolot J, Gruzelier JH. 2001. Associations of P50 suppression and desensitization with perceptual and cognitive features of “unreality” in schizotypy. Biol Psychiatry. 50:441–446. [DOI] [PubMed] [Google Scholar]
- Cromwell HC, Mears RP, Wan L, Boutros NN. 2008. Sensory gating: a translational effort from basic to clinical science. Clin EEG Neurosci. 39:69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahnke R, Yotter RA, Gaser C. 2013. Cortical thickness and central surface estimation. Neuroimage. 65:336–348. [DOI] [PubMed] [Google Scholar]
- Freedman R, Adler LE, Gerhardt GA, Waldo M, Baker N, Rose GM, Drebing C, Nagamoto H, Bickford-Wimer P, Franks R. 1987. Neurobiological studies of sensory gating in schizophrenia. Schizophr Bull. 13:669–678. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. 1999. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 2:861–863. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Hurvitz EA, Brown SH. 2009. Deficits in the ability to use proprioceptive feedback in children with hemiplegic cerebral palsy. Int J Rehabil Res. 32:267–269. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. 1987. Development of cortical circuitry and cognitive function. Child Dev. 58:601–622. [PubMed] [Google Scholar]
- Hanna SE, Rosenbaum PL, Bartlett DJ, Palisano RJ, Walter SD, Avery L, Russell DJ. 2009. Stability and decline in gross motor function among children and youth with cerebral palsy aged 2 to 21 years. Dev Med Child Neurol. 51:295–302. [DOI] [PubMed] [Google Scholar]
- Hodapp M, Klisch C, Berger W, Mall V, Faist M. 2007a. Modulation of soleus H-reflexes during gait in healthy children. Exp Brain Res. 178:252–260. [DOI] [PubMed] [Google Scholar]
- Hodapp M, Klisch C, Mall V, Vry J, Berger W, Faist M. 2007b. Modulation of soleus H-reflexes during gait in children with cerebral palsy. J Neurophysiol. 98:3263–3268. [DOI] [PubMed] [Google Scholar]
- Hoon AH, Stashinko EE, Nagae LM, Lin DD, Keller J, Bastian A, Campbell ML, Levey E, Mori S, Johnston MV. 2009. Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev Med Child Neurol. 51:697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao FJ, Cheng CH, Chen WT, Lin YY. 2013. Neural correlates of somatosensory paired-pulse suppression: a MEG study using distributed source modeling and dynamic spectral power analysis. Neuroimage. 72:133–142. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. 1979. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 163:195–205. [DOI] [PubMed] [Google Scholar]
- Huttunen J, Pekkonen E, Kivisaari R, Autti T, Kähkönen S. 2008. Modulation of somatosensory evoked fields from SI and SII by acute GABA A-agonism and paired-pulse stimulation. Neuroimage. 40:427–434. [DOI] [PubMed] [Google Scholar]
- Jessen F, Kucharski C, Fries T, Papassotiropoulos A, Hoenig K, Maier W, Heun R. 2001. Sensory gating deficit expressed by a disturbed suppression of the P50 event-related potential in patients with Alzheimer’s disease. Am J Psychiatry. 158:1319–1321. [DOI] [PubMed] [Google Scholar]
- Kirby RS, Wingate MS, Van Naarden BK, Doernberg NS, Arneson CL, Benedict RE, Mulvihill B, Durkin MS, Fitzgerald RT, Maenner MJ, et al. 2011. Prevalence and functioning of children with cerebral palsy in four areas of the United States in 2006: a report from the Autism and Developmental Disabilities Monitoring Network. Res Dev Disabil. 32:462–469. [DOI] [PubMed] [Google Scholar]
- Kuo HC, Zewdie E, Ciechanski P, Damji O, Kirton A. 2018. Intervention-induced motor cortex plasticity in hemiparetic children with perinatal stroke. Neurorehabil Neural Repair. 32:941–952. [DOI] [PubMed] [Google Scholar]
- Kurz MJ, Becker KM, Heinrichs-Graham E, Wilson TW. 2015a. Children with cerebral palsy have uncharacteristic somatosensory cortical oscillations after stimulation of the hand mechanoreceptors. Neuroscience. 305:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz MJ, Bergwell H, Spooner R, Baker S, Heinrichs-Graham E, Wilson TW. 2020. Motor beta cortical oscillations are related with the gait kinematics of youth with cerebral palsy. Ann Clin Transl Neurol. 7:2421–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz MJ, Heinrichs-Graham E, Arpin DJ, Becker KM, Wilson TW. 2014. Aberrant synchrony in the somatosensory cortices predicts motor performance errors in children with cerebral palsy. J Neurophysiol. 111:573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz MJ, Heinrichs-Graham E, Becker KM, Wilson TW. 2015b. The magnitude of the somatosensory cortical activity is related to the mobility and strength impairments seen in children with cerebral palsy. J Neurophysiol. 113:3143–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz MJ, Wiesman AI, Coolidge NM, Wilson TW. 2018. Children with cerebral palsy hyper-gate somatosensory stimulations of the foot. Cereb Cortex. 28:2431–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz MJ, Wilson TW. 2011. Neuromagnetic activity in the somatosensory cortices of children with cerebral palsy. Neurosci Lett. 490:1–5. [DOI] [PubMed] [Google Scholar]
- Kurz MJ, Wilson TW, Corr B, Volkman KG. 2012. Neuromagnetic activity of the somatosensory cortices associated with body weight-supported treadmill training in children with cerebral palsy. J Neurol Phys Ther. 36:166–172. [DOI] [PubMed] [Google Scholar]
- Langan J, Kern KL, Hurvitz EA, Brown SH. 2014. Upper-limb position sense deficits in adults with cerebral palsy. Am J Phys Med Rehabil. 93:774–781. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. 2006. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 30:718–729. [DOI] [PubMed] [Google Scholar]
- Lenz M, Tegenthoff M, Kohlhaas K, Stude P, Höffken O, Gatica Tossi MA, Kalisch T, Kowalewski R, Dinse HR. 2012. Increased excitability of somatosensory cortex in aged humans is associated with impaired tactile acuity. J Neurosci. 32:1811–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Jiang H, Bi W, Huang B, Li X, Wang M, Wang X, Zhao H, Cheng Y, Tao X, et al. 2019. Abnormal gray matter structural covariance networks in children with bilateral cerebral palsy. Front Hum Neurosci. 13:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitre NL, Barnett ZP, Key AP. 2012. Novel assessment of cortical response to somatosensory stimuli in children with hemiparetic cerebral palsy. J Child Neurol. 27:1276–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH. 2004. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage. 21:450–455. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. 2003. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 19:1233–1239. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. 2007. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 164:177–190. [DOI] [PubMed] [Google Scholar]
- McCusker MC, Lew BJ, Wilson TW. 2021. Three-year reliability of MEG visual and somatosensory responses. Cereb Cortex. 31:2534–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnozzi AM, Dowson N, Fiori S, Doecke J, Bradley AP, Boyd RN, Rose S. 2016. Alterations in regional shape on ipsilateral and contralateral cortex contrast in children with unilateral cerebral palsy and are predictive of multiple outcomes. Hum Brain Mapp. 37:3588–3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang EW. 2011. Practical aspects of running developmental studies in the MEG. Brain Topogr. 24:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadelis C, Ahtam B, Nazarova M, Nimec D, Snyder B, Grant PE, Okada Y. 2014. Cortical somatosensory reorganization in children with spastic cerebral palsy: a multimodal neuroimaging study. Front Hum Neurosci. 8:725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Marqui RD. 2002. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp Clin Pharmacol Spain. 24:5–12. [PubMed] [Google Scholar]
- Peters R. 2006. Ageing and the brain. Postgrad Med J. 82:84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihko E, Nevalainen P, Vaalto S, Laaksonen K, Mäenpää H, Valanne L, Lauronen L. 2014. Reactivity of sensorimotor oscillations is altered in children with hemiplegic cerebral palsy: a magnetoencephalographic study. Hum Brain Mapp. 35:4105–4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proskovec AL, Spooner RK, Wiesman AI, Wilson TW. 2020. Local cortical thickness predicts somatosensory gamma oscillations and sensory gating: a multimodal approach. Neuroimage. 214:116749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich T, Cassidy J, Menk J, Van Heest A, Krach L, Carey J, Gillick BT. 2017. Stability of stereognosis after pediatric repetitive transcranial magnetic stimulation and constraint-induced movement therapy clinical trial. Dev Neurorehabil. 20:169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J, Mirmiran M, Butler EE, Lin CY, Barnes PD, Kermoian R, Stevenson DK. 2007. Neonatal microstructural development of the internal capsule on diffusion tensor imaging correlates with severity of gait and motor deficits. Dev Med Child Neurol. 49:745–750. [DOI] [PubMed] [Google Scholar]
- Scheck SM, Pannek K, Fiori S, Boyd RN, Rose SE. 2014. Quantitative comparison of cortical and deep grey matter in pathological subtypes of unilateral cerebral palsy. Dev Med Child Neurol. 56:968–975. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. 2003. Mapping cortical change across the human life span. Nat Neurosci. 6:309–315. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Rex D, Kornsand D, Tessner KD, Jernigan TL, Toga AW. 2002. Mapping sulcal pattern asymmetry and local cortical surface gray matter distribution in vivo: maturation in perisylvian cortices. Cereb Cortex. 12:17–26. [DOI] [PubMed] [Google Scholar]
- Spooner RK, Eastman JA, Wiesman AI, Wilson TW. 2020a. Methodological considerations for a better somatosensory gating paradigm: the impact of the inter-stimulus interval. Neuroimage. 220:117048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner RK, Wiesman AI, Mills MS, O'Neill J, Robertson KR, Fox HS, Swindells S, Wilson TW. 2018. Aberrant oscillatory dynamics during somatosensory processing in HIV-infected adults. Neuroimage Clin. 20:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner RK, Wiesman AI, O'Neill J, Schantell MD, Fox HS, Swindells S, Wilson TW. 2020b. Prefrontal gating of sensory input differentiates cognitively impaired and unimpaired aging adults with HIV. Brain Commun. 2:fcaa080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson CM, Wang F, Brookes MJ, Zumer JM, Francis ST, Morris PG. 2012. Paired pulse depression in the somatosensory cortex: associations between MEG and BOLD fMRI. Neuroimage. 59:2722–2732. [DOI] [PubMed] [Google Scholar]
- Strauss D, Brooks J, Rosenbloom L, Shavelle R. 2008. Life expectancy in cerebral palsy: an update. Dev Med Child Neurol. 50:487–493. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J. 2006. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol. 51:1759–1768. [DOI] [PubMed] [Google Scholar]
- Teflioudi EP, Zafeiriou DI, Vargiami E, Kontopoulos E, Tsikoulas I. 2011. Somatosensory evoked potentials in children with bilateral spastic cerebral palsy. Pediatr Neurol. 44:177–182. [DOI] [PubMed] [Google Scholar]
- Thoma RJ, Hanlon FM, Huang M, Miller GA, Moses SN, Weisend MP, Jones A, Paulson KM, Irwin J, Cañive JM. 2007. Impaired secondary somatosensory gating in patients with schizophrenia. Psychiatry Res. 151:189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevarrow MP, Kleinsmith J, Taylor BK, Wilson TW, Kurz MJ. 2020. The somatosensory cortical activity in individuals with cerebral palsy displays an aberrant developmental trajectory. J Physiol. 599:1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi R, Agarwal S, Shah V, Goyel P, Paliwal VK, Rathore RK, Gupta RK. 2010. Correlation of quantitative sensorimotor tractography with clinical grade of cerebral palsy. Neuroradiology. 52:759–765. [DOI] [PubMed] [Google Scholar]
- Trivedi R, Gupta RK, Shah V, Tripathi M, Rathore RK, Kumar M, Pandey CM, Narayana PA. 2008. Treatment-induced plasticity in cerebral palsy: a diffusion tensor imaging study. Pediatr Neurol. 39:341–349. [DOI] [PubMed] [Google Scholar]
- Uusitalo MA, Ilmoniemi RJ. 1997. Signal-space projection method for separating MEG or EEG into components. Med Biol Eng Comput. 35:135–140. [DOI] [PubMed] [Google Scholar]
- Wiesman AI, Wilson TW. 2020. Attention modulates the gating of primary somatosensory oscillations. Neuroimage. 211:116610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Krägeloh-Mann I, Holland SK. 2007. Global and local development of gray and white matter volume in normal children and adolescents. Exp Brain Res. 178:296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingert JR, Burton H, Sinclair RJ, Brunstrom JE, Damiano DL. 2008. Tactile sensory abilities in cerebral palsy: deficits in roughness and object discrimination. Dev Med Child Neurol. 50:832–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotter RA, Dahnke R, Thompson PM, Gaser C. 2011. Topological correction of brain surface meshes using spherical harmonics. Hum Brain Mapp. 32:1109–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]


